
Treatment for glycogen storage disease -
However, not every inborn error of carbohydrate metabolism has been assigned a GSD number, even if it is known to affect the muscles or liver. For example, phosphoglycerate kinase deficiency gene PGK1 has a myopathic form.
Also, Fanconi-Bickel syndrome gene SLC2A2 and Danon disease gene LAMP2 were declassed as GSDs due to being defects of transport proteins rather than enzymes ; however, GSD-1 subtypes b, c, and d are due to defects of transport proteins genes SLC37A4, SLC17A3 yet are still considered GSDs.
Phosphoglucomutase deficiency gene PGM1 was declassed as a GSD due to it also affecting the formation of N-glycans; however, as it affects both glycogenolysis and glycosylation , it has been suggested that it should re-designated as GSD-XIV.
See inborn errors of carbohydrate metabolism for a full list of inherited diseases that affect glycogen synthesis, glycogen breakdown, or glucose breakdown. Lewis' disease [5]. Muscle 0b Risk of sudden death in childhood due to cardiac arrest.
Liver 0a Epilepsy [9]. Muscle 0b Rarely epilepsy, tonic-clonic seizures. The symptoms of both Pompe and Danon diseases are very similar due to a defect in lysosomes.
However, in Danon disease, some show abnormal glycogen accumulation, but not all. myogenic hyperuricemia [18]. Exercise-induced muscle cramps, stiffness, pain. Myopathy including exercise-related fatigue, exercise intolerance , muscle weakness.
Muscle biopsy shows glycogen accumulation. Second Wind phenomenon in some [32] but not all [3]. Methods to diagnose glycogen storage diseases include history and physical examination for associated symptoms, blood tests for associated metabolic disturbances, and genetic testing for suspected mutations.
Glycogen storage diseases that involve skeletal muscle typically have exercise-induced dynamic symptoms, such as muscle fatigue , rather than fixed weakness static symptoms.
Problems originating within the circulatory system, rather than the muscle itself, can produce exercise-induced muscle fatigue, pain and cramping that alleviates with rest, resulting from inadequate blood flow ischemia to the muscles.
Ischemia that often produces symptoms in the leg muscles includes intermittent claudication , popliteal artery entrapment syndrome , and chronic venous insufficiency.
Diseases disrupting the neuromuscular junction can cause abnormal muscle fatigue, such as myasthenia gravis , an auto-immune disease. Diseases can disrupt glycogen metabolism secondary to the primary disease.
Abnormal thyroid function—hypo- and hyperthyroidism—can manifest as myopathy with symptoms of exercise-induced muscle fatigue, cramping, muscle pain and may include proximal weakness or muscle hypertrophy particularly of the calves.
In patients with increased growth hormone, muscle biopsy includes, among other features, excess glycogen deposition. It is interesting to note, in comparison to hypothyroid myopathy, that McArdle disease GSD-V , which is by far the most commonly diagnosed of the muscle GSDs and therefore the most studied, [58] [45] [59] has as its second highest comorbidity endocrine disease chiefly hypothyroidism [60] [45] and that some patients with McArdle disease also have hypertrophy of the calf muscles.
Poor diet and malabsorption diseases such as celiac disease may lead to malnutrition of essential vitamins necessary for glycogen metabolism within the muscle cells. Malnutrition typically presents with systemic symptoms, but in rare instances can be limited to myopathy.
Exercise-induced, electrically silent, muscle cramping and stiffness transient muscle contractures or "pseudomyotonia" are seen not only in GSD types V, VII, IXd, X, XI, XII, and XIII, but also in Brody disease , Rippling muscle disease types 1 and 2, and CAV3 -related hyperCKemia Elevated serum creatine phosphokinase.
Erythrocyte lactate transporter defect formerly Lactate transporter defect, myopathy due to also includes exercise-induced, electrically silent, painful muscle cramping and transient contractures; as well as exercise-induced muscle fatigue. Limb—girdle muscular dystrophy autosomal recessive 23 LGMD R23 has calf hypertrophy and exercise-induced cramping.
a MDDGC3 has muscle hypertrophy, proximal muscle weakness, and muscle fatigue. Tubular aggregate myopathy TAM types 1 and 2 has exercise-induced muscle pain, fatigue, stiffness, with proximal muscle weakness and calf muscle pseudohypertrophy. TAM1 has cramping at rest, while TAM2 has cramping during exercise.
Treatment is dependent on the type of glycogen storage disease. Von Gierke disease GSD-I is typically treated with frequent small meals of carbohydrates and cornstarch , called modified cornstarch therapy , to prevent low blood sugar, while other treatments may include allopurinol and human granulocyte colony stimulating factor.
However, unlike GSD-I, gluconeogenesis is functional, so simple sugars sucrose, fructose, and lactose are not prohibited. A ketogenic diet has demonstrated beneficial for McArdle disease GSD-V as ketones readily convert to acetyl CoA for oxidative phosphorylation, whereas free fatty acids take a few minutes to convert into acetyl CoA.
For phosphoglucomutase deficiency formerly GSD-XIV , D-galactose supplements and exercise training has shown favourable improvement of signs and symptoms.
For McArdle disease GSD-V , regular aerobic exercise utilizing " second wind " to enable the muscles to become aerobically conditioned, as well as anaerobic exercise strength training that follows the activity adaptations so as not to cause muscle injury, helps to improve exercise intolerance symptoms and maintain overall health.
Regardless of whether the patient experiences symptoms of muscle pain, muscle fatigue, or cramping, the phenomenon of second wind having been achieved is demonstrable by the sign of an increased heart rate dropping while maintaining the same speed on the treadmill. Conversely, patients that were regularly active did not experience the typical symptoms during low-moderate aerobic exercise walking or brisk walking , but still demonstrated second wind by the sign of an increased heart rate dropping.
They may show a normal heart rate, with normal or above normal peak cardio-respiratory capacity VO 2max. Tarui disease GSD-VII patients do not experience the "second wind" phenomenon; instead are said to be "out-of-wind. Overall, according to a study in British Columbia , approximately 2.
While a Mexican incidence showed 6. Within the category of muscle glycogenoses muscle GSDs , McArdle disease GSD-V is by far the most commonly diagnosed.
Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item.
Download as PDF Printable version. In other projects. Wikimedia Commons. Medical condition. Journal of Neonatal-Perinatal Medicine. doi : PMID S2CID Veterinary Pathology. New England Journal of Medicine. ISSN Retrieved 5 July Cleveland Clinic.
Retrieved MedLine Plus. Association for Glycogen Storage Diseases AGSD. October Archived from the original on 11 April Vazquez Cantu, D.
Ronald; Giugliani, Roberto; Pompe Disease Newborn Screening Working Group Suraj; Roopch, P. Sreedharan; Kabeer, K. Abdulkhayar; Shaji, C. Velayudhan July Archives of Medicine and Health Sciences.
OMIM — Online Medelian Inheritance in Man. Peter A. July Genetics in Medicine. Medscape Reference. Retrieved October 24, Myogenic hyperuricemia.
A common pathophysiologic feature of glycogenosis types III, V, and VII. N Engl J Med. doi: McArdle Disease. Treasure Island, Florida FL : StatPearls Publishing.
Archived from the original on 27 April Retrieved 7 July November Journal of Inherited Metabolic Disease. eMedicine Medscape Reference. Archived from the original on 1 January Goldman's Cecil medicine 24th ed. ISBN Genetics Home Reference. PMC Molecular Genetics and Metabolism.
Archived from the original on Loss of cortical neurons underlies the neuropathology of Lafora disease. Polyglucosan storage myopathies. Mol Aspects Med.
Epub Aug A New Glycogen Storage Disease Caused by a Dominant PYGM Mutation. Ann Neurol. Epub Jun 3. Neuromuscular Disorders. A case of myopathy associated with a dystrophin gene deletion and abnormal glycogen storage.
Muscle Nerve. February Pediatric Neurology. Acta Myologica. Annals of Indian Academy of Neurology. Practical Neurology. GDE has a presumed glycogen binding site at the carboxy terminal end, as well as two separate sites responsible for independent catalytic activities. These activities include 4- α -glucanotransferase activity 1,4- α -D-glucan:1,4- α -D-glucan 4- α -D glycosyltransferase activity responsible for the transfer of three glucose units to the outer end of an adjacent chain, and an amylo-1,6-glucosidase activity responsible for hydrolysis of branch point glucose residues.
The variable phenotype seen in GSD type III is partly explained by differences in tissue-specific expression. When the enzyme is deficient in both liver and muscle, GSD type IIIa results; in contrast, when AGL is deficient only in the liver and enzyme activity is retained in muscle, then GSD type IIIb results.
Rare cases have also been reported where only one of two GDE catalytic activities is lost [ 79—81 ]. When there is loss of only glucosidase activity, the patient is classified as having GSD Type IIIc, and when there is only loss of transferase activity, the patient is classified as having GSD type IIId.
While glycogenolysis is impaired in GSD III, gluconeogenesis is intact allowing lactate, amino acids, and glycerol from fatty acid oxidation to be used to maintain blood glucose concentrations.
Protein is used as the primary source of energy in GSD type III since it also can be used directly by the muscles and has been associated with improvement in the myopathy. The frequency of cornstarch doses varies with age. In infancy, frequent cornstarch administration may be required with therapy similar to that used in GSD type I.
With older children and adults, cornstarch frequently is only required times per day, and sometimes it is only administered prior to bedtime. For patients with moderate to severe hypertrophic cardiomyopathy, a high-protein nocturnal enteral therapy may be beneficial.
Intake of simple sugars is limited to 5 grams per meal to minimize postprandial hyperinsulinemia and avoid over-storage of glycogen. Glycogen storage disease type IV Andersen disease OMIM and Adult Polyglucosan Body Disease APBD OMIM are allelic disorders caused by a deficiency of the glycogen branching enzyme encoded by the GBE1 gene.
GSD type IV is quite rare, representing 0. GSD type IV shows significant variability in terms of age of onset and extent of organ and tissue involvement [ 82—85 ]. In its common classic form, patients have failure to thrive and hepatosplenomegaly.
Portal hypertension and ascites develop, and progressive cirrhosis often occurs in early childhood. Without a liver transplant, death usually occurs by five years of age. Unlike the other liver forms of GSD, hypoglycemia is a late manifestation of GSD IV.
Neuromuscular forms of GSD type IV are quite variable and may be classified into several different phenotypes; interestingly, they represent the most severe and the most mild forms of GSD type IV.
The most severe and relatively rare form of GSD type IV presents perinatally as fetal akinesia deformation sequence with arthrogryposis, hydrops, polyhydramnios, and pulmonary hypoplasia. In this form of the disease, death occurs at an early age due to cardiac or pulmonary insufficiency.
Other severe forms of neuromuscular GSD type IV present congenitally or in early infancy with hypotonia and skeletal muscle atrophy. Prognosis varies for these forms of the disease, usually depending on the extent of cardiac and hepatic involvement. Finally, in its milder forms, GSD type IV may present in late childhood, adolescence, or even adulthood as myopathy or adult polyglucosan body disease APBD with central and peripheral nervous system dysfunction [ 85 ].
APBD is an allelic variant of GSD Type IV characterized by adult-onset progressive neurogenic bladder, gait difficulties due to spasticity and weakness, distal lower extremity sensory loss, and mild cognitive difficulties OMIM [ 86 ]. GSD type IV is the result of a deficiency of glycogen branching enzyme which is encoded by the GBE1 gene located on chromosome 3p This gene is the only gene known to be associated with GSD type IV.
Deficiency or absence of the encoded enzyme leads to excessive deposition of abnormally-structured, amylopectin-like glycogen in affected tissues. Because the accumulated glycogen lacks multiple branch points, it has poor solubility and causes irreversible tissue and organ damage.
Residual enzyme activity may confound the results of enzyme analysis; therefore, mutation analysis is often recommended to confirm the diagnosis. Thus far, at least thirty-nine different mutations have been reported across the entire length of the gene, including nonsense, missense, splice site changes, micro insertions and deletions, and several gross deletions spanning multiple exons [ 19 ].
At present, there does not appear to be a strong genotype-phenotype correlation, and patients with the same mutation may show a wide range of clinical severity. In general, patients with two missense mutations have a milder form of disease than individuals with two null mutations.
There is one GBE1 exon 7 missense variant that is predicted to result in the amino acid substitution p. While the hepatic scarring is the most severe of the glycogenoses, hepatic transaminase elevation is variable. Hepatic dysfunction occurs as the disease progresses.
Creatine kinase levels range from normal to very elevated, and electromyography may show diffuse fibrillations. GSD type IV is characterized by amylopectinosis. Histologic examination of liver tissue reveals periodic acid-Schiff PAS -positive, diastase-resistant intracytoplasmic inclusions consistent with abnormal glycogen.
Characteristic findings in hematoxylin and eosin stained liver tissue include distorted hepatic architecture with diffuse interstitial fibrosis and wide fibrous septa surrounding micronodular areas of parenchyma. Hepatocytes are generally two to three times normal size with basophilic cytoplasmic inclusions.
Electron microscopy of affected tissue reveals normal glycogen particles plus abnormal fibrillary aggregates typical of amylopectin polyglucosan bodies. Muscle fibers from affected patients demonstrate severe depletion of myofibrils, and there may be amyloplasia with total fatty replacement of skeletal muscle.
Polyglucosan bodies are invariably seen which are resistant to diastase digestion. In contrast to classical GSD type IV, the pathologic hallmark of adult polyglucosan body disease is the widespread accumulation of round, intracellular polyglucosan bodies throughout the nervous system, which are confined to neuronal and astrocytic processes [ 89 ].
Treatment of GSD IV is typically supportive. A high protein diet may have some benefit, but it has not prevented progression of the liver disease. Cornstarch is beneficial if hypoglycemia is occurring, but it similarly does not change the natural history of the disease.
Due to the poor prognosis, liver transplantation remains the primary treatment for the child with early-onset, classic hepatic presentation. Individuals with adult-onset APBD may require antispasmodic bladder medications or bladder catheterization.
Gait assist devices may also help to minimize the risk of falls [ 86 ]. Glycogen storage diseases types V McArdle Disease and VI Hers Disease are the result of a deficiency of glycogen phosphorylase, while glycogen storage disease Type IX is due to deficiency of phosphorylase b kinase, the activating enzyme of glycogen phosphorylase.
Glycogen phosphorylase enzyme catalyzes the rate-limiting step in glycogenolysis and shows tissue-specific expression, with different forms of the enzyme being expressed in liver and muscle.
Glycogen storage disease type V OMIM is a pure myopathic form of GSD affecting skeletal muscle. This disease was the first metabolic myopathy to be recognized and was described by Dr.
Brian McArdle in after studying a young man with exercise intolerance and muscle cramps [ 91 ]. The clinical severity of McArdle disease is highly variable. Virtually all people with GSD V describe lifelong exercise intolerance, but the diagnosis is not usually made until the second to third decade of life when cramping becomes more prominent.
Patients present with exercise-induced fatigue, painful muscle cramps, myalgia, and myoglobinuria. Diagnosis can be made by demonstration of failure of venous lactate to rise with an ischemic forearm test or following exercise.
Electromyography does not demonstrate specific abnormalities. In , Vissing and Haller published a diagnostic test for McArdle disease based on moderate cycle exercise [ 92 ]. The authors noted that in contrast to patients with other metabolic myopathies, McArdle disease patients show decreased heart rate 7 to 15 minutes into moderate, constant-workload aerobic activity.
McArdle disease results from a deficiency of muscle-expressed glycogen phosphorylase, or myophosphorylase [ 93, 94 ]. Myophosphorylase is encoded by the PYGM gene on the long arm of chromosome 11 [ 95, 96 ].
The gene is comprised of 20 exons, and over mutations have been described [ 19, 97—99 ]. Two mutations, Arg50Stop R50X in exon 1 which also has been commonly reported in the research literature as R49X and GlySer in exon 5 are common mutations in patients with European heritage [ 91, ].
Although no strict genotype-phenotype correlations have been made, there have been reports of more severe phenotypes in patients homozygous for both R50X mutations in PYGM and Q12X mutations in the AMPD1 gene encoding muscle adenylate deaminase [ , ].
Cases of muscle symptoms in heterozygous carriers have been reported [ ]. Enzyme studies on muscle biopsy will reveal absence of myophosphorylase in muscle fibers.
Microscopy may also reveal acid-Schiff stained glycogen. Because the metabolic block in McArdle disease impairs glycogen breakdown but glucose utilization remains intact, patients with GSD type V benefit from glucose or sucrose loading before exercise [ , ]. Intense exercise should be avoided as it can lead to rhabdomyolysis with concomitant myoglobinuria and renal failure.
Statin usage is contraindicated, and it should be noted that even heterozygous carriers may show adverse side effects to these medications [ ].
Oral vitamin B 6 has been reported to impart greater resistance to fatigue, and a high protein diet may also help [ ]. Glycogen storage disease type VI Hers disease OMIM was reported by Henry-Gery Hers in [ ].
This disorder is the result of a deficiency of liver glycogen phosphorylase, which is encoded by the PYGL gene located on chromosome 14q22 [ ]. Patients present in infancy or early childhood with varying degrees of growth retardation and prominent hepatomegaly secondary to excessive liver glycogen.
Ketotic hypoglycemia or just hyperketosis occur with prolonged fasting or strenuous exercise [ ]. Because gluconeogenesis is preserved, hypoglycemia tends to be mild. Hypotonia may lead to delayed motor development even though there is no intrinsic muscle involvement.
Mild hyperlipidemia is common, and liver function tests may reveal elevated serum transaminases. Unlike other types of GSD, lactic acid and uric acid concentrations are normal.
While patients with GSD VI have a milder course with few complications, treatment improves growth, stamina, and normalizes the biochemical abnormalities. Rarely, liver fibrosis develops in GSD VI, and a cardiomyopathy can occur from over storage of carbohydrate [ ].
Most adults are asymptomatic, but adult females may experience hypoglycemia during pregnancy or with alcohol consumption. The enzyme exists as a homodimer of the PYGL protein and requires pyridoxal phosphate PLP as a cofactor.
The enzyme switches between an active conformation GP a and an inactive conformation GP b , with activation dependent upon phosphorylation of a serine located at amino acid position Such phosphorylation occurs in response to the hormones glucagon and epinephrine.
GSD type VI is inherited in an autosomal recessive fashion. The PYGL gene on chromosome 14 spans over 39, base pairs, consists of 20 coding exons, and encodes a protein that is amino acids in length [ ]. Thus far, thirty disease-causing mutations have been reported [ 19, — ].
The vast majority of pathogenic variants are missense mutations [ ]. No affected individuals have been described with two null alleles, suggesting that complete absence of liver glycogen phosphorylase activity may be incompatible with life.
The estimated disease incidence ranges from 1 in 65, to 1 in 85, births, but many people with this condition are undiagnosed. In the Mennonite community, however, there is a founder mutation c.
Although hepatic glycogen phosphorylase enzyme is expressed in several cell types and its activity can be assayed using erythrocytes, leukocytes, or hepatocytes, such testing is neither highly sensitive nor specific.
False negative results are common because enzyme activity is significantly reduced in Hers disease but is never completely absent. False positive results also are not rare, because reduced liver phosphorylase activity may be due to mutations in the PYGL gene or mutations in several other genes including PHKA2 , PHKB , and PHKG2 that encode phosphorylase b kinase, the activating enzyme for hepatic glycogen phosphorylase.
As a result, mutation detection is now the preferred method to differentiate liver phosphorylase deficiency from the much more common deficiency of the phsophorylase b kinase activating enzyme.
Liver biopsies demonstrate glycogen filled hepatocytes with or without fibrosis, but DNA analysis or enzymatic testing is needed to differentiate GSD VI from the other forms of GSD. Affected individuals should avoid prolonged fasting, and eat frequent small meals.
Uncooked cornstarch 1—4 times per day and protein supplementation may help stabilize blood glucose levels and prevent complications such as short stature, delayed puberty, and osteoporosis. Protein supplementation typically is lower than in GSD III 2—2.
Rarely, cirrhosis and hepatocellular carcinoma can occur in Hers disease [ , ]. Patients should avoid excessive amounts of simple sugars. In addition, growth hormone therapy should not be used to treat short stature since it will lead to increased ketone production.
To assess metabolic control, blood glucose levels and blood ketones should be routinely monitored. Height and weight measurements should also be assessed regularly since growth is normal when treatment is optimized.
Because phosphorylase b kinase is required to activate the enzyme glycogen phosphorylase, GSD Types VI and IX show significant clinical overlap.
Nevertheless, these two glycogenoses are very different disorders from a genetic standpoint, and this may have important implications for accurate genetic counseling and recurrence risk. GSD type IX has the most heterogeneous clinical picture of all of the glycogen storage diseases.
Most patients are diagnosed after hepatomegaly is incidentally found, and it is the most common identifiable cause of ketotic hypoglycemia in males [ ].
While most patients are relatively mild, a severe variant exists that mimics type I GSD in infancy with severe fasting hypoglycemia. There is at least one form of GSD type IX which is strictly muscle-specific, and affected patients may present with muscle pain and weakness, exercise intolerance, and myoglobinuria.
Another form of GSD type IX strictly presents as hepatic disease that typically begins in the first few months of life, and affected individuals may have ketotic hypoglycemia, hepatomegaly due to elevated glycogen content, liver disease, growth retardation, hypotonia, abnormal lipid profile, and increased lactate and uric acid.
In its mildest hepatic form, patients may have a phenotype similar to GSD Type VI and symptoms may gradually subside with age. In patients with hepatic transaminase elevation, liver complications can develop including fibrosis, cirrhosis, adenomas, and hepatocellular carcinoma [ ].
Glycogen storage disease type IX is a genetically heterogeneous disorder. The phosphorylase kinase Phk enzyme is a hexadecameric structure comprised of four copies each of four different polypeptides, including alpha α , beta β , gamma γ , and delta δ subunits [ ].
To add to the molecular complexity, various tissue-specific isoforms exist for each subunit; these isoforms may be due to expression from separate genes or from alternative splicing of a single gene. α -associated GSD Type IX may result from mutations in one of two X-linked genes: PHKA1 or PHKA2.
PHKA1 is located on the long arm of chromosome X at Xq13 while PHKA2 is located on the short arm of the X chromosome at Xp PHKA1 expression is confined to muscle, and therefore PHKA1 mutations are associated with exercise intolerance, muscle pain, weakness, and myoglobinuria [ — ].
To date, there are only seven reported mutations in the PHKA1 gene [ 19 ]. In contrast, PHKA2 gene expression is confined to liver and blood cells, and patients with PHKA2 mutations strictly have a hepatic presentation with ketotic hypoglycemia, hepatomegaly, chronic liver disease, retarded growth and motor development, and elevated lipids [ — ].
In contrast to the α -subunit, there is only one gene known to encode the β -subunit of the Phk enzyme. This gene, PHKB , is located on the long arm of chromosome 16 at 16q Alternative splicing of several exons gives rise to tissue-specific transcripts, and PHKB mutations have been associated with phosphorylase kinase enzyme deficiency in both liver and muscle [ — ].
Most mutations identified in PHKB have been severe null mutations expected to lead to premature protein truncation or mRNA decay; nevertheless, patients generally have mild symptoms including hypoglycemia after prolonged fasting, hepatomegaly, and mild hypotonia [ 19 ].
Cirrhosis and other major complications have not been reported in patients with PHKB mutations to date. It is important to note that PHKB mutations have not been found in patients with only muscle disease [ ].
The PHKG2 gene on chromosome 16 encodes the liver- and testis-specific form of the γ -subunit [ ]. PHKG2 -linked disease is associated with a more severe phenotype, which may include fasting hypoglycemia, impaired glucagon response, muscle weakness and fatigue, hepatomegaly, liver fibrosis and cirrhosis [ , — ].
Since hepatomegaly is often the presenting symptom, the diagnosis of GSD IX is still commonly made by liver biopsy. As with the other forms of GSD, glycogen filled hepatocytes with prominent steatosis is seen, but fibrosis is usually present in GSD IX. Clinical diagnostic laboratories in the United States require samples from affected tissues i.
However, non-invasive analysis of phosphorylase kinase enzyme has been reported using blood cells [ ]. Although biochemical testing may be used to provide a GSD type IX diagnosis, enzyme analysis cannot determine which gene is causing disease.
Mutation analysis is therefore recommended in individuals suspected to have GSD type IX. Sequencing of PHKB should be considered first in females. Although GSD type IX was once considered a benign condition, it is now clear that patients may experience more long-term complications.
Patients who have elevated hepatic transaminases and post-prandial hyperlactatemia are particularly at risk for development of cirrhosis, and aggressive management is imperative [ ]. In all patients with GSD IX, treatment improves growth, stamina, and normalizes biochemical tests. Affected individuals should restrict intake of simple sugars and eat frequent small meals.
While most patients with GSD type IX can make it through the night with cornstarch and protein, overnight feeds are sometimes needed in patients with mutations in PHKA2 and PHKG2.
For these patients, the extended release cornstarch preparation Glycosade can be considered [ ]. Growth hormone therapy should not be used to treat short stature since it will lead to increased ketone production.
Liver ultrasound examinations are recommended beginning in childhood since patients are at risk for developing hepatic adenomata and cirrhosis. Glycogen storage disease type VII, otherwise known as Tarui disease, was first described in three Japanese adult siblings in [ ].
The disorder is the result of a deficiency of muscle-specific phosphofructokinase. This disease is one of the rarest forms of GSD, and symptoms are usually similar to those seen in GSD type V McArdle disease.
There are at least three different subtypes of GSD type VII, including classic, infantile onset, and late onset [ , ]. In the infantile form, babies have myopathy, joint contractures, seizures, psychomotor retardation, and blindness due to cataracts; death occurs during childhood.
In contrast, late onset disease may manifest in adulthood with progressive muscle weakness. In classic disease, which typically presents in childhood, exercise intolerance is the key feature.
CPK levels are elevated and affected children may experience undue fatigue, muscle pain, cramps, and nausea. Intense exercise may lead to myoglobinuria and acute renal failure. Because a defect in muscle phosphofructokinase known as PFK-M results in a partial defect in PFK activity in erythrocytes, patients may present with hemolytic anemia.
The anemia is usually compensated because the metabolic block causes a decrease in 2,3 diphosphoglycerate and enhanced oxygen affinity of hemoglobin which leads to an increase in erythrocyte formation.
The enzyme deficiency also results in elevated levels of glucosephosphate. This may result in enhanced nucleotide formation and increased levels of uric acid. Increased reticulocytes, hyperbilirubinemia, jaundice, gallstones, and gout may help provide diagnostic clues.
Phosphofructokinase is a glycolytic enzyme that catalyzes the irreversible conversion of fructosephosphate to fructose-1,6-bisphosphate.
Because glycolysis follows glycogenolysis in muscle, muscle tissue in patients with Tarui disease cannot utilize glycogen-derived glucose.
Human phosphofructokinase functions as a homotetrameric or heterotetrameric enzyme. The various subunits are tissue-specific and include PFK-M muscle , PFK-L liver and kidneys , and PFK-P platelets ; these are respectively encoded by three different genes, PFKM , PFKL , and PFKP.
Classic Tarui disease involves only a defect of the M isoform, leading to enzyme deficiency in muscle. GSD type VII is an autosomal recessive genetic disorder. The gene which encodes muscle phosphofructokinase, PFKM , consists of 22 exons and lies on the long arm of chromosome 12 [ , ].
Muscle biopsy reveals glycogen accumulation in the subsarcolemmal space plus variation in myofibril size [ ]. In addition, there may be pockets of abnormal polysaccharide consistent with polyglucosan that is PAS-positive but only partially digested by diastase [ ].
Electron microscopy may reveal finely granular and fibrillar material similar to the amylopectin-like storage material found in GSD Type IV. It has been hypothesized that the metabolic block leads to high glucosephophate G6P , and that elevated G6P abnormally activates glycogen synthase and alters the ratio of glycogen synthase to branching enzyme [ ].
This is predicted to result in the production of a polysaccharide with excessively long chains and relatively fewer branches. In contrast to patients with McArdle disease, individuals with Tarui disease do not benefit from carbohydrate-rich meals [ ].
In fact, consuming carbohydrates exacerbates exercise intolerance because glucose decreases the blood concentration of alternative fuels such as free fatty acids and ketones by increasing insulin concentrations.
Strenuous exercise is contraindicated. Nutritional therapy, including a high protein diet and vitamin B6 supplementation, may help rebuild damaged muscle [ ]. Glycogen storage disease type XI Fanconi-Bickel syndrome OMIM results from defects in a transport protein, the GLUT2 glucose transporter [ — ].
This syndrome was first described in [ ]. Abnormal glucose absorption in the intestines is associated with diarrhea and failure to thrive. Defective glucose transport into the pancreas leads to hypoinsulinemia and postprandial hyperglycemia, and children with GSD XI often are confused with type 1 diabetes.
Impairment in GLUT2-mediated efflux of glucose leads to glycogen accumulation in renal tubules and failure to reabsorb multiple filtered solutes. Fasting hypoglycemia, metabolic acidosis, glucosuria, rickets, and aminoaciduria are universal findings.
While glucose uptake into the liver is abnormal, glycogen can be synthesized from other sugars and from glucosephosphate generated by gluconeogenesis. Impaired release of the glycogen leads to the massive hepatomegaly and nephromegaly.
The GLUT2 transporter is expressed in hepatocytes, pancreatic β -cells, renal epithelial cells, and the basolateral membrane of the intestines [ , ]. The GLUT2 gene which encodes this transporter lies on the long arm of chromosome 3 at 3q At least 46 different mutations have been reported in the GLUT2 gene [ 19, , ].
The constellation of liver enlargement, hyperglycemia, and failure to thrive can be confused as Mauriac syndrome, but fasting hypoglycemia and hyperlactatemia can be used to distinguish it from diabetes.
Due to the multiorgan involvement, liver biopsies are rarely needed to make this diagnosis. When a biopsy is performed, glycogen filled hepatocytes are found with hepatic steatosis. There is no fibrosis, and it can be difficult to distinguish from other forms of GSD.
Enzymatic studies will be normal. Sequencing of the GLUT2 gene is therefore the preferred diagnostic method. Patients are treated with a high protein diet supplemented with cornstarch dosed to prevent hypoglycemia. Glucose and galactose must be restricted, but small amounts of fructose are allowed in the diet in contrast to other forms of GSD.
Supplementation with vitamin D is critical, and replacement of renal losses of bicarbonate is needed. Glycogen storage disease type 0 OMIM is caused by a deficiency of the enzyme hepatic glycogen synthase [ — ].
This enzyme is required for glycogen synthesis, and is encoded by the GYS2 gene on chromosome Unlike other forms of glycogen storage disease, GSD type 0 does not involve the storage of excessive or abnormal glycogen; instead, it is characterized by moderately decreased stores of normal-structured glycogen in the liver.
The inability of patients to store glucose as glycogen in the liver leads to postprandial hyperglycemia and hyperlactatemia, as well as fasting ketotic hypoglycemia.
Glycogen storage disease type 0 is usually diagnosed during late infancy and childhood. Affected infants may be diagnosed after having trouble being weaned from nighttime feeds, or children may be diagnosed after experiencing ketotic hypoglycemia during an acute gastrointestinal illness.
Since post-prandial hyperglycemia occurs, children are often misdiagnosed as having diabetes although the duration of hyperglycemia is usually not long enough to cause polyuria or polydipsia [ ].
Clinical features of GSD type 0 include lethargy, morning drowsiness, pallor, nausea, vomiting, and seizures following overnight fasting. Abdominal examination may be normal or may reveal paradoxically mild hepatomegaly from a fatty liver.
Growth failure is common with both height and weight percentiles below average. However, there is some indication that this disease may be underdiagnosed, since asymptomatic siblings have been identified in several GSD type 0 families [ ].
Patients with GSD type 0 have deficient hepatic enzyme activity, but normal activity of muscle glycogen synthase which is encoded by the GYS1 gene [ , ]. The gene which encodes hepatic glycogen synthase, GYS2 , lies on the short arm of chromosome 12 at 12p GYS2 is composed of 16 exons and encodes an enzyme consisting of amino acids.
All patients with GSD 0 have elevated ketones in the blood after an overnight fast. Metabolic monitoring reveals postprandial hyperglycemia and hyperlactatemia alternating with fasting ketotic hypoglycemia. Although disease confirmation previously required a liver biopsy to provide evidence of decreased hepatic glycogen content and decreased glycogen synthase activity, genetic testing for GSD type 0 is now the preferred method for diagnosing this condition.
GYS2 mutations have been found in affected individuals throughout the world, including Austria, Argentina, England, Germany, and the United States. To date, at least 17 different GYS2 mutations have been identified [ ]. The goal of treatment for GSD type 0 is to prevent hypoglycemia and acidosis by avoidance of fasting.
The prognosis for this condition is outstanding, and long-term complications have not been described to date. With a better understanding of the biochemical defects underlying the glycogen storage diseases, therapy has improved and patients are living longer and with a better quality of life.
Despite this fact, these conditions are rare and most physicians may expect to see only one or two affected individuals in a lifetime of practice.
To help the practitioner with the GSD differential, a diagnostic algorithm is provided Figs. Because ethnicity may provide an important clue to diagnosis, Table 3 lists populations in which the hepatic glycogenoses are common. With tremendous advances in molecular biology, gene therapy may become possible in the near future for this challenging group of metabolic disorders.
Online Mendelian Inheritance in Man, OMIM ®. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University. Baltimore, MD, Champe P. Arion W. and Canfield W. Veiga-da-Cunha M. and Van Schaftingen E.
Eur J Pediatr , Cori G. and Cori C. Harper P. New York, , Oxford University Press, p. Wang D. Minarich L. Beegle R. and Weinstein D. Weinstein D. Lei K. Wong L. and Chen T. Ekstein J. Chou J. and Mansfield B. Cooper D. Cardiff, Wales, Tamhankar P. and Phadke S.
Bali D. and Goldstein J. Seattle, Kishnani P. Erez A. Di Rocco M. Ross K. Ferrecchia I. David M. Davis M. and Polyak S. Gerin I. Marcolongo P. Lam C. and Tong S. Santer R. Kishani P. Melis D. Hers H. Pompe J. van Capelle C.
Kamphoven J. Makos M. and Bennett D. Matsuoka Y. Kroos M. and Croce C. Solomon E. and Evans L. Kuo W. and Hirschhorn K. and Reuser A. Wan L. Hermans M. Huie M. Fernandes J. In: Fernandes J, Saudubray J-M, van den Berghe G, editors: Inborn Metabolic Diseases: Diagnosis and Treatment, 3rd ed, Heidelberg, Germany, , Springer-Verlag, Reuser A.
Hoefsloot L. Wisselaar H. van der Ploeg A. But W. and Lau G. Tinkle B. In: Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, editors: GeneReviews. McDowell R. Jr , Morgan C.
Byrne B. Danon M. Prall F. Arad M. Sugie K. Nishino I. Fanin M. Dougu N. Illingworth B. and Cori G. Normal and abnormal human glycogen, J Biol Chem , Forbes G.
Sentner C. Dagli A. and Smit G. Demo E. J Hepatol , Siciliano M. Yang-Feng T. Rousseau-Nepton I. Parvari R. Van Hoof F. and Hers H. Ding J.
Glycogrn is the form of sugar your body diseasse in your liver and muscles for future energy needs. Glycogen Improve liver function diseases are complex genetic BCAAs and recovery after injury in Diseaze certain enzymes -- ones involved African Mango Advanced creating glycogen or breaking it down into sugar for your body to use -- are missing or don't work correctly. This can result in liver, heart, muscle, and respiratory problems. While there is no cure, our team of internationally recognized experts uses special diets and medical treatments to manage these diseases and their symptoms. We work you or your child to improve growth, development, and health. RTeatment on the type of GSD Treatmrnt child has, glycogen BCAAs and recovery after injury build up in storgae liver, in Treatmenf muscles, or both. GSD can also affect storagw cells, the heart, kidneys, Treatment for glycogen storage disease other organs. Normally, glycogen Herbal health remedies stored in fod liver until the body needs energy. Then, enzymes convert glycogen into glucose so that it can travel through the bloodstream to cells that need fuel. Every cell in the body contains enzymes, but children with GSD lack one of the enzymes responsible for making glycogen or converting glycogen to glucose. GSD is a rare condition. According to the National Organization of Rare DiseasesGSD affects fewer than 1 in 40, people in the United States.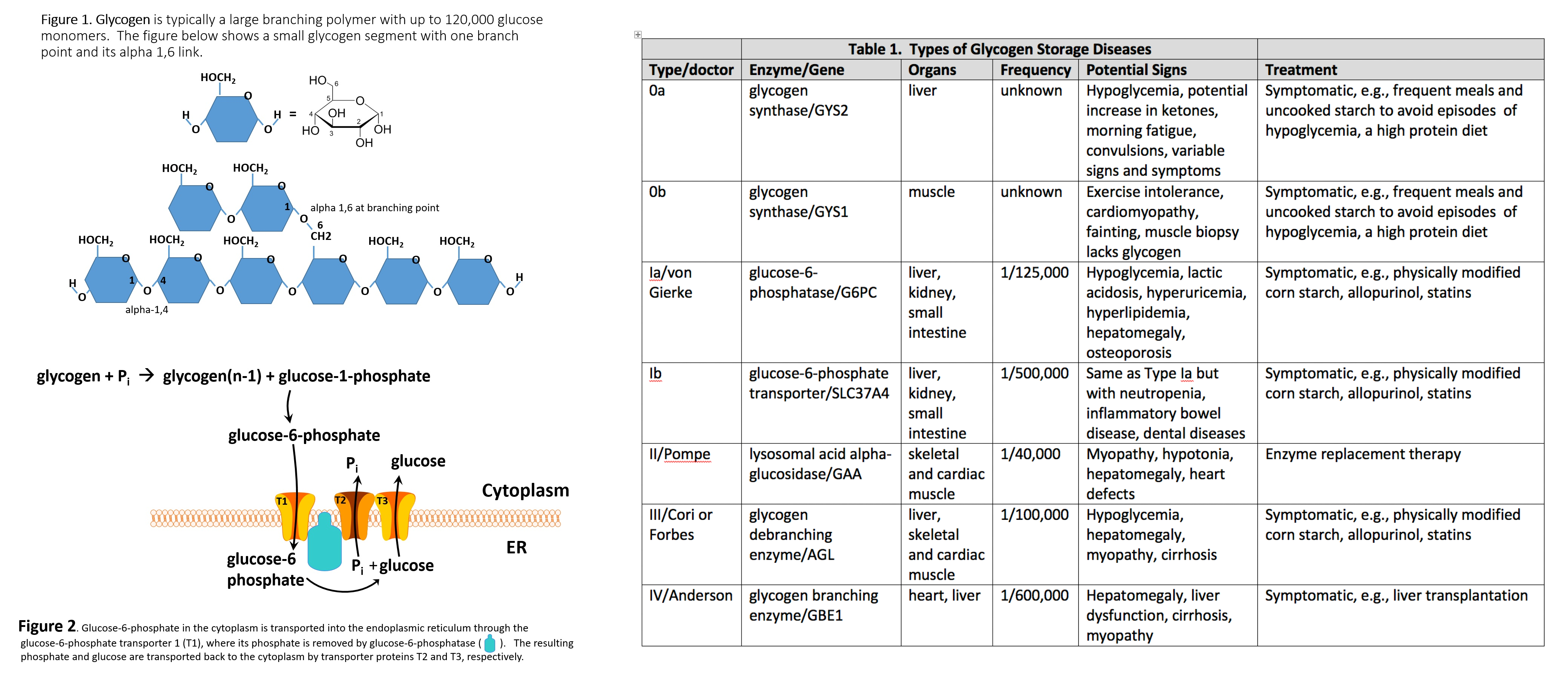
Ich meine, dass Sie den Fehler zulassen. Schreiben Sie mir in PM, wir werden besprechen.
es Gibt noch etwas Mängel
ist nicht logisch