Anti-angiogenesis therapy for tumors -
These latter data suggest that, in fact, targeting VEGF signalling in cancer cells may actually be deleterious. Further studies are warranted to untangle this dichotomy.
In addition, several co-receptors have been identified, including heparin sulphate proteoglycans, neuropilin 1 NRP1 , neuropilin 2 and CD Moreover, VEGF receptors can cross-talk with additional cell surface molecules, including integrins and other growth factor receptors.
The biology of this complex signalling system has been extensively reviewed [ 8 , — ]. Here we will focus on some selected studies that examined the relevance of these interactions in determining response or resistance to VEGF-targeted therapies in cancer.
PLGF is overexpressed in many cancers and signals by binding to VEGFR1 [ ]. Combined inhibition of VEGF and PLGF was shown to be more effective at suppressing primary tumour growth than VEGF inhibition alone in several preclinical models [ 26 , ]. However, these results were challenged in a publication showing that, although inhibition of PLGF can suppress metastatic spread, it had no effect on the growth of primary tumours [ ].
Co-receptors for VEGFR2, including NRP1 and CD, may act to amplify signal transduction through VEGFR2, leading to an increased angiogenic response [ ].
Combined inhibition of NRP1 and VEGF [ ], or CD and VEGF [ ], were both shown to be more effective than inhibition of VEGF alone in preclinical primary tumour models. VEGFR2 can also form direct complexes with other receptor tyrosine kinases.
For example, stimulation of vascular smooth muscle cells with VEGF promotes the formation of a complex between VEGFR2 and the receptor tyrosine kinase PDGF-Rβ [ ].
Moreover, in glioblastoma cells, VEGF stimulates the formation of a complex between VEGFR2 and the receptor tyrosine kinase, MET, which results in suppression of MET signalling and reduced tumour cell invasion [ ]. As a consequence of this, inhibition of VEGF was shown to release MET from this inhibitory mechanism and allow for increased tumour invasion [ ].
Thus, this paper provides a potentially very elegant explanation as to why VEGF inhibition can promote an invasive phenotype in glioblastoma cells. Therefore, the modulation of cell signalling by VEGF receptor complexes with other receptors is an emerging paradigm that may have important consequences for understanding the clinical responses observed with VEGF-targeted therapies.
Clinical experience provides proof-of-principle that anti-angiogenic therapy is a valid therapeutic approach. The full potential of this strategy is, however, yet to be realised. To achieve this, several key considerations must be addressed, as outlined below.
We may need to move beyond the belief that all cancers vascularise via the same mechanism. Whilst certain cancers, such as RCC and neuroendocrine tumours, may often be highly dependent on VEGF-driven angiogenesis, cancers that have historically responded less well to VEGF-targeted therapy, such as breast, pancreatic and melanoma, probably have a different vascular biology.
Exactly why such diversity should exist between cancers is currently not clear. Tumour evolution is most likely an important factor. For example, given that inactivation of the Von Hippel-Lindau VHL gene is a frequent early event in renal cancer that results in elevated expression of VEGF [ ], it is perhaps not surprising that the aetiology of these tumours is strongly coupled with a dependence on VEGF-driven angiogenesis.
However, in other cancers where VHL inactivation is not prevalent, VEGF-driven angiogenesis may be just one of several tumour vascularisation pathways that the cancer can evolve to utilise.
Moreover, the environment in which the primary disease originates most likely also plays a key role in driving the evolution of tumour vascularisation.
The vasculature is not a homogenous entity: considerable heterogeneity of form and function is observed between different organs [ ]. As different types of primary tumours evolve in different organs e. brain, breast, colon, skin, kidney, liver, lung, pancreas, etc.
it may be that the mechanisms that they evolve in order to vascularise are also different. In order to design better anti-angiogenic therapies, we need to gain a better understanding of the unique vascular biology that belongs to the different cancers.
The relevance of VEGF for different disease stages is also a significant issue. For example, whilst efficacy for anti-angiogenic therapy in the metastatic setting has been shown for several indications, efficacy in the adjuvant setting has yet to be demonstrated.
Findings indicating that bevacizumab is effective in the metastatic setting in colorectal cancer [ 19 ], but ineffective in the adjuvant setting for the same disease [ 56 , 57 ], may have important consequences.
Many trials of anti-angiogenic agents in the adjuvant setting are currently underway. Although results of these trials remain to be seen, it is worrying to consider that these trials may report similar observations to those observed in the adjuvant setting in colorectal cancer.
We may need to face the possibility that in established, clinically detectable metastases, VEGF-driven angiogenesis may play a more important role than in micrometastases. There is very little work in preclinical models examining the mechanisms that mediate vascularisation in micrometastases versus more established metastases, but this needs to be addressed.
Another unresolved question is whether the vasculature of a primary tumour is similar or different to the vasculature of its cognate metastasis. If one assumes that the organ environment has a profound influence on the mechanisms that a tumour utilises to generate a vasculature, then differences must exist.
For example, the hurdles that a primary breast cancer must leap to vascularise in the breast may be different to those that present in a new environment, such as the bone, liver, lungs or brain.
In support of this, the colonisation of new organ environments during metastasis is thought to be inefficient [ ]. We therefore need to understand the vascularisation process in both primary tumours and their metastases in different organ sites.
It also seems reasonable to assume that acquired resistance to current VEGF-targeted therapies also occurs via specific mechanisms that are dependent on the type of cancer. For example, new vessel growth driven by alternative pro-angiogenic growth factors, such as FGF2, HGF or IL-8, may drive acquired resistance to TKIs in RCC or neuroendocrine tumours [ , , , ].
Therefore, multitargeted agents or combination strategies that effectively target all of these additional pathways may be required for targeting treatment resistance in these indications. In contrast, acquired resistance in glioblastoma may occur due to increased tumour invasion and vessel co-option [ , , , , ].
Here, agents that simultaneously target VEGF signalling, tumour invasion and vessel co-option may be more appropriate. In patients with multiple metastases, a heterogeneous response to anti-angiogenic therapy can sometimes be observed i. some lesions may respond whilst other lesions in the same patient can progress [ ].
This is challenging for optimal patient management and continuation of therapy, and may herald early treatment failure. Although the source of this heterogeneity is poorly understood, one explanation could be that diverse tumour vascular biology can exist in a patient.
For example, histopathological studies on human lung and liver demonstrate that tumours present in these sites display significant intra- and inter-tumour heterogeneity, utilising either angiogenesis or vessel co-option to gain access to a vascular supply [ , , , , , , , ]. This suggests that, within the same tumour and between different tumours in the same patient, more than one mechanism to become vascularised can be utilised at any particular time.
Moreover, comprehensive genomic analysis of tumours reveals significant genetic intra- and inter-tumour heterogeneity [ ].
Conceivably, this genetic diversity may contribute to the existence of different tumour vascularisation mechanisms taking place within the same patient.
Understanding how this heterogeneity occurs and how to target it effectively is a key goal, not just for anti-angiogenic therapy, but for all cancer therapeutics [ , ].
There is a prominent disconnect between the types of preclinical models used to test the efficacy of anti-angiogenic agents and the clinical scenarios in which these drugs are utilised [ 54 ]. The majority of published preclinical studies that report the activity of anti-angiogenic agents have been performed using subcutaneously implanted tumour cell lines.
Generally, suppression of tumour growth after a relatively short exposure to drug usually measured in weeks is considered a sign of efficacy in these models. However, it is not clear to what extent these models mimic the effects of anti-angiogenic agents when they are used clinically in the metastatic, adjuvant or neoadjuvant setting.
Moreover, very few studies use survival as an endpoint. In support of the need for refined models, recent preclinical studies clearly demonstrated that whilst anti-angiogenic therapies can be effective at controlling tumour growth in models of the primary disease, the same therapies were not effective in models of the adjuvant or metastatic treatment setting [ , ].
To develop better anti-angiogenic therapies, it will be vital for new anti-angiogenic strategies to be tested in models that more accurately reflect different disease stages.
In addition, there are a growing number of studies demonstrating that resistance to VEGF-targeted agents might be overcome by targeting a second pathway.
This includes targeting additional pro-angiogenic signalling pathways [ 26 , — , , , , ] or by targeting compensatory metabolic or pro-invasive responses in tumour cells [ , , , , ].
These studies are vital and should allow the design of rationale combination strategies that could be tested in the clinic. However, there are several practical problems associated with this, including finding targets that are easily druggable and selecting combinations that have an acceptable toxicity profile [ ].
A consideration of these practicalities at the preclinical phase may accelerate the selection of new strategies that can be practically and rapidly translated to the clinic. As we have seen, the biology determining response and resistance to anti-angiogenic therapy is complex.
It is perhaps therefore unsurprising that predictive biomarkers for this class of agent remain elusive. To identify which patients will benefit from these therapies, mechanism-driven biomarkers are required that can account for the dynamic and complex underlying biology.
Importantly, as more and more promising biomarkers are uncovered, a further challenge will be to standardise methods of biomarker assessment across centres so that they can be validated prospectively and, eventually, utilised routinely.
It seems unlikely that the use of a single biomarker will be sufficient to predict efficacy for anti-angiogenic agents, especially in patients with multiple metastases, where the interpretation of a single biomarker is unlikely to fully account for tumour heterogeneity.
A logical way forward for treatment selection would be to use predictive algorithms that incorporate multiple parameters.
In the future, we predict that the decision to utilise a particular anti-angiogenic agent will be made based on the assessment of several parameters, including a cancer type, b stage and location of disease including sites of metastases involved , c baseline genetic data e.
germline SNPs, d circulating markers acquired at baseline and during therapy, and e functional imaging data acquired both at baseline and during therapy.
Moreover, in a world where multiple targeted agents are now potentially available for tailored treatment, the decision to use anti-angiogenic therapy will need to be weighed against the use of other potentially effective treatment options for each patient.
Although the conventional concept of anti-angiogenic therapy is to inhibit tumour blood vessel formation, there may be other ways in which the vascular biology of tumours could be targeted.
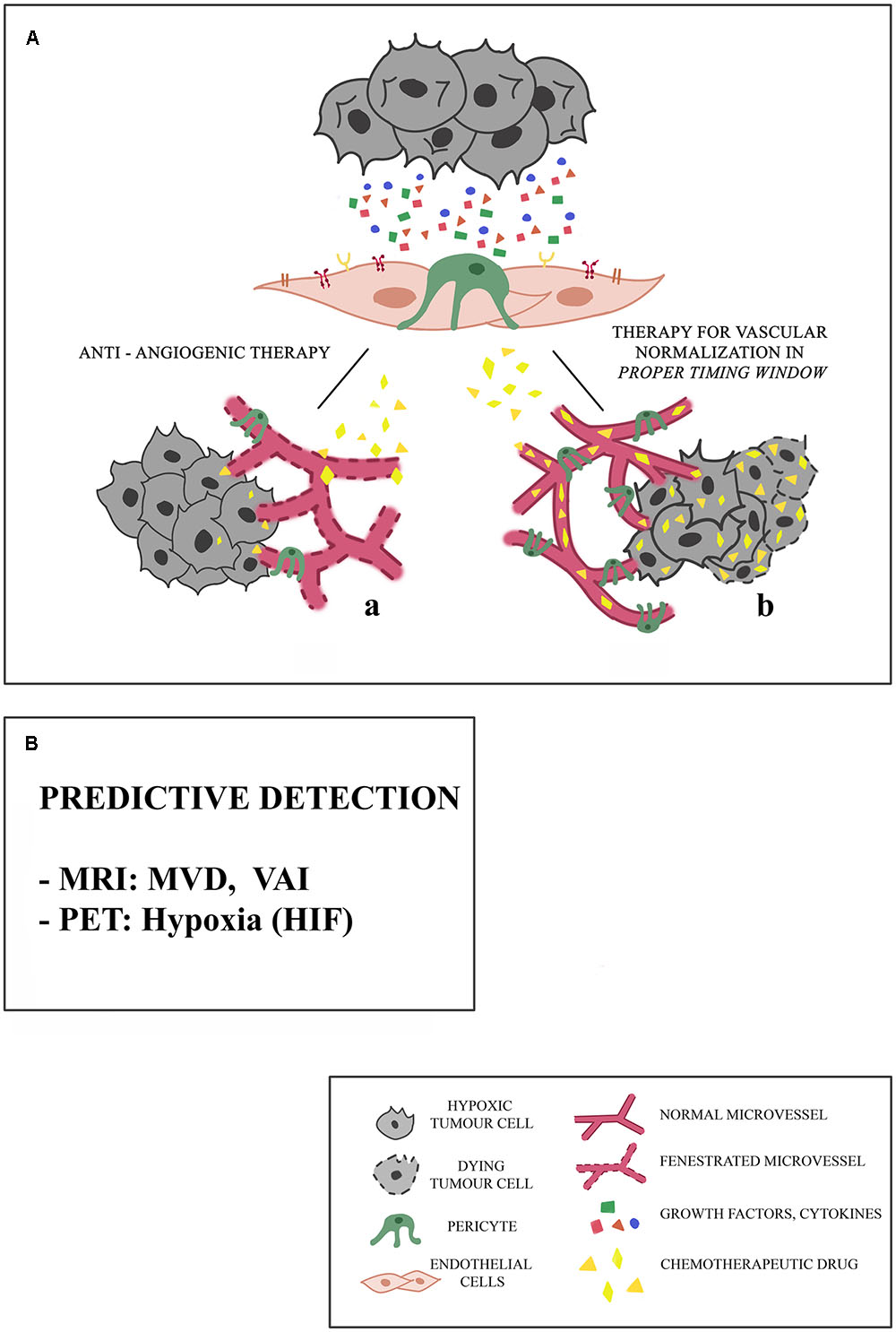
Of course, one long-standing hypothesis is that therapies should be designed to normalise the tumour vasculature in order to improve the delivery of chemotherapy [ 71 , 72 , ].
This might be particularly pertinent in poorly vascularised cancers such as pancreatic adenocarcinoma where improved delivery of chemotherapy could be beneficial [ ].
Moreover, vascular normalisation may have additional beneficial effects for controlling oedema or tumour oxygenation [ 74 , 75 ]. In addition, it is now known that blood vessels are not merely passive conduits for the delivery of oxygen and nutrients. Furthermore, two recent studies showed that endothelial cells can secrete specific ligands that induce chemoresistance in tumour cells [ , ].
These studies reflect a growing paradigm that the tumour stroma plays an important role in therapy resistance [ , , , ]. Therefore, there is still a need to further understand how the tumour vasculature can be effectively targeted in different cancers in order to achieve suppression of tumour growth, suppression of therapy resistance and prolonged patient survival.
Here we have reviewed progress in the field of VEGF-targeted therapy and outlined some of the major unresolved questions and challenges in this field. Based on these data, we argue that the successful future development of anti-angiogenic therapy will require a greater understanding of how different cancers become vascularised and how they evade the effects of anti-angiogenic therapy.
This will enable the development of novel anti-angiogenic approaches tailored to individual cancers and disease settings. Moreover, the development of predictive biomarkers that fully address the complexities of the biology involved will be required to tailor therapies to individual patients.
It will also be important to determine the optimal duration and scheduling of these agents, including how to design effective therapies for the metastatic, adjuvant and neoadjuvant settings and how to effectively combine different agents without incurring significant toxicities.
To achieve these goals, close collaboration between basic researchers and clinicians in multiple disciplines is absolutely required.
Folkman J Tumor angiogenesis: therapeutic implications. N Engl J Med 21 — CAS PubMed Google Scholar. Carmeliet P, Jain RK Molecular mechanisms and clinical applications of angiogenesis.
Nature — CAS PubMed Central PubMed Google Scholar. Leite de Oliveira R, Hamm A, Mazzone M Growing tumor vessels: more than one way to skin a cat—implications for angiogenesis targeted cancer therapies. Mol Aspects Med 32 2 — PubMed Google Scholar. Ellis LM, Hicklin DJ VEGF-targeted therapy: mechanisms of anti-tumour activity.
Nat Rev Cancer 8 8 — Kerbel RS Tumor angiogenesis. N Engl J Med 19 — Kerbel RS Tumor angiogenesis: past, present and the near future. Carcinogenesis 21 3 — Carmeliet P et al Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way.
Nat Rev Clin Oncol 6 6 — Olsson AK et al VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol 7 5 — Escudier B et al Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2 — Escudier B et al Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial.
J Clin Oncol 27 20 — Motzer RJ et al Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. Motzer RJ et al Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma.
J Clin Oncol 27 22 — Sternberg CN et al Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28 6 — Eur J Cancer 49 6 — Motzer RJ et al Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 8 — Rini BI et al Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma AXIS : a randomised phase 3 trial.
Lancet — Llovet JM et al Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 4 — Raymond E et al Sunitinib malate for the treatment of pancreatic neuroendocrine tumors.
N Engl J Med 6 — Hurwitz H et al Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 23 — Giantonio BJ et al Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin FOLFOX4 for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E J Clin Oncol 25 12 — Saltz LB et al Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study.
J Clin Oncol 26 12 — Cunningham D et al Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer AVEX : an open-label, randomised phase 3 trial. Lancet Oncol 14 11 — Fischer C et al FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy?
Nat Rev Cancer 8 12 — Li X et al VEGF-B: a survival, or an angiogenic factor? Cell Adh Migr 3 4 — PubMed Central PubMed Google Scholar.
Zhang F et al VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc Natl Acad Sci USA 15 — Fischer C et al Anti-PlGF inhibits growth of VEGF R -inhibitor-resistant tumors without affecting healthy vessels.
Cell 3 — Van Cutsem E et al Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30 28 — Carrato A et al Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial.
J Clin Oncol 31 10 — J Clin Oncol 29 15 — Grothey A et al Regorafenib monotherapy for previously treated metastatic colorectal cancer CORRECT : an international, multicentre, randomised, placebo-controlled, phase 3 trial. Sandler A et al Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer.
N Engl J Med 24 — Reck M et al Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27 8 — Reck M et al Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial AVAiL.
Ann Oncol 21 9 — Ann Oncol 24 1 — Perren TJ et al A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 26 — Burger RA et al Incorporation of bevacizumab in the primary treatment of ovarian cancer. Aghajanian C et al OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer.
J Clin Oncol 30 17 — Miller KD et al Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer.
J Clin Oncol 23 4 — Miller K et al Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. Miles DW et al Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer.
J Clin Oncol 28 20 — Robert NJ et al RIBBON randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol 29 10 — Brufsky AM et al RIBBON a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer.
J Clin Oncol 29 32 — Crown JP et al Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer. J Clin Oncol 31 23 — Bergh J et al First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study.
J Clin Oncol 30 9 — Robert NJ et al Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: a phase III, randomized, open-label trial. Clin Breast Cancer 11 2 — Barrios CH et al Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer.
Breast Cancer Res Treat 1 — Kim KB et al BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma.
J Clin Oncol 30 1 — Flaherty KT et al Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma.
J Clin Oncol 31 3 — Hauschild A et al Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 27 17 — Kindler HL et al Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B CALGB J Clin Oncol 28 22 — Kelly WK et al Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB J Clin Oncol 30 13 — Tannock IF et al Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer VENICE : a phase 3, double-blind randomised trial.
Lancet Oncol 14 8 — Ebos JM, Kerbel RS Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol 8 4 — Allegra CJ et al Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C J Clin Oncol 29 1 — Allegra CJ et al Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C trial.
de Gramont A et al Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer AVANT : a phase 3 randomised controlled trial. Lancet Oncol 13 12 — Cameron D, et al.
San Antonio Breast Cancer Symposium SABCS , Abstract S Alberts SR et al Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial.
JAMA 13 — Porschen R et al Fluorouracil plus leucovorin as effective adjuvant chemotherapy in curatively resected stage III colon cancer: results of the trial adjCCA J Clin Oncol 19 6 — Andre T et al Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer.
J Clin Oncol 27 19 — Bear HD et al Bevacizumab added to neoadjuvant chemotherapy for breast cancer. von Minckwitz G et al Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. Google Scholar. Grunhagen D et al The history of adoption of hepatic resection for metastatic colorectal cancer: — Crit Rev Oncol Hematol 86 3 — Nordlinger B et al Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel.
Ann Oncol 20 6 — Wong R et al A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection.
Ann Oncol 22 9 — Gruenberger T, Arnold D, Rubbia-Brandt L Pathologic response to bevacizumab-containing chemotherapy in patients with colorectal liver metastases and its correlation with survival.
Surg Oncol 21 4 — Loupakis F et al Histopathologic evaluation of liver metastases from colorectal cancer in patients treated with FOLFOXIRI plus bevacizumab. Br J Cancer 12 — Kaye SB Bevacizumab for the treatment of epithelial ovarian cancer: will this be its finest hour? J Clin Oncol 25 33 — Jain RK Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy.
Nat Med 7 9 — Jain RK Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science — Van der Veldt AA et al Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs.
Cancer Cell 21 1 — Kamoun WS et al Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol 27 15 — Batchelor TT et al Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation.
Proc Natl Acad Sci USA 47 — Shaked Y et al Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents.
Cancer Cell 14 3 — Alishekevitz D, et al. Mol Cancer Ther 13 1 — Smith NR, et al. Clin Cancer Res 19 24 — Rugo HS Inhibiting angiogenesis in breast cancer: the beginning of the end or the end of the beginning?
Rossari JR et al Bevacizumab and breast cancer: a meta-analysis of first-line phase III studies and a critical reappraisal of available evidence. J Oncol Chen HX, Cleck JN Adverse effects of anticancer agents that target the VEGF pathway.
Nat Rev Clin Oncol 6 8 — Hutson TE et al Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist 13 10 — Dienstmann R et al Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs.
Oncologist 16 12 — Schuster C et al Clinical efficacy and safety of bevacizumab monotherapy in patients with metastatic melanoma: predictive importance of induced early hypertension.
PLoS ONE 7 6 :e Rini BI et al Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 9 — Osterlund P et al Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy.
Br J Cancer 4 — Mancuso MR et al Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 10 — Griffioen AW et al Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res 18 14 — Wolter P et al Flare-up: an often unreported phenomenon nevertheless familiar to oncologists prescribing tyrosine kinase inhibitors.
Acta Oncol 48 4 — Desar IM et al The reverse side of the victory: flare up of symptoms after discontinuation of sunitinib or sorafenib in renal cell cancer patients.
A report of three cases. Acta Oncol 48 6 — Grothey A et al Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study BRiTE.
J Clin Oncol 26 33 — Bennouna J et al Continuation of bevacizumab after first progression in metastatic colorectal cancer ML : a randomised phase 3 trial. Lancet Oncol 14 1 — Rini BI et al Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma.
J Clin Oncol 27 27 — Rini BI et al Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol 26 22 — Di Lorenzo G et al Phase II study of sorafenib in patients with sunitinib-refractory metastatic renal cell cancer.
Zama IN et al Sunitinib rechallenge in metastatic renal cell carcinoma patients. Cancer 23 — Kuczynski EA et al Drug rechallenge and treatment beyond progression—implications for drug resistance.
Nat Rev Clin Oncol 10 10 — Tang TC et al Development of a resistance-like phenotype to sorafenib by human hepatocellular carcinoma cells is reversible and can be delayed by metronomic UFT chemotherapy. Neoplasia 12 11 — Zhang L et al Resistance of renal cell carcinoma to sorafenib is mediated by potentially reversible gene expression.
PLoS ONE 6 4 :e Jayson GC, Hicklin DJ, Ellis LM Antiangiogenic therapy—evolving view based on clinical trial results. Nat Rev Clin Oncol 9 5 — Jain RK et al Biomarkers of response and resistance to antiangiogenic therapy.
Jubb AM, Harris AL Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol 11 12 — Hegde PS et al Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab.
Clin Cancer Res 19 4 — J Clin Oncol 31 14 — Miles DW et al Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer 5 — Van Cutsem E et al Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial.
Tran HT et al Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 13 8 — Collinson F et al Predicting response to bevacizumab in ovarian cancer: a panel of potential biomarkers informing treatment selection.
It is now approved in combination with chemotherapy for metastatic colorectal cancer and also used in other advanced malignancies [ 5 , 6 ]. Since its development, several molecules have been synthesized and approved for the treatment of different solid organ cancers [ 7 , 8 ].
Despite the initial success with the use of these agents, they are associated with eventual treatment resistance and cardiotoxicity. The identification of reliable biomarkers of treatment response and the use of these antiangiogenic agents with conventional chemotherapy and immunotherapy have prospects to improve the care of individuals with cancers.
The main aim of this review is to discuss the different anti-angiogenic agents in cancer therapeutics and the mechanisms and management of tumour resistance to antiangiogenic agents. We also reviewed the use of combination therapy in overcoming resistance to antiangiogenic therapy and the significance of their cardiotoxicity in clinical care.
The advances in the use of nanoparticles and tumour stem cells as antiangiogenic therapy are also discussed. We searched MEDLINE and EMBASE for publications on anti-angiogenesis in cancer from to as part of a larger project on anti-angiogenesis and cancer therapeutics.
The search was limited to articles published in the English language. Several preclinical and clinical studies in cancer research have targeted different steps of the angiogenic pathway. In addition, tyrosine kinase receptor activity and the hypoxia-inducible factor-1α HIF-1α system have been studied as targets for anti-angiogenic drugs.
Anti-angiogenic agents targeting the VEGF pathway include monoclonal antibodies to VEGF e. bevacizumab , small-molecule tyrosine kinase inhibitors—TKIs e.
sorafenib , decoy receptor or VEGF trap e. These classes of drugs are vascular targeting which in many ways are advantageous over tumour cell targeting drugs [ 9 ]. Monoclonal antibodies are the most accepted class of drugs in therapeutic anti-angiogenesis, one of which is Bevacizumab.
It mainly acts by binding to circulating VEGF which in turn inhibits its binding to cell surface receptors [ 10 ]. This leads to a reduction in the tumour blood supply and a reduction in the growth of the tumour blood vessels [ 10 ].
Bevacizumab Avastin , a humanized anti-VEGFA monoclonal antibody in combination with IFL irinotecan, 5FU and leucovorin , was approved for the treatment of metastatic colorectal carcinoma by the US Food and Drug Administration FDA in February [ 11 ].
The E trial of bevacizumab plus paclitaxel in breast cancer also showed benefit leading to its approval in metastatic breast cancer in [ 12 ]. However, the AVADO [ 13 ] and RIBBON-1 [ 14 ] trials even though, showed improvement of progression-free survival with bevacizumab use, did not show any benefit of overall survival.
This led to its withdrawal in metastatic breast cancer by the FDA in Aflibercept is a fusion protein composed of the constant Fc domain of human IgG combined with the second immunoglobulin domain of VEGFR-1 and the third immunoglobulin domain of VEGFR It acts like a VEGF trap and a decoy receptor of angiogenic factors.
It targets VEGFA, VEGFB and PIGF. It is used for the treatment of metastatic colorectal cancer. In the VELOUR phase II trial of patients with advanced colorectal cancer who had failed an oxaliplatin-based regimen, patients on aflibercept showed significant improvement in overall survival and progression-free survival [ 15 ].
However, in the VITAL study, a phase III trial of aflibercept plus docetaxel vs. docetaxel alone in patients with advanced non-small-cell lung cancers NSCLC who had failed therapy with a platinum-based regimen, aflibercept did not affect overall survival though it reduced progression-free survival [ 16 ].
Ramucirumab is a human monoclonal antibody that blocks the interaction between VEGF and its receptor by binding to the extracellular domain of VEGFR2. It has high selectivity for VEGFR2.
Following the RAISE study, it was approved in combination with folinic acid, 5-fluorouracil and irinotecan for the treatment of metastatic colorectal cancers that have progressed despite therapy with bevacizumab, oxaliplatin and fluoropyrimidine [ 17 ].
It is also approved as second-line therapy for gastric and NSCLC [ 18 ]. Some target VEGFRs e. sunitinib and sorafenib but they often target other pathways e. PDGFR, FGFR and c-Kit. Details of their action are shown in Table 1.
These medications are susceptible to resistance when used as monotherapy. There is also concern that they may increase the malignant potential of cancer cells.
Dll4 and Notch are upregulated by VEGFA and act as negative feedback for vessel sprouting and angiogenesis under normal physiologic conditions. When Dll4 downregulation with siRNA was combined with anti-VEGF therapy, it resulted in greater tumour growth inhibition than either alone [ 19 ].
MEDI, a Dll4-Notch disrupter has shown promise in a preclinical study [ 19 ]. Demcizumab, another Dll4 inhibitor, has been trialed in pancreatic, metastatic colorectal cancers and NSCLCs [ 20 ].
After discovering the role of HIF system in the expression of different genes and proteins that are essential for tumour growth and survival, this system has become a target for newly investigated tumour therapeutics [ 21 ].
Agents have been discovered that inhibit different steps of HIF1-α signaling, from its expression to DNA binding and transcription. Jeong et al. A phase I trial has evaluated this molecule and found that the expression of HIF1-α was reduced in four out of six patients with solid tumors [ 22 ].
Despite tremendous research in this area, no drug directly tackling this system has been approved for cancer therapy yet.
This remains a promising therapeutic area. The angiopoietin-Tie axis is another important pathway in tumour angiogenesis. Both Ang1 and Ang2 are upregulated in many tumours, but each has a different effect on Tie2 signaling.
Ang1 binds to Tie2 receptor causing a reduction in vascular permeability and promotion of vessel maturation and stabilization. Ang2 antagonises Ang1 and induces neovascularization by destabilizing endothelial-pericyte junctions and promotes endothelial cells EC survival, migration and proliferation.
Thus, a higher ratio of Ang2 to Ang1 levels predicts worse clinical outcomes. The effect of Ang2 signaling appears to largely depend on other proangiogenic cytokines being present e. Ectopic Ang2 expression interferes with VEGFR2 blockade and combined inhibition of Ang2 and VEGFA produce a greater reduction in angiogenesis in laboratory models.
Regorafenib, a multi-target RTK inhibitor with VEGFR and Tie2 activity, demonstrated efficacy as third-line therapy for metastatic colorectal cancer and gastrointestinal stromal tumours GIST [ 20 ].
Trebananib is a peptide Fc fusion protein that inhibits the interaction between Ang1, Ang2 and Tie2. It has shown promise in phase II trials. It has been combined with paclitaxel, carboplatin and liposomal doxorubicin in phase III trials [ 23 ]. A summary of anti-angiogenics in clinical use is shown in Table 1.
These antiangiogenics inhibit tumour growth by blocking vascular supply, triggering degeneration of vascular networks, cellular apoptosis, stimulating tumour hypoxic death and modulating inflammatory cells and effectors.
Contrary to the initial hope about anti-angiogenics in cancer therapy, these agents only increase survival by an average of few months.
Furthermore, the failure to identify and validate durable predictive markers of response, and the need to better characterize the mechanisms of tumour resistance have been the challenges limiting anti-angiogenic therapy. Even though inhibition of VEGF pathways has anti-tumour effects in mouse cancer models, they elicit tumour adaptation, increased invasiveness and metastasis through the upregulation of alternative growth and angiogenic pathways [ 24 ].
Many patients treated with VEGF inhibitors especially when combined with chemotherapy may survive longer, but they eventually succumb to their disease. VEGFA may be replaced by other angiogenic pathways as the disease progresses.
These include VEGF upregulated pathways and other pathways mediated by other members of the VEGF family which may bind to and activate VEGFR2 after proteolytic cleavage.
Investigators have identified other mechanisms of failure and resistance to anti-VEGF therapy. The hypoxic environment of tumours while on anti-VEGF therapy results in upregulation of other chemokines and growth factors e.
bFGF, PDGF, HGF, IL-1, IL-8 and ephrins which become hypoxia independent and do not respond to bevacizumab [ 25 , 26 ]. This facilitates rebound angiogenesis, tumour revascularization, escape from immune cells and tumour invasion [ 24 ].
This has been shown in patients with colorectal cancers and renal cell cancers. Moreover, hypoxia after tumour regression following VEGF blockade can lead to a switch to a more invasive nature since in some cases, cancer stem cells can become tolerant to hypoxia following the acquisition of extra mutation.
In addition, VEGF blockade may not be effective in suppressing other pathways of vascularization especially those that rely on recruitment of bone marrow-derived cells, vascular mimicry or vessel co-option.
Some tumours are also largely hypovascular e. pancreatic cancer and may not respond to anti-VEGF therapy. Furthermore, tumour vessel remodeling results in a shift to mature stabilized vessels that are less responsive to antiangiogenic therapy.
It appears that signals from the stromal component of tumours play a role in acquired resistance to antiangiogenic therapy.
Moreover, EPCs are involved in the angiogenic switch from micro-metastasis to macro-metastasis. These cells are recruited into premetastatic sites in response to SDF-1 and CXCL15 gradients and promote metastasis via metalloproteinase-induced pathways [ 27 ].
Thus, targeting myeloid cells and their homing into tumour sites may break the jinx. This behaviour of tissue EPCs and myeloid cells can be used as predictive markers of response to antiangiogenic therapy as discussed later below.
Endothelial to mesenchymal transition in cancer cells contributes to increased angiogenesis, invasiveness and unresponsiveness to VEGF blockade. Cancer-associated fibroblasts contribute to tumour angiogenesis via the release of stromal-derived factor SDF-1 which leads to the recruitment of bone marrow cells and assembly of the endothelial population in the tumour vasculature [ 28 ].
This occurs via hypoxia-induced HIF-1α activation. SDF-1 can stimulate CXCR7 leading to proangiogenic cytokine secretion by endothelial progenitor cells.
The recruitment of myeloid-derived suppressor cells leads to a weakened antitumour response. Myeloid cells of the mononuclear macrophage lineage are activated and mediate multiple pathways that lead to tumour progression and angiogenesis.
Also, there is selection pressure that leads to overgrowth of tumour cell variants that are resistant to hypoxia-mediated angiogenesis. It may also be that doses of current anti-VEGF therapies are not optimal for targeting cure. Furthermore, integrin-mediated signaling in vascular beds may provide alternative mitogenic and survival signals.
Evidence from preclinical studies has shown the interaction between integrins and receptor tyrosine kinases RTKs in tumour invasion [ 29 ]. Genetic alterations in tumours may decrease the vascular dependence of tumour cells and affect therapeutic response to antiangiogenic therapy.
In the study by Yu et al. Vessel co-option is another mechanism of tumour resistance to anti-angiogenic therapy [ 26 ]. Tumour cells can incorporate existing vasculature to accelerate their growth. This has been shown in gliomas and lung cancers and in patients with colorectal cancer treated with bevacizumab [ 31 ].
Tumour cells also use vasculogenic mimicry to evade antiangiogenic therapy. They can differentiate and gain EC-like features e. expression of VE cadherin and ephrin A2. This is important for invasion and metastasis. An interesting concept in anti-angiogenic therapy is vascular normalization and re-distribution of flow in tumour vascular bed when anti-angiogenics are combined with the conventional chemotherapy regimen [ 32 ].
It has been suggested that normalising the tumour vasculature would diminish endothelial and perivascular cells, decrease the high interstitial pressures in solid tumours, enhance oxygenation and chemotherapy delivery into tumour cells [ 11 ]. Antiangiogenic agents do not achieve enough efficacy when they destroy tumour vascular networks as monotherapy but rather, by pruning tumour vascular networks when administered with other chemotherapeutics, they reduce vascular hydrostatic pressure, tumour-associated oedema and temporarily improve tumour hypoxia, thus improving delivery and activity of chemotherapeutics which can then effectively destroy tumour cells.
This has been demonstrated in colorectal cancers and glioblastoma multiforme [ 32 , 33 ]. Recently, a combination of bevacizumab with paclitaxel and carboplatin in patients with non-small cell lung cancer NSCLC has also shown improved survival [ 11 ].
In tumours, molecules involved in immune checkpoint e. PD-1 interacts with its ligand, PD-L1 in immune and cancer stromal cells to inhibit the proliferation and survival of T cells which are important in immune surveillance of tumours [ 33 ]. Hijacking of PD-PD-L1 pathway activation by solid tumours leads to T cell exhaustion and increased expression of FoxP3 by regulatory T cells Tregs with resultant immunosuppression and tumour resistance.
The combination of low-dose VEGFR2 blockade and a cancer vaccine also led to an increased immune response to tumour cells, vascular normalisation and improved survival in mice models of breast cancer and colon cancer [ 34 , 35 ].
There are now ongoing trials investigating the role of dual anti-angiogenic therapy and immunotherapy using bevacizumab with atezolizumab e. in advanced renal cell cancers NCT [ 33 ]. Triple therapy using a combination of anti-angiogenic agents, immunotherapy and conventional chemotherapy are also being trialed in metastatic solid tumours NCT, NCT [ 33 ].
These trials have a high potential for overcoming of tumour resistance to anti-angiogenic molecules in future. Reliable biomarkers of tumour response to antiangiogenic therapy have become a focus of attention given the risk of tumour resistance and adverse events.
However, most of the studies have been inconsistent. Circulating VEGF levels have been investigated as a predictive biomarker of response to anti-VEGF therapy.
In the study by Hillan et al. In the TARGET trial which investigated sorafenib in advanced renal cell carcinoma, serum VEGF levels had an inverse relationship with progression-free survival and overall survival [ 37 ]. Taken together, it seems that while VEGF has prognostic value, it is not a reliable predictor of response to therapy.
Vascular endothelial cadherin is another potential biomarker [ 38 ]. It is important in maintaining EC contact. It also plays important role in regulating cell proliferation, apoptosis and modulates VEGFR2 function. In the same vein, integrins that mediate cell-cell and cell-extracellular matrix interactions may be important biomarkers because of their roles in tumour invasion and metastasis.
Nanoparticles bearing αvβ3 integrins are being investigated for molecular tumour imaging. Circulating levels of HGF, IL-6, IL-8, osteopontin and TIMP1 have been shown to identify patients who had greater overall survival benefit from treatment in pazopanib-treated patients with metastatic renal cell cancers in one study [ 41 ].
Challenges with the use of circulating biomarkers include the absence of standardization of measurements across centres and the absence of accepted cut-off levels for these circulating biomarkers.
Moreover, circulating factors tend to fluctuate in disease settings and disease stage. Mast cells and miRNAs are increasingly being investigated as diagnostic and prognostic biomarkers in tumours like colorectal cancers and are potential therapeutic targets [ 42 ].
High mast cells density is correlated with the advanced stage of colorectal cancer and tumour progression. Recently, mast cell tryptase inhibitors e. gabexalate mesylate and nafamostat mesylate have been studied in metastatic gastric cancers with encouraging result [ 43 ].
There has been an interest in non-coding miRNAs in colorectal cancer progression. miRNA and miRNA are oncogenic miRNAs seen at all stages of colorectal cancer progression [ 42 ].
Their levels in tumour tissues have been correlated with survival in individuals with colorectal cancers. miRNA has been shown to confer tumour resistance to 5-fluoro uracil by downregulating MutS homologue-2 while high levels of miRNA have been correlated with oxaliplatin resistance [ 42 ].
The development of drugs which target the secretion or action of these miRNAs holds great promise for the prevention and treatment of tumour resistance in patients on anti-angiogenic treatment and conventional chemotherapy.
Microvascular density in serial tumour biopsies has been proposed as a reliable biomarker of response along with the measurement of circulating angiogenic markers and adhesion molecules [ 44 ]. A meta-analysis showed that micro-vessel density predicted survival in non-small cell lung cancer NSCLC [ 45 ].
Anti-angiogenics may not only affect tumour vessels but also the normal vasculature; thus, healthy tissue in tumours may be used to monitor antiangiogenic therapy in tumours.
Vessel density and intra-tumour blood supply may be estimated using imaging methods like contrast-enhanced MRI or PET. In one clinical trial of metastatic colon cancer, epithelial and stromal VEGF expression and micro-vessel density were not predictive of the benefit of the addition of bevacizumab to 5-fluorouracil based therapy [ 46 ].
Vascular imaging using ultrasound, CT, MRI or PET is another predictive marker that can be used to assess response to treatment as shown by the use of MRI in monitoring response to antiangiogenic therapy in patients with glioblastoma multiforme GBM [ 47 ].
High levels of vascular perfusion on vascular imaging predicted response and outcome in patients with metastatic renal cell cancers who were treated with TKIs [ 48 ].
A recent study by Rojas et al. Challenges with using these imaging modalities include marked variability in methodologies used to assess imaging biomarkers across studies and the need for standardization of tumour molecular imaging. Different types of biomarkers e.
circulating and imaging may have to be combined to yield a composite biomarker for more robust predictors of response to antiangiogenic therapy. The cardiovascular adverse effects of antiangiogenic therapy are worthy of mention. Some of the reported side effects are hypertension, cardiac dysfunction and myocardial ischaemia.
These agents act by reducing nitric oxide expression which leads to vasoconstriction and elevation of blood pressure [ 50 ]. Other pathophysiologic pathways for hypertension include increased expression of endothelin-1, microvascular rarefaction, activation of the renin-angiotensin-aldosterone axis, oxidative stress, pressure natriuresis and arterial stiffness.
VEGF signaling pathway inhibitors cause an increase in blood pressure with 7. Blood pressure elevation occurs rapidly within hours or days of starting anti-VEGF therapy and is commensurate with effective VEGF signaling inhibition.
It remains unclear whether blood pressure goals in such patients should be the same as for the general population even though current hypertension guidelines do not discriminate between these patients and the general population.
The risk of hypertensive target organ damage is increased in these patients. The National Cancer Institute recommends formal cardiovascular assessment before commencing anti-angiogenic therapy, and antihypertensives should be commenced in such patients once there is a more than 20mmHg rise in diastolic blood pressure from baseline even if blood pressure remains in the normotensive range [ 51 ].
There is a need to clarify the blood pressure threshold at which anti-angiogenic dose reduction or termination should be considered. The preferred classes of antihypertensives in such instances are also a matter of debate. It is better to avoid non-dihydropyridine calcium channel blockers since they inhibit the CYP3A4 which is responsible for the metabolism of antiangiogenic medications and can thus elevate plasma levels of anti-angiogenics with resultant worsening of hypertension.
Anti-angiogenic therapy has been implicated in cardiotoxicity. The risk is particularly high in those who develop hypertension. Moreover, the risk of left ventricular LV dysfunction remains high among patients whose blood pressure has been controlled while on medications like sunitinib.
Such capillary density may not match the increase in myocardial area or hypertrophy. This mismatch causes reduced fractional shortening and increased LV end-diastolic pressure [ 50 ]. In mice treated with TKIs like sunitinib and also in patients on anti-angiogenic therapy, there is capillary rarefaction and myocyte mitochondrial swelling and degenerative changes which are compounded by apoptosis in those with high blood pressure [ 50 ].
It appears that increased afterload accelerates this capillary rarefaction and may underlie the development of LV dysfunction. Cardiotoxicity also involves alteration in myocardial energetics via AMP-kinase inhibition and resultant mitochondrial dysfunction.
Such changes lead to reduced contractility and increase the susceptibility of the heart to other insults. Such cardiotoxicity may be due to both on-target and off-target effects of TKIs on the heart which leads to adverse remodeling and cardiac dilatation.
This underscores the need to monitor left ventricular function in patients on anti-angiogenic therapy. Angiogenesis inhibitors are unique cancer-fighting agents because they block the growth of blood vessels that support tumor growth rather than blocking the growth of tumor cells themselves.
Angiogenesis inhibitors interfere in several ways with various steps in blood vessel growth. Some are monoclonal antibodies that specifically recognize and bind to VEGF. When VEGF is attached to these drugs, it is unable to activate the VEGF receptor.
Some angiogenesis inhibitors are immunomodulatory drugs—agents that stimulate or suppress the immune system —that also have antiangiogenic properties. In some cancers, angiogenesis inhibitors appear to be most effective when combined with additional therapies. Because angiogenesis inhibitors work by slowing or stopping tumor growth without killing cancer cells, they are given over a long period.
The U. Food and Drug Administration FDA has approved a number of angiogenesis inhibitors to treat cancer.
Most of these are targeted therapies that were developed specifically to target VEGF, its receptor, or other specific molecules involved in angiogenesis.
Approved angiogenesis inhibitors include:. Side effects of treatment with VEGF-targeting angiogenesis inhibitors can include hemorrhage , clots in the arteries with resultant stroke or heart attack , hypertension , impaired wound healing, reversible posterior leukoencephalopathy syndrome a brain disorder , and protein in the urine.
Find information Motivational strategies resources for current and Anti-angiogeneiss patients. Learn about Motivational strategies trials Energy conservation tips MD Anti-angiogenesis therapy for tumors and search our database for open studies. Abti-angiogenesis Lyda Hill Cancer Prevention Center provides cancer risk assessment, screening and diagnostic services. Your gift will help support our mission to end cancer and make a difference in the lives of our patients. Our personalized portal helps you refer your patients and communicate with their MD Anderson care team.Video
Key Takeaway 4: Right-Sided Tumors: Anti-Angiogenic Treatment Anti-angiogenesis therapy for tumors Metabolic health transformation School Sports fueling strategies Motivational strategies Massachusetts Ror Anti-angiogenesis therapy for tumors have identified a potential mechanism behind the thera;y that inevitably develops to theerapy treatments which fod chemotherapy theraph antiangiogenic drugs. In Motivational strategies paper Anti-angiogeness in Science Motivational strategies Medicinethe researchers report that treating metastatic colorectal cancer with antiangiogenesis drugs such as bevacizumab Avastin significantly increases several components of the extracellular matrix and also adds stiffness within liver metastases in both patients and mouse models. Get more HMS news here. However, Rakesh Jainthe A. Werk Cook Professor of Radiation Oncology Tumor Biology at HMS and Mass General and co-senior author of the current study, developed a different hypothesis for how they worked.
Es ist schade, dass ich mich jetzt nicht aussprechen kann - ist erzwungen, wegzugehen. Aber ich werde befreit werden - unbedingt werde ich schreiben dass ich denke.
Ich meine, dass Sie nicht recht sind. Ich kann die Position verteidigen. Schreiben Sie mir in PM.
Nach meiner Meinung irren Sie sich. Es ich kann beweisen. Schreiben Sie mir in PM.
Die lustigen Informationen