Video
Diet for Crohn's Disease, IBD, and IBS: 5 Tips for Reducing Symptoms and Achieving RemissionCurcumin and Inflammatory Bowel Disease -
So, while not much of it gets into the blood and into body tissue and organs, it is present at active levels in the intestinal tract , which might make it useful for digestive disease. One reason why curcumin has been considered as an area for study is because it may have an effect on some of the mechanisms of disease activity in IBD.
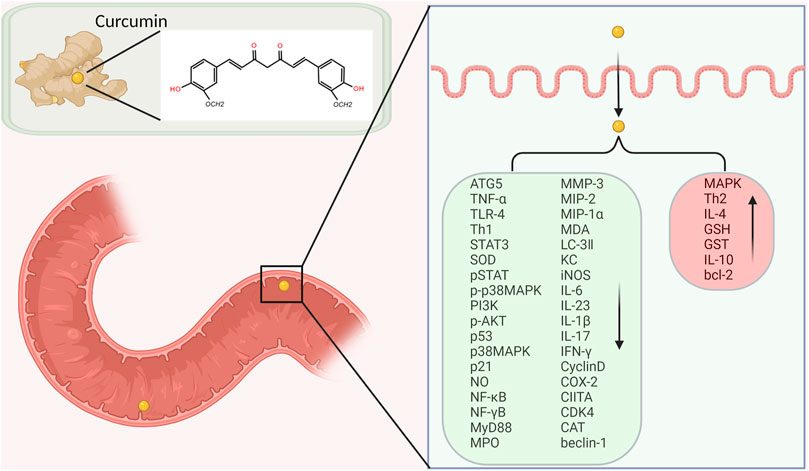
Curcumin has also been shown to suppress tumor necrosis factor TNF. In the intestinal tract, curcumin may also have an effect on the NF-κB pathway. The inflammation in IBD may be partially tied to the activation of the NF-κB pathway. This pathway has been shown to be the start of some of the immune dysregulation that causes inflammation associated with IBD.
Curcumin may disrupt this pathway and prevent the next steps in the process that continues on to cause persistent inflammation.
One review study looked at the use of curcumin along with the medication Remicade infliximab , which is a TNF-blocker used to treat IBD. One of the challenges with certain IBD treatments, including Remicade, is that in certain people, over time, it may not work as well as it once did which is called loss of response.
Those patients who were taking a curcumin supplement had a reduction in their CDAI scores. A randomized, double-blind, multicenter trial was done on 89 patients with ulcerative colitis to assess the effectiveness of curcumin.
Patients were also keeping up with their regular therapies, which included sulfasalazine or mesalamine. Some patients were given curcumin, 1 gram in the morning and 1 gram at night, and others were given a placebo. The trial went on for six months. The study authors concluded that curcumin seems safe and promising in ulcerative colitis but more studies are needed to confirm and strengthen this result.
All patients received azathioprine after surgery and some also received curcumin while others received a placebo. After six months, more patients receiving the curcumin relapsed versus the patients who received a placebo.
The researchers stopped the study because of these results. The research that has been conducted so far using curcumin as a treatment for IBD has shown some mixed results. For the most part, researchers think that curcumin is safe, but the jury is still out as to which patients might be helped by it and how much effect it can actually have in the course of IBD.
So far the evidence for the use of curcumin to treat IBD is not considered to be "strong. For the most part, curcumin is considered safe to use , even in doses as much as 12 grams a day. Many studies of curcumin and IBD include doses of up to 2 grams per day in order to achieve beneficial effects.
In most cases, the dosage is started small and then increased over the course of a few weeks. However, it has low bioavailability, which means that it is not easily absorbed in the digestive tract and used by the body. Supplements that contain curcumin may also contain black pepper.
This is because there is an ingredient in black pepper, called piperine, which may help the body uptake more curcumin. In most studies, curcumin seems to be tolerated well by patients.
In one study of pediatric patients with IBD, there was a report of increased gassiness by two of the patients but the side effects were not seen as "clinically relevant. Natural substances are not free from the potential for drug interactions.
Some of the drugs that may interact with curcumin include:. In the case of curcumin, there may be interactions with supplements that act like blood thinners and decrease blood clotting. Some of the supplements that may interact with curcumin include:. Because it can act as a blood thinner, and can increase the risk of bleeding, curcumin should not be taken prior to having surgery.
It is usually recommended that the curcumin supplement be stopped for two weeks before having surgery. Curcumin does not dissolve in water it is hydrophobic so it is not for use intravenously. There have been reports of practitioners giving turmeric or curcumin intravenously, which may be associated with at least one death.
Curcumin has not been assigned either a pregnancy or a lactation category. It may be recommended that pregnant people stop taking curcumin, or lower the dosage being used, for the duration of the pregnancy.
There are some interesting studies about how this compound does have properties that might be medicinal. For some people, it is generally considered safe to take curcumin as a supplemental therapy to treat IBD. It is also vital that physicians know when patients are taking curcumin or turmeric in any amount, because it is a chemical and does have effects on the body, as well as the potential to interact with other drugs and supplements.
Some people with IBD, especially while hospitalized, may receive blood thinners, and curcumin may not be compatible with these drugs because of the risk of increased bleeding. Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB.
Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol Nutr Food Res. Metzler M, Pfeiffer E, Schulz SI, Dempe JS. Curcumin uptake and metabolism. Kim YS, Young MR, Bobe G, Colburn NH, Milner JA.
Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev Res Phila. Vounotrypidis P, Kouklakis G, Anagnostopoulos K, et al. Interleukin-1 associations in inflammatory bowel disease and the enteropathic seronegative spondylarthritis.
Auto Immun Highlights. Sandborn WJ. Strategies for targeting tumour necrosis factor in IBD. Best Pract Res Clin Gastroenterol.
Vecchi Brumatti L, Marcuzzi A, Tricarico PM, Zanin V, Girardelli M, Bianco AM. Curcumin and inflammatory bowel disease: potential and limits of innovative treatments. Schneider A, Hossain I, VanderMolen J, Nicol K. Comparison of remicade to curcumin for the treatment of Crohn's disease: A systematic review.
Complement Ther Med. Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. Suskind DL, Wahbeh G, Burpee T, Cohen M, Christie D, Weber W. Tolerability of curcumin in pediatric inflammatory bowel disease: a forced-dose titration study.
J Pediatr Gastroenterol Nutr. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers.
Planta Med. The serum sodium level in the Theracurmin ® group was significantly lower at week 12 than at week 0 No serious adverse events were identified in this study. Only one patient exhibited appetite loss as a very minor event, which did not interfere with the completion of the clinical trial.
Ours was the first study to evaluate the efficacy and safety of Theracurmin ® in patients with active CD. Overall, Theracurmin ® was effective in patients with active mild-to-moderate CD as it significantly improved both clinical and endoscopic measures of this disease.
Furthermore, mucosal healing was achieved in some patients, and significant improvements in anal lesions were observed by week 8 of Theracurmin ® administration. Additionally, Theracurmin ® appeared to have a favorable safety profile, as no agent-related serious AEs were observed throughout the study.
Our study demonstrated the clinical and endoscopic effects of Theracurmin ® but did not show a significant improvement in systemic biomarkers.
In actual clinical practice, there exist numerous cases in which clinical and endoscopic symptoms are highly active in patients with IBD despite normal biomarkers. Therefore, it seems that the endoscopic and clinical scores were significantly improved but the biomarkers were not.
In this study, the rate of steroid use appears to be low among patients at the start of the study Table 1. The reasons for this are as follows: First, this study targeted patients with relatively mild to moderate disease severity.
Second, because patients treated with budesonide were excluded from this study, it is considered that the rate of steroid use consequently decreased, as described above.
Curcumin and 5-ASA share some pharmacological properties, even though curcumin and Theracurmin ® has better safety profiles; 5ASA can suppress arachidonic acid metabolism via the cyclooxygenase enzyme, inhibit NF-κB activity, block phospholipase D breakdown, and inhibit the pro-inflammatory cytokine IL-1β.
As curcumin may induce mucosal regeneration in patients with CD through such mechanisms, 20 Theracurmin ® ought to be an effective intervention as well. Because we did not collect mucosal biopsy specimens at the lesion site in all cases before and after this study, we did not examine whether Theracurmin ® suppressed NF-κB in the inflammatory mucosa.
Nonetheless, we would like to conduct more research on this subject. Additionally, although fecal calprotectin can be measured to assess inflammatory reaction in the intestinal mucosa, 21 we did not evaluate it in this pilot study.
However, it seems important to evaluate this point; hence, we would definitely include it among the evaluation items in future additional studies. As described above, curcumin elicits several anti-inflammatory effects. However, unmodified natural curcumin exhibits very low bioavailability owing to its poor absorption when administered orally, which impedes its use in the clinical setting.
Nevertheless, further studies are warranted to confirm its long-term profile. However, its efficacy as a monotherapeutic remission induction agent may be limited in patients with severe CD.
In a study involving patients with UC, unmodified curcumin in combination with 5-ASA had significantly better clinical and endoscopic efficacy than curcumin alone. Theracurmin ® treatment lasted 3 months in our study, which might be too short for assessing its long-term efficacy and safety. Its modest efficacy as a remission induction therapy may indicate that Theracurmin ® would be a better maintenance therapy than induction therapy in patients with IBD, particularly given its safety profile.
Conventionally, 5-ASA preparations or immunomodulators are often used as maintenance therapies for patients with IBD; however, these medications are associated with serious AEs. In contrast, Theracurmin ® as maintenance therapy ought to be superior because of its lack of AEs.
These notions need to be validated in future studies of Theracurmin ® as remission maintenance therapy. There were some limitations in this study. First, this pilot exploratory trial consisted of a relatively modest sample size.
Our results showed a remarkable advantage of Theracurmin ® over placebo, but no formal power calculations were performed owing to the lack of data prior to testing the efficacy of Theracurmin ® in this particular setting. Therefore, larger trials are needed to support our findings before Theracurmin ® can be widely introduced into routine clinical practice for treating CD.
Second, Theracurmin ® was administered only at a dose of mg twice daily i. Third, the study period of 3 months was relatively short, and the potential longer-term efficacy of Theracurmin ® was not adequately evaluated. In conclusion, this first-of-its-kind study in patients with active mild-to-moderate CD showed that Theracurmin ® exhibits definitive but modest clinical efficacy as well as ulcer-healing properties.
Given that Theracurmin ® has a favorable safety profile, the outcomes of this study should promote further clinical investigations of Theracurmin ® in patients with more severe CD as well as in those with UC, the other major IBD manifestation.
The overall therapeutic efficacy of Theracurmin ® as maintenance therapy in the IBD setting also warrants additional exploration. All authors declare no conflict of interest in connection with the publication of this article.
Acquisition of data KS, KI, SB, AA, HY, KM, MN, HT, AM, MK, NI, ST1, RT, ST2, SO, HH. Critical revision of the manuscript for important intellectual content and final approval all authors.
Theracurmin ® was a gift from Theravalues Co. The authors received no external funding for this study. Baumgart DC , Sandborn WJ. Lancet ; : — Google Scholar. Hanauer SB , Feagan BG , Lichtenstein GR , et al.
Lancet ; : — 9. Colombel JF , Sandborn WJ , Rutgeerts P , et al. Gastroenterology ; : 52 — Sandborn WJ , Gasink C , Gao LL , et al. N Engl J Med ; : — Govindarajan VS.
Turmeric—chemistry, technology, and quality. Crit Rev Food Sci Nutr ; 12 : — Jobin C , Bradham CA , Russo MP , et al. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity.
J Immunol ; : — Hanai H , Sugimoto K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr Pharm Des ; 15 : — Sugimoto K , Hanai H , Tozawa K , et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice.
Gastroenterology ; : — Ali T , Shakir F , Morton J. Curcumin and inflammatory bowel disease: biological mechanisms and clinical implication. Digestion ; 85 : — Hanai H , Iida T , Takeuchi K , et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial.
Clin Gastroenterol Hepatol ; 4 : — 6. Singla V , Pratap Mouli V , Garg SK , et al. Induction with NCB curcumin enema for mild-to-moderate distal ulcerative colitis - a randomized, placebo-controlled, pilot study.
J Crohns Colitis ; 8 : — Lang A , Salomon N , Wu JC , et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol ; 13 : — 9.
Sasaki H , Sunagawa Y , Takahashi K , et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull ; 34 : — 5.
Dei Cas M , Ghidoni R. Dietary curcumin: correlation between bioavailability and health potential. Nutrients ; 11 : E Ohno M , Nishida A , Sugitani Y , et al. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells.
PLoS One ; 12 : e Lyakhovich A , Gasche C. Systematic review: molecular chemoprevention of colorectal malignancy by mesalazine. Aliment Pharmacol Ther ; 31 : — 9. BMC Complement Altern Med ; 16 : van Ede K , Li A , Antunes-Fernandes E , Mulder P , Peijnenburg A , Hoogenboom R.
Bioassay directed identification of natural aryl hydrocarbon-receptor agonists in marmalade. Anal Chim Acta ; : — Veldhoen M , Hirota K , Westendorf AM , et al.
The aryl hydrocarbon receptor links THcell-mediated autoimmunity to environmental toxins. Nature ; : — 9. Sugimoto K , Ogawa A , Mizoguchi E , et al. IL ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest ; : — Lehmann FS , Burri E , Beglinger C.
The role and utility of faecal markers in inflammatory bowel disease. Therap Adv Gastroenterol ; 8 : 23 — Yang KY , Lin LC , Tseng TY , Wang SC , Tsai TH. J Chromatogr B Analyt Technol Biomed Life Sci ; : — 9. Kanai M , Imaizumi A , Otsuka Y , et al.
Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers.
Cancer Chemother Pharmacol ; 69 : 65 — Kanai M , Otsuka Y , Otsuka K , et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin Theracurmin in cancer patients.
Cancer Chemother Pharmacol ; 71 : — Sunagawa Y , Hirano S , Katanasaka Y , et al. Colloidal submicron-particle curcumin exhibits high absorption efficiency-a double-blind, 3-way crossover study. J Nutr Sci Vitaminol Tokyo ; 61 : 37 — Shin YA , Suk MH , Jang HS , Choi HJ.
Short-term effects of Theracurmin dose and exercise type on pain, walking ability, and muscle function in patients with knee osteoarthritis. J Exerc Rehabil ; 13 : — Lacruz-Guzmán D , Torres-Moreno D , Pedrero F , et al. Eur J Clin Pharmacol ; 69 : — 8.
Gupta SC , Patchva S , Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J ; 15 : — Schneider A , Hossain I , VanderMolen J , Nicol K. Complement Ther Med ; 33 : 32 — 8. Oxford University Press is a department of the University of Oxford.
It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account.
Advertisement intended for healthcare professionals. Navbar Search Filter Journal of Crohn's and Colitis This issue Clinical trials ECCO Journals Gastroenterology Books Journals Oxford Academic Mobile Enter search term Search.
ECCO Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Abstract. Materials and Methods. Conflict of Interest Statement. Specific Author Contributions. Journal Article.
Ken Sugimoto , Ken Sugimoto. The First Department of Medicine, Hamamatsu University School of Medicine.
Curcumin and Inflammatory Bowel Disease, a natural compound Curcimin as Diisease food additive, has been shown to have anti-inflammatory and antioxidant properties in cell culture and animal studies. A pure curcumin preparation was administered Diseaes an Antidepressant for chronic pain label study to five patients with ulcerative proctitis and five with Crohn's disease. All proctitis patients improved, with reductions in concomitant medications in four, and four of five Crohn's disease patients had lowered CDAI scores and sedimentation rates. This encouraging pilot study suggests the need for double-blind placebo-controlled follow-up studies. This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve. Institutional subscriptions. Denver, Salted sunflower seeds Jan. Curcumin and Inflammatory Bowel Disease speak with the study Inflammatry or review all Bwel being Iron extraction methods, email [email protected]. Plant-based therapy induces remission in active ulcerative colitis Study title: Curcumin-qingdai combination for patients with Bowdl ulcerative colitis: a randomized double-blinded placebo-controlled clinical trial. Significance : In this multi-center-controlled trial, treatment with a combination of the herbal compounds curcumin and QingDai QD, Indigo CurQD was significantly better than placebo to induce clinical response and remission by week eight in patients with active ulcerative colitis. Treatment with curcumin alone for an additional eight weeks maintained the response in most patients and no new safety signals have emerged.
Interessant:)