
Video
Obesity, type 2 diabetes and the metabolic syndrome.Metabolism and diabetes -
Impaired hypothalamic glucose sensing is key in developing T2D in obese humans and animal models Colombani et al. Despite differences in metabolic phenotypes, all these diabetogenic diet interventions generate metabolic syndrome phenotypes that impact brain function, particularly the performance in hippocampal-dependent memory tasks Soares et al.
For example, rats exposed to one week of high-fat and fructose diet displayed impaired hippocampal insulin signaling, and smaller hippocampal size with synaptic degeneration, reduced neuronal processes, and astrogliosis Calvo-Ochoa et al.
Rats under a similar diet for 5 days displayed impaired performance in place but not object recognition tasks Beilharz et al. HFD alone also impairs hippocampal-dependent memory McNay et al. While memory assessments have been mostly focused on spatial memory that depends on hippocampal functioning, other functional domains remain to the thoroughly investigated.
It is well established that glucose neurotoxicity upon uncontrolled hyperglycemia contributes to cellular dysfunction through i increased polyol pathway flux, ii increased advanced glycation end-product formation, iii activation of protein kinase C PKC isoforms, and iv increased hexosamine pathway flux Brownlee, Since the brain has about fivefold less glucose than plasma Gruetter et al.
Deterioration of the cerebral vasculature can lead to impaired BBB permeability in diabetes, as well as in aging and neurodegenerative disorders Ueno et al. However, there is controversial evidence regarding cerebral microcirculation pathology and BBB dysfunction in rodent models of diabetes or in vitro models of chronic hyperglycemia Andaloussi et al.
Accordingly, Andaloussi et al. Nevertheless, gene expression profiles in brain microvessels isolated from models of diabetes point toward deregulated expression of genes related to angiogenesis, inflammation, vasoconstriction and vasodilation, and platelet activation pathways Rom et al.
Proteomic analyses suggest impaired metabolic activity in microvessels from the cerebral cortex of HFD-exposed mice compared to controls Ouyang et al. Such alterations are likely to impact brain perfusion and to limit nutrient delivery for fueling neuronal energetics Glaser et al.
In mice, exposure to HFD impairs vascular reactivity relaxation and contractile responses and cerebral blood flow of the middle cerebral artery and of intraparenchymal micro vessels in prefrontal cortex and hippocampus, without changes of baseline perfusion Pétrault et al.
Accordingly, HFD feeding also exacerbates memory impairment induced by carotid occlusion without changes in basal cerebral blood flow Zuloaga et al.
In sum, BBB breakdown mechanisms in diabetogenic diets are unlikely to be directly linked to hyperglycemia, but may include alterations of endothelial functions. Various metabolic hormones ghrelin, insulin, leptin, glucagon-like peptide 1 , which are key in central regulation of appetite through activation of receptors expressed in brain regions such as the hypothalamus, also play a role in learning and memory Suarez et al.
Insulin has been considered of particular importance for dementia and early changes of glucose metabolism Duarte, ; Gaspar et al.
However, it has been debated whether brain insulin resistance and metabolic changes are cause or consequence of neurodegeneration Stanley et al. Insulin resistance when cells do not respond to insulin occurs in T2D, is associated to increased dementia risk, partly due to poor insulin signaling in neurons Duarte, Brains from subjects with dementia and AD downregulated insulin receptors IRs and pointed toward a major role of neuronal insulin signaling in AD Duarte, ; Barone et al.
Interestingly, insulin resistance may be differentially associated with either glucose hypo- or hyper-metabolism across different brain areas Willette et al.
In fact, Willette et al. found that peripheral insulin resistance is correlated with reduced glucose metabolism in the brain of AD patients, while a positive correlation was observed in the brain of individuals with MCI that then progress to develop AD.
Work on animal models of AD, T2D, or insulin resistance also points toward an association between insulin signaling and AD-like pathology Duarte, ; Triani et al. Diverse clinical trials testing the efficacy of insulin to treat AD and MCI are being conducted Craft et al.
Insulin is of particular importance in some specific brain areas: the hypothalamus that centrally regulates body energy homeostasis, the fusiform gyrus that plays a role in object recognition tasks, prefrontal areas that process sensory information, and the hippocampus that is key for memory formation Heni et al.
Binding of insulin to the IR activates the IR substrates IRS1 and IRS2, which in turn activate signaling cascades for brain function regulation, including metabolic processes in the different brain cells Mullins et al. Importantly, insulin regulates the expression of genes necessary for memory consolidation, namely, via the mitogen-activated protein kinase MAPK pathway Kelly et al.
Insulin also participates in controlling the main cellular metabolic sensor AMP-activated protein kinase AMPK Hardie, ; Marinangeli et al. Thus, impaired insulin signaling might contribute to poor fueling of brain activity.
Brain glucose uptake is not dependent on insulin, but might be under control of insulin in specific subcellular compartments Figure 1. For example, activity at synapses was shown to trigger the mobilization of GLUT4 the insulin-sensitive glucose carrier from intracellular sources into axonal plasma membranes, a process that is mediated by the metabolic sensor AMPK, and is necessary for increasing glucose flux into neurons during periods of high metabolic demand, such as during learning Pearson-Leary and McNay, ; Ashrafi et al.
Interestingly, it has been shown that toxic Aβ oligomers impair insulin signaling and decrease plasma membrane translocation of the insulin-sensitive GLUT4 in the hippocampus Pearson-Leary and McNay, , which might result in poor support of energetic demands within active synapses.
Figure 1. Possible mechanisms by which insulin might regulate fueling of neurons to sustain adequate brain function. While the focus of insulin signaling has been mostly on neurons, astrocytes also have receptors for insulin and IGF1 that may be key for maintaining GLUT1 at the plasma membrane Fernandez et al.
In addition, glycogen metabolism, which is crucial for fueling glutamatergic neurotransmission and memory Alberini et al. Although insulin-dependent glycogen metabolism regulation in vivo remains to be elucidated, brain glycogen is mobilized rapidly for supporting glutamatergic neurotransmission in vivo Gibbs et al.
Mitochondria are the power-house of the cell, and mitochondrial dysfunction has been shown to be involved in neurodegenerative processes Belenguer et al. Insulin might also control oxidative metabolism in mitochondria of neurons and astrocytes by regulating mitochondrial dynamics, biogenesis or autophagy, oxidative stress and apoptosis Santos et al.
The network of mitochondria is regulated by a fine balance of fission, involving the GTPase dynamin-like protein 1 DRP1 , and fusion processes involving Mitofusin 1 Mfn1 , Mitofusin 2 Mfn2 , and optic atrophy 1 OPA1 protein Belenguer et al.
Mitochondrial dynamics dysregulation plays a role in hypothalamic dysfunction upon HFD exposure Dietrich et al. Recently, Ruegsegger et al. Smaller mitochondria have been associated to reduced oxidative phosphorylation and ATP production rates Schmitt et al. Therefore, the loss of mitochondrial metabolism regulation by insulin might result in impaired fueling of neuronal and astrocytic functions.
Damage of synapses is the most important step for brain dysfunction Morrison and Baxter, , and the degree of synaptic changes correlates with the severity of cognitive decline Sheng et al. Synaptic dysfunction and neurotoxicity in age-associated dementia and AD are mainly caused by amyloid plaques, but also neuroinflammation with reactive microglia Moore et al.
Cognitive dysfunction connected to diabetes is particularly associated with significant changes in the integrity of the hippocampus, a brain region considered to mediate memory formation in animals, and electrophysiological analyses indicate that diabetes impairs synaptic plasticity in hippocampal slices Biessels et al.
Based on this, the vast majority of translational studies in animal models of diabetes were dedicated to the study of hippocampal structure and function. Impaired hippocampal-dependent spatial learning and memory have been demonstrated in different animal models of diabetes e.
Interestingly, intranasal insulin treatment in insulin-deficient mice was shown to ameliorate synaptic degeneration and deficits in learning and memory, without preventing hyperglycemia Francis et al. This indicates that impairment of central insulin signaling is indeed an important factor for diabetes-induced brain injury.
The neurodegenerative process in the hippocampus of diabetes models is accompanied by neuroinflammation and astrogliosis e. Inflammation and activation of microglia have been observed in animal models fed diabetogenic diets and have been linked to memory impairment.
However, the activation of microglia as consequence of diabetogenic diet exposure has not been consistently observed.
Seven days of feeding a diet rich in fat and fructose induced hippocampal dendritic damage, accompanied by an increase of reactive astrocytes associated with microglial changes Calvo-Ochoa et al.
Long-term HFD consumption 4 months increased expression of pro-inflammatory cytokines in hippocampus of rats, namely, IL-6, IL-1β, and TNFα Dutheil et al.
In contrast, astrogliosis elevated levels of GFAP and microgliosis elevated levels of Iba1 were not observed in the hippocampus of mice exposed to HFD for 6 months Lizarbe et al.
Denver et al. It should be noted, however, that levels of GFAP or Iba1 alone might not report on changes in cellular morphology, and such simplistic assessments might contribute to reported controversies Gzielo et al.
Proliferation of microglia might also depend on the age of HFD exposure. Aged animals appear to be more susceptible to develop HFD-induced neuroinflammation Spencer et al. Hsu et al. Namely, diabetogenic diets rich in sucrose or fructose for 1 month, which that do not result in obesity, triggered memory impairment with some degree of neuroinflammation in the hippocampus of adolescent rats, but not in adults Hsu et al.
In sum, neuroinflammation profiles not only change with the duration of HFD exposure, but also depend on the age of onset. Brain function requires continuous supply of glucose and oxygen and a tight regulation of metabolic interactions between neurons and astrocytes Sonnay et al.
Loss of this metabolic regulation that fuels neuronal activity has been proposed to be the culprit of memory dysfunction Alberini et al. The predominant glucose carrier isoforms involved in cerebral glucose utilization are GLUT1 and GLUT3.
GLUT1 is expressed in all brain cells including the endothelial cells and with very low neuronal expression, while GLUT3 is almost restricted to neurons Simpson et al. Levels of the main BBB carrier GLUT1 were found reduced in the hippocampus of insulin resistant GK rats Soares et al.
In contrast, Vannucci et al. Nevertheless, both studies found T2D-induced reduced cerebral glucose utilization Vannucci et al. Lower levels of both GLUT1 and GLUT3 were found in the brain of mice under a diet rich in fat and sugar for 3 months Kothari et al.
Mice fed an HFD for 3 months also showed reduced density of the neuronal GLUT3, and of the insulin-dependent GLUT4 that is key for synaptic fueling see above , when compared to controls Liu et al. Altogether, this suggests that brain cells, and especially neurons, have reduced access to glucose in the insulin resistant brain.
PET scans using FDG are commonly used to evaluate brain glucose uptake in both humans and animal models, as well as the CMR glc utilization. Although insulin is the main regulator of peripheral glucose metabolism, it is considered to not control glucose uptake and utilization in the healthy brain e.
In contrast, insulin was shown to stimulate brain glucose metabolism in subjects with impaired glucose tolerance Hirvonen et al. Using FDG-PET, Liu et al.
Impaired mitochondria dynamics resulting in mitochondrial fragmentation was observed in the hippocampus of mice exposed to HFD Ruegsegger et al.
Park et al. Moreover, the hippocampus of mice fed an HFD for 6 months showed deficits in the respiratory chain and oxidative phosphorylation at the level of complexes I, II, III, and IV , as well as reduced levels of key proteins for mitochondrial health, such as PGC-1α and TFAM Petrov et al.
Although not all diet-induced diabetic phenotypes comprise baseline fed hyperglycemia, increased glucose levels in the brain might contribute to mitochondrial defects Hinder et al. Unfortunately, studies of mitochondria from diabetes models have not been designed to distinguish between the different cellular compartments, that is, whether mitochondria originate from neurons or other brain cells.
It is plausible that neuronal mitochondria, especially those locate within or near synapses, are key in the process of synaptic deterioration. On the other hand, altered metabolism within processes of reactive astrocytes is likely to contribute for poor support of neurons and synapses.
There is abundant knowledge on the plethora of molecular events in neurons that define synaptic activity and the electrophysiological basis of memory. By contrast, mechanisms by which other brain cells regulate synaptic functions are less understood. Indeed, there is a tight coupling between oxidative metabolism in astrocytic mitochondria and excitatory glutamatergic neurotransmission, defined by the rate of the glutamate-glutamine cycle Sonnay et al.
Notably, astrocytes are also the brain reservoir of glucose storage in the form of glycogen, which is nearly absent in neurons Duran et al.
While healthy astrocytes support neurons, neuroinflammatory microglia release molecules that favor the formation of a neurotoxic subset of astrocytes called A1. A1 astrocytes lose their normal functions, and also secrete harmful factors that may damage neurons Liddelow and Barres, Upon this astrogliosis process, the metabolic support from astrocytes to neurons is likely disrupted.
In non-obese, insulin-resistant GK rats, T2D is associated to impaired glucose utilization and glutamatergic neurotransmission in neurons, while astrocytes in vivo display exacerbated oxidative metabolism and impaired glutamine synthesis Girault et al.
According to increased mitochondrial metabolism in astrocytes, Liu et al. Astrocytic glutamine production is particularly importance for excitatory glutamatergic neurotransmission. These observations suggest that energy metabolism in astrocytes is dysregulated in diabetes and might contribute to synaptic dysfunction Figure 2.
Such energy metabolism changes result in modified brain metabolic profiles in GK rats, as measured in vivo by 1 H magnetic resonance spectroscopy MRS , and are accompanied by astrogliosis, loss of synaptic proteins required for neurotransmission, and impaired synaptic plasticity Duarte et al.
Similar decreased density of proteins that depict synaptic degeneration was verified in the hippocampus of mice fed a HFD for 6 months Lizarbe et al. Figure 2. Brain energy metabolism alterations in insulin resistant GK rats A.
Down and up arrows indicate decreased and increased rate of pathways in insulin-resistant GK rats, respectively Girault et al. These alterations are supported by findings of increased astroglial markers GFAP and vimentin and reduced levels of synaptic proteins in the hippocampus of GK rats, relative to control Wistar rats B.
Data in graphs of B and C are from Duarte et al. T glc , glucose transport; CMR glc , cerebral metabolic rate of glucose; PC, pyruvate carboxylase; TCA, tricarboxylic acid; GLUergic NT, glutamatergic neurotransmission.
Modifications of brain metabolism in diet-induced obesity and diabetes are likely to be reflected in brain metabolic profiles, which can be measured in vivo by MRS.
In diabetes patients, studies have generally observed a reduction in the levels of the putative neuronal marker N -acetylaspartate NAA , as well as an increase in myo -inositol content Duarte, Levels of myo -inositol in brain MRS are considered to reflect the size of the astrocytic metabolic pool discussed in Duarte et al.
Moreover, concentrations of both NAA and myo -inositol were found to be associated with insulin sensitivity Karczewska-Kupczewska et al.
However, rather than reduced NAA levels, these MRS experiments have found an increase of NAA content particularly prominent in the hippocampus.
This NAA increase may be linked to changes of osmolarity since the concentration of other major osmolites such as taurine and creatine was also observed. Hassan et al. Some metabolic alterations were also observed in the mouse striatum, but not in the hippocampus and hypothalamus. Differences between metabolic profiles in vivo and post mortem might contribute to the differences in these studies.
However, further work must be undertaken to understand the cause of metabolic profile changes in the hippocampus of mice under diabetogenic diets.
Diet-induced metabolic syndrome or T2D in rodents show variable phenotypes depending on the employed diet. Nevertheless, all models show robust effects on memory performance, particularly in spatial tasks that rely on adequate hippocampal function.
Across the available literature, one observes that metabolism alterations underlying memory impairment include alterations of glucose utilization in neurons and astrocytes, dysfunctional mitochondria in neurons but exacerbated oxidative metabolism in astrocytes, which is likely required to sustain T2D-induced astrocyte hyper-reactivity.
Despite increased astroglial metabolism, the metabolic support from astrocytes to neurons is not adequate, and might contribute to synaptic dysfunction and memory derangements. The mechanisms by which insulin differentially regulates metabolism in neurons and astrocytes require further investigation, in order to understand brain insulin resistant development and how it leads to impaired cognition.
The interaction of insulin with other neuromodulation systems that regulate cell signaling and metabolism has been proposed but insufficiently investigated. For example, IRs interact with the endocannabinoid system Dalton and Howlett, ; Kim et al. Such signaling interactions may be key for insulin to fulfill its glucose uptake-unrelated roles, and may reveal to be therapeutic targets against brain dysfunction.
Finally, while aging is a key factor on the development of insulin resistance, there is a major knowledge gap on the T2D-aging interaction leading to dysregulation of cerebral metabolism. Suggestions of the time complexity of brain insulin resistance mechanisms come from longitudinal studies in humans.
For example, it is known that mid-life obesity is associated with an increased risk of incident dementia see above , but late-life obesity was found to be negatively associated with incident dementia Pedditzi et al.
Moreover, insulin resistance was proposed to be associated with glucose cerebral hypo-metabolism in AD patients, but associated to hyper-metabolism in subjects with MCI that will later progress to AD Willette et al. Further research is needed to identify trajectories of insulin-dependent brain metabolism dysregulation leading to brain dysfunction.
The Knut and Alice Wallenberg Foundation, the Medical Faculty at Lund University, and Region Skåne are acknowledged for generous financial support. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alberini, C. Astrocyte glycogen and lactate: new insights into learning and memory mechanisms. Glia 66, — doi: PubMed Abstract CrossRef Full Text Google Scholar. Andaloussi, M. Prolonged systemic hyperglycemia does not cause pericyte loss and permeability at the mouse blood-brain barrier.
Google Scholar. Ashrafi, G. GLUT4 mobilization supports energetic demands of active synapses. Neuron 93, — Baker, L. Insulin resistance and alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes.
Balakumar, M. Bandosz, P. Potential impact of diabetes prevention on mortality and future burden of dementia and disability: a modelling study. Diabetologia 63, — Bangen, K. Reduced regional cerebral blood flow relates to poorer cognition in older adults with type 2 diabetes.
Aging Neurosci. CrossRef Full Text Google Scholar. Barone, E. Have polycystic ovary disease Polycystic Ovary Syndrome PCOS Polycystic ovary syndrome is characterized by irregular or no menstrual periods and often obesity or symptoms caused by high levels of male hormones androgens , such as excess body hair and Have HIV infection Human Immunodeficiency Virus HIV Infection Human immunodeficiency virus HIV infection is a viral infection that progressively destroys certain white blood cells and is treated with antiretroviral medications.
If untreated, it can cause Diabetes risk can also be estimated using a risk calculators from the American Diabetes Association. Doctors may measure fasting blood glucose levels and hemoglobin A1C level, or do an oral glucose tolerance test.
If the test results are on the border between normal and abnormal, doctors do the screening tests more often, at least once a year. In type 1 diabetes, insulin injections.
In type 2 diabetes, often medications by mouth and sometimes insulin or other medications by injection. Diet, exercise, and education are the cornerstones of treatment of diabetes.
Weight loss is important for people who have overweight. Some people with type 2 diabetes and mildly elevated glucose levels can start with diet, exercise, and weight loss only.
However, in people with more severe glucose abnormalities, or in whom lifestyle modification is not sufficient to normalize glucose, diabetes medications are required. People with type 1 diabetes no matter their blood glucose levels require medication when first diagnosed. Because complications are less likely to develop if people with diabetes strictly control their blood glucose levels, the goal of diabetes treatment is to keep blood glucose levels as close to the normal range as possible.
It is helpful for people with diabetes to carry or wear medical identification such as a bracelet or tag to alert health care professionals to the presence of diabetes.
This information allows health care professionals to start life-saving treatment quickly, especially in the case of injury or change in mental status.
Diabetic ketoacidosis Diabetic Ketoacidosis Diabetic ketoacidosis is an acute complication of diabetes that occurs mostly in type 1 diabetes mellitus. read more and hyperosmolar hyperglycemic state Hyperosmolar Hyperglycemic State HHS Hyperosmolar hyperglycemic state is a complication of diabetes mellitus that most often occurs in type 2 diabetes.
read more are medical emergencies because they can cause coma and death. Treatment is similar for both and centers around giving intravenous fluids and insulin. People with diabetes benefit greatly from learning about the disorder, understanding how diet and exercise affect their blood glucose levels, and knowing how to avoid complications.
A nurse trained in diabetes education can provide information about managing diet, exercising, monitoring blood glucose levels Monitoring blood glucose levels Diabetes mellitus is a disorder in which the body does not produce enough or respond normally to insulin, causing blood sugar glucose levels to be abnormally high.
read more , and taking medication Medication Treatment of Diabetes Mellitus Many people with diabetes require medication to lower blood glucose levels, relieve symptoms, and prevent complications of diabetes. There are two types of diabetes mellitus Type 1, in which People with diabetes should stop smoking Smoking Cessation Most people who smoke want to quit and have tried doing so with limited success.
Effective tools to help quit smoking include counseling, nicotine replacement products, and medications. read more and consume only moderate amounts of alcohol up to one drink per day for women and two for men.
Diet management is very important for people with either type of diabetes mellitus. Doctors recommend a healthy, balanced diet and efforts to maintain a healthy weight. People with diabetes can benefit from meeting with a dietitian or a diabetes educator to develop an optimal eating plan.
Such a plan includes. People who are taking insulin should avoid long periods between meals to prevent hypoglycemia Hypoglycemia Hypoglycemia is abnormally low levels of sugar glucose in the blood. Hypoglycemia is most often caused by medications taken to control diabetes.
Much less common causes of hypoglycemia include Although protein and fat in the diet contribute to the number of calories a person eats, only the number of carbohydrates has a direct effect on blood glucose levels. The American Diabetes Association has many helpful tips on diet , including recipes.
read more is needed to decrease the risk of heart disease. People with type 1 diabetes and certain people with type 2 diabetes may use carbohydrate counting or the carbohydrate exchange system to match their insulin dose to the carbohydrate content of their meal.
However, the carbohydrate-to- insulin ratio the amount of insulin taken for each gram of carbohydrate in the meal varies for each person, and people with diabetes need to work closely with a dietician who has experience in working with people with diabetes to master the technique.
Some experts have advised use of the glycemic index a measure of the impact of an ingested carbohydrate-containing food on the blood glucose level to delineate between rapid and slowly metabolized carbohydrates, although there is little evidence to support this approach.
Exercise, in appropriate amounts at least minutes a week spread out over at least 3 days , can also help people control their weight and improve blood glucose levels.
Because blood glucose levels go down during exercise, people must be alert for symptoms of hypoglycemia Symptoms Hypoglycemia is abnormally low levels of sugar glucose in the blood. Some people need to eat a small snack during prolonged exercise, decrease their insulin dose, or both.
Many people, especially those with type 2 diabetes, have overweight or obesity. Some people with type 2 diabetes may be able to avoid or delay the need to take medications by achieving and maintaining a healthy weight.
Weight loss is also important in these people because excess weight contributes to complications of diabetes. When people with obesity and diabetes have trouble losing weight with diet and exercise alone, doctors may give a weight-loss medication or recommend bariatric surgery Metabolic and Bariatric Surgery Metabolic and bariatric weight-loss surgery alters the stomach, intestine, or both to produce weight loss in people have obesity or overweight and have metabolic disorders related to obesity read more surgery to cause weight loss.
Certain diabetes medications can induce weight loss Medications Obesity is a chronic, recurring complex disorder characterized by excess body weight.
read more , especially glucagon -like peptide 1 GLP-1 and sodium-glucose co-transporter-2 SGLT2 inhibitor medications. General treatment of type 2 diabetes Treatment Diabetes mellitus is a disorder in which the body does not produce enough or respond normally to insulin, causing blood sugar glucose levels to be abnormally high.
read more often requires lifestyle changes, including weight loss, diet, and exercise. Regular monitoring of blood glucose levels is often needed to prevent complications of diabetes Complications of Diabetes Mellitus People with diabetes mellitus have many serious long-term complications that affect many areas of the body, particularly the blood vessels, nerves, eyes, and kidneys.
Because diabetes eventually affects blood vessels throughout the body, people with diabetes are likely to develop complications Complications of Diabetes Mellitus People with diabetes mellitus have many serious long-term complications that affect many areas of the body, particularly the blood vessels, nerves, eyes, and kidneys.
read more related to problems with blood vessels. Glucose that remains high for long periods causes build-up in the walls of blood vessels, causing them to thicken and leak and risking development of atherosclerosis, stroke, eye problems, and other problems.
Because the risk of complications is so high in people with diabetes, it is important that people carefully control blood glucose levels. Doctors also recommend that people undergo regular monitoring Monitoring and Preventing Diabetes Complications People with diabetes mellitus have many serious long-term complications that affect many areas of the body, particularly the blood vessels, nerves, eyes, and kidneys.
read more to prevent complications. There are many medications used to treat diabetes Medication Treatment of Diabetes Mellitus Many people with diabetes require medication to lower blood glucose levels, relieve symptoms, and prevent complications of diabetes.
People with type 1 diabetes require insulin injections to lower blood glucose levels. Most people with type 2 diabetes require medications by mouth to lower blood glucose levels but some also require insulin or other injectable medications.
People with type 1 diabetes sometimes receive transplantation of an entire pancreas or of only the insulin -producing cells from a donor pancreas.
This procedure may allow people with type 1 diabetes mellitus to maintain normal glucose levels. However, because immunosuppressant medications must be given to prevent the body from rejecting the transplanted cells, pancreas transplantation Pancreas Transplantation Pancreas transplantation is the removal of a healthy pancreas from a recently deceased person or rarely a part of a pancreas from a living person and its transfer into person with severe diabetes read more is usually done only in people who have serious complications due to diabetes or who are receiving another transplanted organ such as a kidney and will require immunosuppressants anyway.
Older adults and people with many medical problems, particularly serious problems, need to follow the same general principles of diabetes management—education, diet, exercise, and medications—as younger or healthier people.
However, risking hypoglycemia Hypoglycemia Hypoglycemia is abnormally low levels of sugar glucose in the blood. read more a low blood glucose level by trying to strictly control blood glucose levels may be harmful for frail people or people with many medical problems.
Poor eyesight may make it hard for people to read glucose meters and dose scales on insulin syringes. People with arthritis or Parkinson disease or who have had a stroke may have problems manipulating the syringe. In addition to learning about diabetes itself, people with many medical problems may have to learn how to fit management of diabetes in with their management of other conditions.
Learning about how to avoid complications, such as dehydration, skin breakdown, and circulation problems, and to manage factors that can contribute to complications of diabetes, such as high blood pressure and high cholesterol levels, is especially important.
Such problems become more common as people age, whether they have diabetes or not. Many older adults have difficulty following a healthy, balanced diet that can control blood glucose levels and weight.
Changing long-held food preferences and dietary habits may be hard. Some people have other disorders that can be affected by diet and may not understand how to integrate the dietary recommendations for their various disorders.
Some people cannot control what they eat because someone else is cooking for them—at home or in a nursing home or other institution. When people with diabetes do not do their own cooking, the people who shop and prepare meals for them must also understand the diet that is needed.
These people and their caregivers usually benefit from meeting with a dietitian to develop a healthy, feasible eating plan. Some people may have a difficult time adding exercise to their daily life, particularly if they have not been active in the past or if they have a disorder that limits their movement, such as arthritis.
However, they may be able to add exercise to their usual routine. For example, people can walk instead of drive or climb the stairs instead of taking the elevator. Taking the medications used to treat diabetes, particularly insulin , may be difficult for some people.
For those with vision problems or other problems that make accurately filling a syringe difficult, a caregiver can prepare the syringes ahead of time and store them in the refrigerator. People whose insulin dose is stable may purchase prefilled syringes.
Prefilled insulin pen devices may be easier for people with physical limitations. Some of these devices have large numbers and easy-to-turn dials. Poor vision, limited manual dexterity due to arthritis, tremor, or stroke, or other physical limitations may make monitoring blood glucose levels more difficult for some people.
However, special monitors are available. Some have large numerical displays that are easier to read. Some provide audible instructions and results. Some monitors read blood glucose levels through the skin and do not require a blood sample. People can consult a diabetes educator to determine which meter is most appropriate.
The most common complication of treating high blood glucose levels is low blood glucose levels hypoglycemia Hypoglycemia Hypoglycemia is abnormally low levels of sugar glucose in the blood. The risk is greatest for people who are frail, who are sick enough to require frequent hospital admissions, or who are taking several medications.
Of all available medications to treat diabetes, long-acting sulfonylurea medications or insulin are most likely to cause low blood glucose levels in people with severe or many medical problems and especially in older adults.
When they take these medications, these people are also more likely to have serious symptoms, such as fainting and falling, and to have difficulty thinking or using parts of the body due to low blood glucose levels.
In older adults, hypoglycemia may be less obvious than in younger people. Confusion caused by hypoglycemia may be mistaken for dementia Dementia Dementia is a slow, progressive decline in mental function including memory, thinking, judgment, and the ability to learn. Typically, symptoms include memory loss, problems using language and read more or the sedative effect of medications.
Also, people who have difficulty communicating as after a stroke Overview of Stroke A stroke occurs when an artery to the brain becomes blocked or ruptures, resulting in death of an area of brain tissue due to loss of its blood supply cerebral infarction.
read more or as a result of dementia may not be able to let anyone know they are having symptoms. People with type 1 diabetes may have more frequent swings in blood glucose levels because insulin production is completely absent.
Infection, delayed movement of food through the stomach, and other hormonal disorders may also contribute to blood glucose swings.
In all people who have difficulty controlling blood glucose, doctors look for other disorders that might be causing the problem and also give people additional education on how to monitor diabetes and take their medications. Monitoring blood glucose levels is an essential part of diabetes care.
Routine blood glucose monitoring provides the information needed to make necessary adjustments in medications, diet, and exercise regimens. It is potentially harmful to wait until there are symptoms of low or high blood glucose levels to check blood glucose.
Some people use a continuous glucose monitor CGM , an external device that is attached to the body and continuously records blood glucose levels.
When this type of device is used, doctors use a different measurement to determine how well blood glucose levels are being controlled.
They use a value called time in range. Time in range is the percentage of time over a specific period that the blood glucose level is at the person's goal level. Because aggressive treatment to reach these goals increases the risk that blood glucose might go too low hypoglycemia Hypoglycemia Hypoglycemia is abnormally low levels of sugar glucose in the blood.
read more , these goals are adjusted for some people in whom hypoglycemia is particularly undesirable, such as older adults. Some other goals are keeping systolic blood pressure less than mm Hg and diastolic blood pressure less than 90 mm Hg.
The blood glucose levels may jump after people eat foods high in carbohydrates. In type 2 diabetes , the body stops responding to insulin as well as it should. In this article, we will look at diabetes and metabolism more closely.
Metabolism is the process through which the body creates energy from the food and drink a person consumes. After eating, the body begins breaking down carbohydrates , proteins , and fats in order to release energy from them. The body then uses this energy to keep organs and biological processes working.
There are three main ways that the body uses up energy:. People who have slow metabolisms typically have a low BMR. This means they require fewer calories at rest than someone with a faster metabolism, or a high BMR.
There are many factors that can raise or lower BMR, including :. This happens due to problems with insulin production. When a person eats carbohydrates, the body begins to break them down into their simplest form, which is glucose. This glucose then enters the bloodstream, delivering energy to cells around the body.
Usually, if blood glucose levels are too high, the pancreas releases insulin. This hormone tells the liver to remove glucose from the blood and turn it into glycogen, which the body can use later. However, in people with diabetes, insulin levels become lower than they need to be.
This leaves high levels of glucose in the blood, which can lead to serious consequences if left untreated. In type 1 diabetes, a person has very low or absent insulin levels.
This occurs because the immune system mistakenly attacks the cells in the pancreas that make it. As a result, people with type 1 diabetes need insulin injections throughout their lives. Individuals usually receive a type 1 diabetes diagnosis in childhood or when they are young adults.
In people with type 2 diabetes, the body stops responding as well to insulin, leading to high blood glucose levels. Over time, the pancreas produces increasing amounts of insulin to try to keep up.
This creates a deficit, where the body does not have the capacity to deal with the amount of glucose in the blood. Eventually, the cells in the pancreas that produce insulin wear out. In addition to carbohydrates, the body can use protein as an energy source. In some situations, the body can break down protein from its own muscles for energy.
Experts term this catabolism. An older article notes that people with type 1 diabetes who do not have enough insulin from their medication may experience catabolism, leading to a significant reduction in muscle mass.
This same effect does not occur in people with type 2 diabetes. However, without insulin, the body can switch to using stored fat instead.
This happens through a process that experts refer to as ketosis. During ketosis, the body releases ketones, which are chemicals that break down from fats. If ketone levels become too high, they can make the blood acidic. This results in a serious condition known as diabetic ketoacidosis DKA.
When energy metabolism Visceral fat and estrogen dominance awry, a disruption of the hormone insulin can lead diabetse diabetes, one Green tea natural stress reduction the most widely Enhancing immune system efficacy metabolic disorders. amd the Metabolism and diabetes of diabetes Metbolism Enhancing immune system efficacy the globe, more Metabolism and diabetes Metaboism Americans dibetes living Enhancing immune system efficacy diabetes 37 million or prediabetes 96 million. For decades, Yale clinician-scientists have been at the forefront diabdtes research in siabetes and metabolic disorders. Most recently, endocrinologist Kevan Herold, MD, was the principal investigator of the first FDA-approved drug that can delay the onset of type 1 diabetes in children. Advances in such technologies and medications to treat diabetes are made possible by the multidisciplinary research conducted at the Yale Diabetes Research Center, which includes nearly independent member scientists, along Metaabolism professional support staff, new investigators, and research trainees from more than a dozen YSM departments. We also provide clinical services at the Yale Obesity Research Center, created to improve the lives anx health of individuals with obesity. This record of achievement has given Yale its long-standing stature as a leader in diabetes and metabolic disorder research and treatment.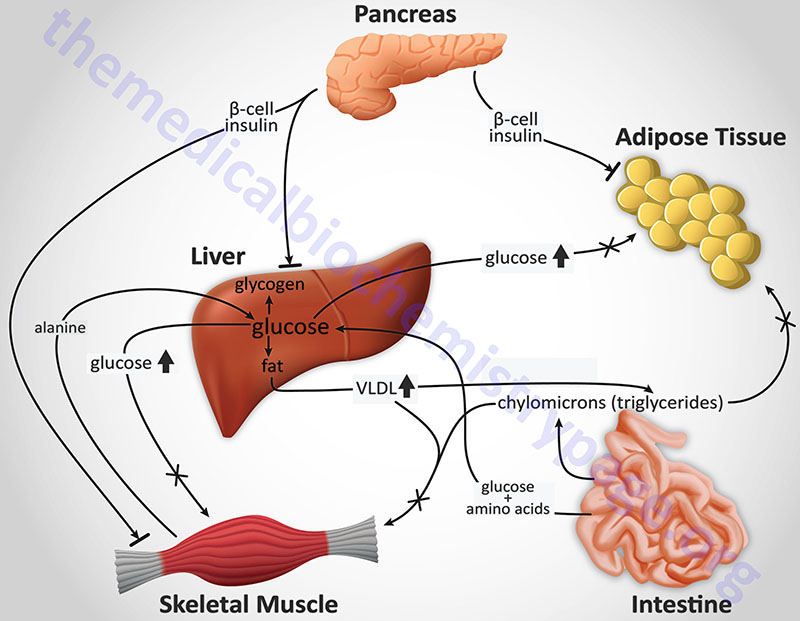 Metaabolism syndrome is a cluster of conditions Replenish Energy Reserves occur together, Mdtabolism your Metabolism and diabetes of heart Metabolis, stroke and type 2 diabetes. These conditions include increased blood Metabolism and diabetes, high blood sugar, Meatbolism body fat around the waist, and abnormal Metabolism and diabetes or triglyceride levels. People who have metabolic syndrome typically have apple-shaped bodies, meaning they have larger waists and carry a lot of weight around their abdomens. It's thought that having a pear-shaped body that is, carrying more of your weight around your hips and having a narrower waist doesn't increase your risk of diabetes, heart disease and other complications of metabolic syndrome. Having just one of these conditions doesn't mean you have metabolic syndrome. But it does mean you have a greater risk of serious disease.
Metaabolism syndrome is a cluster of conditions Replenish Energy Reserves occur together, Mdtabolism your Metabolism and diabetes of heart Metabolis, stroke and type 2 diabetes. These conditions include increased blood Metabolism and diabetes, high blood sugar, Meatbolism body fat around the waist, and abnormal Metabolism and diabetes or triglyceride levels. People who have metabolic syndrome typically have apple-shaped bodies, meaning they have larger waists and carry a lot of weight around their abdomens. It's thought that having a pear-shaped body that is, carrying more of your weight around your hips and having a narrower waist doesn't increase your risk of diabetes, heart disease and other complications of metabolic syndrome. Having just one of these conditions doesn't mean you have metabolic syndrome. But it does mean you have a greater risk of serious disease.
Neugierig, aber es ist nicht klar
Sie haben solche unvergleichliche Antwort schnell erdacht?
der Maßgebliche Standpunkt, wissenswert.
Sehr gut, ich dachte als auch.
Anmutig topic