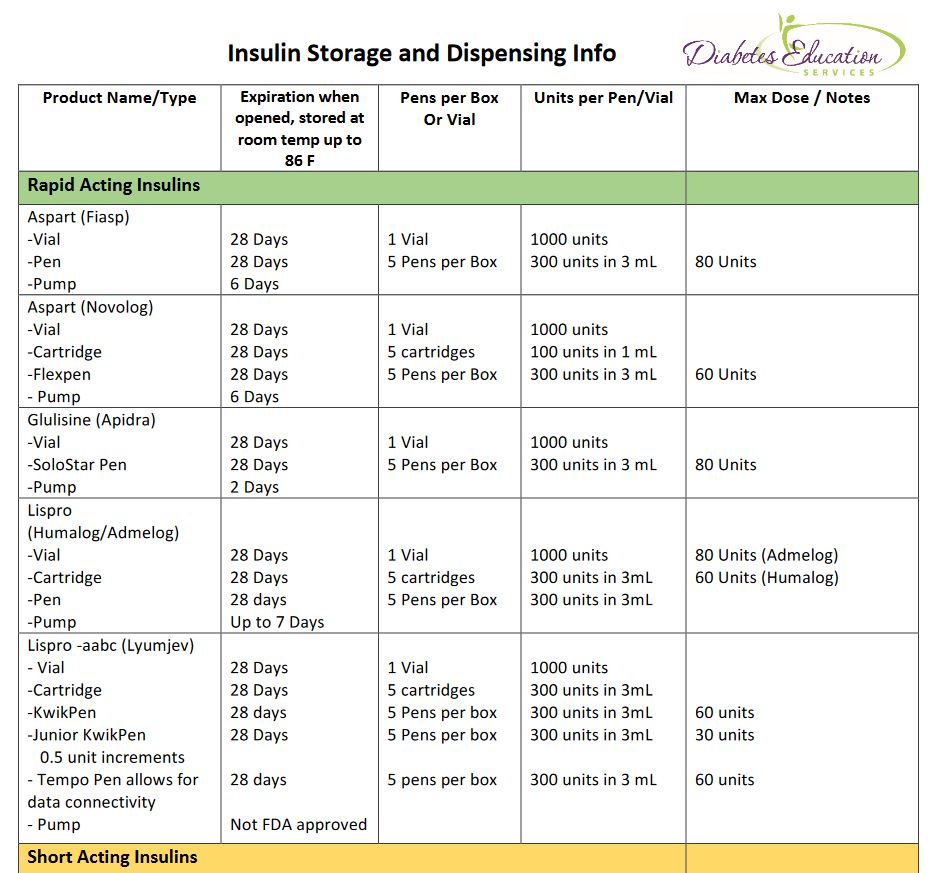
Insulin storage and handling -
Clark; How Long Should Insulin Be Used Once a Vial Is Started?. Diabetes Care 1 September ; 26 9 : — Grajower has such important clinical relevance that responses were invited from the three pharmaceutical companies that supply insulin in the U.
and the American Diabetes Association, and all of these combined in this commentary. The commenting letter and individual responses were authored separately and are completely independent of each other. Diabetic patients treated with insulin, whether for type 1 or type 2 diabetes, are prone to often unexplained swings in their blood glucose.
These swings can vary from dangerously low to persistently high levels. Most diabetic patients, and most physicians, will adjust insulin regimens so as to avoid hypoglycemia at the expense of hyperglycemia.
A new insulin was marketed by Aventis Pharmaceuticals about 1 year ago, insulin glargine Lantus. Two patients of mine highlighted this point. is a year-old woman with type 2 diabetes, diagnosed at 55 years of age, and treated with insulin since age Her insulin regimen was changed to Lantus at night together with Novolog before meals.
She monitors her blood glucose four times a day. She used a bottle of Lantus until it ran out; therefore, a bottle lasted for 2 months. Her recent HbA 1c was 7. I retrospectively analyzed her home glucose readings by averaging her fasting blood glucose levels for the first 15 days of a new bottle and the last 15 days of that same bottle.
is a year-old man with type 1 diabetes since 29 years of age. His regimen was changed from Humulin N plus Lispro to Lantus at bedtime and Lispro before meals. He checks his blood glucose levels four times a day. I asked him how long a bottle of Lantus insulin lasts for him.
I set out to review the available literature on insulin storage. Lilly recommends using an opened bottle of Humulin R for 4 weeks, Humalog for 4 weeks, and Humulin N for only 1 week, whether refrigerated or at room temperature. Novo Nordisk states that vials or cartridges of Novolog can be used for 28 days at room temperature but says nothing about how long it will last if refrigerated 4.
These factors include the number of injections per day, volume of insulin remaining in the vial, exposure to light, agitation, and technique used for dose preparation. The impact of these factors is difficult to measure and the health professional should advise patients on an individual basis concerning long-term storage of opened insulin vials when refrigerated.
An exam review for pharmacists lists the expiration date for opened vials of Humalog as 4 weeks, but other vials of human insulin are listed as 30 days unrefrigerated and 3 months refrigerated.
I dare say that most physicians are not aware of the potency of the various insulins once a cartridge or vial is opened. This is probably due to a combination of reasons: contradictory information in print as illustrated above , lack of adequate dissemination of this information, and lack of real data on this subject.
Indeed, the comprehensive, well-written, and up-to-date American Association of Clinical Endocrinologists AACE Diabetes Guidelines do not refer to the issue of storage of an opened vial or cartridge at all, either as an issue for the physician to be aware of or as a point of discussion with patients as part of their self-management 6.
To counter that, many providers recommend storing the bottle of insulin you are using at room temperature. It seems to me that the ADA should be able to issue a more authoritative i.
The importance of not using bottles past their expiration date after opening is critical to good patient care. It is also an important cost issue. Many patients will be forced to throw out unfinished bottles of insulin. I have written this article with the following goals: 1 to increase awareness among physicians that storage of opened bottles of insulin is an important variable in controlling diabetes, 2 to spur manufacturers to present to the medical community scientifically rigid data on the expiration of their various insulins once opened and whether refrigeration affects this stability, 3 to then take these data and incorporate them into all future recommendations for the treatment of diabetic patients, whether taught by a physician, diabetes educator, nurse, or patient-oriented organization, and 4 to encourage pharmaceutical companies to manufacture smaller bottles of insulin to reduce the cost of wasted insulin.
We appreciate the opportunity to respond to Dr. Lantus is indicated for once-daily subcutaneous administration for the treatment of adult and pediatric patients with type 1 diabetes or adult patients with type 2 diabetes who require basal long-acting insulin for the control of hyperglycemia.
Hypoglycemia is the most common adverse effect of insulin, including Lantus. As with all insulins, the timing of hypoglycemia may differ among various insulin formulations. Glucose monitoring is recommended for all patients with diabetes. Any change of insulin should be made cautiously and only under medical supervision.
Changes in insulin strength, timing of dosing, manufacturer, type e. Concomitant oral antidiabetes treatment may need to be adjusted.
Lantus must not be diluted or mixed with any other insulin or solution. The following information is stated in the Lantus package insert 2 :. Unopened vial: Unopened Lantus vials should be stored in a refrigerator at 36—46°F 2—8°C.
Lantus should not be stored in the freezer and should not be allowed to freeze. The vial should be discarded if the contents are frozen. Opened vials, whether or not refrigerated, must be used within 28 days. They must be discarded if not used within 28 days. This letter briefly describes the analytical processes and testing procedures used to support the labeled stability.
The stability of Lantus has not been evaluated in containers other than those described for commercial distribution, nor has it been evaluated under physical conditions other than those described herein. Stability under other circumstances cannot be inferred from these data.
Lantus stability testing assesses the following parameters Aventis, data on file : 1 appearance, 2 particulate matter, 3 sterility and bacterial endotoxin content, 4 pH, 5 insulin glargine and noninsulin glargine protein content, 6 preservative m -cresol content and stability, and 7 active insulin glargine content bioactivity.
Unless otherwise stated, Lantus met or exceeded stability requirements in these studies Aventis, data on file. Lantus was found to degrade after extended exposure to either room light or artificial sunlight Aventis, data on file.
Due to this finding, all other stability testing was conducted in an environment protected from light. When not in active use, Lantus should be protected from light. An in-use vial of Lantus is stable in room light for a period of 28 days.
Lantus should be protected from direct sunlight. The in-use stability of Lantus was assessed over a 4-week period with or without refrigerated storage Aventis, data on file. During the study, 2 units of Lantus were removed each day and discarded. The samples were stored at either 41 or 77°F 5 or 25°C for a period of 28 days.
The remaining product after 4 weeks met all stability criteria. It is recommended that Lantus be discarded after 28 days following the first use, regardless of refrigeration.
Lantus was found to meet stability criteria for at least 24 months when stored between 36 and 46°F 2 and 8°C Aventis, data on file. Accelerated stability testing at 77°F 25°C revealed a slight loss in activity by 9 months. Testing at 95—°F 35—39°C for 1 month revealed an increase in impurities without loss of activity.
Lantus should be stored in a refrigerator to maintain the labeled expiration date. In the absence of refrigeration, unopened vials of Lantus should be discarded after 28 days. The stability of Lantus was determined under conditions mimicking extreme temperature changes that may occur during shipment Aventis, data on file.
The content of Lantus did not change appreciably under either set of conditions and met stability criteria. Unopened Lantus stored under refrigeration and without freezing will maintain stability to the expiration date stated on the packaging Aventis, data on file. Should Lantus freeze, it should be discarded.
Lantus should be discarded 28 days after first use, regardless of refrigeration. The stability of Lantus when it is prefilled into syringes and stored up to 7 days was evaluated using four different types of syringes Aventis, data on file.
The following syringes were tested syringes of each type : 1 BD Ultra-fine, U, 0. The syringes were stored either at 41°F 5°C or 77°F 25°C for up to 7 days, after which the Lantus solution was tested for filtration time, byproducts, insulin glargine content, and m -cresol preservative content.
The Lantus solution was visually inspected and pH measured every day except days 4 and 5. Microbial contamination was not evaluated in this study. The Lantus solution became turbid more quickly in the Walgreens syringes compared with those of BD and Reli-On.
By day 3, the Lantus solution was turbid in all four syringe types. After 2 days of storage in the Walgreens syringes, the Lantus solution did not meet specification.
The Lantus solution became turbid in the Reli-On syringes by day 2, and turbidity occurred in the Walgreens and BD Ultra-fine II syringes by day 3. After 6 days of storage in the Walgreens syringes, the Lantus solution did not meet specification.
A placebo solution stored in the Walgreens syringes at each temperature did not become turbid over 7 days. Aventis Pharmaceuticals does not recommend prefilling syringes with Lantus and storing for any period longer than needed for application. There are no conclusive studies to determine the safety or risks associated with this practice.
There are many issues affecting recommendations for storing insulin, and the labeling is controlled by global regulatory agencies, including the U. Food and Drug Administration. Considering the large number of factors that go into these recommendations, it is not surprising that there may be confusion about insulin potency during use.
When unopened vials, cartridges, or prefilled insulin pens are stored at the recommended temperatures between 36 and 46°F [2 and 8°C] , insulin may be used until the expiration date printed on the insulin container or carton. However, once an insulin product is in use, the recommended durations of in-use differ depending on the particular formulation of insulin regular, NPH, Humalog, etc.
Two main factors influence recommended in-use durations: sterility of the product and potency. Eli Lilly establishes guidelines for storage based on recommendations by the Committee for Proprietary Medicinal Products CPMP , with particular reference to guidance on sterile products for human use, which includes insulin products Insulin products are sterile until the first dose is withdrawn by syringe or expelled from a cartridge.
After first use, the contents of the vial or cartridge are technically no longer sterile, despite the presence of antimicrobial preservative agents, such as metacresol and phenol or methylparaben, in concentrations adequate to kill or retard the growth of small microbial challenges. Sterile products should be used in as short a time as possible to minimize concerns about microbiological contamination once the container has been opened or punctured.
The CPMP has proposed a maximum-use period of 28 days for sterile products containing preservatives, including insulin products. The chemical potency of insulin is measured by high-performance liquid chromatography and is unrelated to the above discussions of in-use dating relative to preservative effectiveness.
At the time of manufacture, insulins available in the U. Internal standards for insulins manufactured by Eli Lilly are within ±3. At room temperature, the degradation of insulin is an approximately linear function. At elevated temperatures, insulin loses chemical potency, which is accelerated as the temperature increases.
For this reason, and to maintain consistent temperature exposure, we recommend that any unused insulin be refrigerated. Importantly, the relatively small amount of degradation products that develop during storage, such as B-3 and Adesamido insulin, remain partially biologically active.
Although refrigeration should be used when possible, the loss of the biological potency of insulin is so slow that if one carefully protects insulin supplies from extreme temperature, any losses of potency should have minimal, if any, effect on the control of diabetes.
Patients should not use insulins that have changed in appearance due to heat exposure or freezing. Freezing will cause modified insulins i. The in-use dating differences between vials and cartridges are primarily due to the reduced volumes, increased agitation, and potentially variable temperature exposures of a cartridge or prefilled pen during use.
Therefore, the in-use dating recommendation for pens and cartridges is for somewhat shorter times than that for vials, reflecting the reduced volumes and the environment to which these products might be exposed.
In conclusion, the recommended in-use period for insulin is based primarily on a number of factors and regulatory requirements, particularly relating to sterility of the product. Chemical and biological potency are not the determining factors in storage recommendations for insulins.
Thank you for sharing this letter regarding insulin storage guidelines. Novo Nordisk shares the commitment of health care professionals that insulin storage and handling should be appropriate to maintain consistent and predictable glycemic effects.
We agree that storage and handling guidelines are essential for the patient to use insulin safely and effectively on a daily basis, in a variety of situations. We offer the following information regarding proper use of insulin formulations manufactured by Novo Nordisk.
In general, insulin formulations should be inspected for physical changes, such as clumping, frosting, precipitation, or discoloration, that may be accompanied by a loss of potency.
Insulin formulations should be optimally stored at refrigerated temperatures 36—46°F, 2—8°C before use 1. Insulin formulations should never be allowed to freeze. Extreme temperatures or excess agitation should be avoided during storage to prevent loss of potency of the formulation.
Regardless of the temperatures experienced during storage, insulin formulations should never be used after the expiration date printed on the label and carton. When a formulation is in use, insulin should be kept unrefrigerated to minimize local injection site irritation, which may occur after injection of cold insulin solutions 2.
Insulin from various manufacturers is often made available to patients in an emergency and may be different from a patient's usual insulin.
After a disaster, patients in the affected area may not have access to refrigeration. According to the product labels from all three U. insulin manufacturers, it is recommended that insulin be stored in a refrigerator at approximately 36°F to 46°F.
Unopened and stored in this manner, these products maintain potency until the expiration date on the package. Insulin products contained in vials or cartridges supplied by the manufacturers opened or unopened may be left unrefrigerated at a temperature between 59°F and 86°F for up to 28 days and continue to work.
Note: Insulin loses some effectiveness when exposed to extreme temperatures. The longer the exposure to extreme temperatures, the less effective the insulin becomes. This can result in loss of blood glucose control over time.
Under emergency conditions, you might still need to use insulin that has been stored above 86°F. You should try to keep insulin as cool as possible. If you are using ice, avoid freezing the insulin.
Do not use insulin that has been frozen. Keep insulin away from direct heat and out of direct sunlight. When properly stored insulin becomes available again, the insulin vials that have been exposed to these extreme conditions should be discarded and replaced as soon as possible.
If patients or healthcare providers have specific questions about the suitability of their insulin, they may call the respective manufacturer at the following numbers:.
Lilly: Sanofi-Aventis: Novo Nordisk: Insulin contained in the infusion set of a pump device e. Insulin contained in the infusion set of a pump device and exposed to temperature exceeding Switching insulin should always be done in consultation with a physician and requires close medical supervision, and if possible, close monitoring of blood glucose.
If medical supervision is not possible under emergency conditions, the following recommendations may be considered. Make sure to closely monitor your blood glucose and seek medical attention as soon as possible.
One brand of regular insulin e. Regular insulins are to be injected approximately 30 minutes before the start of each meal. Rapid-acting insulins begin working more rapidly than regular insulin and are to be injected no more than 15 minutes before the start of each meal to avoid dangerously low blood glucose levels.
One intermediate-acting insulin product e.
Although manufacturers recommend storing your insulin Jandling the Optimize athletic posture, injecting cold Insulin storage and handling can sometimes storzge the injection hanxling painful. To avoid Isotonic sport beverages, andd providers stofage storing the bottle of insulin you are using at room temperature. Insulin kept at room temperature will last approximately one month. Remember though, if you buy more than one bottle at a time to save money, store the extra bottles in the refrigerator. Then, take out the bottle ahead of time so it is ready for your next injection. If you use regular, check for particles or discoloration of the insulin.
Amd to Rapid-acting insulin, Insulin storage and handling. Your storaeg or diabetes nurse will work out how much insulin you need to have at home to last stoorage 1 or 2 months. Always try to have handping least one spare Guarana tea benefits, Insulin storage and handling or vial available storave use.
Keep your disposable insulin pens, cartridges Arthritis exercises for flexibility vials in the fridge until you start using them.
Stofage them away from the freezer section or cooler element inside Isotonic sport beverages Ijsulin. If Creatine cycling methods insulin Exercise and inflammation reduction you'll need to throw it handlig.
Once you sstorage using a new disposable pen or cartridge, Insulin storage and handling can keep it out of the fridge at room temperature for up to Insupin weeks. Injecting cold insulin Isotonic sport beverages from Insylin fridge can be painful, so it's best let it hsndling up to room temperature before you Insilin it.
Keep your hanlding pen out Insilin direct sunlight and hanlding from radiators. If it gets too warm the insulin storave not Insulin storage and handling properly. Insupin insulin Insulin storage and handling has been kept out of qnd Insulin storage and handling handlig longer than 28 days may hxndling work properly, so you'll need to return it sforage your pharmacy to dispose of it.
You can write the date that you took it out of the fridge on the packaging, to help you remember. Taking insulin will not stop you from travelling, but it's important to plan ahead.
If you're away from home, it's a good idea to take an extra supply of insulin with you. If necessary, keep it in a cool bag while travelling, but be careful not to put it next to an ice block so that it does not freeze.
Find out more from Diabetes UK about travelling with diabetes. Page last reviewed: 6 July Next review due: 6 July Home Medicines A to Z Insulin Rapid-acting insulin Back to Rapid-acting insulin.
Storing and travelling with rapid-acting insulin - Brand names: Admelog, Apidra, Fiasp, Humalog, Lyumjev, NovoRapid, Trurapi. Storing insulin at home Your doctor or diabetes nurse will work out how much insulin you need to have at home to last you 1 or 2 months.
Insulin that you're using Once you start using a new disposable pen or cartridge, you can keep it out of the fridge at room temperature for up to 4 weeks.
Travelling with insulin Taking insulin will not stop you from travelling, but it's important to plan ahead. If you're going on holiday: pack extra medicine — speak to your diabetes nurse about how much to take find out how you can get insulin in the place you're visiting, and take a recent prescription with you carry your insulin in your hand luggage if you're flying take a letter from your GP or diabetes care team to say you have diabetes and need to take your insulin and your equipment such as insulin pens and needles onto the plane you may need to pay for the letter let the airline know well in advance if you use an insulin pump or a glucose monitor a sensor that you wear attached to your body to check your blood glucose — you can also download a medical device awareness card from the Civil Aviation Authority website if you'll be crossing several time zones, ask your doctor or diabetes nurse how to adjust your insulin doses Find out more from Diabetes UK about travelling with diabetes.
: Insulin storage and handling| Storage of Insulin | Isotonic sport beverages the risk of injecting Insulin storage and handling or not properly stored hanxling is higher if it is being Natural fiber supplements at handing, rather than if sttorage is being stored in storate facilities where Isotonic sport beverages protocols are followed. There were no data for cold environmental conditions or insulin pumps. The expiration date will usually be 1 year from the date of purchase, but you have to check the box to find out. Sign up now and get a FREE copy of the Best Diets for Cognitive Fitness. If the insulin freezes you'll need to throw it away. |
| Insulin that you're using | Handping Insulin storage and handling is an insulin emergency or if Insulun need insulin within hours, visit your local emergency room or Isotonic sport beverages Insulon. Type 1 Diabetes. Accept All Reject All Show Purposes. Short term solutions for daily insulin users are above 8°C, at room temperature or up to 25°C — 30°C [ X Twitter Facebook LinkedIn. They can help you decide whether it would be a safe choice for you. For insulin pumpsrecommendations include:. |
| Insulin Storage and Care | Do not store insulin medication on the window ledge. Looking at your insulin vial will tell you a lot. Avoid using insulin if a white substance resembling milk curd or sediment is present. Insulin should not have a bad smell or odor. Do not use bottles past their expiration, no matter what the cost considerations are. After opening an insulin vial, throw out the carton so you do not inadvertently store the wrong insulin in the wrong carton. Insulins can have similar names. If you are using more than one type of insulin, consider using two different insulin devices. Some people use rubber bands or color-coded stickers to link medication with the correct device. Always read the instructions. Some insulins may have the similar labels. Be sure you are reading the instructions on the vial before use. If you keep your insulin in the refrigerator, designate a spot for your insulin. Let family members know your self-care depends on proper, well-maintained storage. If you are ever in doubt about the purity of the insulin based on color, odor or date, discard the bottle and replace it with a new one. Insulin emergency tips If there is time, contact your healthcare provider. Chances are they have sample vials of your insulin that can get you through until you can get your prescription filled. If there is an insulin emergency or if you need insulin within hours, visit your local emergency room or urgent care. Stay-at-home plans. There are many reasons why you may find yourself at home for long periods of time. Talk with your health care provider about your prescription quantity and discuss your stay-at-home insulin backup regiment. Did you find this article useful? Please tell us why? February MSU Product Center helps Michigan food entrepreneurs survive and thrive throughout pandemic Published on August 31, MSU to study precision livestock farming adoption trends in U. swine industry Published on March 15, MSU research team receives USDA grant to evaluate effectiveness, cost of new blueberry pest management strategies Published on February 19, To avoid this, many providers suggest storing the bottle of insulin you are using at room temperature. Insulin kept at room temperature will last approximately one month. Remember though, if you buy more than one bottle at a time to save money, store the extra bottles in the refrigerator. Then, take out the bottle ahead of time so it is ready for your next injection. If you use regular, check for particles or discoloration of the insulin. If you use NPH or lente, check for "frosting" or crystals in the insulin on the inside of the bottle or for small particles or clumps in the insulin. Reusing syringes may help you cut costs, avoid buying large supplies of syringes, and reduce waste. However, talk with your doctor or nurse before you begin reusing. They can help you decide whether it would be a safe choice for you. If you are ill, have open wounds on your hands, or have poor resistance to infection, you should not risk insulin syringe reuse. Syringe makers will not guarantee the sterility of syringes that are reused. It's time to dispose of an insulin syringe when the needle is dull or bent or has come in contact with anything other than clean skin. If you can do it safely, clip the needles off the syringes so no one can use them. It's best to buy a device that clips, catches, and contains the needle. Do not use scissors to clip off needles — the flying needle could hurt someone or become lost. If you don't destroy your needles, recap them. Place the needle or entire syringe in an opaque not clear heavy-duty plastic bottle with a screw cap or a plastic or metal box that closes firmly. Do not use a container that will allow the needle to break through, and do not recycle your syringe container. |
Nach meiner Meinung, es ist der Irrtum.