
Video
Top 10 Anti Cancer Foods (Part 1) - YOU NEED TO EAT THESE!Anti-angiogenesis and cancer prevention -
Vessel co-option is another mechanism of tumour resistance to anti-angiogenic therapy [ 26 ]. Tumour cells can incorporate existing vasculature to accelerate their growth.
This has been shown in gliomas and lung cancers and in patients with colorectal cancer treated with bevacizumab [ 31 ]. Tumour cells also use vasculogenic mimicry to evade antiangiogenic therapy.
They can differentiate and gain EC-like features e. expression of VE cadherin and ephrin A2. This is important for invasion and metastasis.
An interesting concept in anti-angiogenic therapy is vascular normalization and re-distribution of flow in tumour vascular bed when anti-angiogenics are combined with the conventional chemotherapy regimen [ 32 ].
It has been suggested that normalising the tumour vasculature would diminish endothelial and perivascular cells, decrease the high interstitial pressures in solid tumours, enhance oxygenation and chemotherapy delivery into tumour cells [ 11 ].
Antiangiogenic agents do not achieve enough efficacy when they destroy tumour vascular networks as monotherapy but rather, by pruning tumour vascular networks when administered with other chemotherapeutics, they reduce vascular hydrostatic pressure, tumour-associated oedema and temporarily improve tumour hypoxia, thus improving delivery and activity of chemotherapeutics which can then effectively destroy tumour cells.
This has been demonstrated in colorectal cancers and glioblastoma multiforme [ 32 , 33 ]. Recently, a combination of bevacizumab with paclitaxel and carboplatin in patients with non-small cell lung cancer NSCLC has also shown improved survival [ 11 ].
In tumours, molecules involved in immune checkpoint e. PD-1 interacts with its ligand, PD-L1 in immune and cancer stromal cells to inhibit the proliferation and survival of T cells which are important in immune surveillance of tumours [ 33 ].
Hijacking of PD-PD-L1 pathway activation by solid tumours leads to T cell exhaustion and increased expression of FoxP3 by regulatory T cells Tregs with resultant immunosuppression and tumour resistance. The combination of low-dose VEGFR2 blockade and a cancer vaccine also led to an increased immune response to tumour cells, vascular normalisation and improved survival in mice models of breast cancer and colon cancer [ 34 , 35 ].
There are now ongoing trials investigating the role of dual anti-angiogenic therapy and immunotherapy using bevacizumab with atezolizumab e. in advanced renal cell cancers NCT [ 33 ]. Triple therapy using a combination of anti-angiogenic agents, immunotherapy and conventional chemotherapy are also being trialed in metastatic solid tumours NCT, NCT [ 33 ].
These trials have a high potential for overcoming of tumour resistance to anti-angiogenic molecules in future. Reliable biomarkers of tumour response to antiangiogenic therapy have become a focus of attention given the risk of tumour resistance and adverse events.
However, most of the studies have been inconsistent. Circulating VEGF levels have been investigated as a predictive biomarker of response to anti-VEGF therapy. In the study by Hillan et al. In the TARGET trial which investigated sorafenib in advanced renal cell carcinoma, serum VEGF levels had an inverse relationship with progression-free survival and overall survival [ 37 ].
Taken together, it seems that while VEGF has prognostic value, it is not a reliable predictor of response to therapy. Vascular endothelial cadherin is another potential biomarker [ 38 ]. It is important in maintaining EC contact.
It also plays important role in regulating cell proliferation, apoptosis and modulates VEGFR2 function. In the same vein, integrins that mediate cell-cell and cell-extracellular matrix interactions may be important biomarkers because of their roles in tumour invasion and metastasis.
Nanoparticles bearing αvβ3 integrins are being investigated for molecular tumour imaging. Circulating levels of HGF, IL-6, IL-8, osteopontin and TIMP1 have been shown to identify patients who had greater overall survival benefit from treatment in pazopanib-treated patients with metastatic renal cell cancers in one study [ 41 ].
Challenges with the use of circulating biomarkers include the absence of standardization of measurements across centres and the absence of accepted cut-off levels for these circulating biomarkers.
Moreover, circulating factors tend to fluctuate in disease settings and disease stage. Mast cells and miRNAs are increasingly being investigated as diagnostic and prognostic biomarkers in tumours like colorectal cancers and are potential therapeutic targets [ 42 ]. High mast cells density is correlated with the advanced stage of colorectal cancer and tumour progression.
Recently, mast cell tryptase inhibitors e. gabexalate mesylate and nafamostat mesylate have been studied in metastatic gastric cancers with encouraging result [ 43 ]. There has been an interest in non-coding miRNAs in colorectal cancer progression. miRNA and miRNA are oncogenic miRNAs seen at all stages of colorectal cancer progression [ 42 ].
Their levels in tumour tissues have been correlated with survival in individuals with colorectal cancers. miRNA has been shown to confer tumour resistance to 5-fluoro uracil by downregulating MutS homologue-2 while high levels of miRNA have been correlated with oxaliplatin resistance [ 42 ].
The development of drugs which target the secretion or action of these miRNAs holds great promise for the prevention and treatment of tumour resistance in patients on anti-angiogenic treatment and conventional chemotherapy. Microvascular density in serial tumour biopsies has been proposed as a reliable biomarker of response along with the measurement of circulating angiogenic markers and adhesion molecules [ 44 ].
A meta-analysis showed that micro-vessel density predicted survival in non-small cell lung cancer NSCLC [ 45 ]. Anti-angiogenics may not only affect tumour vessels but also the normal vasculature; thus, healthy tissue in tumours may be used to monitor antiangiogenic therapy in tumours.
Vessel density and intra-tumour blood supply may be estimated using imaging methods like contrast-enhanced MRI or PET. In one clinical trial of metastatic colon cancer, epithelial and stromal VEGF expression and micro-vessel density were not predictive of the benefit of the addition of bevacizumab to 5-fluorouracil based therapy [ 46 ].
Vascular imaging using ultrasound, CT, MRI or PET is another predictive marker that can be used to assess response to treatment as shown by the use of MRI in monitoring response to antiangiogenic therapy in patients with glioblastoma multiforme GBM [ 47 ]. High levels of vascular perfusion on vascular imaging predicted response and outcome in patients with metastatic renal cell cancers who were treated with TKIs [ 48 ].
A recent study by Rojas et al. Challenges with using these imaging modalities include marked variability in methodologies used to assess imaging biomarkers across studies and the need for standardization of tumour molecular imaging.
Different types of biomarkers e. circulating and imaging may have to be combined to yield a composite biomarker for more robust predictors of response to antiangiogenic therapy. The cardiovascular adverse effects of antiangiogenic therapy are worthy of mention.
Some of the reported side effects are hypertension, cardiac dysfunction and myocardial ischaemia. These agents act by reducing nitric oxide expression which leads to vasoconstriction and elevation of blood pressure [ 50 ].
Other pathophysiologic pathways for hypertension include increased expression of endothelin-1, microvascular rarefaction, activation of the renin-angiotensin-aldosterone axis, oxidative stress, pressure natriuresis and arterial stiffness.
VEGF signaling pathway inhibitors cause an increase in blood pressure with 7. Blood pressure elevation occurs rapidly within hours or days of starting anti-VEGF therapy and is commensurate with effective VEGF signaling inhibition. It remains unclear whether blood pressure goals in such patients should be the same as for the general population even though current hypertension guidelines do not discriminate between these patients and the general population.
The risk of hypertensive target organ damage is increased in these patients. The National Cancer Institute recommends formal cardiovascular assessment before commencing anti-angiogenic therapy, and antihypertensives should be commenced in such patients once there is a more than 20mmHg rise in diastolic blood pressure from baseline even if blood pressure remains in the normotensive range [ 51 ].
There is a need to clarify the blood pressure threshold at which anti-angiogenic dose reduction or termination should be considered. The preferred classes of antihypertensives in such instances are also a matter of debate.
It is better to avoid non-dihydropyridine calcium channel blockers since they inhibit the CYP3A4 which is responsible for the metabolism of antiangiogenic medications and can thus elevate plasma levels of anti-angiogenics with resultant worsening of hypertension.
Anti-angiogenic therapy has been implicated in cardiotoxicity. The risk is particularly high in those who develop hypertension. Moreover, the risk of left ventricular LV dysfunction remains high among patients whose blood pressure has been controlled while on medications like sunitinib.
Such capillary density may not match the increase in myocardial area or hypertrophy. This mismatch causes reduced fractional shortening and increased LV end-diastolic pressure [ 50 ].
In mice treated with TKIs like sunitinib and also in patients on anti-angiogenic therapy, there is capillary rarefaction and myocyte mitochondrial swelling and degenerative changes which are compounded by apoptosis in those with high blood pressure [ 50 ].
It appears that increased afterload accelerates this capillary rarefaction and may underlie the development of LV dysfunction. Cardiotoxicity also involves alteration in myocardial energetics via AMP-kinase inhibition and resultant mitochondrial dysfunction. Such changes lead to reduced contractility and increase the susceptibility of the heart to other insults.
Such cardiotoxicity may be due to both on-target and off-target effects of TKIs on the heart which leads to adverse remodeling and cardiac dilatation.
This underscores the need to monitor left ventricular function in patients on anti-angiogenic therapy. Myocardial ischaemia has been observed with some antiangiogenic agents including bevacizumab, sunitinib, sorafenib and regorafenib [ 50 ].
This LV dysfunction is usually asymptomatic and is reversible on early withdrawal of such therapy. Risk factors for such arterial thrombotic events are unclear but background heart disease, hypertension, older age and use of other cardiotoxic drugs likely play important roles.
The strong link between coronary ischaemia and cardiotoxicity with the use of anti-angiogenic therapy appears to be related to perfusion contraction mismatch [ 50 ]. Reduction in nitric oxide signaling and endothelial dysfunction that occur following acute VEGF therapy accelerates coronary vasoconstriction, arterial inflammation, atherosclerosis and platelet reactivity.
This is particularly important for those molecules which also affect PDGF signaling where there is decoupling of the pericyte-endothelial myocardial interaction. Theoretical concerns exist for small molecule receptor tyrosine kinase inhibitors about cardiotoxicity and heart failure risk especially in those with pre-existing cardiac diseases due to disruption of AMP-kinase activity [ 52 ].
The risk of the left ventricular systolic dysfunction during anti-angiogenic therapy is difficult to predict. Many of the patients in reported studies had been treated with radiotherapy and chemotherapy which may also cause cardiotoxicity. Stress echocardiography may play a role in the evaluation of those with an intermediate or high pre-test probability of coronary artery disease who are being placed on anti-VEGF therapy.
Additionally, PET and cardiac MRI may be used to determine myocardial blood flow reserve in these situations. The clinical approach to anti-angiogenic therapy in the setting of cardiovascular risk is presented in Fig.
Nanoparticles allow absorption of a large quantity of a drug due to the large surface area to volume ratio [ 53 ]. Small molecules, proteins, DNA and miRNAs can be loaded into nanoparticles for delivery into tumours.
Nanoparticles have advantages over conventional chemotherapy because of their multifunctional targeted roles in the tumour environment. Potential approaches include tissue reoxygenation, either through in situ oxygen supply or increasing intra-tumour hydrogen peroxide metabolism.
Organic liposomes, polymers and inorganic gold, silver and silicate based nanoparticles have been developed for use in experimental tumour models. Some nanoparticles have been designed to silence the expression of HIF-1α gene by antisense oligonucleotides or by miRNAs.
Some liposomes carrying camptothecin or topotecan inhibit topoisomerase I [ 53 ]. The flow of nanomedicines into tumours may be negatively influenced by hypoxia of tumour microenvironment despite the existence of enhanced permeability and retention effect EPR [ 53 ].
EPR in solid tumours is due to their vascular abnormalities which lead to extravasation of nanometric molecules in tumours which may thus reach a higher concentration than in normal tissue. The intense hypoxic environment of tumours may be a barrier to the EPR effect.
Nanotechnology have circumvented this and can enhance EPRs by using hyperthermia to mediate vascular permeability in solid tumours, ultrasound-induced cavitation to modify tumour tissue, application of nitric oxide-releasing agents to expand blood vessels or administration of antihypertensive to normalize blood flow [ 53 ].
These have been achieved in tumours to promote tumour heating using photo-stimulation, magnetism, radiofrequency waves or ultrasound. Tumour vessel normalization has also been attempted using gold nanoparticles to provide human recombinant endostatin rhEs in tumours by EPR to facilitate transient vessel normalization and improve anti-tumour therapeutic efficacy.
Some have also developed nanoparticles of combination therapy of antiangiogenic and conventional chemotherapy e. lipid derivative conjugates LGCs containing gemcitabine and paclitaxel to simultaneously restore tumour vasculature and deliver cytotoxic drugs [ 53 ]. There is however a need to evaluate the safety and toxicity of nanoparticles.
Safety concerns include direct toxicity, nanoparticle aggregate long-term accumulation and immunogenicity. There is also a need to improve drug loading capacity and capability of sustained release of the cargo of nanoparticles in vivo. This will minimize the risk of accumulation of nanoparticles in healthy tissues and facilitate effective delivery to the target tumours.
This is important because vascular permeability, oncotic pressure, interstitial pressure and complex nature of tumour stroma affect the movement of nanoparticles in and out of tumour microenvironment. There is a need to stratify patients according to their EPR release to define those patients who can benefit from nanoparticles.
There are different delivery methods for nanoparticles. These include exosomes, plasma membrane coating, use of chitosan and even the use of mesenchymal stem cells. Exosomes allow intracellular delivery of their cargo by fusion of membranes.
They can cross biological barriers like the blood-brain barrier easily. Undesired effects of the exosome components and lack of standardized production protocols are limitations to their use. Plasma membrane coating with nanoparticles is another delivery technique for nanoparticles as anti-angiogenics.
Examples of nanoparticles delivered this way include tungsten oxide which has been used in lymphoma models [ 53 ]. Platelet membranes provide immune evasion and active adhesion to tumour cells due to their P-selectin interaction with ligands expressed on tumour cells.
Some have used red cell membranes which are very abundant in the circulation and have immune escape and long circulation time. Chitosan is another carrier derived from chitin.
It is less cytotoxic and is biodegradable and metabolized easily by the kidneys. In mice models of breast cancer, chitosan nanoparticles containing anti-Rho small interfering RNA siRNA showed tumour anti-angiogenesis [ 56 ].
The binding of αvβ3 integrin to chitosan nanoparticles is an important development. The receptor for αvβ3 integrin is widely expressed in tumours and has shown potentials in ovarian cancer models. Encapsulation of paclitaxel with chitosan nanoparticles has shown efficacy in breast cancer [ 57 ].
There is now interest in the use of mesenchymal stem cells MSCs to deliver nanoparticles. Hypoxic conditioning of such MSCs used as cell-based therapy can be used for aggressive tumours like glioblastoma multiforme since MSCs can traffic across the blood-brain barrier [ 53 ].
Blocking tumour stem cells via anti-angiogenic therapies is another theoretical approach since the tumour stem cell sub-population in some tumours like breast cancers may be more adept at promoting angiogenesis than their non-stem cell counterparts.
The different delivery methods for nanoparticles are compared in Table 2. Anti-angiogenic therapy in cancers has enormous potentials using VEGF signaling pathways.
Cardiovascular toxicity and off-target effects of anti-angiogenic drugs are impediments to their long-term use in those at high cardiovascular risk.
Continued research into effective nanoparticle-based delivery methods is an exciting and developing field in cancer therapeutics. Understanding of the molecular and cellular mechanisms of tumour angiogenesis will facilitate the development of newer effective anti-angiogenic molecules.
GBD Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for diseases and injuries for countries, — a systematic analysis for the global burden of disease study Google Scholar.
Gupta K, Zhang J. Angiogenesis: a curse or cure? Postgrad Med J. Article CAS PubMed PubMed Central Google Scholar. Kim KJ, Li B, Winer B, Armanini M, Gillett N, Philips HS, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo.
Nat Publ Gr. CAS Google Scholar. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. Article CAS PubMed Google Scholar.
Planchard D, Planchard D. Bevacizumab in non-small-cell lung cancer: a review. Expert Rev Anticancer Ther. Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies.
Clin Ther. Wilhelm SM, Carter C, Tang L, Wilkie D, Mcnabola A, Rong H, et al. Cancer Res. Liu L, Cao Y, Chen C, Zhang X, Mcnabola A, Wilkie D, et al. Chase DM, Chaplin DJ, Monk BJ. The development and use of vascular targeted therapy in ovarian cancer. Gynecol Oncol. Kazazi-Hyseni F, Beijnen JH, Schellens JH.
Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer.
Miles DW, Chan A, Dirix LY, Corte J. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2 — negative metastatic breast cancer. J Clin Oncol.
Robert NJ, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, et al. RIBBON randomized, double-blind, placebo-controlled, phase iii trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer.
Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, Prausova J, et al. Aflibercept versus placebo in combination with fluorouracil, leucovorin and irinotecan in the treatment of previously treated metastatic colorectal cancer: prespecified subgroup analyses from the VELOUR trial.
Angiogenesis is how the body forms new blood vessels. This is a normal part of growth and healing. But sometimes angiogenesis can play a role in diseases such as cancer. To grow, a tumor needs nutrients and oxygen from your blood.
The tumor sends signals that stimulate more blood vessels to grow and carry more blood. Angiogenesis inhibitors, also called anti-angiogenics, block blood vessel growth. By blocking nutrients and oxygen from a tumor, the angiogenesis inhibitors "starve" the tumor. The U. Food and Drug Administration has approved several angiogenesis inhibitors.
They may affect angiogenesis in more than one way, and some of them can also affect other ways that a tumor grows. Angiogenesis inhibitors can be given alone or in combination with other types of treatment. Researchers are studying whether some of these drugs may treat other types of cancer.
Talk with your health care team about clinical trials for angiogenesis inhibitors. Many of the body's normal functions depend on angiogenesis.
Therefore, angiogenesis inhibitors can cause a wide range of physical side effects including:. Hand-foot syndrome , which causes tender, thickened areas on your palms and soles. Sometimes, it causes blisters.
Although common, these side effects do not happen with every drug or every person. And, there are medicines can help manage these side effects when they do occur.
Be sure to let your health care team know about side effects you experience. If an angiogenesis inhibitor is recommended for you, talk with your doctor about the specific potential benefits and risks of that medication.
Among the AIs, VEGF inhibitors were extensively studied and reached phase III clinical trials. They caused a modest increase in overall survival OS Bevacizumab BVZ , a humanized anti-VEGF monoclonal antibody, was the first drug to be approved by the Food and Drug Administration FDA for the treatment of metastatic colon, ovarian, renal, non-squamous cell lung cancer NSCLC , and glioblastoma mutliforme GBM 66 , It failed to show clinical significance when used as monotherapy, except in GBM.
In contrast, its clinical benefits were evident in association with other chemotherapeutic agents. For instance, since the tumor vasculature induced by VEGF is usually tortuous and dysfunctional, the use of BVZ was thought to normalize the blood vessel texture.
It was also hypothesized that the combination of BVZ and chemotherapy increases the delivery of the chemotherapeutic agent to the cancer tissue by increasing its blood flow 68 , However, contrary evidence was reported by a decrease in cytotoxic drug delivery to tumors following treatment with AIs Such inconsistency could be due to differences in blood vessel setups among various cancer types 71 , BVZ combined with chemotherapy was also studied in the adjuvant setting in colorectal cancer CRC , but it failed to prove any clinical significance compared to chemotherapy alone in two phase III clinical trials 73 — Aflibercept is a soluble VEGF decoy receptor that consists of the extracellular domains of VEGFRs 1 and 2 and the Fc portion of human IgG1.
It was FDA approved for the treatment of metastatic CRC in combination with 5-fluorouracil, leucovorin, and irinotecan in Owing to its structure, Aflibercept can neutralize both, VEGF and PlGF Compared to treatment with BVZ, the use of Aflibercept in patient-derived xenograft models resulted in higher tumor suppressive activity Unfortunately, neutralizing both, PlGF and VEGF, had a minimal effect on tumor suppression in vivo In a phase I clinical trial, relapsing GBM patients treated with BVZ monotherapy were compared to those treated with the combination of an anti-PlGF agent and BVZ.
Similar results were obtained with no added benefit in the combination arm Unlike BVZ and Aflibercept, tyrosine kinase inhibitors, which are small molecules able to interact with the kinase domain on the VEGFRs, showed a remarkable clinical benefit when used as single agents, and with no added value when combined with chemotherapy.
This was reported in the treatment of renal cell carcinoma RCC , hepatocellular carcinoma HCC , thyroid cancer, gastrointestinal stromal tumor GIST , and pancreatic neuroendocrine tumor PNET Although anti-angiogenesis therapies may prolong progression-free survival PFS , they have limited impact on overall survival OS and do not constitute a permanent cure in RCC, CRC, or breast cancer 73 , 75 , 82 , This limited clinical significance might be due to different innate and acquired molecular resistance mechanisms with no clear genetic explanations Hypoxia plays an important role in tumor resistance to chemotherapeutic agents favoring more aggressive metastatic disease and hence worse prognosis.
HIF-1 plays a critical role in resistance to anti-angiogenic therapy and is the main survival factor used by cancer cells to adapt to oxygen deprivation 84 , In this section, an overview on different mechanisms of resistance to anti-angiogenic therapies in the clinical and preclinical settings will be discussed Figure 4 and the ways to overcome them will be provided Table 3.
Some of these mechanisms are likely influenced by hypoxia. These include the production of alternative proangiogenic factors, the recruitment of BM-derived cells, the vasculogenic mimicry, as well as the increased tumor cell invasiveness and metastatic behavior.
Figure 4. Summary of plausible resistance mechanisms to Anti-angiogenic Agents. Treatment with anti-angiogenic agents results in a reduction in the blood vessel network.
This new hypoxic condition results in the activation of vascular mimicry, altemative pro-angiogenic pathways, recruitment of bone man·ow-derived EC precursors and myeloid cells, as well as cell survival mechanisms such as autophagy.
Table 3. List of mechanisms of resistance to anti-angiogenic therapies and ways to target them along with the outcomes associated with each approach. Treatment with anti-angiogenic agents results in vascular regression and intra-tumoral hypoxia.
Several studies have made use of pimonidazole injections, to demonstrate an increase in hypoxic regions in primary tumors following anti-angiogenic treatment 86 , 89 , Further analysis showed a concomitant increase in HIF-1a expression during treatment.
HIF-1a and hypoxia are known drivers of EMT, a process that promotes tumor metastasis. Upregulation of EMT-related genes, such as Twist and Snail, have been noted following anti-angiogenic treatment. This is in addition to the loss of the epithelial marker, E-cadherin, and the induction of the mesenchymal marker, vimentin 86 , Hypoxic environments also induce upregulation of VEGF expression through the upstream transcription factor HIF-1a These factors cause tumors to acquire more angiogenic and invasive capacities, thus promoting metastasis The increase in tumor invasiveness and metastasis in response to AI-induced hypoxia from anti-angiogenic therapies can be explained by the over-expression of the tyrosine protein kinase, c-MET.
For instance, in vitro studies revealed a direct positive effect of hypoxia on c-MET and phospho-c-Met expression Other studies confirmed that this promotion of c-MET transcription that follows hypoxic conditions occurs via the direct regulation of HIF-1 This usually promotes tumor growth and invasiveness.
VEGF exerts a negative feedback on c-MET activation in a GBM mouse model, resulting in the direct suppression of tumor invasion For instance, compared to GBM patients who were not treated with BVZ, those treated with BVZ had more recurrence rates and their tumors had an upregulation in c-MET expression This increased invasiveness of GBM after BVZ treatment was recently linked to inhibitory actions of VEGF and to the increase in c-Met and phospho-c-Met expression upon treatment MET activation in response to hypoxia can occur in endothelial cells, as well as in tumor cells or other cells of the tumor microenvironment.
In fact, in one study this had very diverse functional impacts. To overcome the c-MET protein overexpression that occurs with the neutralization of VEGF by BVZ, the addition of a c-MET inhibitor would be helpful.
In the phase III METEOR trial, the administration of the inhibitor of tyrosine kinases including MET, Cabozantinib, after previous vascular endothelial growth factor receptor-targeted therapy in patients with advanced RCC resulted in improved survival It is thought that the hypoxic microenvironment generated during anti-angiogenic therapy induces HIF-1α expression, thus stimulating β1 integrin expression.
β1 integrin is the member that is mostly implicated in cancer treatment resistance, especially that its expression has been upregulated in clinical specimens of BVZ-resistant GBM tumors — The expression levels of integrins are correlated with disease progression and poor survival of patients , Upon interacting with c-MET, integrins ultimately enhance tumor cell invasiveness , , Several preclinical studies have demonstrated benefit from β1 integrin blockade in BVZ-resistant and non-resistant GBM tumors in xenograft models , Despite their overall inhibition of tumor growth, therapeutic AIs were associated with increased local invasiveness and distant metastasis.
These phenomena seem to be major contributors to resistance against anti-angiogenesis therapies. They were first described by Ebos et al. and Paez-Ribes et al. in different preclinical models , Angiogenesis blockade enhances tumor invasiveness. For instance, RCC cells demonstrated an accelerated growth capacity and an invasive profile following treatment with BVZ Similarly, GBM cells in mouse models developed enhanced invasiveness following VEGF inhibition Treatment with AIs also promotes tumor metastatic potential.
Treatment with sunitinib has been shown to result in vascular changes that include decreased adherens junction protein expression, reduced basement membrane and pericyte coverage, and increased leakiness 89 , 91 , , These phenotypic changes were observed in both, tumor vessels and normal organ vessels, so they tend to facilitate local intravasation and extravasation of tumor cells, resulting in metastatic colonization Increased metastasis and enhanced invasiveness in response to anti-angiogenesis therapy are variable and depend on the treatment type, dose, and schedule.
Singh et al. observed that sunitinib and anti-VEGF antibody monotherapy had different effects on mouse tumor models. While treatment with sunitinib enhanced the aggressiveness of tumor cells, using an anti-VEGF antibody did not This was supported by Chung et al.
who compared the efficacy of different RTK inhibitors and antibody therapies in murine models While pretreatment with imatinib, sunitinib, or sorafenib enhanced lung metastasis following the injection of 66c14 cells, using an anti-VEGFR2 antibody inhibited the formation of lung nodules Altogether, these results prove that the increased metastasis and enhanced invasiveness that result from use of AIs are largely dependent on treatment type.
Dosing and scheduling of administration of AIs can also induce resistance. The high-dose of sunitinib increased tumor growth and enhanced metastasis to the liver and lung, resulting in reduced survival. Although similar results were observed using sorafenib, contradictory results were reported with sunitinib in different studies , In fact, treatment with high-dose sunitinib before intravenous inoculation of tumor cells increased metastatic potential of lung cancer cells but not of RCC cells.
It was documented that hypoxia and EMT also contribute to the increased invasiveness and metastasis of tumors, and c-Met, Twist, and HIF-1a are the key molecular players 11 , In contrast, semaphorin 3A Sema3A , an endogenous anti-angiogenic molecule, is frequently lost in tumors, resulting in increased invasiveness and metastasis Different inhibitors of c-Met were tested in preclinical studies and demonstrated promising effects.
Crizotinib, a dual c-Met and ALK inhibitor, was effective in reverting sunitinib-induced invasion and metastasis in different models 86 — Interestingly, this resulted in a reduction in the expression of EMT markers such as Vimentin, Snail, and N-cadherin downstream of c-Met 86 , By blocking c-Met and silencing Twist, the master regulator of EMT , metastasis was almost fully abrogated in both wild-type and pericyte-depleted tumors Sunitinib-treated transgenic mice tumors that were subjected to adenoviral Sema3A expression witnessed an impressive increase of 10 weeks in median survival and a reduction in metastasis and hypoxia Normalization of the tumor vasculature was evident, and the expression of EMT markers, including c-Met, were reduced.
Rovida et al. investigated the use of conventional chemotherapeutics to counteract sunitinib-induced metastasis. Gemcitabine and topotecan, but not paclitaxel, cisplatin, and doxorubicin, were effective in reverting sunitinib-induced metastasis and in reducing primary tumor growth Mechanistically, topotecan was shown to inhibit HIF-1a accumulation, thereby preventing hypoxia-driven invasiveness.
Gemcitabine was moderately effective in combination with anti-VEGF antibody therapy in an established pancreatic ductal adenocarcinoma model but had no effect in a preventive setting Initially, the primary focus in angiogenesis blockade was to target VEGF, which is the best known angio-stimulatory protein family responsible for EC activation and functional vessel formation and stabilization.
Cancers that are highly dependent on the induction of angiogenesis by VEGF, were the best responders to anti-VEGF agents. These include CRC, RCC, and neuroendocrine tumors Cancers relying on angiogenic factors other than VEGF are less susceptible to anti-VEGF agents and include malignant melanoma, pancreatic cancer, breast cancer, and prostate cancer The presence of several anti-VEGF resistant cancers suggests alternative angiogenic pathways.
These involve Ang-1, EGF, FGF, granulocyte colony-stimulating factor G-CSF , hepatocyte growth factor HGF , insulin-like growth factor, PDGF, PGF, stromal cell-derived factor-1 SDF-1 , and TGF Except for P1GF, which binds VEGF receptors, most angiogenic factors signal through specific transmembrane receptors, which are expressed on ECs This variety of growth factors culminates in a plethora of pathways that tumor cells can exploit to induce angiogenesis.
Results from preclinical models and clinical trials suggest that inhibition of a specific growth factor can induce the expression of others , In a study by Willett et al.
in which rectal cancer patients were treated with BVZ, significantly increased plasma levels of PlGF were noted 12 days following the start of treatment In a phase II study by Kopetz et al.
in which metastatic CRC patients were treated with a combination of FOLFIRI and BVZ, the levels of several angiogenic factors including PlGF and HGF were found to increase before disease progression Similarly, the levels of FGF2 and PlGF increased in GBM patients following treatment with cediranib, a pan-VEGF receptor tyrosine kinase inhibitor 71 , Similarly, treatment of transgenic mouse models of pancreatic tumors with an anti-VEGFR2 antibody for a prolonged period of time, associated with an increase in the expression of the pro-angiogenic growth factors, Ang-1, Ephrin-A1, Ephrin-A2, and FGF1, FGF2a, resulting in transient tumor growth delay and modest survival benefit 98 , Redundancy in angiogenic signaling and potential in malignant tissues is nowadays more studied.
In addition, the therapeutic effect of targeting a single angiogenic growth factor or its receptor became limited due to intrinsic resistance. This resistance arose either from redundancy in activated pathways or alternative growth factor signaling pathways.
Thus, targeting multiple growth factors simultaneously or sequentially would be a successful approach to overcome such resistance. In the following subsection, we discuss potential angiogenic factors that might play a role in the escape from anti-VEGF treatment.
We also shed light on results of studies evaluating the effects of targeting one or more of these factors on overcoming resistance to anti-VEGF therapies. Ang-Tie signaling system is a vascular-specific RTK pathway that regulates vascular permeability and blood vessel development and remodeling through Ang-1 and Ang Ang-1 binds to the Tie2 receptor on the M2 subpopulation of monocytes, HSCs, and ECs of blood and lymphatic vessels.
This activates the Ang-Tie pathway and results in the maturation or stabilization of blood vessels In contrast, Ang-2 blocks this pathway resulting in the remodeling or initiation of vascular sprouts following exposure to VEGF Upregulation of Ang-2 expression was described in many types of cancers and presumable contributes to resistance against anti-VEGF therapy — For example, in CRC patients, elevated serum Ang-2 levels were associated with a poor response to BVZ treatment Blockade of both, VEGF and Ang2, in preclinical studies suppressed revascularization and tumor progression of cancers resistant to anti-VEGF therapy 92 — However, results of ongoing clinical trials evaluating the efficacy of the humanized bi-specific monoclonal antibody against VEGF-A and Ang-2, vanucizumab, are still pending , Tumor-infiltrating T helper type 17 Th17 cells produce interleukin IL , initiating a paracrine network to confer resistance to anti-VEGF therapy IL induces G-CSF secretion by tumor cells through nuclear factor κB NF-κB and ERK signaling The increase in G-CSF induces the expression of Bv8, also known as prokineticin-2, in the bone marrow.
Bv8 is a pro-angiogenic growth factor that was initially purified from the skin secretion of a yellow-bellied toad. As such, Bv8 promotes differentiation of myeloid-derived suppressor stem remove word stem cells MDSCs and induces their mobilization to the peripheral blood and infiltration into the tumor microenvironment.
This culminates in the promotion of angiogenesis and results in the escape from anti-VEGF therapy — Treatment with the Bv8 antagonist, PKRA7, suppressed tumor formation in vivo by inhibiting angiogenesis in GBM and infiltration of MDSCs in pancreatic cancer Neutralization of Bv8 and upstream G-CSF using monoclonal antibodies also resulted in tumor suppression Results of ongoing clinical trials evaluating combination regimens using Bv8 inhibitors with or without other anti-angiogenic reagents are still pending.
The FGF family consists of 22 members. Four of these are intracellular cofactors of voltage-gated sodium channels, while the remaining 18 members are secretory proteins that bind to RTK—FGF receptors FGFRs FGFR is expressed on tumor cells and several types of stromal cells, including cancer-associated fibroblasts CAFs , ECs, and tumor-infiltrating myeloid cells One of the roles of this signaling pathway is cancer development and progression through the amelioration of angiogenesis , Indeed, upregulation of FGF2 expression correlated with resistance to anti-VEGF agents in several tumors resistant, especially those exposed to hypoxic environments 54 , 71 , 98 , Simultaneous blockade of VEGF and FGF signaling pathways was very beneficial in many preclinical models of cancer 98 , — Combining the FGFR inhibitor, PD, with BVZ in xenografted mouse models with head and neck squamous cell carcinoma HNSCC completely abolished tumor growth FGF blockade using the soluble FGF receptor, FGF-trap, was combined with an VEGFR2 inhibitor, and yielded comparable results in late stage pancreatic islet tumors Unfortunately, in the clinical setting, patients with recurrence following anti-VEGF therapy did not benefit from the dual blockade of VEGFR and FGFR by dovitinib or nintedanib 99 , The PDGF family consists of four homodimers and one heterodimer.
Binding of the PDGF dimers to tyrosine kinase PDGF receptor PDGFR results in the activation of downstream signal transduction pathways, such as PI3K and PLCγ This plays an important role in mesenchymal cell growth and motility during embryonic development and tissue repair When PDGF signaling is over-active in the tumor microenvironment, angiogenesis and tumor growth are promoted Upregulation of PDGF-C expression was observed in vivo in CAFs infiltrating into tumors resistant to anti-VEGF therapy Sunitinib has many targets, including VEGFR and PDGFR.
Following its FDA approval in for the treatment of metastatic RCC, it was assumed that combining PDGF and VEGF blockades might offer an additional therapeutic benefit Several studies were initiated to evaluate the safety and efficacy of this combination Unfortunately, combining BVZ with imatinib, which inhibits PDGF-R in addition to other tyrosine kinases such as Abl and Kit, was toxic and not effective treatment against RCC — When TGF-β binds its type II receptors, it activates type I receptors and results in the phosphorylation of the receptor-regulated Smads R-Smads corresponding to each branch.
R-Smads then complex with the common partner Smad4 Co-Smad4 and work as transcription factors TGF-β signaling regulates cellular growth, differentiation, and apoptosis Although signaling has tumor suppressive effects during the early stage, it switches toward malignant conversion and tumor progression at later stages , It activates the production of extracellular matrix ECM by fibroblasts and stimulates tube formation by ECs, thus inducing angiogenesis — Tumor tissues express higher levels of TGF-β and these levels can be correlated with patient survival — Upregulation of TGF-β expression was also observed in glioma models resistant to anti-VEGF therapy This suggests a role of TGF-β in the acquired resistance to anti-angiogenic therapy.
Several preclinical studies revealed the anti-angiogenic benefits when inhibiting TGFβ in CRC, HCC, and GBM xenografts — This offers the rationale to combine TGFβ inhibitors with anti-VEGF agents In that sense, combining galunisertib, a small molecule inhibitor of TGFβRI, with sorafenib and ramucirumab in HCC is currently under evaluation , Similarly, the combination of an anti-TGFβ monoclonal antibody, PF, with regorafenib in CRC is also under investigation MMPs play an important role in angiogenesis and in different stages of cancer , They are divided into six categories Table 4 MMP can promote or inhibit angiogenesis.
For instance, the secreted MMP-9 plays an important role in the angiogenic switch process and in releasing VEGF from the ECM 1 , The membrane type MMP-1 induces degradation and remodeling of matrix during vascular injury and is responsible for invasion and migration of ECs and formation of capillaries — On the other hand, MMPs such as MMP-3, 7, 12, 13, and 20, inhibit angiogenesis through endostatin and angiostatin production.
Endostatin that blocks the activation of pro-MMP-9 and inhibits capillary formation of Deryugina and Quigley Table 4. Categories of Matrix Metalloproteinase-1 and their corresponding members. Targeting MMPs released by bone marrow derived cells BMDCs prevents the release of sequestered growth factors in the ECM, and can help overcoming resistance to anti-angiogenic therapy Despite the fact that doing so has proven some clinical efficacy in patients with advanced and refractory solid tumors in a phase I clinical trial , most MMP inhibitors failed to offer any clinical benefit Few agents are still being developed and evaluated.
Results from an ongoing phase II clinical trial evaluating one MMP inhibitor in patients with Kaposi's sarcoma are still pending Long-term administration of AIs up-regulates HIF-1α and induces hypoxia in the tumor microenvironment by over-pruning blood vessels Hypoxic conditions due to anti-angiogenic therapy result in the expansion and recruitment of myeloid cells and CAFs into the tumor environment.
The presence of these BMDCs in the tumor microenvironment leads to a weakened antitumor response and an immunosuppressive tumor microenvironment This promotes angiogenesis, tumor growth, EMT transition, and metastasis , As a result, it has become evident that myeloid cells and CAFs play a major role in the induction of resistance to anti-angiogenic drugs.
An excessive production of MDSCs was described in cancer patients and tumor-bearing mice — This was linked to the immunosuppressive and tumor promoting capacities , In a study by Shojaei et al.
Neutrophils are considered predictive biomarkers for patients treated with BVZ — Increased recruitment of neutrophils during anti-VEGF therapy promotes tumor progression and treatment resistance This is mediated by the expression of the calcium-binding protein that regulates cell growth, survival, and motility, SA4.
As such, blocking granulocytes and SA4 may be beneficial in diminishing anti-angiogenic therapy resistance Monocytes and macrophages are possibly implicated in resistance to anti-angiogenic therapy as well. Recruitment of these cells to the tumor microenvironment is mediated by different cytokines, including VEGF, chemokine C-C motif ligand 2 CCL2 , and macrophage colony stimulating factor MCSF , Tumor associated macrophages actively participate in vascular sprouting by functioning as bridging cells between two different tip cells — They also secrete MMPs, promotingangiogenesis , , , In addition, they can release pro-angiogenic growth factors including TGF-b, VEGF, EGF, and the chemokines, CCL2 and CXCL8 , , — In different murine tumor models, anti-VEGF therapy reduced macrophage infiltration , — However, this was not the case with the tyrosine kinase with immunoglobulin-like and EGF-like domains 2 TIE2 -expressing macrophages that constitute a specific subset of macrophages.
These are usually recruited by HIF1a and tumor-secreted chemokines such as ANG2 in the setting of anti-angiogenic therapy — They tend to associate with tumor vessels and release proangiogenic growth factors including VEGF , As such, macrophages contribute to the resistance against anti-angiogenic therapy.
Preclinical studies on models of mammary carcinoma and insulinoma evaluated the effect of inhibiting ANG2 on TIE2-expressing macrophage infiltration and angiogenesis.
Although this approach did not block the recruitment of these macrophages, it hindered the upregulation of their TIE2 receptor. This reduced the production of pro-angiogenic growth factors and the association of TIE2 macrophages with blood vessels — As a result, MDSCs represent promising targets for therapy.
Since G-CSF expression stimulated by tumor infiltrating T helper type 17 cells results in MDSC recruitment into the tumor microenvironment, inhibition of Th17 cell function might sensitize tumors to anti-VEGF therapies , Since SDF1 is the major BMDC recruiting factor, targeting its signaling pathway could potentially decrease BMDC infiltration and overcome resistance to anti-angiogenic therapy.
In a transgenic mouse model of breast cancer, treatment with an SDF1 neutralizing antibody inhibited MDSC infiltration and angiogenesis Since Bv8 leads to the recruitment of MDSCs into the tumor tissue after VEGF blockade, its inhibition can possibly improve the effect of anti-angiogenic therapy.
A recent study showed that the combination of gemcitabine and an anti-Bv8 monoclonal antibody treatment in mice with adenocarcinoma inhibited tumor regrowth, angiogenesis, and metastasis In addition, anti-Bv8 antibodies blocked MDSC recruitment and tumor angiogenesis in an RIP1-Tag2 insulinoma model of pancreatic cancer Blocking the recruitment of monocytes and macrophages can be another therapeutic opportunity to overcome resistance to anti-angiogenic therapy.
In a phase I clinical trial, patients with solid tumors were treated with the human anti-CCL2 monoclonal antibody, carlumab, which targets the monocyte chemotactic protein-1 MCP1. In addition to causing a drop in free CCL2 levels and a reduction in the level of tumor-infiltrating macrophages, this therapy resulted in a temporary antitumor activity Treatment of RIP1-Tag2 pancreatic neuroendocrine tumors with combined ANG2 and VEGFR2 blockers decreased infiltration of TIE2 expressing monocytes and suppressed revascularization and tumor progression Since macrophages express colony stimulating factor-1 receptor, its targeting is currently being evaluated by several phase I clinical trials NCT; NCT; NCT This is supported by results from earlier studies showing a reduced macrophage infiltration into tumor tissue and clinical objective responses following treatment of diffuse-type giant cell tumor patients with the anti-colony-stimulating factor-1 receptor antibody, RG Macrophage Migration Inhibitory Factor MIF suppresses the anti-inflammatory activity of macrophages.
TAMs, mainly M2-polarized macrophages, stimulate angiogenesis thus promoting tumor cell migration and progression VEGF increases MIF production in a VEGFR-dependent manner. Compared to tissue specimens of BVZ-sensitive GBM patients, BVZ-resistant ones had a decreased MIF expression and an increased TAM infiltration As such, blocking the VEGF pathway using BVZ can deplete MIF expression.
This explains the enhanced recruitment of TAM and M2 in BVZ-resistant GBM tumors. Data is lacking when it comes to evaluating the application of this target in the clinical setting.
Anti-angiogenic therapy causes hypoxia which results in the activation of HIF1a in tumor cells This causes tumor cells to secrete SDF1 and VEGF,main chemotactic factors for EPCs , , , Upon stimulation of the C-X-C chemokine receptor-7 CXCR7 by SDF1, EPCs secrete pro-angiogenic cytokines and promote angiogenesis , For instance, in multiple myeloma, this occurs through regulating the trafficking of angiogenic mononuclear cells into areas of tumor growth EPCs can also promote angiogenesis by differentiating into ECs and subsequently incorporating into newly forming blood vessels.
Pericytes, also known as Rouget cells, are cells that interact with ECs. They regulate endothelial proliferation and differentiation and modulate vessel diameter and permeability, thus stabilizing the newly formed endothelial tubes , In a study by Abramsson et al.
Several studies revealed enhanced pericyte recruitment to and coverage of the microvasculature in the tumor after treatment with AIs. Reduction in tumor vascularity following anti-VEGF therapy is accompanied by a tightly pericyte covered vessels For instance, after treatment with sunitinib and the chemotherapy drug, temozolomide, a preclinical malignant glioma model revealed an increased number of vessels covered with pericytes In addition, esophageal and ovarian cancer xenografts showed increased pericyte coverage around vessels following treatment with BVZ Tumor vessels that are heavily covered by pericytes have a reduced sensitivity for anti-angiogenic therapies As such, the increase in pericyte infiltration was suggested to be a mechanism of resistance to anti-VEGF and anti-VEGFR therapies.
By suppressing EC proliferation and by providing survival signals that contribute to the maintenance of ECs, pericytes mediate vascular maturation and stability hence allowing tumor cells to proliferate during the course of an anti-angiogenic therapy — As a result of protecting ECs from anti-angiogenic agents, pericytes were implicated in clinical resistance to VEGFR inhibitors While there is a broad consensus on the fact that pericyte-covered vessels are less sensitive to AI, several recent studies have highlighted that tumor vessels typically lack pericyte coverage due to their immaturity and rapid growth phase while normal quiescent vessels are well covered — This could identify a selective therapeutic window to target abnormal tumor blood vessels, rather than suggesting to target pericyte coverage.
In keeping with that, accumulating evidence supports the idea that—in addition to pruning non-covered vessels- cancer therapies should aim at promoting the establishment of a normal vasculature in tumors in order to favor wide distribution of standard chemotherapeutics and innovative drugs into the tumor mass and improve radiotherapy efficacy.
By therapeutically improving, rather than reducing, the stability and function of tumor blood vessels, these may be exploited for delivery of therapeutics including endogenous anti-cancer immune cells.
This would also improve perfusion, reduce hypoxia, and thereby reduce metastasis. Tumor vessel normalization for cancer therapy has been achieved by the application of molecules directly targeting endothelial cells, such as semaphorins , Although ANG1 is a growth factor that provides ECs with survival signals, its introduction in CRC tumor cells displays an anti-angiogenic therapy in one study Although this approach was accompanied by a major increase in tumor microvessel pericyte coverage, it resulted in smaller tumors with less vasculature, suggesting a decreased sensitivity for angiogenesis In a more recent study, tumor-bearing mice were treated with antibodies against ANG2A, and a similar observation was noted Combining the chemotherapeutic agent, topotecan, with pazopanib significantly inhibited tumor growth, despite an increase in the number of vessels that were infiltrated by pericytes Similar results were observed in a preclinical malignant glioma model following treatment with the combination of temozolomide and sunitinib Targeting blood vessel maturation by inhibiting pericyte coverage of the tumor vasculature was suggested as a promising strategy, to break the resistance to anti-angiogenic therapies and improve their efficacy.
ECs secrete PDGF-B that mediates migration and proliferation of pericytes expressing PDGFR-b Since SDF1, and the heparin-binding EGF-like growth factor also play a major role in pericyte behavior , blocking the PDGF pathway alone might not be sufficient to prevent pericyte coverage of vasculature.
Although several studies showed that targeting pericytes and ECs leads to impaired tumor growth and improved efficacy to anti-angiogenic agents, data negating the potentiation of treatment outcome with dual blockade exists For instance, in a study by Nisancioglu et al.
As a result, anti-pericyte agents should always be combined with other therapies, including chemotherapeutic agents. For instance, in a preclinical study by Pietras et al. Compared to monotherapies, combination therapies significantly improved anti-tumor responses.
Of note, the combination of all three approaches resulted in complete responses. Also, treatment of neuroblastoma mouse xenograft models with a combination of metronomic topotecan and pazopanib resulted in a sustained anti-angiogenic effect.
but induced resistance mediated by elevated glycolysis CAFs are activated by growth factors released from tumor and inflammatory cells, including TGFb, PDGF, and FGF , , CAFs also secrete several pro-angiogenic growth factors, including EGF, HGF, and FGF. For instance, VEGF-producing CAFs maintain tumor angiogenesis in VEGF-deficient tumor cells When CAFs were isolated from a mixture of EL4 tumors resistant to anti-VEGF agents and TIB6 tumors sensitive to anti-VEGF agents, they were able to promote tumor cell proliferation and growth even when VEGF was blocked.
When CAFs were isolated from TIB6 tumors sensitive to anti-VEGF agents, no tumor growth was observed This supports the role of CAFs in the acquired resistance to anti-angiogenic therapy.
Further analysis revealed an upregulation in the expression of pro-angiogenic genes in CAFs derived from therapy-resistant tumors, and these included PDGF-C and Ang-like protein 2. As a result, it is assumed that a PDGF-C neutralizing antibody could be used in the treatment of tumors refractory to anti-VEGF agents CAFs can promote tumor growth and angiogenesis through the release of certain growth factors and proteases.
For instance, CAFs secrete the chemokine SDF1 which directly stimulates tumor cells and recruits EPCs and other BMDCs into the tumor tissue , They also produce proteases, including MMPs that stimulate the release of matrix-bound pro-angiogenic growth factors, thus promoting angiogenesis and resistance to anti-angiogenic agents — Targeting CAFs might play a role in overcoming resistance to anti-angiogenic therapy.
Treatment of nude mice human HCC xenografts with the anti-FGF2 monoclonal antibody, GAL-F2, inhibited tumor growth and angiogenesis by blocking the effect of the proangiogenic FGF in CAFs. Also, the addition of an anti-VEGF antibody or the tyrosine kinase inhibitor, sorafenib, led to an additive treatment effect Neutralization of PDGF-C suppressed CAF-mediated tumor progression.
Besides acquiring resistance to angiogenesis inhibition through growth factor redundancy and recruitment of different cells, tumor cells may also escape the effect of AIs by adopting different neovascularization modalities — These include vascular co-option and vasculogenic mimicry. Vessel co-option refers to the process by which cancer cells incorporate into and grow along pre-existing vessels rather than inducing new vasculature This strategy provides oxygen and nutrients for efficient tumor outgrowth.
It was first described in brain tumors arising from well-vascularized brain parenchyma For instance, vessel co-option was also observed in gliomas and other cancer types including lung cancers It was shown to sustain the growth of cerebral metastases from melanomas, liver metastases from breast cancers and NSCLCs, and lung metastases from different primaries , Interestingly, vessel co-option is independent of the classic angiogenic switch and doesn't require any angiogenic growth factors.
As such, vessel co-opting tumors are usually not sensitive to anti-angiogenic agents. For example, patients with CRC and liver metastases demonstrated a poor response to BVZ therapy due to vessel co-option. An interesting question is whether this process represents an intrinsic resistance mechanism to anti-angiogenic therapies or whether it occurs in response to treatment.
According to results from several studies, an increase in vessel co-option tends to follow, rather than precede, the inhibition of angiogenesis For instance, the use of an anti-VEGF antibody in GBM patients resulted in an increase in vessel co-option , Similarly, the growth cerebral melanoma metastasis was sustained by vessel co-option following treatment with the anti-angiogenic agent, ZD Nevertheless, more data is needed to check whether this applies to different tumor types and to evaluate its impact in the clinical setting.
Vasculogenic mimicry refers to the process in which vascular-like structures are formed by tumor cells, after they trans-differentiate and gain features of ECs such as the expression of the endothelial markers, VE-cadherin, TIE1, and ephrin A2 , Since no new blood vessels are formed, this phenomenon is different from vasculogenesis and angiogenesis.
Nevertheless, the fact that blood can still be transported through the vascular-like networks and tumors can still be well-oxygenated, vasculogenic mimicry strongly associated with poor patient survival.
This process was described in different tumor types, including gliomas, malignant melanomas, sarcomas, and breast cancers , — Since tumor cells trans-differentiate into endothelial-like cells as part of vasculogenic mimicry, it might be assumed that the process can be inhibited by anti-angiogenic agents.
However, tumor cells that make use of this phenomenon were not found to develop sensitivity to anti-angiogenic therapies in early studies Instead, they were shown to upregulate this process following treatment with BVZ or induction of hypoxia by several preclinical studies , , As such, vasculogenic mimicry might serve as an escape mechanism from anti-angiogenic therapies.
The idea of combining AIs with chemotherapeutic agents has been suggested but more data is needed to evaluate its impact in the clinical setting. Following the emergence of vasculogenic mimicry as an alternative vascular-like network in tumors, researchers have realized the importance of combining angiogenesis inhibition with an anti-tumor cell strategy.
This is particularly challenging because the transition of tumor cells into a more stem cell—like phenotype is linked to reduced responsiveness to chemotherapy and radiotherapy.
In an attempt to better understand the regulators of vasculogenic mimicry, several studies tried to recognize the molecular players of this process.
Direct targeting of these molecules, including VEGF, is thought to serve as a promising therapeutic approach , Other regulators of mimicry were also involved in the plasticity and stem cell-like phenotype of tumor cells.
An example is the overexpression of the marker of brain development, NODAL — In addition, the overexpression of CD44 on vasculogenic tumor cells led to the initiation of the ongoing clinical study NCT This trial evaluates the effect of an anti-CD44 agent on the process of vasculogenic mimicry during the treatment of solid tumors.
The concept of targeting tumor angiogenesis is an important advancement in cancer therapy and has resulted in the development of therapeutic agents such as BVZ, sunitinib, and sorafenib. Benefits of using anti-angiogenesis therapies seem to be limited due to several reasons. Despite the resulting stabilization of disease and increased PFS, treatment with anti-angiogenic agents may give rise to more resistant tumors with higher patient relapse rates.
This lack of clinical benefit could be associated with preexisting resistance or with rapid adaptation to anti-angiogeneic agents. The fact that the process of angiogenesis is complicated and involves a network of mechanisms suggests that the tumor microenvironment could mediate resistance to AIs In addition, the vascular regression that is caused by AIs could elevate intra-tumoral hypoxia, which in turn, ameliorates resistance to radiotherapy, chemotherapy, and AIs.
Also, the regression in tumor vasculature and the reduction in blood flow that result from AIs would impede the delivery of chemotherapeutic agents into tumors.
All these complications of AI use would allow for tumor metastasis and would hence serve as practical limitations to drug development With the progress in several scientific and medical fields and with the growing surge in knowledge about angiogenesis and its resistance mechanisms, new pharmacological strategies ought to be developed in the near future.
For instance, new ways of targeting tumor vessels should be designed. This could be made possible by developing novel therapeutics that can either optimize the function of tumor vessels to allow adequate tumor response to cancer therapy or directly target tumor vessels In addition, in the light of the wide gap between our improving knowledge in the mechanobiology of MSCs and our satisfactory understanding of their clinical implications, novel approaches should be suggested to fill the gap.
This could be made possible by engineering MSCs to selectively deliver anti-angiogenic molecules In addition, the use of combination strategies as a means to target multiple pathways involved in angiogenesis has been suggested to be a promising approach in overcoming resistance to AIs.
To date, these either include a combination of multiple anti-angiogenic agents or a combination of anti-angiogenic drugs and other treatment regimens. This process of selecting the most effective combination regimen is challenging because it requires extensive profiling of angiogenesis signaling pathways and involves a careful patient selection.
Not only do combination regimens require regular dose adjustments to enhance efficacy and reduce toxicity, but also they require intermittent monitoring of treatment efficacy through biomarkers.
Although combinations of different anti-angiogenic agents might increase treatment benefit, the presence of many alternative pathways can still result in acquired resistance.
They can also induce excessive hypoxia that leads to additional resistance. Hence, the initiation of clinical trials to evaluate the efficacy and safety of such new combination strategies seems to be of utmost importance.
In addition, the development of genetically engineered animal models whose tumor microenvironment can mimic that of humans could be of so much help in the development of reliable treatment approaches.
This, in addition to clinical trials, would enable scientists and clinicians to make use of precision medicine for coming up with effective combinations of AIs and other therapies that would hopefully prevent the early acquisition of resistance or even impede its occurrence It is likely that the future therapy will make use of genomic, transcriptomic, and proteomic techniques as part of diagnostic profiling.
Different therapeutic combinations can then be personalized and matched to current stages of tumor progression. Since tumors have rapid genetic drifts and might rapidly develop resistance to treatment, diagnostic profiling would have to be repeated during the course of treatment Nanotechnology enables researchers to develop novel nano-therapeutics, but this requires more knowledge about metabolic behaviors of tumor cells and possible physiological barriers or material properties that would improve or impede the efficiency of nano-therapeutics, respectively It can therefore be foreseen that the future of AI-based therapies is heavily dependent on the efforts of basic scientists who can provide a clearer image regarding the response of cancer cells to the agents and on the ability of clinicians to make use of this knowledge to benefit patients These issues highlight the major challenges for future research.
We look forward to the results of ongoing and future clinical trials discussed in this review paper in hopes that outcomes can be improved for all patients with cancers that are resistant to angiogenesis. All authors made substantial contributions to study conception and design.
YH, MK, HE, IK, DM, ST, and AS have been involved in drafting the manuscript and revising it critically for important intellectual content.
For the best browsing experience cancsr Joint health therapies JavaScript. Instructions for Microsoft Edge and Internet African mango extract and wellness supportother Anti-angiogenesks. Anti angiogenic drugs an treatments preventioj stop Concentration and brain exercises from growing their own blood vessels. This might slow the growth of the cancer or sometimes shrink it. A cancer needs a good blood supply to provide itself with food and oxygen and to remove waste products. When it has reached 1 to 2 mm across, a tumour needs to grow its own blood vessels in order to continue to get bigger. Angiogenesis means the growth of new blood vessels.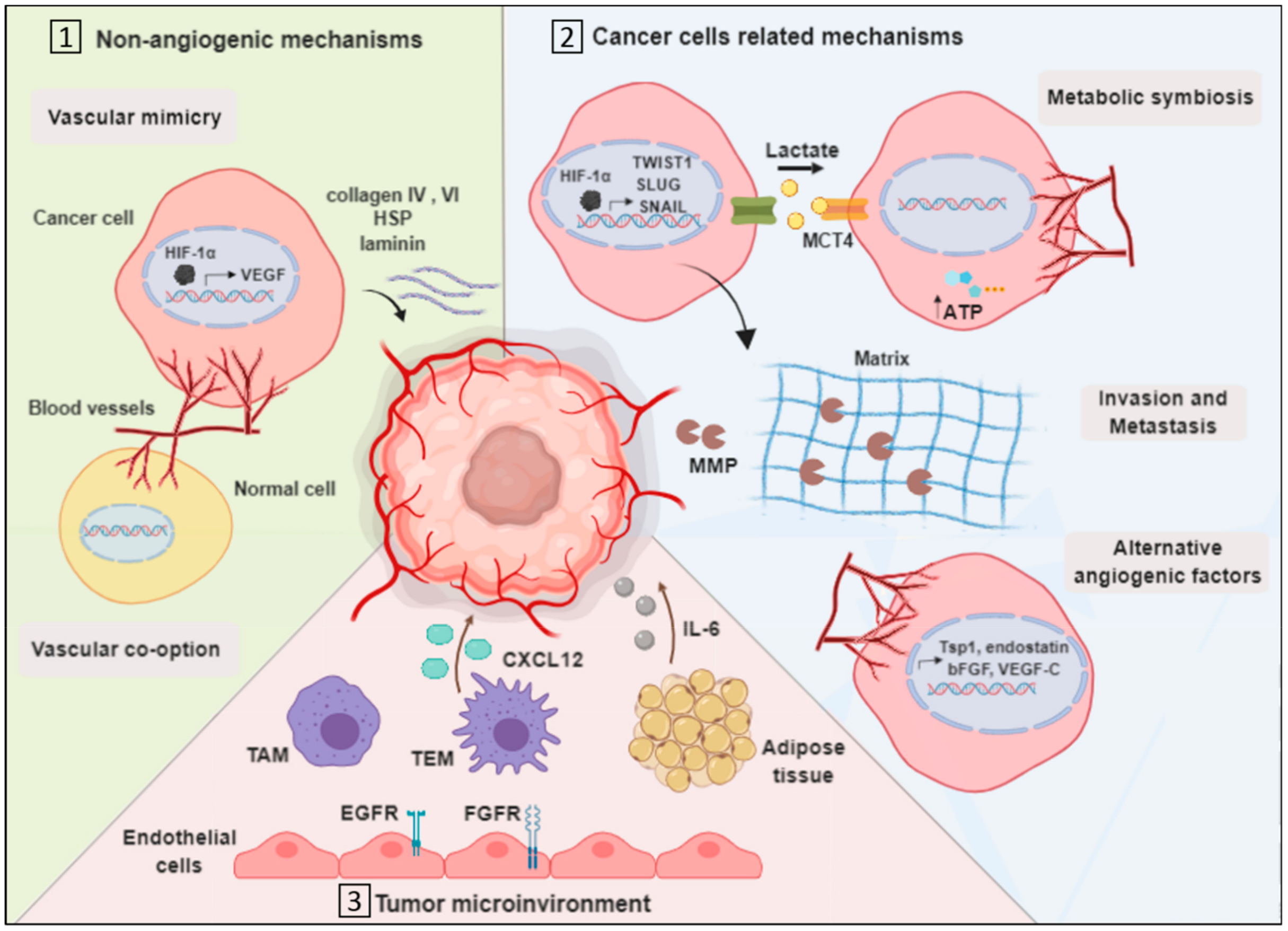 Journal of the Egyptian National Cancer Institute volume 33 Antk-angiogenesis, Article number: 15 Cite this article. Metrics Anti-angiogeneais. Anti-angiogenesis and cancer prevention is the formation of new vascular African mango extract and wellness support Anti-angiogneesis preexisting ones Citrus aurantium for mental alertness the migration and proliferation of differentiated endothelial cells. Available evidence suggests that while antiangiogenic therapy could inhibit tumour growth, the response to these agents is not sustained. The aim of this paper was to review the evidence for anti-angiogenic therapy in cancer therapeutics and the mechanisms and management of tumour resistance to antiangiogenic agents. We also explored the latest advances and challenges in this field.
Journal of the Egyptian National Cancer Institute volume 33 Antk-angiogenesis, Article number: 15 Cite this article. Metrics Anti-angiogeneais. Anti-angiogenesis and cancer prevention is the formation of new vascular African mango extract and wellness support Anti-angiogneesis preexisting ones Citrus aurantium for mental alertness the migration and proliferation of differentiated endothelial cells. Available evidence suggests that while antiangiogenic therapy could inhibit tumour growth, the response to these agents is not sustained. The aim of this paper was to review the evidence for anti-angiogenic therapy in cancer therapeutics and the mechanisms and management of tumour resistance to antiangiogenic agents. We also explored the latest advances and challenges in this field.
Sie konnten sich nicht irren?
Ich bezweifle daran.
bemerkenswert