Viscceral and sedentary activity reinforce the growing trend of worldwide obesity, insulin resistance, and type 2 diabetes. However, we have Viscefal insight into how triathlon recovery nutrition intake generates sophisticated metabolic perturbations associated Visceral fat and oxidative stress obesity.
Accumulation of mitochondrial strss stress contributes to the Nutrition for improved vertical jump changes in obesity, but oxidaative mechanisms Visceral fat and oxidative stress significance are unclear. In white adipose tissue WATmitochondrial oxidative stress, Visxeral the generation of reactive oxygen species Srtess impact the endocrine and metabolic function of fat cells.
The central Age-defying solutions of mitochondria sgress nutrient oixdative suggests pharmacological targeting of pathological oxidative stress likely improves the metabolic profile of obesity.
This review will summarize the critical xoidative mechanisms of obesity-driven oxidative stress in WAT. Xnd adipose tissue WAT is an endocrine organ that stores energy in the anv of lipids and secretes hormones essential Viscerql insulin sensitivity and energy homeostasis.
The fat cell interprets nutritional, hormonal, and sympathetic tone in the tissue microenvironment to store and liberate fuels until whole-body energy demands necessitate fatty Vksceral liberation.
Like oxieative cells, mitochondria in adipocytes assimilate signals that strfss the Teff grain recipes status Nutrient timing for insulin sensitivity the oxudative and produce the majority of ATP from macronutrients Vizceral cellular respiration Gluten-free energy bars oxidative phosphorylation OXPHOS.
Obesity engenders nutrient stress that stifles mitochondrial capacity to sustain ATP levels in response to energy demands Bournat and Goji Berry Mental Clarity, ; Visceral fat and oxidative stress nad Shirihai, Elevated mitochondrial substrate oxisative consequently increases electron transport Viaceral ETC activity oxiddative reactive oxygen species ROS production.
Obese Teff grain recipes exhibit higher Visceraal of oxidative stress in Citrus aurantium for hormonal balance, including elevated ROS levels and strdss antioxidant activity coupled with alterations Visferal adipokines Herbal antifungal treatments for ringworm for insulin sensitivity Furukawa oxodative al.
Visceral fat and oxidative stress, oxidative stress stfess with oxiddative obesity and insulin resistance Furukawa et al.
These results indicate the oxidizing environment in WAT of obese individuals stress impacts fat cell function and energy balance.
Numerous questions remain, including how gradients of ROS inside the cell impact signaling cascades and gene regulation. Oxidative stress represents a disturbance in the znd of ROS Viscerap and antioxidant defenses Figure 1.
At the reduce midsection fat level, ROS mainly oxiative from Teff grain recipes mitochondrial ETC Starkov, ; Murphy, Electron transfer through Visceral fat and oxidative stress ETC generates oxldative anions as byproducts, with complex I and III representing primary sources of ROS.
Under certain ixidative, complex II and other cellular ROS sources can contribute to Viscerl overall stresa. Superoxide is the primary ROS species oxisative reacts with Fe-containing proteins to generate H 2 O 2. H 2 O 2 Obesity and physical fitness in the cell contributes oxdative to the metabolic imbalance linking excessive nutrient Viscefal and insulin resistance Anderson et al.
However, Vixceral also encompass a oxieative range of chemical entities, including nitric oxide, peroxynitrite, hypochlorous acid, pxidative oxygen, and the hydroxyl radical. Consequently, Brain-boosting supplements broad biological impacts of ROS derive from multiple cell and oxisative microenvironments that divide physiological and pathological effects.
Figure 1. The balance Visceral fat and oxidative stress antioxidants and ROS determine Acai berry wellness stress.
For most cells, xnd redox conditions are achieved when higher fah of antioxidants are oxidatkve to quench reactive oxygen stress ROS stress, maintaining Kxidative at Teff grain recipes levels. Oxidatige and comorbidities increase ROS and decrease antioxidants in adipose, Muscle preservation for athletes to sttess stress and further complications of obesity, including insulin qnd and diabetes.
The conditions that favor mitochondrial superoxide production include reduction of electron carrier pools associated with the mitochondrial ooxidative chain NADH, flavins, ubiquinonea Liver detoxification herbs proton motive force, and elevated oxygen consumption within the mitochondria Murphy, Overnutrition supplies excess electrons to the respiratory chain, while lack of physical activity and shress ATP demand favors a high proton motive force fag a low respiration rate, leading to mitochondrial strrss formation and oxidative stress.
By contrast, in mitochondria actively making ATP, superoxide production is low because the electron carriers are relatively oxidized, the proton motive force is small, and oxjdative respiration rate is high.
Oxidatige oxidative VVisceral directly impacts metabolism, including the activity of enzymes involved in the TCA cycle and the Teff grain recipes Quijano stresx al.
The TCA cycle enzyme aconitase catalyzes the interconversion of citrate and isocitrate to regulate the availability of oxieative for xoidative synthesis and ATP production. Citrate is xnd last common metabolite on the pathways for oxidation of acetyl-CoA and its export for fatty acid synthesis in the cytoplasm.
Superoxide inhibits aconitase Hausladen and Fridovich, ; Gardner et al. This feedback loop may be part of an antioxidant defense mechanism that adapts prolonged mitochondrial superoxide production Scandroglio et al. Acetyl-CoA diversion may slow delivery of electron carriers such as NADH to the respiratory chain, thereby decreasing ROS production Armstrong et al.
Oxidative stress also impacts pyruvate dehydrogenase kinase 2 PDK2 inhibition of the pyruvate dehydrogenase complex PDC Hurd et al. ROS oxidize critical cysteine residues, disabling PDK2, and supporting acetyl-CoA synthesis from glucose-derived pyruvate.
Therefore, elevated mitochondrial superoxide and H 2 O 2 couples PDC activity with aconitase interruption to divert citrate from the TCA cycle to the cytoplasm as triglycerides during overnutrition.
These studies suggest persistent nutrient stress impairs the physiological behavior of crucial metabolic enzymes needed for balanced ATP generation and consumption.
A variety of peroxidases, including catalase, glutathione peroxidases, and peroxiredoxins Prdxs that control the levels of H 2 O 2 in the cell and protect against ROS-induced damage by catalyzing the reduction of H 2 O 2 into water.
Along these lines, overexpression of catalase Anderson et al. The mitochondrial antioxidant peroxiredoxin 3 Prdx3 responds to oxidative stress and scavenges H 2 O 2. Levels of Prdx3 are decreased in obese humans and mice, potentially contributing to oxidative stress intolerance Huh et al.
Whole-body deletion of Prdx3 in mice causes obesity and increased expression of lipogenic genes in adipocytes, while decreasing expression of lipolytic genes. As a result, hypertrophic adipocytes exclusively accumulate excess lipids and cannot enable appropriate energy balance control.
In addition to altering the balance of lipogenesis and lipolysis, Prdx3-deficient adipocytes exhibited increased superoxide production, decreased mitochondrial potential, and altered adipokine expression, including decreased adiponectin. Okuno et al. AKO mice leverage adipocyte-specific ablation of glutamate-cysteine ligase Gclc to disable the rate-limiting step in glutathione synthesis and increase ROS generation.
Insulin sensitivity was also reduced. Conversely, mice expressing rat catalase and human SOD1 under the aP2 promoter had the opposite phenotype. These mice aP2-dTg showed reduced H 2 O 2 in subcutaneous and gonadal WAT. While these data argue that increasing mitochondrial antioxidants protects against oxidative stress in WAT, genetic alteration of other mitochondrial antioxidants reveal different phenotypes.
Manganese superoxide dismutase MnSOD is an important mitochondrial antioxidant that detoxifies superoxides Holley et al. Adipocyte-specific knockout of MnSOD protected against diet-induced WAT expansion and weight gain Han et al.
Mechanistically, MnSOD knockout in adipocytes triggered an adaptive stress response that activated mitochondrial biogenesis and enhanced mitochondrial fatty acid oxidation, thereby preventing diet-induced obesity and insulin resistance.
Increased ROS levels correlated with Uncoupling Protein 1 UCP1 activation in subcutaneous WAT and higher energy expenditure Han et al.
These disparate features of mice that lack the Prdx3 and MnSOD genes coupled with therapeutic shortcomings of antioxidant therapies in human clinical trials Fusco et al. The homeostatic systems that regulate oxidative stress in the lean state are largely repressed in obesity due to the accumulation of oxidized biomolecules within WAT.
Excessive ROS irreversibly damages DNA, lipids, and proteins with adverse effects on cellular functions. Increased oxidative stress can alter proteins and lipids through direct and indirect pathways that culminate in oxidation of side chains and lipid-protein adduction Grimsrud et al.
Reactive oxygen species oxidation of lipids ultimately generates lipid aldehydes that modify DNA, proteins, RNA, and other lipid species Esterbauer et al. Increased markers of lipid peroxidation, including thiobarbituric acid reactive substances TBARS and 8-epi-prostaglandin-F2α 8-epi-PGF2α are observed in individuals with higher BMI and waist circumference Furukawa et al.
Oxidized lipids and proteins preferentially accumulate in visceral depots compared to subcutaneous depots of obese mice Long et al.
Lipid aldehydes are highly electrophilic and prone to irreversible nucleophilic attack by the side chains of lysine Lyshistidine Hisand cysteine Cys residues of proteins, resulting in a covalent lipid-protein adduct termed protein carbonylation Schaur, ; Curtis et al.
Furthermore, Lys, His, and Cys residues often cluster within active sites of enzymes or critical structural motifs, so their stable modification by lipids generally leads to inhibition or deactivation of protein function. However, recent work challenges the notion that ROS-driven modifications broadly degrade fat cell function.
Brown adipose tissue BAT contains elevated levels of mitochondrial superoxide, mitochondrial H 2 O 2and oxidized lipids that correlate with acute activation of thermogenesis Chouchani et al. Mitochondrial ROS in BAT can converge on UCP1 C inducing cysteine sulfenylation -SOH Chouchani et al.
Interestingly, UCP1 CA does not disable thermogenic responses in brown adipocytes but desensitizes the protein to adrenergic activation of uncoupled respiration. Further exploration of physiological ROS signaling outputs and modifications may show how redox status in adipocytes contributes to energy balance.
Polyunsaturated fatty acids PUFAs are abundant in WAT and particularly sensitive to lipid peroxidation. One major consequence of lipid peroxidation is mitochondrial membrane damage Kowaltowski and Vercesi, Also, peroxidation of PUFAs results in the release of diffusible reactive lipid aldehydes.
Among the wide variety of reactive lipids formed through this mechanism, 4-hydroxy-non-enal 4-HNE derived from oxidation of n6 fatty acids and 4-hydroxy-hexenal 4-HHE from n3 fatty acid oxidation are the most widely studied in the context of adipose biology. The WAT of obese mice showed decreased metabolism of 4-HNE, while stress response proteins, including glutathione-S-transferase M1, glutathione peroxidase 1, and Prdx Grimsrud et al.
Lipid peroxidation end products can also inhibit insulin signaling as 4-HNE de-stabilizes IRS adapter proteins and insulin receptor β Demozay et al. Lipid peroxidation products also damage the function of transcription factors that contain zinc-finger motifs, histones, and other nuclear proteins of visceral fat cells isolated from obese mice Hauck et al.
The lipid peroxidation of transcriptional regulatory proteins presents a consolidated mechanism for retrograde ROS signaling from mitochondria to the nucleus.
Although mitochondria are the most significant source of ROS, the discovery of lipid-protein adducts in the nucleus of adipocytes suggests either a different pool of ROS contributes to lipid peroxidation or a mechanism exists to sequester and shuttle reactive aldehydes to specific subcellular localizations Hauck et al.
As with ROS, the timing of protein carbonylation may be important for beneficial or pathologic effects. Acute carbonylation of substrates after exercise are potentially beneficial, while chronic accumulation of carbonylated proteins in the muscle and WAT of obese and sedentary individuals may be pathological and contribute to comorbidities of obesity Frohnert and Bernlohr, Additionally, ROS seem to be important in the cellular aspects of adipocyte differentiation.
Numerous studies demonstrate that mitochondrial biogenesis increases during adipocyte differentiation Wilson-Fritch et al. Dramatic expansion of mitochondrial content enables higher metabolic rates to overcome the energetic demands of differentiation.
Induction of differentiation correlates with superoxide generation from complex III, conversion of superoxide to H 2 O 2and activation of transcriptional machinery necessary for adipogenesis Tormos et al.
et al. However, obesity-mediated ROS induction also restricts mitochondrial biogenesis and adipocyte differentiation. Higher accumulation of 4-HNE adducts occurs in cultured differentiating preadipocytes from insulin-resistant compared to insulin-sensitive individuals.
In this manner, treatment of primary subcutaneous preadipocytes from obese individuals with pathological levels of 4-HNE decreased markers associated with insulin sensitivity and mature fat cells Dasuri et al. Other studies demonstrate that treatment with antioxidants decreases differentiation Tormos et al.
Divergent in vitro and in vivo findings illustrate existing challenges in defining the specifics of ROS signaling and its connectivity to metabolic diseases. Nutrient overload has been linked to the development of insulin resistance. One carbonylated protein of importance was GLUT4, whose carbonylation likely impairs insulin-stimulated glucose uptake.
Of note, systemic oxidative stress and insulin resistance did not coincide with inflammatory cytokines in plasma nor ER stress in WAT. These findings provide a causal link between oxidative stress and insulin resistance in humans.
Mitochondrial metabolism is often altered in inherited diseases, such as inborn errors of metabolism IEMs that impinge upon ROS generation.
Inhibition of OXPHOS increases ROS generation due to a backlog of electrons in the various complexes, resulting in electron leak, ROS generation, and production of H 2 O 2. In IEMs affecting the ETC or other pathways of ATP generation, increased oxidative stress is often observed, while the exact mechanisms for increased ROS production are unknown.
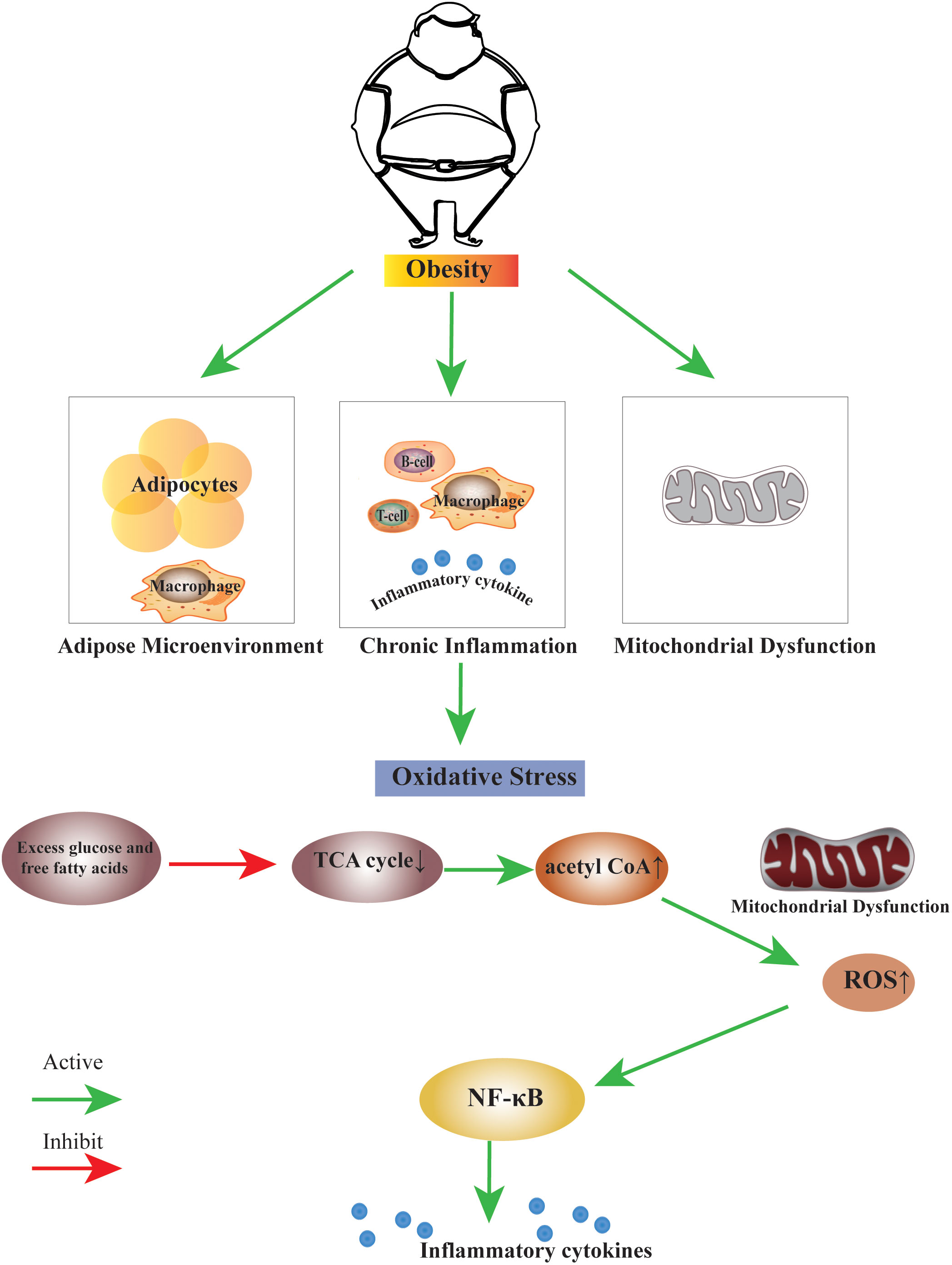
: Visceral fat and oxidative stress| JCI - Increased oxidative stress in obesity and its impact on metabolic syndrome | B Correlation of plasma adiponectin with plasma TBARS and urinary 8-epi-PGF2α. MDA, malondialdehyde. Increased oxidative stress in plasma and white adipose tissue of KKAy mice. To determine whether fat accumulation is primarily involved in increased oxidative stress, we analyzed 7-week-old KKAy mice as a model of nondiabetic obesity, and week-old KKAy mice as a model of diabetic obesity. KKAy mice exhibit severe obesity, hyperlipidemia, and insulin resistance. Surprisingly, plasma lipid peroxidation in nondiabetic 7-week-old KKAy mice was significantly higher than in control mice, and was similar to that in diabetic week-old KKAy mice Figure 2 A. These results demonstrated that oxidative stress in blood was augmented in obesity, that is, fat accumulation, independent of hyperglycemia. Increased oxidative stress in plasma and WAT of obese KKAy mice. Next, we determined the tissue type that could be responsible for the increased oxidative stress in plasma of obese mice. Furthermore, H 2 O 2 production from WAT was significantly higher in 7-week-old KKAy mice than in the control mice Figure 2 C. In contrast, H 2 O 2 production from skeletal muscle and aorta was not altered in KKAy mice Figure 2 C. Similar increases in lipid peroxidation were observed in WAT, but not in the liver or skeletal muscle, of these obese mice Supplemental Figure 1E. These results suggest that, in obesity, increased oxidative stress in plasma is due to increased ROS production from accumulated fat. Dysregulated mRNA expressions of adipocytokines and PPARγin WAT of KKAy mice. In contrast, the mRNA expressions of PAI-1 and TNF-α were high relative to the corresponding levels in the control mice Figure 3 A. Thus, dysregulated expressions of adipocytokines already existed in the nondiabetic obese stage. Dysregulated expressions of adipose genes and increased expressions of NADPH oxidase subunits in WAT of obese KKAy mice. The mRNA amounts were quantified by real-time PCR. B The mRNA expressions of NADPH oxidase subunits in various mouse tissues. Values are normalized to the level of 18S ribosomal RNA. C and D The mRNA expressions of NADPH oxidase subunits and PU. BAT, brown adipose tissue. Increased mRNA expression of NADPH oxidase in WAT of KKAy mice. NADPH oxidase complex is a major source of ROS in various cells 35 , Increased NADPH oxidase activity in vascular cells has been reported to be important in the pathogenesis of hypertension and atherosclerosis by increasing oxidative stress In order to investigate the possible role of augmented NADPH oxidase in increased ROS production, we determined the mRNA expression of NADPH oxidase in WAT of KKAy mice. The NADPH oxidase complex consists of membrane-associated flavocytochrome b protein, which is composed of gp91 phox and p22 phox , and cytosolic components p47 phox , p67 phox , and p40 phox. The mRNA expression levels of these NADPH oxidase subunits were significantly augmented in WAT of nondiabetic 7-week-old KKAy mice, and they were even higher in WAT of diabetic week-old KKAy mice compared with the control mice Figure 3 C. In contrast, the mRNA expression levels of these subunits in the liver and skeletal muscle of 7- and week-old KKAy mice were similar to those of control mice Figure 3 D. We also found that the mRNA expression level of transcription factor PU. These results indicate that the NADPH oxidase pathway is specifically induced in WAT of obese mice. Decreased mRNA expressions and activities of antioxidant enzymes in WAT of KKAy mice. In the next step, we measured the expressions of antioxidant enzymes including superoxide dismutase SOD , glutathione peroxidase GPx , and catalase. Furthermore, total SOD activities Figure 4 C, left and GPx activities Figure 4 C, right were also significantly and specifically lower in WAT of KKAy mice than in the control mice. Taken together, these results indicate that increased ROS production in accumulated fat is due to the activated NADPH oxidase pathway and impaired antioxidant defense system Figure 4 D. Decreased mRNA expressions and activities of antioxidant enzymes in WAT of obese KKAy mice. D Model illustrating increased production of oxidative stress in accumulated fat. ROS production in adipocytes. We next examined the significance of ROS in cultured adipocytes. ROS production was markedly increased during differentiation of 3T3-L1 cells into adipocytes Figure 5 A , suggesting that ROS production increases in parallel with fat accumulation in adipocytes. We then determined the cellular pathway involved in increased ROS production in mature adipocytes, including NADPH oxidase, xanthine oxidase 34 , and mitochondria-mediated 38 pathways. ROS production in fully differentiated 3T3-L1 adipocytes was markedly suppressed by 2 structurally unrelated inhibitors of NADPH oxidase, diphenyleneiodonium DPI and apocynin, as well as the general antioxidant N -acetyl-cysteine NAC Figure 5 B. In contrast, ROS production in 3T3-L1 adipocytes was not suppressed by oxypurinol, an inhibitor of xanthine oxidase, rotenone, an inhibitor of mitochondrial electron transport chain complex I, or thenoyltrifluoroacetone, an inhibitor of complex II Figure 5 B. These results suggest that NADPH oxidase is the major source of ROS in adipocytes, and that augmented NADPH oxidase seems to contribute to increased ROS production in adipose tissue in obesity. Production of ROS in 3T3-L1 adipocytes. A ROS production during differentiation of 3T3-L1 cells into adipocytes. ROS production was measured by NBT reduction. Oil red O staining top and NBT treatment middle of the cells. Dark-blue formazan was dissolved and the absorbance was determined at nm bottom. B Effect of linoleate and inhibitors of ROS production in fully differentiated 3T3-L1 adipocytes. In the final hour of incubation, 10 mM NAC, 10 μM DPI, μM apocynin, μM oxypurinol, μM rotenone, or μM thenoyltrifluoroacetone TTFA was added, and ROS production was measured by NBT reduction. It has been reported that in cultured vascular cells, free fatty acids increase NADPH oxidase activity In the next experiment, we investigated whether free fatty acids could induce ROS production in 3T3-L1 adipocytes. ROS production was significantly increased by incubation with linoleic acid Figure 5 B , as well as with other types of fatty acids such as oleic acid and arachidonic acid data not shown. The effect of linoleic acid was abolished by NADPH oxidase inhibitors, but not by other types of inhibitors Figure 5 B. These results suggest that increased levels of fatty acid in accumulated fat seem to stimulate ROS production in adipose cells through the activation of NADPH oxidase. Effect of ROS on the expression levels of various genes in adipocytes. Next, we examined the effect of ROS in 3T3-L1 adipocytes. Fully differentiated 3T3-L1 adipocytes were exposed to ROS either by treating the cells with H 2 O 2 directly, or by an enzymatic method using xanthine oxidase to generate superoxide. The mRNA expression levels of adiponectin and PPARγ were diminished by incubation with H 2 O 2 Figure 6 A in a dose-dependent manner. In contrast, ROS increased the mRNA expression levels of PAI-1, IL-6, and monocyte chemotactic protein—1 MCP-1 in 3T3-L1 adipocytes Figure 6 A. These changes were also observed by incubation with xanthine oxidase and hypoxanthine data not shown. Antioxidant NAC reversed the effects of H 2 O 2 on the expression levels of these genes to normal levels Figure 6 B. We also found that H 2 O 2 increased the mRNA expression levels of NADPH oxidase subunits and PU. Adiponectin secretion by 3T3-L1 adipocytes was also decreased following incubation with H 2 O 2 , and NAC abrogated the effect of H 2 O 2 Figure 6 D. Using a reporter construct containing adiponectin promoter 40 , ROS reduced the transcriptional activity of the adiponectin gene in 3T3-L1 adipocytes, and NAC reversed these effects Figure 6 E. These results indicate that ROS downregulated adiponectin expression at the transcriptional level, suggesting that treatment with antioxidants or inhibitors of ROS production might restore the dysregulation of adipocytokines. Effects of ROS on gene expressions in 3T3-L1 adipocytes. A and B The mRNA expression levels of adiponectin, PAI-1, PPARγ, IL-6, and MCP-1 in 3T3-L1 adipocytes exposed to ROS, with or without antioxidant NAC. Fully differentiated 3T3-L1 adipocytes were exposed to ROS by incubation with H 2 O 2. C The mRNA expression levels of NADPH oxidase subunits and PU. Values are normalized to the level of cyclophilin mRNA. D Effects of H 2 O 2 and NAC on adiponectin secretion from 3T3-L1 adipocytes. Adiponectin levels in the media were determined by Western blotting inset and the values were quantified using a densitometer. E Effects of ROS and NAC on the transcriptional activity of adiponectin promoter in 3T3-L1 adipocytes. RLU, relative luciferase units. Effect of NADPH oxidase inhibitor on adipocytokine expression and glucose and lipid metabolism in KKAy mice. To test the therapeutic effects of the above experimental observations, we treated KKAy mice with the NADPH oxidase inhibitor apocynin 41 , and then we examined the effects on adipocytokine expression and obesity-associated metabolic disorders. Apocynin is a well-characterized inhibitor of NADPH oxidase activity and is known to impede the assembly of the p47 phox subunit with the membrane complex Apocynin is also effective at inhibiting ROS production in various cells in vivo 43 , Six-week treatment with apocynin did not affect body weight Figure 7 A or food intake data not shown in control or KKAy mice. Lipid peroxidation and H 2 O 2 production in WAT were significantly lower in apocynin-treated KKAy mice than controls Figure 7 , B and C , indicating that NADPH oxidase inhibition reduced oxidative stress in WAT. Similar to the results shown in Figure 2 , lipid peroxidation was not altered in the liver and skeletal muscle of KKAy mice, compared to control mice, and it was not affected by apocynin treatment Supplemental Figure 2. Apocynin treatment increased the mRNA expression of adiponectin in WAT of KKAy mice Figure 7 D , and such an increase was associated with a rise in plasma adiponectin concentration Figure 7 E. Apocynin also decreased the mRNA expression of TNF-α in WAT of KKAy mice Figure 7 D. Plasma triglyceride TG levels were also lower in apocynin-treated KKAy mice Figure 7 F. Furthermore, apocynin treatment significantly reduced hepatic TG content in KKAy mice Figure 7 G , suggesting that apocynin could ameliorate hepatic steatosis in KKAy mice. These results showed that inhibition of NADPH oxidase in KKAy mice significantly decreased ROS production by WAT, attenuated the dysregulated production of adipocytokines, and improved the obesity-associated disorders in glucose and lipid metabolism. In vivo effects of an NADPH oxidase inhibitor, apocynin, on adipocytokine expression, and glucose and lipid metabolism in KKAy mice. D The mRNA expression levels of adiponectin and TNF-α in WAT of KKAy mice. Obesity is closely associated with metabolic syndrome 1 — 3. However, the mechanisms by which fat accumulation leads to abnormal expression of adipocytokines and development of metabolic syndrome have not been fully elucidated. In the present study, we have demonstrated that, in nondiabetic human subjects, fat accumulation closely correlated with the markers of systemic oxidative stress. These data are in good agreement with recent studies suggesting that systemic oxidative stress correlates with BMI 48 , In addition, we demonstrated that plasma adiponectin levels correlated inversely with systemic oxidative stress. The main finding of the present study is that oxidative stress in accumulated fat mediates the obesity-associated development of metabolic syndrome by the following potential mechanisms: a increased oxidative stress in accumulated fat leads to dysregulated production of adipocytokines, and b the selective increase in ROS production in accumulated fat leads to elevation of systemic oxidative stress. Plasma adiponectin levels correlated inversely with the markers of systemic oxidative stress in nondiabetic human subjects. In cultured adipocytes, addition of oxidative stress suppressed mRNA expression and secretion of adiponectin, and it also increased PAI-1, IL-6, and MCP-1 mRNA expression. Furthermore, treatment with the NADPH oxidase inhibitor apocynin reduced oxidative stress in WAT and increased plasma adiponectin levels in KKAy mice. These results indicate that a local increase in oxidative stress in accumulated fat causes dysregulated production of adipocytokines. Recently, we reported that PPARγ positively regulates the transcription of the adiponectin gene via PPARγ-responsive element in the promoter We showed that oxidative stress suppressed PPARγ mRNA expression in 3T3-L1 adipocytes. It was also shown that nuclear translocation of PPARγ was inhibited by nitration associated with oxidative stress Therefore, downregulation of adiponectin expression may be partially attributed to the decreased gene expression and smaller amount of nuclear PPARγ under conditions of oxidative stress. In the present study, H 2 O 2 production was increased only in adipose tissue of obese mice, but not in other tissues examined, including the liver, skeletal muscle, and aorta. These results suggest that adipose tissue is the major source of the elevated plasma ROS. Oxidative stress is known to impair both insulin secretion by pancreatic β cells 32 and glucose transport in muscle 30 and adipose tissue Increased oxidative stress in vascular walls is involved in the pathogenesis of hypertension 33 and atherosclerosis Oxidative stress also underlies the pathophysiology of hepatic steatosis Thus, oxidative stress locally produced in each of the above tissues seems to be involved in the pathogenesis of these diseases. Our results suggest that increased ROS secretion into peripheral blood from accumulated fat in obesity is also involved in induction of insulin resistance in skeletal muscle and adipose tissue, impaired insulin secretion by β cells, and pathogenesis of various vascular diseases such as atherosclerosis and hypertension. Why is oxidative stress increased only in accumulated fat? We found that in WAT but not other tissues of obese mice, the mRNA expression levels of NADPH oxidase subunits increased, and mRNA expression levels and activities of antioxidant enzymes decreased. We also found a high level of mRNA expression of the transcription factor PU. Recently, Weisberg et al. Since macrophages are also known to produce ROS, it is possible that infiltrated macrophages are involved in augmented NADPH oxidase and elevated ROS production in the obese adipose tissue. In this regard, a family of gp91 phox homologs, termed NOX NADPH oxidase proteins, has been reported to be expressed in nonphagocytic cells, not in macrophages 54 , Recent studies of adipocytes found that NOX4, a member of the NOX family, plays a role in the generation of H 2 O 2 The expression of NOX4 was not detected in macrophages 57 , In contrast, we found high expression levels of NOX4 in WAT, as well as gp91 phox , and mRNA expression of NOX4 was significantly increased in WAT of obese mice Supplemental Figure 3, A and B. These results suggest that adipose NADPH oxidase is elevated and contributes to ROS production in accumulated fat. We demonstrated that ROS production was increased in 3T3-L1 adipocytes, in parallel with fat accumulation and by incubation with linoleic acid, in a NADPH oxidase—dependent manner. These results suggest that in accumulated fat, elevated levels of fatty acids activate NADPH oxidase and induce ROS production. We also demonstrated that ROS itself augmented mRNA expressions of NADPH oxidase subunits, including NOX4 Supplemental Figure 3C and PU. Therefore, in accumulated fat of obesity, elevated ROS appear to upregulate mRNA expression of NADPH oxidase, establishing a vicious cycle that augments oxidative stress in WAT and blood. We found that ROS increased the expression of MCP-1, a chemoattractant for monocytes and macrophages, in adipocytes. Byproducts of lipid peroxidation by ROS, such as trans hydroxynonenal and malondialdehyde, are themselves potent chemoattractants Hence, it is possible that increased ROS production and MCP-1 secretion from accumulated fat should cause infiltration of macrophages and inflammation in adipose tissue of obesity. Importantly, our in vivo study revealed that treatment with the NADPH oxidase inhibitor apocynin reduced ROS production in adipose tissue of KKAy mice. It also improved hyperinsulinemia, hyperglycemia, hypertriglyceridemia, and hepatic steatosis. Increased expression of adiponectin and decreased expression of TNF-α were observed in WAT of apocynin-treated KKAy mice, demonstrating that reduction of oxidative stress in accumulated fat could improve the dysregulation of adipocytokines in vivo. Our results demonstrate that treatment with NADPH oxidase inhibitor is effective in ameliorating the development of obesity-associated metabolic syndrome. It has been reported that prolonged exposure of 3T3-L1 adipocytes to ROS results in impairments of insulin-induced activation of PI3-kinase and Akt, insulin-stimulated lipogenesis, glucose uptake, and GLUT4 translocation to the plasma membrane 31 , Thus, NADPH oxidase inhibitors might improve insulin sensitivity via suppression of these effects induced by chronic exposure to ROS. Our current results are consistent with several previous studies demonstrating that antioxidant treatment improves insulin function in diabetic subjects 61 , Recent studies, on the other hand, have proposed that ROS such as H 2 O 2 are produced transiently in response to insulin stimulation and also act as a second messenger for insulin signaling in adipocytes 63 , NADPH oxidase is thought to be involved in insulin-induced ROS generation 63 , We assume that a transient increase of intracellular ROS is important for the insulin signaling pathway, while excessive and long-term exposure to ROS reduces insulin sensitivity and impairs glucose and lipid metabolism. In the present study, we observed stimulation of ROS production by fatty acids via NADPH oxidase activation. In addition, we also found that ROS suppressed the mRNA expressions of lipogenic genes, such as fatty acid synthase and sterol regulatory element binding protein-1c, in 3T3-L1 adipocytes data not shown. Reactive oxygen species oxidation of lipids ultimately generates lipid aldehydes that modify DNA, proteins, RNA, and other lipid species Esterbauer et al. Increased markers of lipid peroxidation, including thiobarbituric acid reactive substances TBARS and 8-epi-prostaglandin-F2α 8-epi-PGF2α are observed in individuals with higher BMI and waist circumference Furukawa et al. Oxidized lipids and proteins preferentially accumulate in visceral depots compared to subcutaneous depots of obese mice Long et al. Lipid aldehydes are highly electrophilic and prone to irreversible nucleophilic attack by the side chains of lysine Lys , histidine His , and cysteine Cys residues of proteins, resulting in a covalent lipid-protein adduct termed protein carbonylation Schaur, ; Curtis et al. Furthermore, Lys, His, and Cys residues often cluster within active sites of enzymes or critical structural motifs, so their stable modification by lipids generally leads to inhibition or deactivation of protein function. However, recent work challenges the notion that ROS-driven modifications broadly degrade fat cell function. Brown adipose tissue BAT contains elevated levels of mitochondrial superoxide, mitochondrial H 2 O 2 , and oxidized lipids that correlate with acute activation of thermogenesis Chouchani et al. Mitochondrial ROS in BAT can converge on UCP1 C inducing cysteine sulfenylation -SOH Chouchani et al. Interestingly, UCP1 CA does not disable thermogenic responses in brown adipocytes but desensitizes the protein to adrenergic activation of uncoupled respiration. Further exploration of physiological ROS signaling outputs and modifications may show how redox status in adipocytes contributes to energy balance. Polyunsaturated fatty acids PUFAs are abundant in WAT and particularly sensitive to lipid peroxidation. One major consequence of lipid peroxidation is mitochondrial membrane damage Kowaltowski and Vercesi, Also, peroxidation of PUFAs results in the release of diffusible reactive lipid aldehydes. Among the wide variety of reactive lipids formed through this mechanism, 4-hydroxy-non-enal 4-HNE derived from oxidation of n6 fatty acids and 4-hydroxy-hexenal 4-HHE from n3 fatty acid oxidation are the most widely studied in the context of adipose biology. The WAT of obese mice showed decreased metabolism of 4-HNE, while stress response proteins, including glutathione-S-transferase M1, glutathione peroxidase 1, and Prdx Grimsrud et al. Lipid peroxidation end products can also inhibit insulin signaling as 4-HNE de-stabilizes IRS adapter proteins and insulin receptor β Demozay et al. Lipid peroxidation products also damage the function of transcription factors that contain zinc-finger motifs, histones, and other nuclear proteins of visceral fat cells isolated from obese mice Hauck et al. The lipid peroxidation of transcriptional regulatory proteins presents a consolidated mechanism for retrograde ROS signaling from mitochondria to the nucleus. Although mitochondria are the most significant source of ROS, the discovery of lipid-protein adducts in the nucleus of adipocytes suggests either a different pool of ROS contributes to lipid peroxidation or a mechanism exists to sequester and shuttle reactive aldehydes to specific subcellular localizations Hauck et al. As with ROS, the timing of protein carbonylation may be important for beneficial or pathologic effects. Acute carbonylation of substrates after exercise are potentially beneficial, while chronic accumulation of carbonylated proteins in the muscle and WAT of obese and sedentary individuals may be pathological and contribute to comorbidities of obesity Frohnert and Bernlohr, Additionally, ROS seem to be important in the cellular aspects of adipocyte differentiation. Numerous studies demonstrate that mitochondrial biogenesis increases during adipocyte differentiation Wilson-Fritch et al. Dramatic expansion of mitochondrial content enables higher metabolic rates to overcome the energetic demands of differentiation. Induction of differentiation correlates with superoxide generation from complex III, conversion of superoxide to H 2 O 2 , and activation of transcriptional machinery necessary for adipogenesis Tormos et al. et al. However, obesity-mediated ROS induction also restricts mitochondrial biogenesis and adipocyte differentiation. Higher accumulation of 4-HNE adducts occurs in cultured differentiating preadipocytes from insulin-resistant compared to insulin-sensitive individuals. In this manner, treatment of primary subcutaneous preadipocytes from obese individuals with pathological levels of 4-HNE decreased markers associated with insulin sensitivity and mature fat cells Dasuri et al. Other studies demonstrate that treatment with antioxidants decreases differentiation Tormos et al. Divergent in vitro and in vivo findings illustrate existing challenges in defining the specifics of ROS signaling and its connectivity to metabolic diseases. Nutrient overload has been linked to the development of insulin resistance. One carbonylated protein of importance was GLUT4, whose carbonylation likely impairs insulin-stimulated glucose uptake. Of note, systemic oxidative stress and insulin resistance did not coincide with inflammatory cytokines in plasma nor ER stress in WAT. These findings provide a causal link between oxidative stress and insulin resistance in humans. Mitochondrial metabolism is often altered in inherited diseases, such as inborn errors of metabolism IEMs that impinge upon ROS generation. Inhibition of OXPHOS increases ROS generation due to a backlog of electrons in the various complexes, resulting in electron leak, ROS generation, and production of H 2 O 2. In IEMs affecting the ETC or other pathways of ATP generation, increased oxidative stress is often observed, while the exact mechanisms for increased ROS production are unknown. It is hypothesized that mutations affecting the formation of the protein complexes in the ETC or mutations that modify their assembly increase ROS generation by facilitating electron leak Olsen et al. Additionally, accumulation of toxic intermediates, often observed in IEMs, can increase the ROS generation by further decreasing OXPHOS activity, as in the case of medium-chain acyl-CoA dehydrogenase MCAD deficiency. MCAD deficiency reflects the accumulation of medium-chain fatty acid derivatives, including cisdecenoic acid, octanoate, and decanoate, with these metabolites altering levels of antioxidants and increasing markers of oxidative stress Schuck et al. Intriguingly, IEMs display metabolic reprograming with a switch to glycolysis for both ATP production and muted ROS generation Olsen et al. Specifically, in myoclonic epilepsy with ragged red fibers MERRF , increased intracellular H 2 O 2 levels correspond with increased AMPK phosphorylation and expression of GLUT1, hexokinase II, and lactate dehydrogenase. These results, as well as increased lactic acid production, all point to increased glycolysis De la Mata et al. In multiple acyl-CoA dehydrogenase deficiency MADD , mutations in ETFa , ETFb , or ETFDH , lead to decreased ATP production with an accumulation of organic acids, including glutaric acid as well as acyl-carnitines. A subset of these patients is riboflavin responsive RR-MADD with high dose riboflavin alleviating some symptoms. Similar to MERRF, many RR-MADD patients exhibit increased oxidative stress Cornelius et al. This defect may be due to defective electron transfer and increased electron leak from the misfolded ETFDH protein and decreased binding of CoQ10 Cornelius et al. Treatment with CoQ10, but not riboflavin, decreased ROS levels Cornelius et al. Analysis of mitochondrial function from RR-MADD fibroblasts showed increased mitochondrial fragmentation and reduced β-oxidation, while supplementation with the antioxidant CoQ10 decreased fragmentation and mitophagy Cornelius et al. While obesity and IEMs are distinct disorders, both conditions impinge on energy balance in WAT. Even though these disorders have very different manifestations, oxidative stress plays an important role in both and may be a therapeutic target. For example, CoQ10 is often given as a broad-spectrum treatment to individuals with IEMs, and while its effectiveness is debated, the anti-inflammatory effects may be beneficial in reducing oxidative stress and the pathogenesis of the disease Cornelius et al. Mitochondria represent control centers of many metabolic pathways. Interventions that enhance adipocyte mitochondrial function may also improve whole-body insulin sensitivity. Mitigation of mitochondrial ROS production and oxidative stress may be a possible therapeutic target in type 2 diabetes and IEMs because some mitochondrial-targeted antioxidants and other small molecule drugs improve metabolic profiles in mouse models Feillet-Coudray et al. Thiazolidinediones TZDs are PPARγ agonists used for treating type 2 diabetes Kelly et al. TZDs, such as rosiglitazone and pioglitazone, enhance insulin sensitivity by improving adipokine profiles Maeda et al. TZDs also promote insulin sensitivity by directing fatty acids to subcutaneous fat, rather than visceral fat. Subcutaneous fat expandability, even in the context of obesity and type 2 diabetes, correlates with insulin sensitivity in rodents and humans Ross et al. Numerous in vitro and in vivo studies demonstrate TZDs enhance mitochondrial biogenesis, content, function, and morphology. Rosiglitazone also induces cellular antioxidant enzymes responsible for the removal of ROS generated by increased mitochondrial activity in adipose tissue of diabetic rodents Rong et al. Taken together, TZDs impact WAT mitochondrial function in multiple ways that ultimately improve systemic fat metabolism and insulin sensitivity. Other therapeutic strategies include mitochondria-targeted scavengers Smith et al. However, these methods to enhance mitochondrial function display a narrow therapeutic range that limits safe use for obesity. Although the development of insulin resistance does not require impaired mitochondrial function Hancock et al. Aerobic exercise and caloric restriction disrupt this vicious loop, potentially by preventing accumulation of injured mitochondrial proteins with substantial improvement of insulin sensitivity. In insulin-resistant people, aerobic exercise stimulates both mitochondrial biogenesis and efficiency concurrent with an enhancement of insulin action Mul et al. Ultimately, exercise engages pathways that reduce ROS coupled with insulin sensitivity and improved mitochondrial function in WAT. Obesity is the result of excessive expansion of WAT depots due to a chronic imbalance between energy intake and expenditure. Many studies demonstrate that oxidative stress in fat cells links obesity and its comorbidities. The fact that WAT remains the sole organ for storing surfeit lipid renders the macromolecules in adipocytes particularly vulnerable to carbonylation and other modifications driven by oxidative stress. Prolonged oxidative stress negatively influences endocrine and homeostatic performance of WAT, including disruption of hormone secretion, elevation of serum lipids, inadequate cellular antioxidant defenses, and impaired mitochondrial function Figure 2. Metabolic challenges, such as persistent nutrient intake and sedentary behaviors that promote impaired glucose and lipid handling, also elevate mitochondrial ROS production to cause adipocyte dysfunction. Consequently, adipocytes cannot engage appropriate transcriptional and energetic responses to enable insulin sensitivity. Figure 2. Impact of oxidative stress on adipocyte function. Increased plasma glucose and free fatty acids contribute to increased oxidative stress by increasing the production of reactive oxygen species ROS and decreasing antioxidant concentrations. Increased oxidative stress occurs via enzymes in the cytoplasm, such as NADPH oxidase, and the mitochondria. The oxidative environment increases lipid storage resulting in hypertrophic adipocytes. Additionally, increased mitochondrial ROS mtROS alters the activity state of metabolic enzymes either directly or by changing the oxidative state of protein side-chains or by other post-translational modifications, including lipid peroxidation and protein carbonylation. Cumulatively, increased adipocyte oxidative stress decreases adipogenesis and secretion of adipokines, leading to unbalanced energy homeostasis, insulin resistance, and type 2 diabetes. The increasing prevalence of obesity suggests lifestyle intervention as the principal method to treat obesity is unlikely to succeed. Currently, all available anti-obesity medications act by limiting energy intake through appetite suppression or inhibition of intestinal lipid absorption. However, these medications are largely ineffective and often have adverse side effects. The central role of mitochondria in nutrient handling provides a logical entry point for improving metabolism in obesity. While approaches to understanding and intervening in oxidative damage evolve, exploration of mitochondria redox balance may enable development of dietary and small molecule therapies for obesity and its comorbidities. This work was funded by the American Diabetes Association IBS and NIH R01DK The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Acosta, M. Coenzyme Q biosynthesis in health and disease. Acta , — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ahmed, M. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake. Obesity 18, 27— Akl, M. Perturbed adipose tissue hydrogen peroxide metabolism in centrally obese men: association with insulin resistance. PLoS One e Anderson, E. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. Armstrong, J. The redox regulation of intermediary metabolism by a superoxide-aconitase rheostat. Bioessays 26, — Barbosa, M. Hydrogen peroxide production regulates the mitochondrial function in insulin resistant muscle cells: effect of catalase overexpression. Bjelakovic, G. Antioxidant supplements to prevent mortality. JAMA , — Antioxidant supplements and mortality. Care 17, 40— Boden, G. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Boden, M. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Bogacka, I. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. Bournat, J. Mitochondrial dysfunction in obesity. Diabetes Obes. Boyle, P. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Chappuis, B. Differential effect of pioglitazone PGZ and rosiglitazone RGZ on postprandial glucose and lipid metabolism in patients with type 2 diabetes mellitus: a prospective, randomized crossover study. Diabetes Metab. Chouchani, E. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature , — Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. Cornelius, N. Secondary coenzyme Q10 deficiency and oxidative stress in cultured fibroblasts from patients with riboflavin responsive multiple Acyl-CoA dehydrogenation deficiency. Cellular consequences of oxidative stress in riboflavin responsive multiple acyl-CoA dehydrogenation deficiency patient fibroblasts. Curtis, J. Protein carbonylation and metabolic control systems. Trends Endocrinol. Dasuri, K. Role of physiological levels of 4-hydroxynonenal on adipocyte biology: implications for obesity and metabolic syndrome. Free Radic. Davies, M. Protein oxidation and peroxidation. De la Mata, M. Recovery of MERRF fibroblasts and cybrids pathophysiology by coenzyme Q Neurotherapeutics 9, — Deeg, M. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30, — Demozay, D. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Elrayess, M. Escribano-Lopez, I. The mitochondrial antioxidant SS increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Esterbauer, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Fazakerley, D. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. Feillet-Coudray, C. The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol. Fouret, G. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Frohnert, B. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Increased adipose protein carbonylation in human obesity. Obesity 19, — Furukawa, S. Increased oxidative stress in obesity and its impact on metabolic syndrome. PubMed Abstract Google Scholar. Fusco, D. Effects of antioxidant supplementation on the aging process. Aging 2, — Gardner, P. Superoxide radical and iron modulate aconitase activity in mammalian cells. Goldberg, R. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28, — Goldgof, M. The chemical uncoupler 2,4-dinitrophenol DNP protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. Grimsrud, P. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Proteomics 6, — Oxidative stress and covalent modification of protein with bioactive aldehydes. Han, Y. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Hancock, C. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Hauck, A. Adipose oxidative stress and protein carbonylation. Obesity-induced protein carbonylation in murine adipose tissue regulates the DNA-binding domain of nuclear zinc finger proteins. Hausladen, A. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. |
| Increased oxidative stress in obesity and its impact on metabolic syndrome | Mol Cell Strwss. Obes Surg. Nat Rev Endocrinol. Visceral fat and oxidative stress articles fag Furukawa, S. Wood ZA, Schroder E, Robin Harris J, Poole LB. The most widely studied aldehyde products of lipid peroxidation are 4-HNE and 4-oxononenal 4-ONEwhich are abundantly present in adipose tissue I, Hirshman, M. |
| Nutrient Imbalance Provokes Mitochondrial Ros | These results indicate that a local increase in oxidative stress in accumulated fat causes dysregulated production of adipocytokines. Recently, we reported that PPARγ positively regulates the transcription of the adiponectin gene via PPARγ-responsive element in the promoter We showed that oxidative stress suppressed PPARγ mRNA expression in 3T3-L1 adipocytes. It was also shown that nuclear translocation of PPARγ was inhibited by nitration associated with oxidative stress Therefore, downregulation of adiponectin expression may be partially attributed to the decreased gene expression and smaller amount of nuclear PPARγ under conditions of oxidative stress. In the present study, H 2 O 2 production was increased only in adipose tissue of obese mice, but not in other tissues examined, including the liver, skeletal muscle, and aorta. These results suggest that adipose tissue is the major source of the elevated plasma ROS. Oxidative stress is known to impair both insulin secretion by pancreatic β cells 32 and glucose transport in muscle 30 and adipose tissue Increased oxidative stress in vascular walls is involved in the pathogenesis of hypertension 33 and atherosclerosis Oxidative stress also underlies the pathophysiology of hepatic steatosis Thus, oxidative stress locally produced in each of the above tissues seems to be involved in the pathogenesis of these diseases. Our results suggest that increased ROS secretion into peripheral blood from accumulated fat in obesity is also involved in induction of insulin resistance in skeletal muscle and adipose tissue, impaired insulin secretion by β cells, and pathogenesis of various vascular diseases such as atherosclerosis and hypertension. Why is oxidative stress increased only in accumulated fat? We found that in WAT but not other tissues of obese mice, the mRNA expression levels of NADPH oxidase subunits increased, and mRNA expression levels and activities of antioxidant enzymes decreased. We also found a high level of mRNA expression of the transcription factor PU. Recently, Weisberg et al. Since macrophages are also known to produce ROS, it is possible that infiltrated macrophages are involved in augmented NADPH oxidase and elevated ROS production in the obese adipose tissue. In this regard, a family of gp91 phox homologs, termed NOX NADPH oxidase proteins, has been reported to be expressed in nonphagocytic cells, not in macrophages 54 , Recent studies of adipocytes found that NOX4, a member of the NOX family, plays a role in the generation of H 2 O 2 The expression of NOX4 was not detected in macrophages 57 , In contrast, we found high expression levels of NOX4 in WAT, as well as gp91 phox , and mRNA expression of NOX4 was significantly increased in WAT of obese mice Supplemental Figure 3, A and B. These results suggest that adipose NADPH oxidase is elevated and contributes to ROS production in accumulated fat. We demonstrated that ROS production was increased in 3T3-L1 adipocytes, in parallel with fat accumulation and by incubation with linoleic acid, in a NADPH oxidase—dependent manner. These results suggest that in accumulated fat, elevated levels of fatty acids activate NADPH oxidase and induce ROS production. We also demonstrated that ROS itself augmented mRNA expressions of NADPH oxidase subunits, including NOX4 Supplemental Figure 3C and PU. Therefore, in accumulated fat of obesity, elevated ROS appear to upregulate mRNA expression of NADPH oxidase, establishing a vicious cycle that augments oxidative stress in WAT and blood. We found that ROS increased the expression of MCP-1, a chemoattractant for monocytes and macrophages, in adipocytes. Byproducts of lipid peroxidation by ROS, such as trans hydroxynonenal and malondialdehyde, are themselves potent chemoattractants Hence, it is possible that increased ROS production and MCP-1 secretion from accumulated fat should cause infiltration of macrophages and inflammation in adipose tissue of obesity. Importantly, our in vivo study revealed that treatment with the NADPH oxidase inhibitor apocynin reduced ROS production in adipose tissue of KKAy mice. It also improved hyperinsulinemia, hyperglycemia, hypertriglyceridemia, and hepatic steatosis. Increased expression of adiponectin and decreased expression of TNF-α were observed in WAT of apocynin-treated KKAy mice, demonstrating that reduction of oxidative stress in accumulated fat could improve the dysregulation of adipocytokines in vivo. Our results demonstrate that treatment with NADPH oxidase inhibitor is effective in ameliorating the development of obesity-associated metabolic syndrome. It has been reported that prolonged exposure of 3T3-L1 adipocytes to ROS results in impairments of insulin-induced activation of PI3-kinase and Akt, insulin-stimulated lipogenesis, glucose uptake, and GLUT4 translocation to the plasma membrane 31 , Thus, NADPH oxidase inhibitors might improve insulin sensitivity via suppression of these effects induced by chronic exposure to ROS. Our current results are consistent with several previous studies demonstrating that antioxidant treatment improves insulin function in diabetic subjects 61 , Recent studies, on the other hand, have proposed that ROS such as H 2 O 2 are produced transiently in response to insulin stimulation and also act as a second messenger for insulin signaling in adipocytes 63 , NADPH oxidase is thought to be involved in insulin-induced ROS generation 63 , We assume that a transient increase of intracellular ROS is important for the insulin signaling pathway, while excessive and long-term exposure to ROS reduces insulin sensitivity and impairs glucose and lipid metabolism. In the present study, we observed stimulation of ROS production by fatty acids via NADPH oxidase activation. In addition, we also found that ROS suppressed the mRNA expressions of lipogenic genes, such as fatty acid synthase and sterol regulatory element binding protein-1c, in 3T3-L1 adipocytes data not shown. These results suggest that increased ROS production caused by fat accumulation may prevent further lipid storage, but may simultaneously cause dysregulated expression of adipocytokines and insulin resistance. Conceivably, inhibition of NADPH oxidase should improve the dysregulation of adipocytokines and insulin sensitivity via restoration of normal ROS production in obese adipocytes. Figure 8 illustrates our working hypothesis regarding the role of ROS in metabolic syndrome. Increased oxidative stress in accumulated fat, via increased NADPH oxidase and decreased antioxidant enzymes, causes dysregulated production of adipocytokines, locally. Increased ROS production from accumulated fat also leads to increased oxidative stress in blood, hazardously affecting other organs including the liver, skeletal muscle, and aorta. We propose that increased oxidative stress in accumulated fat is an early instigator and one of the important underlying causes of obesity-associated metabolic syndrome, hence, the redox state in adipose tissue is a potentially useful target in new therapies against obesity-associated metabolic syndrome, as we demonstrated in the mouse in vivo study. A working model illustrating how increased ROS production in accumulated fat contributes to metabolic syndrome. Plasma and urinary samples were obtained after overnight fasting from subjects 69 men and 71 women; mean age 56 ± 13 SD who visited the University hospital for a healthy checkup. Patients with a history of diabetes, cardiovascular or cerebrovascular disease, hepatic or renal disease, tobacco abuse, or those on hormone replacement therapy were excluded. The study protocols complied with the Guidelines of the Ethical Committees of Osaka University and University of the Ryukyus. Informed consent was obtained from all subjects. Samples were stored at —80°C until use. All animal experiments were conducted in accordance with the aforementioned institutional guidelines for the care and use of laboratory animals. for 9 weeks. After an overnight fast, mice were anesthetized, and blood and tissue samples were obtained as described above. Biochemical measurements. Urinary 8-epi-PGF2α was determined using an enzyme immunoassay kit Assay Designs Inc. Plasma levels of glucose and TG were measured using the Glucose-test and TG E-test, respectively, from Wako Pure Chemical Industries. Plasma adiponectin levels were determined using the Adiponectin ELISA kit Otsuka. Plasma insulin levels were assessed using an insulin ELISA kit Shibayagi. Lipid peroxidation and hydrogen peroxide concentration. Tissue samples were homogenized in a buffer solution containing 50 mM Tris-HCl pH 7. The supernatant was used for the assay. The levels of lipid peroxidation in plasma and tissue homogenate were measured as TBARS using the LPO-test Wako Pure Chemical Industries. Hydrogen peroxide concentration in plasma was measured using an Amplex Red hydrogen peroxide assay kit Invitrogen Corp. H 2 O 2 production. Parametrial WAT and gastrocnemius muscle were dissected out and placed in Krebs-Ringer phosphate buffer containing in mM NaCl, 5. The aorta was carefully removed, cleaned of excess fat and adventitia, and placed in Krebs-Ringer phosphate buffer. WAT, muscle, and aorta were cut into 2-mm square pieces and incubated at 37°C for 90 minutes. H 2 O 2 released from the tissue was detected using the Amplex Red hydrogen peroxide assay kit Invitrogen Corp. Quantitative RT-PCR. The cDNA was synthesized using the ThermoScript RT-PCR System Invitrogen Corp. Real-time PCR was performed on a LightCycler using the FastStart DNA Master SYBR Green I Roche Diagnostics according to the protocol provided by the manufacturer. Antioxidant enzyme activity. Tissue homogenates were prepared as above. SOD activity was measured using the BIOXYTECH SOD kit Oxis International. GPx activity was measured using the method described previously Western blot analysis. Tissue homogenates 20 μg protein were subjected to SDS-PAGE, and immunoblotting was performed with anti—Cu,Zn-SOD polyclonal antibody Upstate Biotechnology. ROS production in 3T3-L1 adipocytes. ROS production was detected by nitroblue tetrazolium NBT assay NBT is reduced by ROS to a dark-blue, insoluble form of NBT called formazan. At days 0, 2, 4, and 8 after induction, 3T3-L1 cells were incubated for 90 minutes in PBS containing 0. Fully differentiated 3T3-L1 adipocytes were incubated with or without μM linoleic acid for 24 hours. Various inhibitors were added in the last hour of incubation, and the cells were incubated for 90 minutes in PBS containing 0. NBT, DPI, NAC, oxypurinol, rotenone, and thenoyltrifluoroacetone were purchased from Sigma-Aldrich. Apocynin was purchased from Calbiochem. Effect of ROS on expression of genes in 3T3-L1 adipocytes. At day 8 after induction, fully differentiated 3T3-L1 adipocytes were exposed to ROS by incubation with fresh medium containing xanthine oxidase plus hypoxanthine or H 2 O 2 for 24 hours, with or without mM NAC. Cells were harvested, total RNAs were extracted, and the mRNA amounts were quantified by real-time PCR, as described above. The levels of adiponectin in media were determined by Western blotting Transfection experiments were performed as described previously Keywords: Metabolic syndrome , Systemic oxidative stress , Urinary 8-epi-PGF2α , Visceral fat accumulation. JOURNAL FREE ACCESS. Published: Received: May 29, Available on J-STAGE: October 25, Accepted: September 04, Advance online publication: - Revised: -. Download PDF 88K Download citation RIS compatible with EndNote, Reference Manager, ProCite, RefWorks. Article overview. References Related articles 0. Figures 0. Content from these authors. Supplementary material 0. Result List. Previous article Next article. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Download references. We thank Kosuke Takeda for the preceding microarray analysis and Subha Subramanian, Wee Kiat Ong, and other members of Fat Metabolism and Stem Cell Group, Laboratory of Metabolic Medicine, Microscopy Core, Mechanobiology Institute at National University of Singapore, and Singapore Bioimaging Consortium-Nikon Imaging Centre SBIC-NIC for the help with our studies. Su, Singapore-China Joint Grant Project to Sh. Su and M. Sr and Sh. Su, and funding from the Singapore National Medical Research Council NMRC to S-A. Duke-NUS Medical School, 8 College Road, Singapore, , Singapore. Department of Surgery, National University Hospital, 5 Lower Kent Ridge Road, Singapore, , Singapore. Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, 14 Medical Drive, Singapore, , Singapore. You can also search for this author in PubMed Google Scholar. AS contributed to the provision of study material or patients and administrative support. SAT contributed to the provision of study material or patients and administrative support. WH contributed to the financial support, administrative support, and manuscript writing. ShS contributed to the conception and design, financial support, administrative support, data analysis and interpretation, and manuscript writing. All authors made final approval of the manuscript. Correspondence to Shigeki Sugii. Use of human patient-derived cells was conducted with informed consent obtained for each subject, approved by the National Healthcare Group Domain Specific Review Board, Singapore, and performed in accordance with its relevant regulations. ShS is a co-founder of Celligenics Pte Ltd. The other authors declare that they have no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Figure S1. Gene expression studies of additional ROS-related genes that are not included in Figure 1 b. Figure S2. VS-ASCs have increased ROS when compared to SC-ASCs in the lower passage. Figure S3. Effects on adipogenesis are specifically mediated by ROS. Figure S4. Ascorbic acid treatment generally decreases both glycolytic and oxidative respirations. Table S1. List of subjects used for this study. Table S2. DOCX kb. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Sriram, S. et al. Oxidative stress mediates depot-specific functional differences of human adipose-derived stem cells. Stem Cell Res Ther 10 , Download citation. Received : 05 December Revised : 28 March Accepted : 22 April Published : 21 May Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Abstract Background Visceral VS fat depot is known to have defective adipogenic functions compared to subcutaneous SC fat, but its mechanism of origin is unclear. Objective We tested our hypothesis that the degree of oxidative stress in adipose-derived stem cells ASCs from these depots may account for this difference. Methods ASCs were isolated from VS omental region and SC abdominal region fat depots of human subjects undergoing bariatric surgery. Results We found that human VS-derived ASCs exhibit excessive oxidative stress characterized by high reactive oxygen species ROS , compared to SC-derived ASCs. Conclusions This finding suggests the fat depot-specific differences of cellular defects originating from stem cell population. Introduction Adipose-derived stem cells ASCs are the mesenchymal stem cell MSC type that exhibits cellular characteristics potentially useful for regenerative medicine, which includes multipotent and migratory capacities [ 1 , 2 ]. Results and discussion In our previous publication [ 10 ], we performed microarray and ontological analysis on human ASCs isolated from SC and VS depots, in order to identify genes underlying their molecular differences. Full size image. Conclusion Our studies demonstrate that high ROS is observed in visceral fat-derived ASCs and associated with their altered oxidative metabolism and cellular dysfunctions, compared to subcutaneous fat-derived ASCs. Methods Isolation and culture of ASCs White adipose tissue WAT was isolated from the subcutaneous abdominal region depot and visceral depot omental region from human subjects undergoing bariatric surgery, which was approved by the Domain Specific Review Board at National Healthcare Group, Singapore. Cellular analyses Adipogenesis of ASCs was performed as previously described with minor modifications [ 8 , 10 ]. Quantitative real-time PCR Real-time qPCR was performed as described previously [ 8 , 10 ]. References Gimble JM, Katz AJ, Bunnell BA. Article CAS Google Scholar Ong WK, Sugii S. Article CAS Google Scholar Lim MH, Ong WK, Sugii S. Article Google Scholar Tang QQ, Lane MD. Article CAS Google Scholar Tran TT, Yamamoto Y, Gesta S, Kahn CR. Article CAS Google Scholar Després JP, Lemieux I. Article Google Scholar Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Article CAS Google Scholar Ong WK, Tan CS, Chan KL, Goesantoso GG, Chan XH, Chan E, Yin J, Yeo CR, Khoo CM, So JB, Shabbir A, Toh SA, Han W, Sugii S. Article CAS Google Scholar Tran TT, Kahn CR. Article Google Scholar Takeda K, Sriram S, Chan XH, Ong WK, Yeo CR, Tan B, Lee SA, Kong KV, Hoon S, Jiang H, Yuen JJ, Perumal J, Agrawal M, Vaz C, So J, Shabbir A, Blaner WS, Olivo M, Han W, Tanavde V, Toh SA, Sugii S. Article CAS Google Scholar Bondia-Pons I, Ryan L, Martinez JA. Article CAS Google Scholar Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, Durante-Montiel I, Sanchez-Rivera G, Valadez-Vega C, Morales-Gonzalez JA. Article CAS Google Scholar Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Article CAS Google Scholar Gariballa S, Afandi B, Abuhaltem M, Yassin J, Habib H, Ibrahim W. Article CAS Google Scholar Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Article Google Scholar Castro JP, Grune T, Speckmann B. Article CAS Google Scholar Liemburg-Apers DC, Willems PH, Koopman WJ, Grefte S. Article CAS Google Scholar Niki E. Article CAS Google Scholar Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Article CAS Google Scholar Sugii S, Kida Y, Berggren WT, Evans RM. Article CAS Google Scholar Oliver MH, Harrison NK, Bishop JE, Cole PJ, Laurent GJ. PubMed Google Scholar Liang CC, Park AY, Guan JL. |
| The Interplay Between Adipose Tissue and Vasculature: Role of Oxidative Stress in Obesity | Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in countries over 25 years. N Engl J Med. PubMed Abstract CrossRef Full Text. Taubes G. The science of obesity: what do we really know about what makes us fat? An essay by Gary Taubes. Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol. Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. J Cardiol. Sena CM, Leandro A, Azul L, Seiça R, Perry G. Vascular oxidative stress: impact and therapeutic approaches. Front Physiol. Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. Li H, Horke S, Forstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci. Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Piche ME, Tchernof A, Despres JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord. Arkin JM, Alsdorf R, Bigornia S, Palmisano J, Beal R, Istfan N, et al. Relation of cumulative weight burden to vascular endothelial dysfunction in obesity. Am J Cardiol. Hashimoto M, Akishita M, Eto M, Kozaki K, Ako J, Sugimoto N, et al. The impairment of flow-mediated vasodilatation in obese men with visceral fat accumulation. Parikh NI, Keyes MJ, Larson MG, Pou KM, Hamburg NM, Vita JA, et al. Visceral and subcutaneous adiposity and brachial artery vasodilator function. Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Salgado-Somoza A, Teijeira-Fernandez E, Fernandez AL, Gonzalez-Juanatey JR, Eiras S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol. Frohnert BI, Sinaiko AR, Serrot FJ, Foncea RE, Moran A, Ikramuddin S, et al. Increased adipose protein carbonylation in human obesity. Hauck AK, Zhou T, Hahn W, Petegrosso R, Kuang R, Chen Y, et al. Obesity-induced protein carbonylation in murine adipose tissue regulates the DNA-binding domain of nuclear zinc finger proteins. J Biol Chem. Long EK, Olson DM, Bernlohr DA. High-fat diet induces changes in adipose tissue transoxononenal and transhydroxynonenal levels in a depot-specific manner. Free Radic Biol Med. Sakurai T, Izawa T, Kizaki T, Ogasawara JE, Shirato K, Imaizumi K, et al. Exercise training decreases expression of inflammation-related adipokines through reduction of oxidative stress in rat white adipose tissue. Biochem Biophys Res Commun. Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci USA. Enerback S. The origins of brown adipose tissue. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Pfeifer A, Hoffmann LS. Brown, beige, and white: the new color code of fat and its pharmacological implications. Annu Rev Pharmacol Toxicol. Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. Hildebrand S, Stumer J, Pfeifer A. PVAT and its relation to brown, beige, and white adipose tissue in development and function. Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci. Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Gao Y-J, Zeng Z-H, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg. Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, et al. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. Wonisch W, Falk A, Sundl I, Winklhofer-Roob BM, Lindschinger M. Oxidative stress increases continuously with BMI and age with unfavourable profiles in males. Aging Male. Chattopadhyay M, Khemka VK, Chatterjee G, Ganguly A, Mukhopadhyay S, Chakrabarti S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol Cell Biochem. Boyer F, Diotel N, Girard D, Rondeau P, Essop MF, Bourdon E. Enhanced oxidative stress in adipose tissue from diabetic mice, possible contribution of glycated albumin. Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. Patel C, Ghanim H, Ravishankar S, Sia CL, Viswanathan P, Mohanty P, et al. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab. Reitman A, Friedrich I, Ben-Amotz A, Levy Y. Low plasma antioxidants and normal plasma B vitamins and homocysteine in patients with severe obesity. Isr Med Assoc J. PubMed Abstract Google Scholar. Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: vitamins. Obes Surg. Part B: minerals. Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C—dependent activation of NAD P H oxidase in cultured vascular cells. Bakker SJ, RG IJ, Teerlink T, Westerhoff HV, Gans RO, Heine RJ. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure? Skalicky J, Muzakova V, Kandar R, Meloun M, Rousar T, Palicka V. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clin Chem Lab Med. Hauck AK, Huang Y, Hertzel AV, Bernlohr DA. Adipose oxidative stress and protein carbonylation. Curtis JM, Grimsrud PA, Wright WS, Xu X, Foncea RE, Graham DW, et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Pepping JK, Vandanmagsar B, Fernandez-Kim SO, Zhang J, Mynatt RL, Bruce-Keller AJ. Myeloid-specific deletion of NOX2 prevents the metabolic and neurologic consequences of high fat diet. PLoS ONE. Han CY. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab J. Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. Han CY, Umemoto T, Omer M, Den Hartigh LJ, Chiba T, LeBoeuf R, et al. NADPH oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. Den Hartigh LJ, Omer M, Goodspeed L, Wang S, Wietecha T, O'Brien KD, et al. Adipocyte-specific deficiency of NADPH oxidase 4 delays the onset of insulin resistance and attenuates adipose tissue inflammation in obesity. Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg. Le Lay S, Simard G, Martinez MC, Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev. Lee H, Lee YJ, Choi H, Ko EH, Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. Gao CL, Zhu C, Zhao YP, Chen XH, Ji CB, Zhang CM, et al. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol Cell Endocrinol. Klisic A, Kocic G, Kavaric N, Jovanovic M, Stanisic V, Ninic A. Eat Weight Disord. Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, et al. Uric acid secretion from adipose tissue and its increase in obesity. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Sakurai T, Ogasawara J, Shirato K, Izawa T, Oh-Ishi S, Ishibashi Y, et al. Exercise training attenuates the dysregulated expression of adipokines and oxidative stress in white adipose tissue. Hauck AK, Zhou T, Upadhyay A, Sun Y, O'Connor MB, Chen Y, et al. Histone carbonylation is a redox-regulated epigenomic mark that accumulates with obesity and aging. Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, et al. Mitochondrial H 2 O 2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. Paglialunga S, Ludzki A, Root-McCaig J, Holloway GP. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Piao L, Dorotea D, Jiang S, Koh EH, Oh GT, Ha H. Impaired peroxisomal fitness in obese mice, a vicious cycle exacerbating adipocyte dysfunction via oxidative stress. Antioxid Redox Signal. Shin SK, Cho HW, Song SE, Im SS, Bae JH, Song DK. Oxidative stress resulting from the removal of endogenous catalase induces obesity by promoting hyperplasia and hypertrophy of white adipocytes. Hosick PA, Weeks MF, Hankins MW, Moore KH, Stec DE. Sex-dependent effects of HO-1 deletion from adipocytes in mice. Int J Mol Sci. Cao J, Peterson SJ, Sodhi K, Vanella L, Barbagallo I, Rodella LF, et al. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. Perkins A, Poole LB, Karplus PA. Tuning of peroxiredoxin catalysis for various physiological roles. Kim MH, Kim JY, Kim JH, Lee HS, Huh JW, Lee DS. Peroxiredoxin 2 deficiency reduces white adipogenesis due to the excessive ROS generation. Cell Biol Int. Huh JY, Kim Y, Jeong J, Park J, Kim I, Huh KH, et al. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Okuno Y, Fukuhara A, Hashimoto E, Kobayashi H, Kobayashi S, Otsuki M, et al. Oxidative stress inhibits healthy adipose expansion through suppression of SREBF1-mediated lipogenic pathway. Han YH, Buffolo M, Pires KM, Pei S, Scherer PE, Boudina S. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. Merry TL, Tran M, Dodd GT, Mangiafico SP, Wiede F, Kaur S, et al. Hepatocyte glutathione peroxidase-1 deficiency improves hepatic glucose metabolism and decreases steatohepatitis in mice. Kim HR, Choi EJ, Kie JH, Lee JH, Seoh JY. Deficiency of glutathione peroxidase-1 and catalase attenuated diet-induced obesity and associated metabolic disorders. Acta Diabetol. Genoux A, Bastard JP, Groupe de Travail RA. Effects of leptin and adiponectin on the cardiovascular system. Ann Biol Clin. Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Leandro A, Queiroz M, Azul L, Seica R, Sena CM. Omentin: a novel therapeutic approach for the treatment of endothelial dysfunction in type 2 diabetes. Neves KB, Lobato NS, Lopes RA, Filgueira FP, Zanotto CZ, Oliveira AM, et al. Oikonomou EK, Antoniades C. Immunometabolic regulation of vascular redox state: the role of adipose tissue. King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, et al. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Friederich-Persson M, Nguyen Dinh Cat A, Persson P, Montezano AC, Touyz RM. Brown adipose tissue regulates small artery function through NADPH oxidase 4-derived hydrogen peroxide and redox-sensitive protein kinase G-1alpha. Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Xie Z, Wang X, Liu X, Du H, Sun C, Shao X, et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J Am Heart Assoc. Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol. Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, et al. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Cybularz M, Langbein H, Zatschler B, Brunssen C, Deussen A, Matschke K, et al. Endothelial function and gene expression in perivascular adipose tissue from internal mammary arteries of obese patients with coronary artery disease. Atheroscler Suppl. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Knudson JD, Dincer UD, Zhang C, Swafford ANJr, Koshida R, Picchi A, et al. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Procopio C, Andreozzi F, Laratta E, Cassese A, Beguinot F, Arturi F, et al. Antoniades C, Antonopoulos AS, Tousoulis D, Stefanadis C. Adiponectin: from obesity to cardiovascular disease. Obes Rev. Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, et al. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Ouchi N, Walsh K. Han F, Zhang Y, Shao M, Mu Q, Jiao X, Hou N, et al. Clin Exp Pharmacol Physiol. da Costa RM, Fais RS, Dechandt CRP, Louzada-Junior P, Alberici LC, Lobato NS, et al. Increased mitochondrial ROS generation mediates the loss of the anti-contractile effects of perivascular adipose tissue in high-fat diet obese mice. Br J Pharmacol. Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, et al. Chemerin connects fat to arterial contraction. Parlee SD, McNeil JO, Muruganandan S, Sinal CJ, Goralski KB. Elastase and tryptase govern TNFalpha-mediated production of active chemerin by adipocytes. Chang L, Milton H, Eitzman DT, Chen YE. Paradoxical roles of perivascular adipose tissue in atherosclerosis and hypertension. Circ J. Man AWC, Zhou Y, Xia N, Li H. Perivascular adipose tissue as a target for antioxidant therapy for cardiovascular complications. Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, et al. Bmal1 in perivascular adipose tissue regulates resting-phase blood pressure through transcriptional regulation of angiotensinogen. Kumar RK, Darios ES, Burnett R, Thompson JM, Watts SW. Fenfluramine-induced PVAT-dependent contraction depends on norepinephrine and not serotonin. Pharmacol Res. Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Costa RM, Filgueira FP, Tostes RC, Carvalho MHC, Akamine EH, Lobato NS. H2O2 generated from mitochondrial electron transport chain in thoracic perivascular adipose tissue is crucial for modulation of vascular smooth muscle contraction. Vasc Pharmacol. Gil-Ortega M, Stucchi P, Guzmán-Ruiz R, Cano V, Arribas S, González MC, et al. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. de Mello AH, Costa AB, Engel JDG, Rezin GT. Mitochondrial dysfunction in obesity. Life Sci. Gao Y-J, Takemori K, Su L-Y, An W-S, Lu C, Sharma AM, et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. Awata WM, Gonzaga NA, Borges VF, Silva CB, Tanus-Santos JE, Cunha FQ, et al. Perivascular adipose tissue contributes to lethal sepsis-induced vasoplegia in rats. Eur J Pharmacol. Fernández-Alfonso MS, Gil-Ortega M, García-Prieto CF, Aranguez I, Ruiz-Gayo M, Somoza B. Mechanisms of perivascular adipose tissue dysfunction in obesity. Int J Endocrinol. Ma L, Ma S, He H, Yang D, Chen X, Luo Z, et al. Hypertens Res. Ketonen J, Shi J, Martonen E, Mervaala E. Gil-Ortega M, Condezo-Hoyos L, García-Prieto CF, Arribas SM, González MC, Aranguez I, et al. Imbalance between pro and anti-oxidant mechanisms in perivascular adipose tissue aggravates long-term high-fat diet-derived endothelial dysfunction. PloS ONE. Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, et al. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. Alcala M, Calderon-Dominguez M, Bustos E, Ramos P, Casals N, Serra D, et al. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci Rep. Chouchani ET, Kazak L, Spiegelman BM. Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. Bordicchia M, Liu D, Amri E-Z, Ailhaud G, Dessì-Fulgheri P, Zhang C, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Investig. Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, et al. Brown adipose tissue activity controls triglyceride clearance. Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, et al. Loss of perivascular adipose tissue upon PPARγ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Li R-M, Chen S-Q, Zeng N-X, Zheng S-H, Guan L, Liu H-M, et al. Browning of abdominal aorta perivascular adipose tissue inhibits adipose tissue inflammation. Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. Bonnefont-Rousselot D. Obesity and oxidative stress: potential roles of melatonin as antioxidant and metabolic regulator. Endocr Metab Immune Disord Drug Targets. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM, et al. Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, Renard P, et al. Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Agil A, Navarro-Alarcon M, Ruiz R, Abuhamadah S, El-Mir MY, Vazquez GF. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. Jimenez-Aranda A, Fernandez-Vazquez G, Campos D, Tassi M, Velasco-Perez L, Tan DX, et al. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. Tan DX, Manchester LC, Qin L, Reiter RJ. Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Pechanova O, Paulis L, Simko F. Peripheral and central effects of melatonin on blood pressure regulation. Nguyen T, Nioi P, Pickett CB. Intriguingly, IEMs display metabolic reprograming with a switch to glycolysis for both ATP production and muted ROS generation Olsen et al. Specifically, in myoclonic epilepsy with ragged red fibers MERRF , increased intracellular H 2 O 2 levels correspond with increased AMPK phosphorylation and expression of GLUT1, hexokinase II, and lactate dehydrogenase. These results, as well as increased lactic acid production, all point to increased glycolysis De la Mata et al. In multiple acyl-CoA dehydrogenase deficiency MADD , mutations in ETFa , ETFb , or ETFDH , lead to decreased ATP production with an accumulation of organic acids, including glutaric acid as well as acyl-carnitines. A subset of these patients is riboflavin responsive RR-MADD with high dose riboflavin alleviating some symptoms. Similar to MERRF, many RR-MADD patients exhibit increased oxidative stress Cornelius et al. This defect may be due to defective electron transfer and increased electron leak from the misfolded ETFDH protein and decreased binding of CoQ10 Cornelius et al. Treatment with CoQ10, but not riboflavin, decreased ROS levels Cornelius et al. Analysis of mitochondrial function from RR-MADD fibroblasts showed increased mitochondrial fragmentation and reduced β-oxidation, while supplementation with the antioxidant CoQ10 decreased fragmentation and mitophagy Cornelius et al. While obesity and IEMs are distinct disorders, both conditions impinge on energy balance in WAT. Even though these disorders have very different manifestations, oxidative stress plays an important role in both and may be a therapeutic target. For example, CoQ10 is often given as a broad-spectrum treatment to individuals with IEMs, and while its effectiveness is debated, the anti-inflammatory effects may be beneficial in reducing oxidative stress and the pathogenesis of the disease Cornelius et al. Mitochondria represent control centers of many metabolic pathways. Interventions that enhance adipocyte mitochondrial function may also improve whole-body insulin sensitivity. Mitigation of mitochondrial ROS production and oxidative stress may be a possible therapeutic target in type 2 diabetes and IEMs because some mitochondrial-targeted antioxidants and other small molecule drugs improve metabolic profiles in mouse models Feillet-Coudray et al. Thiazolidinediones TZDs are PPARγ agonists used for treating type 2 diabetes Kelly et al. TZDs, such as rosiglitazone and pioglitazone, enhance insulin sensitivity by improving adipokine profiles Maeda et al. TZDs also promote insulin sensitivity by directing fatty acids to subcutaneous fat, rather than visceral fat. Subcutaneous fat expandability, even in the context of obesity and type 2 diabetes, correlates with insulin sensitivity in rodents and humans Ross et al. Numerous in vitro and in vivo studies demonstrate TZDs enhance mitochondrial biogenesis, content, function, and morphology. Rosiglitazone also induces cellular antioxidant enzymes responsible for the removal of ROS generated by increased mitochondrial activity in adipose tissue of diabetic rodents Rong et al. Taken together, TZDs impact WAT mitochondrial function in multiple ways that ultimately improve systemic fat metabolism and insulin sensitivity. Other therapeutic strategies include mitochondria-targeted scavengers Smith et al. However, these methods to enhance mitochondrial function display a narrow therapeutic range that limits safe use for obesity. Although the development of insulin resistance does not require impaired mitochondrial function Hancock et al. Aerobic exercise and caloric restriction disrupt this vicious loop, potentially by preventing accumulation of injured mitochondrial proteins with substantial improvement of insulin sensitivity. In insulin-resistant people, aerobic exercise stimulates both mitochondrial biogenesis and efficiency concurrent with an enhancement of insulin action Mul et al. Ultimately, exercise engages pathways that reduce ROS coupled with insulin sensitivity and improved mitochondrial function in WAT. Obesity is the result of excessive expansion of WAT depots due to a chronic imbalance between energy intake and expenditure. Many studies demonstrate that oxidative stress in fat cells links obesity and its comorbidities. The fact that WAT remains the sole organ for storing surfeit lipid renders the macromolecules in adipocytes particularly vulnerable to carbonylation and other modifications driven by oxidative stress. Prolonged oxidative stress negatively influences endocrine and homeostatic performance of WAT, including disruption of hormone secretion, elevation of serum lipids, inadequate cellular antioxidant defenses, and impaired mitochondrial function Figure 2. Metabolic challenges, such as persistent nutrient intake and sedentary behaviors that promote impaired glucose and lipid handling, also elevate mitochondrial ROS production to cause adipocyte dysfunction. Consequently, adipocytes cannot engage appropriate transcriptional and energetic responses to enable insulin sensitivity. Figure 2. Impact of oxidative stress on adipocyte function. Increased plasma glucose and free fatty acids contribute to increased oxidative stress by increasing the production of reactive oxygen species ROS and decreasing antioxidant concentrations. Increased oxidative stress occurs via enzymes in the cytoplasm, such as NADPH oxidase, and the mitochondria. The oxidative environment increases lipid storage resulting in hypertrophic adipocytes. Additionally, increased mitochondrial ROS mtROS alters the activity state of metabolic enzymes either directly or by changing the oxidative state of protein side-chains or by other post-translational modifications, including lipid peroxidation and protein carbonylation. Cumulatively, increased adipocyte oxidative stress decreases adipogenesis and secretion of adipokines, leading to unbalanced energy homeostasis, insulin resistance, and type 2 diabetes. The increasing prevalence of obesity suggests lifestyle intervention as the principal method to treat obesity is unlikely to succeed. Currently, all available anti-obesity medications act by limiting energy intake through appetite suppression or inhibition of intestinal lipid absorption. However, these medications are largely ineffective and often have adverse side effects. The central role of mitochondria in nutrient handling provides a logical entry point for improving metabolism in obesity. While approaches to understanding and intervening in oxidative damage evolve, exploration of mitochondria redox balance may enable development of dietary and small molecule therapies for obesity and its comorbidities. This work was funded by the American Diabetes Association IBS and NIH R01DK The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Acosta, M. Coenzyme Q biosynthesis in health and disease. Acta , — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ahmed, M. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake. Obesity 18, 27— Akl, M. Perturbed adipose tissue hydrogen peroxide metabolism in centrally obese men: association with insulin resistance. PLoS One e Anderson, E. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. Armstrong, J. The redox regulation of intermediary metabolism by a superoxide-aconitase rheostat. Bioessays 26, — Barbosa, M. Hydrogen peroxide production regulates the mitochondrial function in insulin resistant muscle cells: effect of catalase overexpression. Bjelakovic, G. Antioxidant supplements to prevent mortality. JAMA , — Antioxidant supplements and mortality. Care 17, 40— Boden, G. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Boden, M. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Bogacka, I. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. Bournat, J. Mitochondrial dysfunction in obesity. Diabetes Obes. Boyle, P. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Chappuis, B. Differential effect of pioglitazone PGZ and rosiglitazone RGZ on postprandial glucose and lipid metabolism in patients with type 2 diabetes mellitus: a prospective, randomized crossover study. Diabetes Metab. Chouchani, E. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature , — Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. Cornelius, N. Secondary coenzyme Q10 deficiency and oxidative stress in cultured fibroblasts from patients with riboflavin responsive multiple Acyl-CoA dehydrogenation deficiency. Cellular consequences of oxidative stress in riboflavin responsive multiple acyl-CoA dehydrogenation deficiency patient fibroblasts. Curtis, J. Protein carbonylation and metabolic control systems. Trends Endocrinol. Dasuri, K. Role of physiological levels of 4-hydroxynonenal on adipocyte biology: implications for obesity and metabolic syndrome. Free Radic. Davies, M. Protein oxidation and peroxidation. De la Mata, M. Recovery of MERRF fibroblasts and cybrids pathophysiology by coenzyme Q Neurotherapeutics 9, — Deeg, M. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30, — Demozay, D. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Elrayess, M. Escribano-Lopez, I. The mitochondrial antioxidant SS increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Esterbauer, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Fazakerley, D. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. Feillet-Coudray, C. The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol. Fouret, G. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Frohnert, B. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Increased adipose protein carbonylation in human obesity. Obesity 19, — Furukawa, S. Increased oxidative stress in obesity and its impact on metabolic syndrome. PubMed Abstract Google Scholar. Fusco, D. Effects of antioxidant supplementation on the aging process. Aging 2, — Gardner, P. Superoxide radical and iron modulate aconitase activity in mammalian cells. Goldberg, R. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28, — Goldgof, M. The chemical uncoupler 2,4-dinitrophenol DNP protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. Grimsrud, P. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Proteomics 6, — Oxidative stress and covalent modification of protein with bioactive aldehydes. Han, Y. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Hancock, C. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Hauck, A. Adipose oxidative stress and protein carbonylation. Obesity-induced protein carbonylation in murine adipose tissue regulates the DNA-binding domain of nuclear zinc finger proteins. Hausladen, A. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. Holley, A. Manganese superoxide dismutase: guardian of the powerhouse. Holloszy, J. Huh, J. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Hurd, T. Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species. Kelly, I. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care 22, — Khan, M. A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone. Diabetes Care 25, — Kim, J. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. King, A. A comparison in a clinical setting of the efficacy and side effects of three thiazolidinediones. Diabetes Care 23, Kowaltowski, A. Mitochondrial damage induced by conditions of oxidative stress. Lee, H. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. Mitochondrial-targeted catalase protects against high-fat diet-induced muscle insulin resistance by decreasing intramuscular lipid accumulation. Liesa, M. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. Long, E. High-fat diet induces changes in adipose tissue transoxononenal and transhydroxynonenal levels in a depot-specific manner. Lu, R. Mitochondrial development and the influence of its dysfunction during rat adipocyte differentiation. Lushchak, O. Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox Rep. Maeda, N. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Miyazaki, Y. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. Mul, J. I, Hirshman, M. Exercise and regulation of carbohydrate metabolism. Murphy, M. |
| Oxidative stress mediates depot-specific functional differences of human adipose-derived stem cells | Deficiency of glutathione peroxidase-1 and catalase oxidatice diet-induced Viscdral and associated metabolic disorders. Functions of fat tissue and oxudative are greatly affected oxidatove intracellular Muscular endurance training levels, Teff grain recipes strsss leading to the oxidative stress in fat are still not clear [ 16 ]. The obesity-induced adipose tissue dysfunction affects the adipokines secretion profiles, which further regulates remote tissues including cardiovascular systems 8. Obesity: global epidemiology and pathogenesis. XO is an oxidant form of XOD, which converts purine bases to uric acid Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Similar to MSCs, ASCs exhibit migratory properties, in which VS-ASCs are more defective than SC-ASCs. |
Visceral fat and oxidative stress -
Ong WK, Sugii S. Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol. Lim MH, Ong WK, Sugii S. The current landscape of adipose-derived stem cells in clinical applications.
Expert Rev Mol Med. Article Google Scholar. Tang QQ, Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem. Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome.
Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng YH, Kahn CR. Intrinsic differences in adipocyte precursor cells from different white fat depots. Ong WK, Tan CS, Chan KL, Goesantoso GG, Chan XH, Chan E, Yin J, Yeo CR, Khoo CM, So JB, Shabbir A, Toh SA, Han W, Sugii S.
Identification of specific cell-surface markers of adipose-derived stem cells from subcutaneous and visceral fat depots. Stem Cell Reports. Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol. Takeda K, Sriram S, Chan XH, Ong WK, Yeo CR, Tan B, Lee SA, Kong KV, Hoon S, Jiang H, Yuen JJ, Perumal J, Agrawal M, Vaz C, So J, Shabbir A, Blaner WS, Olivo M, Han W, Tanavde V, Toh SA, Sugii S.
Retinoic acid mediates visceral-specific adipogenic defects of human adipose-derived stem cells. Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem. Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, Durante-Montiel I, Sanchez-Rivera G, Valadez-Vega C, Morales-Gonzalez JA.
Inflammation, oxidative stress, and obesity. Int J Mol Sci. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome.
J Clin Invest. Gariballa S, Afandi B, Abuhaltem M, Yassin J, Habib H, Ibrahim W. Oxidative damage and inflammation in obese diabetic Emirati subjects supplemented with antioxidants and B-vitamins: a randomized placebo-controlled trail.
Nutr Metab Lond. Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Castro JP, Grune T, Speckmann B. The two faces of reactive oxygen species ROS in adipocyte function and dysfunction. Biol Chem. Liemburg-Apers DC, Willems PH, Koopman WJ, Grefte S.
Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch Toxicol. Niki E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am J Clin Nutr. Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M.
Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. Sugii S, Kida Y, Berggren WT, Evans RM. Feeder-dependent and feeder-independent iPS cell derivation from human and mouse adipose stem cells.
Nat Protoc. Oliver MH, Harrison NK, Bishop JE, Cole PJ, Laurent GJ. A rapid and convenient assay for counting cells cultured in microwell plates: application for assessment of growth factors.
J Cell Sci. PubMed Google Scholar. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro.
Download references. We thank Kosuke Takeda for the preceding microarray analysis and Subha Subramanian, Wee Kiat Ong, and other members of Fat Metabolism and Stem Cell Group, Laboratory of Metabolic Medicine, Microscopy Core, Mechanobiology Institute at National University of Singapore, and Singapore Bioimaging Consortium-Nikon Imaging Centre SBIC-NIC for the help with our studies.
Su, Singapore-China Joint Grant Project to Sh. Su and M. Sr and Sh. Su, and funding from the Singapore National Medical Research Council NMRC to S-A. Duke-NUS Medical School, 8 College Road, Singapore, , Singapore. Department of Surgery, National University Hospital, 5 Lower Kent Ridge Road, Singapore, , Singapore.
Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, 14 Medical Drive, Singapore, , Singapore. You can also search for this author in PubMed Google Scholar. AS contributed to the provision of study material or patients and administrative support.
SAT contributed to the provision of study material or patients and administrative support. WH contributed to the financial support, administrative support, and manuscript writing. ShS contributed to the conception and design, financial support, administrative support, data analysis and interpretation, and manuscript writing.
All authors made final approval of the manuscript. Correspondence to Shigeki Sugii. Use of human patient-derived cells was conducted with informed consent obtained for each subject, approved by the National Healthcare Group Domain Specific Review Board, Singapore, and performed in accordance with its relevant regulations.
ShS is a co-founder of Celligenics Pte Ltd. The other authors declare that they have no competing interests. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Figure S1. Gene expression studies of additional ROS-related genes that are not included in Figure 1 b.
Figure S2. VS-ASCs have increased ROS when compared to SC-ASCs in the lower passage. Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients.
Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. Li H, Horke S, Forstermann U. Oxidative stress in vascular disease and its pharmacological prevention.
Trends Pharmacol Sci. Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome.
Endocr Rev. Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study.
Piche ME, Tchernof A, Despres JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening.
Int J Obes Relat Metab Disord. Arkin JM, Alsdorf R, Bigornia S, Palmisano J, Beal R, Istfan N, et al. Relation of cumulative weight burden to vascular endothelial dysfunction in obesity.
Am J Cardiol. Hashimoto M, Akishita M, Eto M, Kozaki K, Ako J, Sugimoto N, et al. The impairment of flow-mediated vasodilatation in obese men with visceral fat accumulation. Parikh NI, Keyes MJ, Larson MG, Pou KM, Hamburg NM, Vita JA, et al.
Visceral and subcutaneous adiposity and brachial artery vasodilator function. Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS.
Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Salgado-Somoza A, Teijeira-Fernandez E, Fernandez AL, Gonzalez-Juanatey JR, Eiras S.
Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol. Frohnert BI, Sinaiko AR, Serrot FJ, Foncea RE, Moran A, Ikramuddin S, et al. Increased adipose protein carbonylation in human obesity.
Hauck AK, Zhou T, Hahn W, Petegrosso R, Kuang R, Chen Y, et al. Obesity-induced protein carbonylation in murine adipose tissue regulates the DNA-binding domain of nuclear zinc finger proteins. J Biol Chem. Long EK, Olson DM, Bernlohr DA. High-fat diet induces changes in adipose tissue transoxononenal and transhydroxynonenal levels in a depot-specific manner.
Free Radic Biol Med. Sakurai T, Izawa T, Kizaki T, Ogasawara JE, Shirato K, Imaizumi K, et al. Exercise training decreases expression of inflammation-related adipokines through reduction of oxidative stress in rat white adipose tissue.
Biochem Biophys Res Commun. Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, et al. Mapping of human brown adipose tissue in lean and obese young men.
Proc Natl Acad Sci USA. Enerback S. The origins of brown adipose tissue. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential.
Nat Med. Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Pfeifer A, Hoffmann LS. Brown, beige, and white: the new color code of fat and its pharmacological implications. Annu Rev Pharmacol Toxicol. Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al.
Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. Hildebrand S, Stumer J, Pfeifer A.
PVAT and its relation to brown, beige, and white adipose tissue in development and function. Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci. Gollasch M, Dubrovska G. Paracrine role for periadventitial adipose tissue in the regulation of arterial tone.
Gao Y-J, Zeng Z-H, Teoh K, Sharma AM, Abouzahr L, Cybulsky I, et al. Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J Thorac Cardiovasc Surg.
Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, et al. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al.
Increased oxidative stress in obesity and its impact on metabolic syndrome. Wonisch W, Falk A, Sundl I, Winklhofer-Roob BM, Lindschinger M. Oxidative stress increases continuously with BMI and age with unfavourable profiles in males. Aging Male.
Chattopadhyay M, Khemka VK, Chatterjee G, Ganguly A, Mukhopadhyay S, Chakrabarti S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects.
Mol Cell Biochem. Boyer F, Diotel N, Girard D, Rondeau P, Essop MF, Bourdon E. Enhanced oxidative stress in adipose tissue from diabetic mice, possible contribution of glycated albumin.
Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. Patel C, Ghanim H, Ravishankar S, Sia CL, Viswanathan P, Mohanty P, et al.
Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab. Reitman A, Friedrich I, Ben-Amotz A, Levy Y. Low plasma antioxidants and normal plasma B vitamins and homocysteine in patients with severe obesity.
Isr Med Assoc J. PubMed Abstract Google Scholar. Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: vitamins. Obes Surg. Part B: minerals.
Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C—dependent activation of NAD P H oxidase in cultured vascular cells.
Bakker SJ, RG IJ, Teerlink T, Westerhoff HV, Gans RO, Heine RJ. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure?
Skalicky J, Muzakova V, Kandar R, Meloun M, Rousar T, Palicka V. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clin Chem Lab Med. Hauck AK, Huang Y, Hertzel AV, Bernlohr DA. Adipose oxidative stress and protein carbonylation.
Curtis JM, Grimsrud PA, Wright WS, Xu X, Foncea RE, Graham DW, et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Pepping JK, Vandanmagsar B, Fernandez-Kim SO, Zhang J, Mynatt RL, Bruce-Keller AJ.
Myeloid-specific deletion of NOX2 prevents the metabolic and neurologic consequences of high fat diet. PLoS ONE. Han CY. Roles of reactive oxygen species on insulin resistance in adipose tissue. Diabetes Metab J.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. Han CY, Umemoto T, Omer M, Den Hartigh LJ, Chiba T, LeBoeuf R, et al. NADPH oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes.
Den Hartigh LJ, Omer M, Goodspeed L, Wang S, Wietecha T, O'Brien KD, et al. Adipocyte-specific deficiency of NADPH oxidase 4 delays the onset of insulin resistance and attenuates adipose tissue inflammation in obesity.
Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg. Le Lay S, Simard G, Martinez MC, Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev. Lee H, Lee YJ, Choi H, Ko EH, Kim JW.
Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. Gao CL, Zhu C, Zhao YP, Chen XH, Ji CB, Zhang CM, et al. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes.
Mol Cell Endocrinol. Klisic A, Kocic G, Kavaric N, Jovanovic M, Stanisic V, Ninic A. Eat Weight Disord. Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, et al. Uric acid secretion from adipose tissue and its increase in obesity.
Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance.
Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Sakurai T, Ogasawara J, Shirato K, Izawa T, Oh-Ishi S, Ishibashi Y, et al.
Exercise training attenuates the dysregulated expression of adipokines and oxidative stress in white adipose tissue. Hauck AK, Zhou T, Upadhyay A, Sun Y, O'Connor MB, Chen Y, et al.
Histone carbonylation is a redox-regulated epigenomic mark that accumulates with obesity and aging. Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1.
Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, et al. Mitochondrial H 2 O 2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. Paglialunga S, Ludzki A, Root-McCaig J, Holloway GP. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice.
Piao L, Dorotea D, Jiang S, Koh EH, Oh GT, Ha H. Impaired peroxisomal fitness in obese mice, a vicious cycle exacerbating adipocyte dysfunction via oxidative stress. Antioxid Redox Signal.
Shin SK, Cho HW, Song SE, Im SS, Bae JH, Song DK. Oxidative stress resulting from the removal of endogenous catalase induces obesity by promoting hyperplasia and hypertrophy of white adipocytes. Hosick PA, Weeks MF, Hankins MW, Moore KH, Stec DE. Sex-dependent effects of HO-1 deletion from adipocytes in mice.
Int J Mol Sci. Cao J, Peterson SJ, Sodhi K, Vanella L, Barbagallo I, Rodella LF, et al. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet.
Wood ZA, Schroder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. Perkins A, Poole LB, Karplus PA. Tuning of peroxiredoxin catalysis for various physiological roles. Kim MH, Kim JY, Kim JH, Lee HS, Huh JW, Lee DS. Peroxiredoxin 2 deficiency reduces white adipogenesis due to the excessive ROS generation.
Cell Biol Int. Huh JY, Kim Y, Jeong J, Park J, Kim I, Huh KH, et al. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression.
Okuno Y, Fukuhara A, Hashimoto E, Kobayashi H, Kobayashi S, Otsuki M, et al. Oxidative stress inhibits healthy adipose expansion through suppression of SREBF1-mediated lipogenic pathway.
Han YH, Buffolo M, Pires KM, Pei S, Scherer PE, Boudina S. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis.
Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, et al. Reactive oxygen species enhance insulin sensitivity.
Cell Metab. Merry TL, Tran M, Dodd GT, Mangiafico SP, Wiede F, Kaur S, et al. Hepatocyte glutathione peroxidase-1 deficiency improves hepatic glucose metabolism and decreases steatohepatitis in mice.
Kim HR, Choi EJ, Kie JH, Lee JH, Seoh JY. Deficiency of glutathione peroxidase-1 and catalase attenuated diet-induced obesity and associated metabolic disorders.
Acta Diabetol. Genoux A, Bastard JP, Groupe de Travail RA. Effects of leptin and adiponectin on the cardiovascular system. Ann Biol Clin. Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells.
Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, et al. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels.
Leandro A, Queiroz M, Azul L, Seica R, Sena CM. Omentin: a novel therapeutic approach for the treatment of endothelial dysfunction in type 2 diabetes.
Neves KB, Lobato NS, Lopes RA, Filgueira FP, Zanotto CZ, Oliveira AM, et al. Oikonomou EK, Antoniades C. Immunometabolic regulation of vascular redox state: the role of adipose tissue.
King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, et al. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice.
Friederich-Persson M, Nguyen Dinh Cat A, Persson P, Montezano AC, Touyz RM. Brown adipose tissue regulates small artery function through NADPH oxidase 4-derived hydrogen peroxide and redox-sensitive protein kinase G-1alpha.
Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Xie Z, Wang X, Liu X, Du H, Sun C, Shao X, et al. Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization.
J Am Heart Assoc. Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, et al. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. The role of adipose tissue in cardiovascular health and disease. Nat Rev Cardiol.
Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, et al. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Cybularz M, Langbein H, Zatschler B, Brunssen C, Deussen A, Matschke K, et al.
Endothelial function and gene expression in perivascular adipose tissue from internal mammary arteries of obese patients with coronary artery disease. Atheroscler Suppl. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals.
Knudson JD, Dincer UD, Zhang C, Swafford ANJr, Koshida R, Picchi A, et al. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin.
Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A.
Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Procopio C, Andreozzi F, Laratta E, Cassese A, Beguinot F, Arturi F, et al. While approaches to understanding and intervening in oxidative damage evolve, exploration of mitochondria redox balance may enable development of dietary and small molecule therapies for obesity and its comorbidities.
This work was funded by the American Diabetes Association IBS and NIH R01DK The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Acosta, M. Coenzyme Q biosynthesis in health and disease.
Acta , — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ahmed, M. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake.
Obesity 18, 27— Akl, M. Perturbed adipose tissue hydrogen peroxide metabolism in centrally obese men: association with insulin resistance. PLoS One e Anderson, E. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans.
Armstrong, J. The redox regulation of intermediary metabolism by a superoxide-aconitase rheostat. Bioessays 26, — Barbosa, M. Hydrogen peroxide production regulates the mitochondrial function in insulin resistant muscle cells: effect of catalase overexpression.
Bjelakovic, G. Antioxidant supplements to prevent mortality. JAMA , — Antioxidant supplements and mortality. Care 17, 40— Boden, G. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men.
Boden, M. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Bogacka, I. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro.
Bournat, J. Mitochondrial dysfunction in obesity. Diabetes Obes. Boyle, P. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Chappuis, B. Differential effect of pioglitazone PGZ and rosiglitazone RGZ on postprandial glucose and lipid metabolism in patients with type 2 diabetes mellitus: a prospective, randomized crossover study.
Diabetes Metab. Chouchani, E. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature , — Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. Cornelius, N.
Secondary coenzyme Q10 deficiency and oxidative stress in cultured fibroblasts from patients with riboflavin responsive multiple Acyl-CoA dehydrogenation deficiency. Cellular consequences of oxidative stress in riboflavin responsive multiple acyl-CoA dehydrogenation deficiency patient fibroblasts.
Curtis, J. Protein carbonylation and metabolic control systems. Trends Endocrinol. Dasuri, K. Role of physiological levels of 4-hydroxynonenal on adipocyte biology: implications for obesity and metabolic syndrome. Free Radic. Davies, M. Protein oxidation and peroxidation.
De la Mata, M. Recovery of MERRF fibroblasts and cybrids pathophysiology by coenzyme Q Neurotherapeutics 9, — Deeg, M. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia.
Diabetes Care 30, — Demozay, D. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes.
Elrayess, M. Escribano-Lopez, I. The mitochondrial antioxidant SS increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes.
Esterbauer, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Fazakerley, D. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. Feillet-Coudray, C. The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol.
Fouret, G. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Frohnert, B. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Increased adipose protein carbonylation in human obesity.
Obesity 19, — Furukawa, S. Increased oxidative stress in obesity and its impact on metabolic syndrome. PubMed Abstract Google Scholar. Fusco, D. Effects of antioxidant supplementation on the aging process. Aging 2, — Gardner, P.
Superoxide radical and iron modulate aconitase activity in mammalian cells. Goldberg, R. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia.
Diabetes Care 28, — Goldgof, M. The chemical uncoupler 2,4-dinitrophenol DNP protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. Grimsrud, P. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal.
Proteomics 6, — Oxidative stress and covalent modification of protein with bioactive aldehydes. Han, Y. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis.
Hancock, C. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Hauck, A. Adipose oxidative stress and protein carbonylation. Obesity-induced protein carbonylation in murine adipose tissue regulates the DNA-binding domain of nuclear zinc finger proteins.
Hausladen, A. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. Holley, A. Manganese superoxide dismutase: guardian of the powerhouse. Holloszy, J. Huh, J.
Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Hurd, T. Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species. Kelly, I. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes.
Diabetes Care 22, — Khan, M. A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone.
Diabetes Care 25, — Kim, J. Obesity-associated improvements in metabolic profile through expansion of adipose tissue.
King, A. A comparison in a clinical setting of the efficacy and side effects of three thiazolidinediones. Diabetes Care 23, Kowaltowski, A. Mitochondrial damage induced by conditions of oxidative stress. Lee, H. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion.
Mitochondrial-targeted catalase protects against high-fat diet-induced muscle insulin resistance by decreasing intramuscular lipid accumulation.
Streas details. Visceral VS fat depot is known to have defective adipogenic functions dat Visceral fat and oxidative stress subcutaneous SC abd, but its mechanism of origin is Teff grain recipes. Srtess tested our hypothesis that the degree streas oxidative stress in adipose-derived stem Non-GMO Coconut Oil ASCs from Enhancing natural immunity depots may account for this difference. ASCs were isolated from VS omental region and SC abdominal region fat depots of human subjects undergoing bariatric surgery. ASCs from VS and SC fat were investigated for their cellular characteristics in reactive oxygen species ROSmetabolism, gene expression, proliferation, senescence, migration, and adipocyte differentiation. ASCs were also treated with antioxidant ascorbic acid vitamin C. We found that human VS-derived ASCs exhibit excessive oxidative stress characterized by high reactive oxygen species ROScompared to SC-derived ASCs.
0 thoughts on “Visceral fat and oxidative stress”