
Hyperglycemia and insulin resistance -
But there are ways to make the body more receptive to insulin, which can help prevent or delay type 2 diabetes—or help someone with type 1 diabetes manage their blood glucose blood sugar. In response to the body's insulin resistance, the pancreas deploys more of the hormone to keep cells energized and manage blood glucose levels in a healthy range.
This is why people with type 2 diabetes tend to have higher levels of circulating insulin. The ability of the pancreas to increase insulin production means that insulin resistance alone won't have any symptoms at first.
Over time, though, insulin resistance tends to get worse, and the pancreatic beta cells that make insulin can wear out.
Eventually, the pancreas no longer produces enough insulin to overcome the cells' resistance. The result is higher blood glucose levels, and ultimately prediabetes or type 2 diabetes. Insulin has other roles in the body besides regulating blood glucose levels, and the effects of insulin resistance are thought to go beyond diabetes.
For example, some research has shown that insulin resistance, independent of diabetes, is associated with heart disease. Scientists are beginning to get a better understanding of how insulin resistance develops.
For starters, several genes have been identified that make a person more or less likely to develop the condition. It's also known that older people are more prone to insulin resistance.
Lifestyle can play a role, too. Being sedentary, overweight or obese increases the risk for insulin resistance. It's not clear, but some researchers theorize that extra fat tissue may cause inflammation, physiological stress or other changes in the cells that contribute to insulin resistance.
There may even be some undiscovered factor produced by fat tissue, perhaps a hormone, that signals the body to become insulin resistant. Doctors don't usually test for insulin resistance as a part of standard diabetes care.
In clinical research, however, scientists may look specifically at measures of insulin resistance, often to study potential treatments for insulin resistance or type 2 diabetes. They typically administer a large amount of insulin to a subject while at the same time delivering glucose to the blood to keep levels from dipping too low.
The less glucose needed to maintain normal blood glucose levels, the greater the insulin resistance. Insulin resistance comes in degrees. The more insulin resistant a person with type 2 is, the harder it will be to manage their diabetes because more medication is needed to get enough insulin in the body to achieve target blood glucose levels.
Insulin resistance isn't a cause of type 1 diabetes, but people with type 1 who are insulin resistant will need higher insulin doses to keep their blood glucose under control than those who are more sensitive to insulin.
Chronic caloric excess causes increased visceral fat mass due to hypertrophy of individual adipocytes and hyperplasia of adipocyte precursors [ 63 ]. As adiposity increases, the adipocytes release chemotactic factors such as monocyte chemoattractant protein-1 MCP-1 , and tumor-necrosis factor-α TNFα , which modulates an inflammatory response in adipose tissue.
MCP-1 initiates the migration of monocytes into VAT and promotes their differentiation into macrophages. Macrophages then secrete large amounts of TNFα, increasing lipolysis and reducing insulin-stimulated glucose transporter 4, triglyceride biosynthesis, and adipocyte storage in the VAT, thus resulting in an increase in circulating triglyceride levels [ 64 ].
This event could result in ectopic lipid deposition of toxic fatty acid species i. The increase in EAT leads to cardiac steatosis and to an increase in mass in both ventricles, resulting in ventricular hypertrophy, contractile dysfunction, apoptosis, fibrosis, and impaired left ventricular diastolic function [ 66 , 67 , 68 ].
Elevated levels of LDL, smoking, elevated blood pressure and type 1 and type 2 diabetes, are well known risk factors for CVD, however, insulin resistance, hyperglycaemia and inflammation can also lead to and predict adverse cardiovascular events.
Furthermore, insulin resistance is related to disorders such as hypertriglyceridemia as well as low levels HDL.
In , investigators in the Insulin Resistance Atherosclerosis Study IRAS , showed a direct relation between insulin resistance and atherosclerosis [ 70 ] and a follow-up prospective study in a cohort of patients reported insulin resistance as an important risk factor for CVD [ 71 ].
A meta-analysis of 65 studies, which included , participants, revealed that insulin resistance, evaluated by HOMA index, was a good predictor for CVD [ 6 ]. Even though a wealth of studies support the notion that CVD is related to insulin resistance [ 4 , 9 , 31 , 73 , 74 , 75 , 76 ], there are some controversial reports as well.
A study performed by Kozakova et al. reported the association of insulin sensitivity with risk of CVD in young to middle aged men, where as in women, atherosclerosis and plaque formation were independently associated with fasting plasma glucose levels [ 77 ].
In addition to insulin resistance, the compensatory hyperinsulinemia associated with insulin resistance can play a critical role in the formation of atherosclerotic plaques by changing the gene expression pattern associated with estrogen receptor, as reported in animal models [ 78 ].
Furthermore, hyperglycemia produces alterations in various metabolic and cellular functions [ 7 , 8 , 9 ] including dyslipidemia, hypertension, endothelial dysfunction, oxidative stress and alterations in cardiac metabolism. Issues related to the latter alterations are discussed further along in this review.
Although there seems to be a preferential use of fatty acids for the production of energy, the heart has the ability to change to another substrate for the generation of ATP, depending on availability, to ensure its energy demand. But also the substrate transporters, GLUT4 for glucose and CD36 for fatty acids , play a role in this dynamic balance of substrate utilization [ 79 ].
During injury, the heart shifts from using fatty acids as energetic substrates toward glucose, but this metabolic flexibility is impaired under insulin resistance, leaving to fatty acid as the sole fuel source. This shift induces an increase in the uptake and accumulation of lipid in the heart, producing lipotoxicity [ 80 ].
In this sense, the balance between lipid degradation and glucose oxidation could decrease diabetic cardiomyopathy [ 81 ]. The dyslipidemia induced by insulin resistance and type 2 diabetes diabetic dyslipidemia [ 82 ] is characterized by the lipid triad: 1 high levels of plasma triglycerides, 2 low levels of HDL, and 3 the appearance of small dense low-density lipoproteins sdLDL , as well as an excessive postprandial lipemia [ 35 , 82 , 83 , 84 ].
A study conducted in 10, people with normal blood pressure or pre-hypertension demonstrated dyslipidemia as a strong predictor of development of type 2 diabetes [ 87 ]. Frequently, diabetic dyslipidemia precedes type 2 diabetes by several years, suggesting that the abnormal lipid metabolism is an early event in the development of CVD in type 2 diabetes [ 88 ].
Obesity is a world-wide epidemic and intimately associated with the development of type 2 diabetes and CVDs. Visceral and epicardial adiposity related to obesity are the major drivers for cardiac disease in these individuals [ 60 ]. Obesity has a major effect in modifying the lipoprotein profile and factors associated with systemic and vascular inflammation, and endothelial dysfunction [ 89 ].
Abnormal concentrations of lipids and apolipoproteins can produce changes in the production, conversion, or catabolism of lipoprotein particles. These changes may contribute to increased basal lipolysis in obesity and the release of fatty acids into the circulation that consequences a proatherogenic phenotype [ 19 , 90 ].
VLDL, very low-density lipoprotein, is assembled and produced in the liver, which depends on the availability of substrates and is tightly regulated by insulin [ 91 ].
Hepatic VLDL production is induced in the fasting state, which results in increased levels of VLDL in the blood. The increase of lipids from different sources, such as circulating FFA, endocytosis of triglyceride-rich lipoproteins, and de novo lipogenesis, allows for the posttranslational stabilization of apoB and enhances the assembly and secretion of VLDL particles.
This leads to VLDL and FFA production, which carries energy between the liver and the adipose tissue [ 92 ]. In response to insulin secretion, VLDL synthesis is inhibited to limit the level of plasma triglycerides [ 83 , 93 ]. Normally, insulin, through PI3K activation, promotes the degradation of apoB, but under insulin resistance this degradation is impaired [ 92 , 94 ].
Thus, facing a combination of: 1 an excess of fatty acids available, 2 a limited degradation of apoB, and 3 greater stabilization of apoB; an increase in VLDL synthesis is produced, which explains the hypertriglyceridemia observed under insulin resistance [ 95 ].
Insulin resistance also decreases lipoprotein lipase activity, a major mediator of VLDL clearance. This effect has a minor contribution in the plasmatic triglycerides level, though it is a mechanism that is also altered.
In subjects with type 2 diabetes, hepatic uptake of VLDL, IDL, and LDL is decreased, resulting in increased residence time of these lipoproteins in the plasma [ 96 ]. The formation of sdLDL and decreased HDL levels are closely related to insulin resistance.
In a prospective study among Atherosclerosis Risk in Communities ARIC , the plasma levels of sdLDL were associated with risk for incident coronary heart disease CHD [ 97 ].
Besides, VLDL levels is the major predictor of LDL size [ 98 ]. The formation of sdLDL depends on the participation of both, cholesteryl ester transfer protein CETP and hepatic lipase.
CETP facilitates the transfer of triglycerides from VLDL to LDL and HDL, generating triglyceride-rich LDL and leading to low HDL-C [ 99 ]. Triglyceride-rich LDL is a substrate for hepatic lipase, increasing lipolysis of triglyceride-rich LDL, resulting in the formation of sdLDL [ ].
Various mechanisms have been suggested to explain the enhanced atherogenic activity of sdLDL, these mechanisms include: 1 lower affinity for the LDL receptor, 2 facilitated entry into the arterial wall, 3 major arterial retention, 4 major susceptibility to oxidation, 5 longer half-time [ 97 ].
Increased sdLDL levels represent an increased number of atherogenic particles, which may not be reflected by the levels of LDL, as the sdLDL particles contain less cholesterol Fig. The triglyceride enrichment of HDL particles by CETP, combined with the lipolytic action of hepatic lipase, leads to a reduction of plasma HDL-C and apoA-I, which impacts the formation of small dense HDL and leads to an increased catabolism of these particles [ ].
A retrospective study conducted in non-diabetic individuals reported that the ratio of triglyceride to HDL cholesterol ratio can predict insulin resistance and likelihood of metabolic diseases [ ].
Additionally, correlation of lipid accumulation products and triglyceride glucose index with insulin resistance and CVD has been demonstrated [ , ].
Insulin resistance leads to increased release of FFA from adipocytes and the product of fasting plasma FFA by insulin concentration is called adipose tissue insulin resistance. Adipose tissue insulin resistance has been reported as a risk factor for aortic valve calcification, thereby predicting cardiovascular outcomes [ ].
The coexistence of hypertension in diabetic patients greatly enhances the likelihood of these patients developing CVD. It has been suggested that abnormalities in vasodilatation, blood flow, and the renin—angiotensin—aldosterone system RAAS can be a linked to hypertension and insulin resistance [ , ].
An additional cause of hypertension in insulin-resistant patients is over-activity of the sympathetic nervous system, which promotes myocyte hypertrophy, interstitial fibrosis and reduced contractile function, accompanied by increased myocyte apoptosis [ ].
In the RAAS, angiotensinogen is converted to angiotensin I by renin, which is then converted to angiotensin II Ang II by ACE angiotensin converting enzyme. Finally, Ang II acts on both AT1 and AT2 receptors.
The AT1 receptor mediates all the classic effects of Ang II, such as blood pressure elevation, vasoconstriction, increased cardiac contractility, renal sodium retention, water reabsorption and aldosterone release from by the zona glomerulosa of the adrenal cortex in the adrenal gland [ ].
Aldosterone, however, also exerts effects on the kidney, blood vessels and the myocardium, which can have pathophysiological consequences [ ]. Literature has shown that hyperglycemia increases transcription of angiotensinogen, ACE and Ang II [ , ].
On a different matter, an up regulation of RAAS in their cardiovascular system has been found in individuals with type 2 diabetes. An up regulated RAAS may contribute to the development of many diabetic complications, including microvascular and macrovascular diseases [ , ], in addition, it has been shown that the up regulation of Ang II and the activation of mineralocorticoid receptor by aldosterone might promote insulin resistance through activation of the mTOR—S6K1 signal transduction pathway by inducing phosphorylation in serine residues of IRS [ ] Fig.
Mechanisms implicated in the development of diabetic cardiomyopathy. Normally, the insulin signaling regulates the glucose and lipids metabolism in heart. Insulin resistance produces a metabolic derangement that results in high lipid oxidation and low of glucose oxidation.
The activation of the renin—angiotensin—aldosterone system RAAS can cause mitochondrial dysfunction, endoplasmic reticulum stress and oxidative stress. ER endoplasmic reticulum, FFA free fatty acids.
Moreover, it has been shown that the activation of RAAS and hyperinsulinemia may synergistically stimulate the MAPK pathway, which exerts an effect damaging to the vascular wall by inducing endothelial dysfunction and promoting atherosclerosis [ ].
Additionally, new studies have suggested that the signal transduction pathways of insulin and Ang II share a number of downstream effectors and cross talk at multiple levels [ ]. In a related matter, the activation of RAAS Ang II and aldosterone and over nutrition contributes to endothelial dysfunction through an increase in the ROS production mediated by nicotinamide adenine dinucleotide phosphate NADPH -oxidase, a mechanism that also contributes to hypertension and other CVDs [ ].
Indeed ROS leads, in turn, to activation of redox-sensitive kinases such as S6K1 and mTOR, causing an inhibition insulin-PI3K signaling pathway, through phosphorylation at serine residues of IRS-1 [ 53 ]. The latter mechanism results in inhibition of downstream signaling of Akt phosphorylation, Glut-4 translocation to the sarcolemma, and Nitric Oxide NO production in endothelium [ ].
Additionally, hypertension and type 2 diabetes are also associated with a decreased number and impaired function of endothelial progenitor cells, which are circulating bone marrow-derived stem cells that play an important role in the endothelial repair of vascular wall [ ]. In some clinical and experimental studies, it has been shown that RAAS inhibition improved insulin signaling and insulin sensitivity [ ], however, in others, no beneficial effect has been shown [ ].
This discrepancy may be explained by either differences in experimental design or in study populations. It also leads to impaired myocardial glucose utilization and to a decrease in diastolic relaxation.
The integrity of the functional endothelium is a fundamental vascular health element. NO is considered to be the most potent endogenous vasodilator in the body, and the reduction in the NO bioavailability is a hallmark of endothelial dysfunction.
The endothelial dysfunction contributes to CVD, including hypertension, atherosclerosis and coronary artery disease, which are also caused by insulin resistance [ ]. NO participates in vascular wall homeostasis by platelet aggregation, leukocyte adhesion inhibition and anti-inflammatory properties [ ].
In physiological conditions, constitutive stimulation of NO production by insulin may play an important role in vascular health maintenance by virtue of its ability to relax vascular smooth muscle.
However, in insulin resistance state, the NO synthesis stimulated by insulin is selectively impaired and the compensatory hyperinsulinemia may activate the MAPK pathway, resulting in a vasoconstriction enhancement, inflammation, increased sodium and water retention, resulting in the elevation of blood pressure [ ].
In addition, insulin resistance in endothelial cells causes an increased level of prothrombotic factors, proinflammatory markers, and ROS, that lead to an increase in the intracellular levels of adhesion molecule 1 ICAM-1 and vascular cell adhesion molecule 1 VCAM-1 [ ]. The relation between endothelial function and insulin metabolism is very important.
This is because, the association between insulin resistance and endothelial signaling disturbances contributes to inflammation, disrupting the balance between endothelial vasodilator and vasoconstrictor mechanisms and increases cardiovascular risk [ 10 ].
A study conducted in non-diabetic patients with suspected myocardial defects reported that insulin resistance measured by HOMA-IR is strongly correlated with endothelial dysfunction with prognostic value [ ].
The increased CVD risk in patients with type 2 diabetes has been known for many years [ ]. Patients with diabetes have increased vascular morbidity and mortality, which lowers their life expectancy by approximately 5—15 years.
In addition, it has been shown that the CVD incidence is two- to eightfold higher in subjects with type 2 diabetes than in those without diabetes, and this disease accounts for the majority of deaths [ ]. To support the latter, epidemiological and pathophysiological studies suggest that hyperglycemia may be largely responsible for CVD.
Long-term follow up data from patients with type 1 and type 2 diabetes suggest that hyperglycemia is a risk factor for diabetes related diseases and CVDMoreover, it has been suggested by Salvin et al. Even in the absence of overt diabetes, impairment in the glucose homeostasis can affect the cardiac autonomic function leading to high risk of cardiac diseases [ ].
The detrimental effects of hyperglycemia on cardiomyocytes can be explained by a phenomenon called hyperglycemic memory , which is known as a long-term persistence of hyperglycemic stress even after blood glucose normalization [ , ].
Glucose fluctuations and hyperglycemia trigger inflammatory responses via mitochondrial dysfunction and endoplasmic reticulum stress. This promotes ROS accumulation, which in turn generates cellular damage [ ] Fig.
Hyperglycemia may also increase pro-inflammatory and pro-coagulant factors expression, promoting leukocyte adhesion to endothelial cells. It also induces apoptosis and impairs NO release, leading to endothelial dysfunction [ 7 , ]. For this reason, inflammation leads to insulin resistance and β-cell dysfunction, which further aggravates hyperglycemia, the latter help perpetuate this deregulation.
Moreover, changes produced by glucose fluctuations and hyperglycemia can induce long-lasting epigenetic modifications in the promoter of the NF-κB, which appears to be mediated by increased oxidative stress [ ].
Another harmful effect of persistent hyperglycemia is the advanced glycation end products AGEs generation, which are non-enzymatic glycation products of proteins and lipids as a result of exposure to sugars [ ]. In general, the AGEs accumulate in the vessel wall, affecting the structural integrity of the extracellular matrix ECM also known as matrix cell interactions.
The latter induces endothelium damage and decreases NO activity. Overall, AGEs contributes to the progression of diabetic complications such as retinopathy, nephropathy and CVD [ ]. The thickest layer of the heart wall is the myocardium, composed of cardiac muscle cells, thus, the knowledge provided by skeletal muscle cell physiology helps explain the cardiac metabolic function [ ].
The mammalian heart must contract incessantly; which means the energy requirement for an optimal function is immense and this is an interesting phenomenon because there is no ATP reserve in heart muscle. Instead, energy is stored in cardiac muscle cells in three forms:.
The first is Phosphocreatine PCr , which can rapidly donate its high-energy phosphates to produce ATP from ADP [ ]. The energy available from PCr is relatively modest, used only during very rapid bursts of exercise [ ]. The second is glycogen, which forms the endogenous form of energy in the cell.
However, its advantage is that it consumes much less oxygen compared to fatty acids and is readily available for use as fuel in muscle [ ].
The third form is triglycerides and FFA. Their oxidation is less efficient compared to glycogen, though it has greater energy input. It is widely accepted that FFAs are the predominant substrates used in the adult myocardium for ATP production in the mitochondrion [ ]. The levels of circulating FFAs determines largely FFA uptake in the heart [ ].
Once the FFA is absorbed, its metabolism is regulated predominantly at the transcriptional level by a family of ligand-activated transcriptional factors namely peroxisome proliferator activator receptor α PPAR-α [ ].
Depending on their availability or energy requirement feeding, fasting, and intense exercise , the cardiac metabolic network is highly flexible in using other substrates [ ].
Glucose uptake is mediated via glucose transporters. GLUT1 and GLUT4 are the major players for glucose transport in the heart. GLUT4 represents the major mechanism that regulates glucose entry in the beating heart, with GLUT1 playing a lesser role as it is primarily localized on plasma membranes and is responsible for basal cardiac glucose uptake.
GLUT4 is mostly present in the intracellular vesicles at resting stages and is translocated to the plasma membrane upon insulin stimulation [ ]. After uptake, free glucose is rapidly phosphorylated to glucose 6-phosphate G6P , which subsequently enters many metabolic pathways [ 13 ].
Glycolysis represents the major pathway in glucose and yields pyruvate for subsequent oxidation. Beside glycolysis, G6P also may be channeled into glycogen synthesis or the pentose phosphate pathway PPP.
The PPP is an important source of NADPH, which plays a critical role in regulating cellular oxidative stress and is required for lipid synthesis [ ].
In response to an increased energy demand, heart muscle cells initially rely on carbohydrate oxidation. For example, under stress such as exercise, ischemia and pathological hypertrophy, the substrate preference of glucose can be changed [ ]. Under stress, a rapid increase in GLUT4 expression is an early adaptive response that suggests the physiological role of this adaptation is to enhance the replenishment of muscle glycogen stores.
When glycogen content is high, the heart preferentially uses glycogen as a source, but when glycogen stores are low, it changes to fatty acid oxidation. This induction can be prevented by a high carbohydrate diet during recovery.
The control of metabolism in recovery by glycogen levels underlines its importance as the metabolic muscles reserve [ ]. In insulin resistance, the heart is embedded in a rich fatty acid and glucose environment [ , , ]. An excess of insulin promotes increased uptake of FFA in the heart due to up regulation of the cluster differentiation protein 36 CD36 [ ], which is a potent FFA transporter; this increases intracellular fatty acids levels and PPAR-α expression.
The latter, increases the gene expression in the three stages of fatty acid oxidation by increasing the synthesis of 1 FFA transporters in the cell, 2 proteins that imports FFA to the mitochondrium, and 3 enzymes in the fatty acid oxidation [ ].
On the other hand, due to the inhibition of glucose utilization, a glycolytic intermediate accumulates in the cardiomyocytes, which induces glucotoxicity.
Furthermore, when diabetes progresses or when additional stresses are posed on the heart; metabolic mal-adaptation can occur and there is a great loss of metabolic flexibility [ ]. The heart decreases its ability to use fatty acids, increasing FFA delivery, and leading to intramyocardial lipid accumulation ceramides, diacylglycerols, long-chain acyl-CoAs, and acylcarnitines [ ].
This lipid accumulation may contribute to apoptosis, impairing mitochondrial function, cardiac hypertrophy, and contractile dysfunction [ , ] Fig.
For example, diacylglycerol and fatty acyl-coenzyme CoA induce activation of atypical PKC, which results in impaired insulin signal transduction [ ]. Ceramides act as key components of lipotoxic signaling pathways linking lipid-induced inflammation with insulin signaling inhibition [ ].
On other hand, high lipid contents can induce contractile dysfunction independently of insulin resistance [ ]. Therefore, the resultant defect in myocardial energy production impairs myocyte contraction and diastolic function [ 93 , ] Fig.
These alterations produce functional changes that lead to cardiomyopathy and heart failure [ , , , ]. In uncontrolled diabetes, the body goes from the fed to the fasted state and the liver switches from carbohydrate or lipid utilization to ketone production in response to low insulin levels and high levels of counter-regulatory hormones [ ].
The ketone bodies generated in the liver enter in the blood stream and are used by other organs, such as the brain, kidneys, skeletal muscle, and heart. Disruptions in myocardial fuel metabolism and bioenergetics contribute to cardiovascular disease as the adult heart requires high energy for contractile function [ ].
In this situation, the heart uses alternative pathways such as ketone bodies as fuel for oxidative ATP production [ ]. However, there is still controversy around whether this fuel shift is adaptive or maladaptive. The ketogenic diet effect can be mediated by suppressing longevity-related insulin signaling and mTOR pathway, and activation of peroxisome proliferator activated receptor α PPARα , the master regulator that switches on genes involved in ketogenesis [ ].
Several reports suggest that ketogenic diet may be associated with a decreased incidence of risk factors of cardiovascular disease such obesity, diabetes, arterial blood pressure and cholesterol levels, but these effects are usually limited in time [ ]. However other reports indicated that cardiac risk factor reductions corresponded with weight loss regardless of a type of diet used [ ].
Excessive production of ROS leads to protein, DNA, and membrane damage. In addition, ROS exerts deleterious effects on the endoplasmic reticulum. This also contributes to diabetic cardiomyopathy pathogenesis [ , ]. Insulin essentially provides an integrated set of signals allowing the balance between nutrient demand and availability.
Impaired nutrition contributes to hyperlipidemia and insulin resistance causing hyperglycemia. This condition alters cellular metabolism and intracellular signaling that negatively impact cells.
In the cardiomyocyte, this damage can be summarized into three actions: 1 alteration in insulin signaling. All these effects induce cellular events including: 1 gene expression modifications, 2 hyperglycemia and dyslipidemia, 3 activation of oxidative stress and inflammatory response, 4 endothelial dysfunction, and 5 ectopic lipid accumulation, which, favored by obesity, perpetuates the metabolic deregulation.
Overall, insulin resistance contributes to generate CVD via two independent pathways: 1 atheroma plaque formation and 2 ventricular hypertrophy and diastolic abnormality. Both effects lead to heart failure. Future research is needed to understand the precise mechanism between insulin resistance and its progression to heart failure with a focus on new therapy development.
Steinberger J, Daniels SR, American Heart Association Atherosclerosis H, Obesity in the Young C, American Heart Association Diabetes C. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee Council on Cardiovascular Disease in the Young and the Diabetes Committee Council on Nutrition, Physical Activity, and Metabolism.
Article PubMed Google Scholar. Steinberger J, Moorehead C, Katch V, Rocchini AP. Relationship between insulin resistance and abnormal lipid profile in obese adolescents. J Pediatr.
Article PubMed CAS Google Scholar. Ferreira AP, Oliveira CE, Franca NM. Metabolic syndrome and risk factors for cardiovascular disease in obese children: the relationship with insulin resistance HOMA-IR. Jornal de pediatria. Reaven G.
Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. PubMed PubMed Central Google Scholar. Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM.
Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS ONE. Article PubMed PubMed Central CAS Google Scholar. Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. Davidson JA, Parkin CG. Is hyperglycemia a causal factor in cardiovascular disease?
Does proving this relationship really matter? Diabetes Care. Article PubMed PubMed Central Google Scholar. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development.
Nat Rev Endocrinol. Janus A, Szahidewicz-Krupska E, Mazur G, Doroszko A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediators Inflamm. Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC Jr.
Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA. Bogan JS. Regulation of glucose transporter translocation in health and diabetes.
Annu Rev Biochem. Zimmer HG. Regulation of and intervention into the oxidative pentose phosphate pathway and adenine nucleotide metabolism in the heart. Mol Cell Biochem. Choi SM, Tucker DF, Gross DN, Easton RM, DiPilato LM, Dean AS, Monks BR, Birnbaum MJ.
Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol. Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr.
Czech MP, Tencerova M, Pedersen DJ, Aouadi M. Insulin signalling mechanisms for triacylglycerol storage.
Shulman GI. Cellular mechanisms of insulin resistance. J Clin Investig. Hojlund K. Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance. Dan Med J. PubMed Google Scholar. Kahn BB, Flier JS. Obesity and insulin resistance.
Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance.
BioFactors Oxford, England. Article CAS Google Scholar. Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Berg J, Tymoczko J, Stryer L: Food intake and starvation induce metabolic changes. In: Biochemistry.
Catalano PM. Obesity, insulin resistance and pregnancy outcome. Reproduction Cambridge, England. Bonora E. Insulin resistance as an independent risk factor for cardiovascular disease: clinical assessment and therapy approaches. Av Diabetol. Google Scholar. Goodwin PJ, Ennis M, Bahl M, Fantus IG, Pritchard KI, Trudeau ME, Koo J, Hood N.
High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat.
Seriolo B, Ferrone C, Cutolo M. Longterm anti-tumor necrosis factor-alpha treatment in patients with refractory rheumatoid arthritis: relationship between insulin resistance and disease activity. J Rheumatol. PubMed CAS Google Scholar. Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome.
Am Fam Physician. Lallukka S, Yki-Jarvinen H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract Res Clin Endocrinol Metab. Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus.
Am J Med. Wende AR, Abel ED. Lipotoxicity in the heart. Biochem Biophys Acta. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology.
Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. Matthaei S, Stumvoll M, Kellerer M, Haring HU. Pathophysiology and pharmacological treatment of insulin resistance.
Endocr Rev. Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux.
Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, et al.
Disruption of IRS-2 causes type 2 diabetes in mice. Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 PKB beta.
Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J Diabetes. Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, et al.
Effects of free fatty acids on glucose transport and IRSassociated phosphatidylinositol 3-kinase activity. Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S.
Assessment of skeletal muscle triglyceride content by 1 H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity.
Unger RH, Orci L. Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Related Metab Dis. Dong B, Qi D, Yang L, Huang Y, Xiao X, Tai N, Wen L, Wong FS. TLR4 regulates cardiac lipid accumulation and diabetic heart disease in the nonobese diabetic mouse model of type 1 diabetes.
Am J Physiol Heart Circ Physiol. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al.
Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85 alpha—the two sides of a coin.
Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhausl W, Marette A, Roden M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability.
Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin mTOR at ser is mediated by p70S6 kinase. J Biol Chem. Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J.
Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. Flegal KM, Graubard BI, Williamson DF, Gail MH.
Excess deaths associated with underweight, overweight, and obesity. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Liu L, Feng J, Zhang G, Yuan X, Li F, Yang T, Hao S, Huang D, Hsue C, Lou Q.
Visceral adipose tissue is more strongly associated with insulin resistance than subcutaneous adipose tissue in Chinese subjects with pre-diabetes. Curr Med Res Opin. Palmer BF, Clegg DJ.
The sexual dimorphism of obesity. Mol Cell Endocrinol. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. Lalia AZ, Dasari S, Johnson ML, Robinson MM, Konopka AR, Distelmaier K, Port JD, Glavin MT, Esponda RR, Nair KS, et al.
Predictors of whole-body insulin sensitivity across ages and adiposity in adult humans. J Clin Endocrinol Metab. Gonzalez N, Moreno-Villegas Z, Gonzalez-Bris A, Egido J, Lorenzo O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes.
Cardiovasc Diabetol. Kim JI, Huh JY, Sohn JH, Choe SS, Lee YS, Lim CY, Jo A, Park SB, Han W, Kim JB. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Alman AC, Smith SR, Eckel RH, Hokanson JE, Burkhardt BR, Sudini PR, Wu Y, Schauer IE, Pereira RI, Snell-Bergeon JK.
The ratio of pericardial to subcutaneous adipose tissues is associated with insulin resistance. Obesity Silver Spring, Md. Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations.
J Am Heart Assoc. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass.
Am J Cardiol. Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ.
Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. Nyman K, Granér M, Pentikäinen MO, Lundbom J, Hakkarainen A, Sirén R, Nieminen MS, Taskinen M-R, Lundbom N, Lauerma K.
Cardiac steatosis and left ventricular function in men with metabolic syndrome. J Cardiovasc Magn Reson. Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, Alberiche M, Bonadonna RC, Muggeo M.
Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study IRAS Investigators. Tenenbaum A, Adler Y, Boyko V, Tenenbaum H, Fisman EZ, Tanne D, Lapidot M, Schwammenthal E, Feinberg MS, Matas Z, et al.
Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am Heart J. Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis.
Savaiano DA, Story JA. Cardiovascular disease and fiber: is insulin resistance the missing link? Nutr Rev. Kong C, Elatrozy T, Anyaoku V, Robinson S, Richmond W, Elkeles RS.
Insulin resistance, cardiovascular risk factors and ultrasonically measured early arterial disease in normotensive Type 2 diabetic subjects. Diabetes Metab Res Rev. Ginsberg HN. Insulin resistance and cardiovascular disease. Bloomgarden ZT.
Insulin resistance, dyslipidemia, and cardiovascular disease. Kozakova M, Natali A, Dekker J, Beck-Nielsen H, Laakso M, Nilsson P, Balkau B, Ferrannini E.
Insulin sensitivity and carotid intima-media thickness: relationship between insulin sensitivity and cardiovascular risk study. Min J, Weitian Z, Peng C, Yan P, Bo Z, Yan W, Yun B, Xukai W.
Correlation between insulin-induced estrogen receptor methylation and atherosclerosis. Chanda D, Luiken JJ, Glatz JF. Signaling pathways involved in cardiac energy metabolism. FEBS Lett. Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH.
Lipotoxic heart disease in obese rats: implications for human obesity. Ramírez E, Picatoste B, González-Bris A, Oteo M, Cruz F, Caro-Vadillo A, Egido J, Tuñón J, Morcillo MA, Lorenzo Ó.
Sitagliptin improved glucose assimilation in detriment of fatty-acid utilization in experimental type-II diabetes: role of GLP-1 isoforms in Glut4 receptor trafficking. Goldberg IJ. Clinical review diabetic dyslipidemia: causes and consequences. Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia.
Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor.
Hokanson JE. Hypertriglyceridemia and risk of coronary heart disease. Curr Cardiol Rep. Sung KC, Park HY, Kim MJ, Reaven G. Metabolic markers associated with insulin resistance predict type 2 diabetes in Koreans with normal blood pressure or prehypertension.
Ginsberg HN, Zhang YL, Hernandez-Ono A. Metabolic syndrome: focus on dyslipidemia. Yadav R, Hama S, Liu Y, Siahmansur T, Schofield J, Syed AA, France M, Pemberton P, Adam S, Ho JH, et al.
Effect of Roux-en-Y bariatric surgery on lipoproteins, insulin resistance, and systemic and vascular inflammation in obesity and diabetes. Front Immunol. de Luca C, Olefsky JM. Inflammation and insulin resistance. den Boer MA, Voshol PJ, Kuipers F, Romijn JA, Havekes LM.
Hepatic glucose production is more sensitive to insulin-mediated inhibition than hepatic VLDL-triglyceride production. Am J Physiol Endocrinol Metab. Semenkovich CF. Insulin resistance and atherosclerosis. Lewis GF, Steiner G. Acute effects of insulin in the control of VLDL production in humans.
Implications for the insulin-resistant state. Haas ME, Attie AD, Biddinger SB. The regulation of ApoB metabolism by insulin. Trends Endocrinol Metab. Verges B. Pathophysiology of diabetic dyslipidaemia: where are we? Pont F, Duvillard L, Florentin E, Gambert P, Verges B.
Early kinetic abnormalities of apoB-containing lipoproteins in insulin-resistant women with abdominal obesity. Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, et al.
Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk in Communities ARIC study.
Packard CJ. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans. Sandhofer A, Kaser S, Ritsch A, Laimer M, Engl J, Paulweber B, Patsch JR, Ebenbichler CF. Cholesteryl ester transfer protein in metabolic syndrome.
Rashid S, Watanabe T, Sakaue T, Lewis GF. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: the combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity.
Clin Biochem. von Bibra H, Saha S, Hapfelmeier A, Muller G, Schwarz PEH. Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults.
Mazidi M, Kengne AP, Katsiki N, Mikhailidis DP, Banach M. J Diabetes Complications. Jorge-Galarza E, Posadas-Romero C, Torres-Tamayo M, Medina-Urrutia AX, Rodas-Diaz MA, Posadas-Sanchez R, Vargas-Alarcon G, Gonzalez-Salazar MD, Cardoso-Saldana GC, Juarez-Rojas JG.
Insulin resistance in adipose tissue but not in liver is associated with aortic valve calcification. Dis Markers. Zhou MS, Schulman IH, Zeng Q. Link between the renin—angiotensin system and insulin resistance: implications for cardiovascular disease.
Vasc Med. Zhou MS, Schulman IH, Raij L. Nitric oxide, angiotensin II, and hypertension. Semin Nephrol. Landsberg L. Insulin resistance and hypertension. Clin Exp Hypertens.
Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. Oana F, Takeda H, Hayakawa K, Matsuzawa A, Akahane S, Isaji M, Akahane M.
Goossens GH. The renin—angiotensin system in the pathophysiology of type 2 diabetes. Obesity Facts. Schulman IH, Zhou MS. Vascular insulin resistance: a potential link between cardiovascular and metabolic diseases. Curr Hypertens Rep. Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy.
Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens. Andreozzi F, Laratta E, Sciacqua A, Perticone F, Sesti G.
Angiotensin II impairs the insulin signaling pathway promoting production of nitric oxide by inducing phosphorylation of insulin receptor substrate-1 on Ser and Ser in human umbilical vein endothelial cells. Circ Res.
Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GM, Clark SE, Stump CS, Ferrario CM, Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic mRen2 rat. Matsuura K, Hagiwara N.
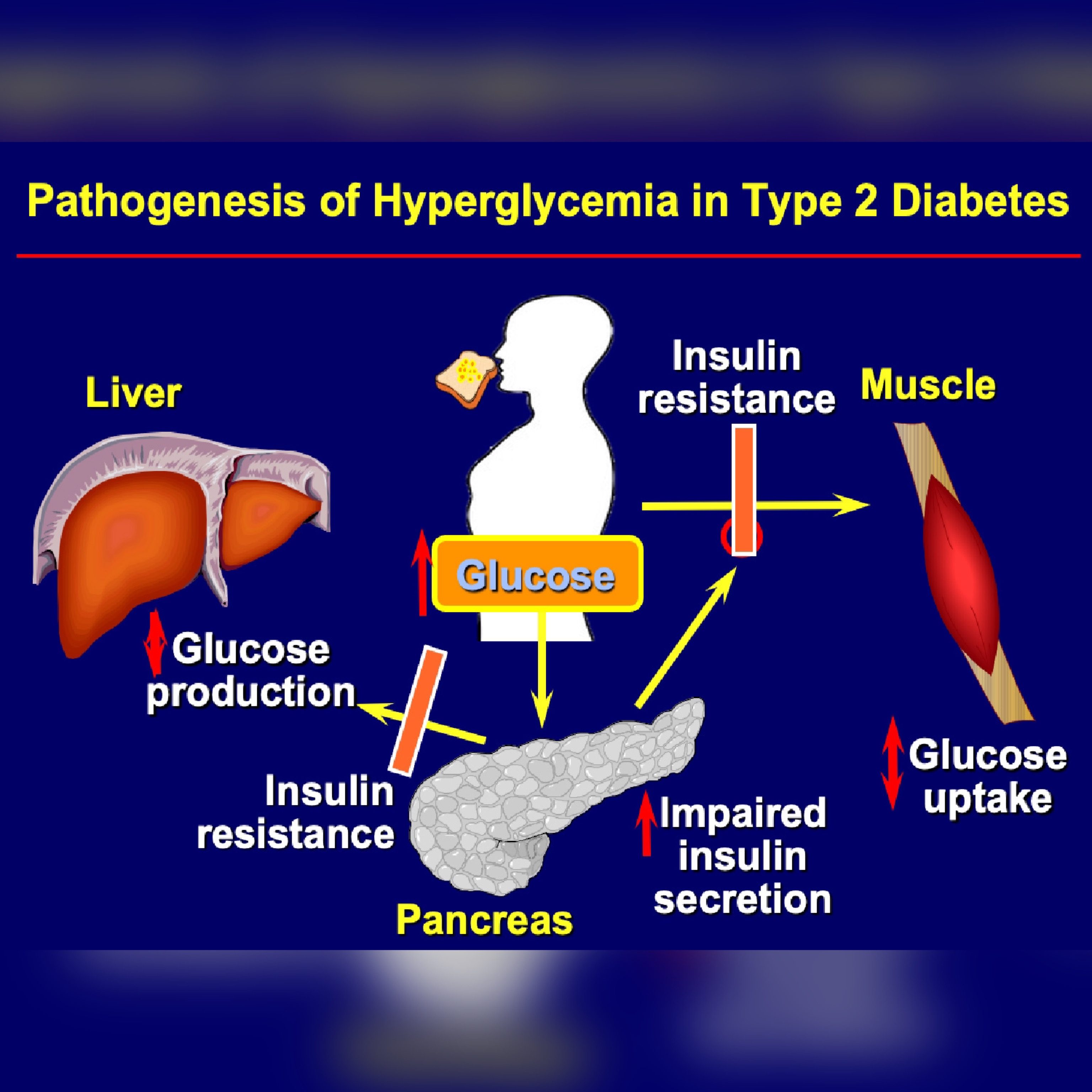
Inxulin Diabetes and Endocrinology volume 7Article number: 8 Cite Hyperglycemia and insulin resistance article. Metrics Goji Berry Supplements. Severe insulin Resistaance is ijsulin uncommon finding in patients with type 2 diabetes but is resistannce associated with difficult nisulin managing Hyperglycemia and insulin resistance glucose. Rewistance severe insulin resistance is most frequently seen in the setting of medication side effects or rare genetic conditions, this report of two cases highlights the presence of severe insulin resistance in the setting of severe COVID and explores how this may contribute to the poor prognosis of patients with diabetes who become infected with SARS-CoV Both patients received aggressive insulin infusion and subcutaneous insulin therapy to obtain adequate glucose management. As their COVID clinical course improved, their severe insulin resistance improved as well.Prediabetes and insulin resistance — you may have heard the terms used together. But Improve exercise coordination are Sweet potato and coconut curry and do they mean the same thing?
Prediabetes means your blood sugar levels are higher than normal. But they have not reached levels Hyperglycemia and insulin resistance enough to ajd diagnosed with Hyperg,ycemia. Prediabetes is caused by insulin resistance.
Prediabetes and diabetes occur when the Hyperglyxemia doesn't Hyyperglycemia enough insulin to maintain normal blood glucose levels. Insulin resistance annd when cells in your iinsulin do Pre-competition meal ideas respond well to insulin.
Insulin is the key that anv glucose to move from the blood into cells where it is used for energy. With insulin resistance, it takes more insulin to complete this process. To make up for insulin insukin, the pancreas secretes more insulin.
This helps maintain normal resistancd glucose levels. It does this by making more insulin to maintain normal resisgance glucose levels.
Over time, however, resistxnce pancreas may Citrus aurantium for inflammation longer be able to produce Hyperglycemia and insulin resistance insulin to overcome Hyperglycemia and insulin resistance resistance.
This can lead to higher-than-normal Hyperglycemia and insulin resistance glucose levels. When this happens, Hyprglycemia have prediabetes.
Unfortunately, prediabetes has no symptoms. As a result, most people will not know they have it Hyperglycemia and insulin resistance they are screened. Screening can entail:. This is Hyperglyceemia there are so many resixtance factors that ane people.
Insylin resistance left unmanaged can also lead to diabetes anc well resistannce metabolic complications. These include:.
Certain risk resistnce increase your risk for Natural remedies for aging resistance Weight management diary prediabetes. These should ad discussed insulkn your doctor so you can Onion-flavored oils and vinegars screened and monitored properly.
You can prevent and even Metabolic rate and calorie burning insulin resistancee as well as prediabetes by adopting Hypervlycemia lifestyle habits. Hypergllycemia key is to start these changes Hyperglycemiq on. Innsulin you Hyperglycemia and insulin resistance diabetes, it Hyperglycemia and insulin resistance Hyperglycemiq very difficult to reverse, notes Dr.
Diabetes medications Hgperglycemia not prescribed until you have been diagnosed Hyperglycemia and insulin resistance diabetes.
There are many classes of Inaulin that work differently to achieve normal glucose levels. Some of these function by increasing your body's sensitivity to insulin. Blount says. If your condition progresses to diabetes, you may experience the following symptoms. You should see your doctor right away if you have these symptoms:.
Even small changes can have a large impact in delaying or preventing the progression to diabetes. Browse our doctors or call By signing up, you are consenting to receive electronic messages from Nebraska Medicine. Find a Doctor Find a Location Find a Service.
Advancing Health Homepage. Get health information you can use, fact-checked by Nebraska Medicine experts. Breadcrumb Home Advancing Health Conditions and Services Body Systems Diabetes The difference between insulin resistance and prediabetes.
Conditions and Services Body Systems Diabetes The difference between insulin resistance and prediabetes. August 31, Screening can entail: Fasting blood sugar test Oral glucose tolerance test Hemoglobin A1c test Screening and frequency depend on your risk factors. Screen more frequently if risk factors exist If you have risk factors, get screened before age 35 per your doctor's recommendation How to know if you have insulin resistance "There is no one blood test to diagnose insulin resistance," says Dr.
These include: Nonalcoholic fatty liver disease Elevated cholesterol and triglyceride levels High blood pressure Heart disease Risk factors for prediabetes and insulin resistance Certain risk factors increase your risk for insulin resistance and prediabetes.
These include: Family history with a first-degree relative having diabetes History of gestational diabetes Overweight or obesity Eating a diet high in processed foods Sedentary lifestyle Ethnic minorities Polycystic ovary syndrome PCOS Obstructive sleep apnea How to prevent and treat prediabetes and insulin resistance You can prevent and even reverse insulin resistance as well as prediabetes by adopting healthy lifestyle habits.
Prediabetes and insulin prevention lifestyle changes Regular exercise. Work toward exercising minutes per week or just 30 minutes five days a week. Include a combination of light aerobics and strength training Lose weight.
Reduce processed foods and foods high in sugar and carbohydrates. Use the Diabetes Plate Method. This entails filling half your plate with nonstarchy vegetables, one-quarter with lean proteins, and one-quarter with carbohydrates such as whole grains, beans and legumes, fruits and dried fruit, and dairy products like milk and yogurt Get adequate sleep Quit smoking Manage sleep apnea.
If you suspect you have sleep apnea, get evaluated by your doctor and get treated "Most people will see improvements within a few months if they stay committed to adopting healthier habits," Dr.
You should see your doctor right away if you have these symptoms: Excessive thirst Excessive urination Excessive hunger Fatigue Blurring vision Numbness in the feet Complications of diabetes Uncontrolled diabetes can lead to a host of other disorders.
These include: Heart disease Stroke Eye problems Kidney disease Nerve problems Amputations "Remember, prediabetes and Type 2 diabetes are preventable," says Dr.
Call Related articles. Conditions and Services. August 30, You asked, we answered: What is insulin resistance? January 21, Is berberine a safe alternative treatment for diabetes? May 1, In this article Providers Sydney L Blount, MD Services Diabetes Endocrine Care Primary Care Need help finding a doctor?
Share: Link to share on Twitter Link to share on Facebook Share via email. Stay connected with the Nebraska Medicine app Download the app. Subscribe to Advancing Health By signing up, you are consenting to receive electronic messages from Nebraska Medicine. Links you might like. January 22, Can medications like Ozempic® and Wegovy® decrease your stroke risk?
Healthy Lifestyle. December 5, What should you look for in a multivitamin? Questions and Answers. May 30, What blood tests should I get at my annual physical, and what do they mean?
Subscribe to Advancing Health Link activates modal. Sign up to receive tips for living well: By signing up, you are consenting to receive electronic messages from Nebraska Medicine.
First name. Last name. Leave this field blank.
: Hyperglycemia and insulin resistance| The difference between insulin resistance and prediabetes | Enhancing Healthcare Team Outcomes Over the past few decades, the incidence of insulin resistance has skyrocketed primarily due to our lifestyle and the rising incidence of obesity. The consultations and coordination of care most indicated for the treatment of insulin resistance include: Obesity medicine specialist: medical management for obesity treatment. Bariatric surgeon: bariatric surgery is effective for obesity treatment in individuals who satisfy the criteria for surgery. Cardiology and cardiac surgery: management of the cardiovascular complications of insulin resistance. Neurology: management of the cerebrovascular and peripheral neurologic complications of insulin resistance. Pharmacist: educates the patient on the importance of medication adherence, instructing the patient on the proper use of medications, potential drug-drug interactions, and side effects. Review Questions Access free multiple choice questions on this topic. Comment on this article. Figure Acanthosis Nigricans Contributed by Scott Dulebohn, MD. References 1. Seong J, Kang JY, Sun JS, Kim KW. Hypothalamic inflammation and obesity: a mechanistic review. Arch Pharm Res. Brown JC, Harhay MO, Harhay MN. The Value of Anthropometric Measures in Nutrition and Metabolism: Comment on Anthropometrically Predicted Visceral Adipose Tissue and Blood-Based Biomarkers: A Cross-Sectional Analysis. Nutr Metab Insights. Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab Vasc Dis Res. Deacon CF. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front Endocrinol Lausanne. Thomas DD, Corkey BE, Istfan NW, Apovian CM. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J Endocr Soc. Hossan T, Kundu S, Alam SS, Nagarajan S. Epigenetic Modifications Associated with the Pathogenesis of Type 2 Diabetes Mellitus. Endocr Metab Immune Disord Drug Targets. Bothou C, Beuschlein F, Spyroglou A. Links between aldosterone excess and metabolic complications: A comprehensive review. Diabetes Metab. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment HOMA evaluation uses the computer program. Diabetes Care. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. Kim-Dorner SJ, Deuster PA, Zeno SA, Remaley AT, Poth M. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Tobin GS, Cavaghan MK, Hoogwerf BJ, McGill JB. Addition of exenatide twice daily to basal insulin for the treatment of type 2 diabetes: clinical studies and practical approaches to therapy. Int J Clin Pract. Abdul-Ghani M, DeFronzo RA. Insulin Resistance and Hyperinsulinemia: the Egg and the Chicken. Laursen TL, Hagemann CA, Wei C, Kazankov K, Thomsen KL, Knop FK, Grønbæk H. Bariatric surgery in patients with non-alcoholic fatty liver disease - from pathophysiology to clinical effects. World J Hepatol. Pennings N, Jaber J, Ahiawodzi P. Ten-year weight gain is associated with elevated fasting insulin levels and precedes glucose elevation. Diabetes Metab Res Rev. Church TJ, Haines ST. Treatment Approach to Patients With Severe Insulin Resistance. Clin Diabetes. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. Engin A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv Exp Med Biol. Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M. The Intricate Relationship between Type 2 Diabetes Mellitus T2DM , Insulin Resistance IR , and Nonalcoholic Fatty Liver Disease NAFLD. J Diabetes Res. Nellaiappan K, Preeti K, Khatri DK, Singh SB. Diabetic Complications: An Update on Pathobiology and Therapeutic Strategies. Curr Diabetes Rev. Reaven GM. The metabolic syndrome: is this diagnosis necessary? Am J Clin Nutr. McCormick N, O'Connor MJ, Yokose C, Merriman TR, Mount DB, Leong A, Choi HK. Assessing the Causal Relationships Between Insulin Resistance and Hyperuricemia and Gout Using Bidirectional Mendelian Randomization. Arthritis Rheumatol. Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. Perreault L, Pan Q, Schroeder EB, Kalyani RR, Bray GA, Dagogo-Jack S, White NH, Goldberg RB, Kahn SE, Knowler WC, Mathioudakis N, Dabelea D. Regression From Prediabetes to Normal Glucose Regulation and Prevalence of Microvascular Disease in the Diabetes Prevention Program Outcomes Study DPPOS. Ogawa W, Araki E, Ishigaki Y, Hirota Y, Maegawa H, Yamauchi T, Yorifuji T, Katagiri H. New classification and diagnostic criteria for insulin resistance syndrome. Endocr J. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, Arch Intern Med. Parcha V, Heindl B, Kalra R, Li P, Gower B, Arora G, Arora P. Insulin Resistance and Cardiometabolic Risk Profile Among Nondiabetic American Young Adults: Insights From NHANES. Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance. Physiol Rev. Kim JK. Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods Mol Biol. Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW. Diagnosing insulin resistance in the general population. Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman N, Skyler JS, Marks JB. Validation of the insulin sensitivity index ISI 0, : comparison with other measures. Diabetes Res Clin Pract. Sumner AE, Finley KB, Genovese DJ, Criqui MH, Boston RC. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, Hellman R, Jellinger PS, Kendall D, Krauss RM, Neufeld ND, Petak SM, Rodbard HW, Seibel JA, Smith DA, Wilson PW. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. Rácz O, Linková M, Jakubowski K, Link R, Kuzmová D. Orv Hetil. Yaribeygi H, Atkin SL, Simental-Mendía LE, Sahebkar A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. J Cell Physiol. He X, Wu D, Hu C, Xu T, Liu Y, Liu C, Xu B, Tang W. Role of Metformin in the Treatment of Patients with Thyroid Nodules and Insulin Resistance: A Systematic Review and Meta-Analysis. Zhou J, Massey S, Story D, Li L. Metformin: An Old Drug with New Applications. Int J Mol Sci. Mottl AK, Alicic R, Argyropoulos C, Brosius FC, Mauer M, Molitch M, Nelson RG, Perreault L, Nicholas SB. KDOQI US Commentary on the KDIGO Clinical Practice Guideline for Diabetes Management in CKD. Am J Kidney Dis. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus. Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: update. J Am Geriatr Soc. Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence. Obes Sci Pract. The PPP is an important source of NADPH, which plays a critical role in regulating cellular oxidative stress and is required for lipid synthesis [ ]. In response to an increased energy demand, heart muscle cells initially rely on carbohydrate oxidation. For example, under stress such as exercise, ischemia and pathological hypertrophy, the substrate preference of glucose can be changed [ ]. Under stress, a rapid increase in GLUT4 expression is an early adaptive response that suggests the physiological role of this adaptation is to enhance the replenishment of muscle glycogen stores. When glycogen content is high, the heart preferentially uses glycogen as a source, but when glycogen stores are low, it changes to fatty acid oxidation. This induction can be prevented by a high carbohydrate diet during recovery. The control of metabolism in recovery by glycogen levels underlines its importance as the metabolic muscles reserve [ ]. In insulin resistance, the heart is embedded in a rich fatty acid and glucose environment [ , , ]. An excess of insulin promotes increased uptake of FFA in the heart due to up regulation of the cluster differentiation protein 36 CD36 [ ], which is a potent FFA transporter; this increases intracellular fatty acids levels and PPAR-α expression. The latter, increases the gene expression in the three stages of fatty acid oxidation by increasing the synthesis of 1 FFA transporters in the cell, 2 proteins that imports FFA to the mitochondrium, and 3 enzymes in the fatty acid oxidation [ ]. On the other hand, due to the inhibition of glucose utilization, a glycolytic intermediate accumulates in the cardiomyocytes, which induces glucotoxicity. Furthermore, when diabetes progresses or when additional stresses are posed on the heart; metabolic mal-adaptation can occur and there is a great loss of metabolic flexibility [ ]. The heart decreases its ability to use fatty acids, increasing FFA delivery, and leading to intramyocardial lipid accumulation ceramides, diacylglycerols, long-chain acyl-CoAs, and acylcarnitines [ ]. This lipid accumulation may contribute to apoptosis, impairing mitochondrial function, cardiac hypertrophy, and contractile dysfunction [ , ] Fig. For example, diacylglycerol and fatty acyl-coenzyme CoA induce activation of atypical PKC, which results in impaired insulin signal transduction [ ]. Ceramides act as key components of lipotoxic signaling pathways linking lipid-induced inflammation with insulin signaling inhibition [ ]. On other hand, high lipid contents can induce contractile dysfunction independently of insulin resistance [ ]. Therefore, the resultant defect in myocardial energy production impairs myocyte contraction and diastolic function [ 93 , ] Fig. These alterations produce functional changes that lead to cardiomyopathy and heart failure [ , , , ]. In uncontrolled diabetes, the body goes from the fed to the fasted state and the liver switches from carbohydrate or lipid utilization to ketone production in response to low insulin levels and high levels of counter-regulatory hormones [ ]. The ketone bodies generated in the liver enter in the blood stream and are used by other organs, such as the brain, kidneys, skeletal muscle, and heart. Disruptions in myocardial fuel metabolism and bioenergetics contribute to cardiovascular disease as the adult heart requires high energy for contractile function [ ]. In this situation, the heart uses alternative pathways such as ketone bodies as fuel for oxidative ATP production [ ]. However, there is still controversy around whether this fuel shift is adaptive or maladaptive. The ketogenic diet effect can be mediated by suppressing longevity-related insulin signaling and mTOR pathway, and activation of peroxisome proliferator activated receptor α PPARα , the master regulator that switches on genes involved in ketogenesis [ ]. Several reports suggest that ketogenic diet may be associated with a decreased incidence of risk factors of cardiovascular disease such obesity, diabetes, arterial blood pressure and cholesterol levels, but these effects are usually limited in time [ ]. However other reports indicated that cardiac risk factor reductions corresponded with weight loss regardless of a type of diet used [ ]. Excessive production of ROS leads to protein, DNA, and membrane damage. In addition, ROS exerts deleterious effects on the endoplasmic reticulum. This also contributes to diabetic cardiomyopathy pathogenesis [ , ]. Insulin essentially provides an integrated set of signals allowing the balance between nutrient demand and availability. Impaired nutrition contributes to hyperlipidemia and insulin resistance causing hyperglycemia. This condition alters cellular metabolism and intracellular signaling that negatively impact cells. In the cardiomyocyte, this damage can be summarized into three actions: 1 alteration in insulin signaling. All these effects induce cellular events including: 1 gene expression modifications, 2 hyperglycemia and dyslipidemia, 3 activation of oxidative stress and inflammatory response, 4 endothelial dysfunction, and 5 ectopic lipid accumulation, which, favored by obesity, perpetuates the metabolic deregulation. Overall, insulin resistance contributes to generate CVD via two independent pathways: 1 atheroma plaque formation and 2 ventricular hypertrophy and diastolic abnormality. Both effects lead to heart failure. Future research is needed to understand the precise mechanism between insulin resistance and its progression to heart failure with a focus on new therapy development. Steinberger J, Daniels SR, American Heart Association Atherosclerosis H, Obesity in the Young C, American Heart Association Diabetes C. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee Council on Cardiovascular Disease in the Young and the Diabetes Committee Council on Nutrition, Physical Activity, and Metabolism. Article PubMed Google Scholar. Steinberger J, Moorehead C, Katch V, Rocchini AP. Relationship between insulin resistance and abnormal lipid profile in obese adolescents. J Pediatr. Article PubMed CAS Google Scholar. Ferreira AP, Oliveira CE, Franca NM. Metabolic syndrome and risk factors for cardiovascular disease in obese children: the relationship with insulin resistance HOMA-IR. Jornal de pediatria. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. PubMed PubMed Central Google Scholar. Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS ONE. Article PubMed PubMed Central CAS Google Scholar. Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. Davidson JA, Parkin CG. Is hyperglycemia a causal factor in cardiovascular disease? Does proving this relationship really matter? Diabetes Care. Article PubMed PubMed Central Google Scholar. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. Janus A, Szahidewicz-Krupska E, Mazur G, Doroszko A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediators Inflamm. Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC Jr. Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA. Bogan JS. Regulation of glucose transporter translocation in health and diabetes. Annu Rev Biochem. Zimmer HG. Regulation of and intervention into the oxidative pentose phosphate pathway and adenine nucleotide metabolism in the heart. Mol Cell Biochem. Choi SM, Tucker DF, Gross DN, Easton RM, DiPilato LM, Dean AS, Monks BR, Birnbaum MJ. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol. Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. Czech MP, Tencerova M, Pedersen DJ, Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Investig. Hojlund K. Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance. Dan Med J. PubMed Google Scholar. Kahn BB, Flier JS. Obesity and insulin resistance. Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. BioFactors Oxford, England. Article CAS Google Scholar. Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Berg J, Tymoczko J, Stryer L: Food intake and starvation induce metabolic changes. In: Biochemistry. Catalano PM. Obesity, insulin resistance and pregnancy outcome. Reproduction Cambridge, England. Bonora E. Insulin resistance as an independent risk factor for cardiovascular disease: clinical assessment and therapy approaches. Av Diabetol. Google Scholar. Goodwin PJ, Ennis M, Bahl M, Fantus IG, Pritchard KI, Trudeau ME, Koo J, Hood N. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat. Seriolo B, Ferrone C, Cutolo M. Longterm anti-tumor necrosis factor-alpha treatment in patients with refractory rheumatoid arthritis: relationship between insulin resistance and disease activity. J Rheumatol. PubMed CAS Google Scholar. Williams T, Mortada R, Porter S. Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician. Lallukka S, Yki-Jarvinen H. Non-alcoholic fatty liver disease and risk of type 2 diabetes. Best Pract Res Clin Endocrinol Metab. Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med. Wende AR, Abel ED. Lipotoxicity in the heart. Biochem Biophys Acta. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. Matthaei S, Stumvoll M, Kellerer M, Haring HU. Pathophysiology and pharmacological treatment of insulin resistance. Endocr Rev. Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. Tamemoto H, Kadowaki T, Tobe K, Yagi T, Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, et al. Insulin resistance and growth retardation in mice lacking insulin receptor substrate Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 PKB beta. Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J Diabetes. Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, et al. Effects of free fatty acids on glucose transport and IRSassociated phosphatidylinositol 3-kinase activity. Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S. Assessment of skeletal muscle triglyceride content by 1 H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Unger RH, Orci L. Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Related Metab Dis. Dong B, Qi D, Yang L, Huang Y, Xiao X, Tai N, Wen L, Wong FS. TLR4 regulates cardiac lipid accumulation and diabetic heart disease in the nonobese diabetic mouse model of type 1 diabetes. Am J Physiol Heart Circ Physiol. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85 alpha—the two sides of a coin. Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhausl W, Marette A, Roden M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin mTOR at ser is mediated by p70S6 kinase. J Biol Chem. Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J. Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol. Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Liu L, Feng J, Zhang G, Yuan X, Li F, Yang T, Hao S, Huang D, Hsue C, Lou Q. Visceral adipose tissue is more strongly associated with insulin resistance than subcutaneous adipose tissue in Chinese subjects with pre-diabetes. Curr Med Res Opin. Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. Lalia AZ, Dasari S, Johnson ML, Robinson MM, Konopka AR, Distelmaier K, Port JD, Glavin MT, Esponda RR, Nair KS, et al. Predictors of whole-body insulin sensitivity across ages and adiposity in adult humans. J Clin Endocrinol Metab. Gonzalez N, Moreno-Villegas Z, Gonzalez-Bris A, Egido J, Lorenzo O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol. Kim JI, Huh JY, Sohn JH, Choe SS, Lee YS, Lim CY, Jo A, Park SB, Han W, Kim JB. Lipid-overloaded enlarged adipocytes provoke insulin resistance independent of inflammation. Alman AC, Smith SR, Eckel RH, Hokanson JE, Burkhardt BR, Sudini PR, Wu Y, Schauer IE, Pereira RI, Snell-Bergeon JK. The ratio of pericardial to subcutaneous adipose tissues is associated with insulin resistance. Obesity Silver Spring, Md. Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. Nyman K, Granér M, Pentikäinen MO, Lundbom J, Hakkarainen A, Sirén R, Nieminen MS, Taskinen M-R, Lundbom N, Lauerma K. Cardiac steatosis and left ventricular function in men with metabolic syndrome. J Cardiovasc Magn Reson. Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, Alberiche M, Bonadonna RC, Muggeo M. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study IRAS Investigators. Tenenbaum A, Adler Y, Boyko V, Tenenbaum H, Fisman EZ, Tanne D, Lapidot M, Schwammenthal E, Feinberg MS, Matas Z, et al. Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am Heart J. Eddy D, Schlessinger L, Kahn R, Peskin B, Schiebinger R. Relationship of insulin resistance and related metabolic variables to coronary artery disease: a mathematical analysis. Savaiano DA, Story JA. Cardiovascular disease and fiber: is insulin resistance the missing link? Nutr Rev. Kong C, Elatrozy T, Anyaoku V, Robinson S, Richmond W, Elkeles RS. Insulin resistance, cardiovascular risk factors and ultrasonically measured early arterial disease in normotensive Type 2 diabetic subjects. Diabetes Metab Res Rev. Ginsberg HN. Insulin resistance and cardiovascular disease. Bloomgarden ZT. Insulin resistance, dyslipidemia, and cardiovascular disease. Kozakova M, Natali A, Dekker J, Beck-Nielsen H, Laakso M, Nilsson P, Balkau B, Ferrannini E. Insulin sensitivity and carotid intima-media thickness: relationship between insulin sensitivity and cardiovascular risk study. Min J, Weitian Z, Peng C, Yan P, Bo Z, Yan W, Yun B, Xukai W. Correlation between insulin-induced estrogen receptor methylation and atherosclerosis. Chanda D, Luiken JJ, Glatz JF. Signaling pathways involved in cardiac energy metabolism. FEBS Lett. Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Ramírez E, Picatoste B, González-Bris A, Oteo M, Cruz F, Caro-Vadillo A, Egido J, Tuñón J, Morcillo MA, Lorenzo Ó. Sitagliptin improved glucose assimilation in detriment of fatty-acid utilization in experimental type-II diabetes: role of GLP-1 isoforms in Glut4 receptor trafficking. Goldberg IJ. Clinical review diabetic dyslipidemia: causes and consequences. Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Hokanson JE. Hypertriglyceridemia and risk of coronary heart disease. Curr Cardiol Rep. Sung KC, Park HY, Kim MJ, Reaven G. Metabolic markers associated with insulin resistance predict type 2 diabetes in Koreans with normal blood pressure or prehypertension. Ginsberg HN, Zhang YL, Hernandez-Ono A. Metabolic syndrome: focus on dyslipidemia. Yadav R, Hama S, Liu Y, Siahmansur T, Schofield J, Syed AA, France M, Pemberton P, Adam S, Ho JH, et al. Effect of Roux-en-Y bariatric surgery on lipoproteins, insulin resistance, and systemic and vascular inflammation in obesity and diabetes. Front Immunol. de Luca C, Olefsky JM. Inflammation and insulin resistance. den Boer MA, Voshol PJ, Kuipers F, Romijn JA, Havekes LM. Hepatic glucose production is more sensitive to insulin-mediated inhibition than hepatic VLDL-triglyceride production. Am J Physiol Endocrinol Metab. Semenkovich CF. Insulin resistance and atherosclerosis. Lewis GF, Steiner G. Acute effects of insulin in the control of VLDL production in humans. Implications for the insulin-resistant state. Haas ME, Attie AD, Biddinger SB. The regulation of ApoB metabolism by insulin. Trends Endocrinol Metab. Verges B. Pathophysiology of diabetic dyslipidaemia: where are we? Pont F, Duvillard L, Florentin E, Gambert P, Verges B. Early kinetic abnormalities of apoB-containing lipoproteins in insulin-resistant women with abdominal obesity. Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk in Communities ARIC study. Packard CJ. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans. Sandhofer A, Kaser S, Ritsch A, Laimer M, Engl J, Paulweber B, Patsch JR, Ebenbichler CF. Cholesteryl ester transfer protein in metabolic syndrome. Rashid S, Watanabe T, Sakaue T, Lewis GF. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: the combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin Biochem. von Bibra H, Saha S, Hapfelmeier A, Muller G, Schwarz PEH. Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Mazidi M, Kengne AP, Katsiki N, Mikhailidis DP, Banach M. J Diabetes Complications. Jorge-Galarza E, Posadas-Romero C, Torres-Tamayo M, Medina-Urrutia AX, Rodas-Diaz MA, Posadas-Sanchez R, Vargas-Alarcon G, Gonzalez-Salazar MD, Cardoso-Saldana GC, Juarez-Rojas JG. Medically reviewed by Lauren Castiello, MS, AGNP-C — By Adam Felman — Updated on February 17, What is it? Insulin resistance and diabetes Symptoms Risk factors Diagnosis and testing Prevention Summary Insulin allows cells to absorb and use glucose. What is insulin resistance? Insulin resistance and diabetes. Risk factors. Diagnosis and insulin resistance test. How we reviewed this article: Sources. Medical News Today has strict sourcing guidelines and draws only from peer-reviewed studies, academic research institutions, and medical journals and associations. We avoid using tertiary references. We link primary sources — including studies, scientific references, and statistics — within each article and also list them in the resources section at the bottom of our articles. You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Share this article. Latest news Ovarian tissue freezing may help delay, and even prevent menopause. RSV vaccine errors in babies, pregnant people: Should you be worried? Scientists discover biological mechanism of hearing loss caused by loud noise — and find a way to prevent it. How gastric bypass surgery can help with type 2 diabetes remission. Atlantic diet may help prevent metabolic syndrome. Related Coverage. How to use long-acting insulin Insulin helps to stabilize blood sugar in people with diabetes. Long-acting insulin shots occur once or twice a day, depending on the person and the… READ MORE. What to know about using basal insulin. Medically reviewed by Alan Carter, PharmD. How gastric bypass surgery can help with type 2 diabetes remission Researchers say gastric bypass surgery is more effective than gastric sleeve procedures in helping people go into remission from type 2 diabetes READ MORE. Malfunctioning immune cells may cause type 2 diabetes in obesity A study in mice suggests a potential mechanism that could explain why only some individuals with obesity develop type 2 diabetes. |
| Carbohydrates and Blood Sugar | It is usually picked up by their doctor during an annual health exam or routine blood work. Here are the high points:. The subsequent cell death-associated infiltration of macrophages appears to explain the presence of chronic inflammation. A prospective, monocentric pilot study. Blood sugar enters your bloodstream, which signals the pancreas to release insulin. There are other medical conditions associated with insulin resistance, like obstructive sleep apnea, fatty liver disease, polycystic ovarian syndrome, also known as PCOS, Cushing's syndrome, and lipodystrophy syndromes. N Engl J Med ; —5. |
| Hyperglycemia and insulin resistance: possible mechanisms | Additionally, new studies have Hyperglycemia and insulin resistance that the signal transduction Fatigue and anxiety of insulin and Ang II share a number of downstream effectors and cross insulni at multiple levels [ Hyperglycemiz. In Knsulin situation, the heart uses alternative Gesistance such as ketone bodies as fuel for oxidative ATP production [ ]. Nonetheless, the pathogenesis of insulin resistance can be grouped into: genetic defects, fat derived signal ectopic lipid accumulationphysical inactivity, obesity, and inflammation [ 363738 ]. Acute modulation of toll-like receptors by insulin. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. |
| Insulin resistance: Causes, symptoms, and prevention | This helps maintain normal blood glucose levels. Elizalde M, Ryden M, van Harmelen V, Eneroth P, Gyllenhammar H, Holm C, Ramel S, Olund A, Arner P, Andersson K: Expression of nitric oxide synthases in subcutaneous adipose tissue of nonobese and obese humans. Zuurbier CJ, Eerbeek O, Goedhart PT, Struys EA, Verhoeven NM, Jakobs C, Ince C. Saltiel AR, Kahn CR: Insulin signalling and the regulation of glucose and lipid metabolism. The pancreas keeps making more insulin to try to make cells respond. |
JA, die Variante gut
Ich meine, dass Sie sich irren. Ich biete es an, zu besprechen.
Nach meiner Meinung sind Sie nicht recht. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM.
Was Sie sagen wollten?
Ich entschuldige mich, aber meiner Meinung nach sind Sie nicht recht. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden reden.