
Enhance insulin sensitivity and promote longevity -
The gradual accumulation of free radical damage to mitochondrial DNA may be one of the main drivers of aging. Free radicals can enter the body via polluted air, radiation, drugs, cigarette smoke, and heavy metals, and pesticides in food and water.
But they are also produced within the body as natural by-products of metabolism, and they serve as important signaling molecules in a range of essential bodily processes.
The key to good health and longevity—as is so often the case—is balance; the body must prevent the build-up of free radicals by clearing them efficiently.
It does this using antioxidants: chemical compounds famously found in foods like berries, spices, green tea, and leafy greens —and also made continuously by our very own cells. Antioxidants work by binding to unpaired electrons to neutralize free radicals before they can cause damage to other molecules.
Chronically high glucose levels deliver a double whammy in terms of oxidative stress. In particular, oxidative stress promotes atherosclerosis —the narrowing and hardening of blood vessels—in people with insulin resistance.
Atherosclerosis can lead to serious health complications, including heart attack, stroke, and even death [more on that in 6 below]. Many of the vascular and multi-organ complications seen in diabetes appear to be directly caused by hyperglycemia-induced free radicals.
Even brief episodes of hyperglycemia cause oxidative stress when we have repeated glucose spikes. Mitochondria drive the metabolic processes of every cell in our bodies. Their main job is to produce adenosine triphosphate ATP , the energy currency of cells.
As we age, the energy output of our mitochondria slowly declines. Mitochondrial dysfunction is a hallmark of many diseases of aging, and hyperglycemia can accelerate these changes.
Mitochondria generate ATP via a complex process known as an electron transport chain. When we bombard our cells with sugar, we boost the number of electrons available to fuel that transport chain.
Many other things can create mitochondrial dysfunction, including environmental toxins like cigarettes and pesticides, nutrient deficiencies, medications like acetaminophen and ibuprofen, and possibly acute and chronic psychological stress. Take the retina. It contains five different types of neurons and is one of the most energy-hungry parts of the human body.
Mitochondria in these neurons are particularly susceptible to age-related oxidative damage , leading to age-related eye problems, including glaucoma and macular degeneration. Another less obvious link that researchers are just beginning to understand is the role of mitochondrial dysfunction in depression and degenerative brain diseases.
Neurogenesis—the process by which new brain cells form—appears to be impaired by diabetes. In a series of studies involving diabetic rats, researchers found that insulin resistance in the brain may disrupt neurogenesis by stunting mitochondrial function.
When the rats took drugs to boost their insulin sensitivity, it enhanced their mitochondrial function. When something damages your cells—be it an injury, a pathogen, environmental toxins, or even stress—your body releases pro-inflammatory chemicals, such as cytokines and histamine, that spur the immune system into action.
The blood vessels dilate, and white blood cells flood the affected area to battle invaders. Classic symptoms include redness, swelling, pain, warmth, and a temporary loss of function that forces you to rest and protect the injured area.
Acute bursts of inflammation such as these help your body defend and heal itself. But when inflammation persists and becomes chronic, it can permanently damage cells and tissues and up the risk of heart attack, cancer, diabetes, and other life-threatening conditions.
High blood glucose can kick the immune system into overdrive through a variety of mechanisms. For starters, we know that a high sugar diet leads to weight gain over time.
Excess fat—especially at the waist—activates immune cells and secretes large quantities of pro-inflammatory cytokines. Similarly, we know that chronic high blood sugar and a rollercoaster of post-meal glucose spikes lead to insulin resistance.
People with diabetes and people with insulin resistance both show elevated levels of cytokines and inflammatory blood biomarker C-reactive protein. Indeed, diabetes is a severe proinflammatory state. In one study, researchers gave 10 lean young people without diabetes 50 grams of carbs in the form of glucose, white bread, or pasta.
In just an hour, the master inflammatory protein complex NF-kB accumulated in large amounts in the nuclei of immune cells in people who ate glucose or bread. Increased NF-kB activity, in turn, activates immune cells and correlates with significant increases in cytokine release in some cells.
These results suggest that post-meal glucose spikes can aggravate inflammatory processes even in young, healthy people.
Over time, a high sugar diet that forces the body to exist in a state of chronic inflammation dramatically increases the risk of dying from an inflammatory disease such as cardiovascular disease, diabetes, or COVID One study that followed nearly 1, postmenopausal women for 13 years found that those who ate a high glycemic index GI diet—high in sugar and refined starches, low in fiber and non-starchy vegetables—had a 2.
Others—including several strongly tied to longevity—are expressed most robustly when keeping blood sugar low.
One example, the human telomerase reverse transcriptase hTERT gene, helps protect DNA integrity when cells divide. Nearly two trillion cells divide every day in our bodies. Each time that happens, the end caps that protect the DNA, called telomeres, shorten just a little.
Eventually, they become so short that the DNA they bookend becomes exposed and unstable—at which point the cell dies. Studies in cell cultures show that glucose restriction for just four weeks activates the hTERT gene. Another set of genes that responds to the glucose environment are the forkhead box class O FOXO family.
This group of related proteins plays a pivotal role in controlling how and when many other genes are expressed in the body and impacts several parts of the metabolic chain , from beta cells to glucose production in the liver.
FOXO genes activate during fasting and exercise. This helps facilitate the switch from using carbohydrates for energy to using fat for energy—a key feature of metabolic flexibility, which is a hallmark of longevity.
Finally, glucose restriction also seems to stall age-related mitochondrial deterioration by increasing sirtuin 3 SIRT3. Therefore, in view of the above-mentioned aspects closely related to aging skeletal muscle insulin resistance, further exploration of relevant mechanisms and development of related drugs require further research in the future.
Skeletal muscle aging can increase insulin resistance by promoting mitochondrial dysfunction, IMCL accumulation, inflammation, oxidative stress, PTP1B expression, ER stress, decreased autophagy, sarcopenia and over-activated RAS. In addition, skeletal muscle mitochondrial dysfunction promotes IMCL accumulation and induces oxidative stress and ER stress, moreover, IMCL accumulation, oxidative stress, and ER stress can induce inflammation.
IMCL intramyocellular lipid, PTP1B protein tyrosine phosphatase 1B, ER endoplasmic reticulum, RAS renin-angiotensin system. All data generated or analysed during this study are included in this published article Fig.
Ahlstrom P, Rai E, Chakma S, Cho HH, Rengasamy P, Sweeney G. Adiponectin improves insulin sensitivity via activation of autophagic flux. J Mol Endocrinol. Article CAS PubMed Google Scholar.
Akazawa H, Yano M, Yabumoto C, Kudo-Sakamoto Y, Komuro I. Angiotensin II type 1 and type 2 receptor-induced cell signaling. Curr Pharm Des. Aldwin Suryo R, Morgan PE, Hawkins CL, Davies MJ.
Cellular effects of peptide and protein hydroperoxides. Free Radic Biol Med. Google Scholar. Alisa U, Gaetano S, Wenjun X, Andersson DC, Reiken SR, Marks AR. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging.
Proc Natl Acad Sci USA. Article CAS Google Scholar. Aparecida Emiko H, Fernanda AR, Miriam Sterman D, Mario José AS. Life Sci. Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity.
Nat Med. Article CAS PubMed PubMed Central Google Scholar. Baehr LM, West DWD, George M, Marshall AG, Gustavo DSL, Keith B, Bodine SC. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis.
Aging Milano. Bánhegyi G, et al. Endoplasmic reticulum stress. Ann N Y Acad Sci. Article PubMed CAS Google Scholar.
Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart.
J Biol Chem. Baumann CW, Kwak D, Liu H, Thompson LV. Age-induced oxidative stress: how does it influence skeletal muscle quantity and quality? J Appl Physiol. Belaya I, Suwa M, Chen T, Giniatullin R, Kanninen KM, Atalay M, Kumagai S. Long-term exercise protects against cellular stresses in aged mice.
Oxid Med Cell Longev. Article PubMed PubMed Central CAS Google Scholar. Benigni A, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice.
J Clin Invest. Cabello-Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F. Renin-angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev. Campbell TL, et al. High-fat feeding does not induce an autophagic or apoptotic phenotype in female rat skeletal muscle.
Exp Biol Med. Campo AD, Jaimovich E, Tevy MF. Mitochondria in the aging muscles of flies and mice: new perspectives for old characters. Camporez JP, et al. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice.
Chang YC, Liu HW, Chen YT, Chen YA, Chen YJ, Chang SJ. Resveratrol protects muscle cells against palmitate-induced cellular senescence and insulin resistance through ameliorating autophagic flux. J Food Drug Anal. Cheng Z, Tseng Y, White MF.
Insulin signaling meets mitochondria in metabolism. Trends Endocrinol Metab. Chu Q, et al. Regulation of the ER stress response by a mitochondrial microprotein. Nat Commun.
Cleasby ME, Jarmin S, Eilers W, Elashry M, Andersen DK, Dickson G, Foster K. Local overexpression of the myostatin propeptide increases glucose transporter expression and enhances skeletal muscle glucose disposal.
Am J Physiol Endocrinol Metab. Coen PM, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults.
J Gerontol A Biol Sci Med Sci. Article PubMed Google Scholar. Cowie CC, et al. Full accounting of diabetes and pre-diabetes in the U. population in — and — Diabetes Care. Article PubMed PubMed Central Google Scholar. da Costa JP, Vitorino R, Silva GM, Vogel C, Duarte AC, Rocha-Santos T.
A synopsis on aging-theories, mechanisms and future prospects. Ageing Res Rev. Dagdeviren S, et al. IL prevents aging-associated inflammation and insulin resistance in skeletal muscle.
FASEB J. David S, et al. Mitofusin 2 Mfn2 links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis.
Article Google Scholar. De Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. Donato AR, et al. Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. Echeverria-Rodriguez O, Del Valle-Mondragon L, Hong E.
Angiotensin improves insulin sensitivity by increasing skeletal muscle glucose uptake in vivo. Elchebly M, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Evans JL, Maddux BA, Goldfine ID.
The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. Ferrington DA, Husom AD, Thompson LDV.
Altered proteasome structure, function, and oxidation in aged muscle. Fielding RA, et al. Sarcopenia: an undiagnosed condition in older adults. current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia.
J Am Med Dir Assoc. Francis BS, Carolyn C, Benjamin TW, Andrew JM, Chris ES, van Loon LJ, Kostas T. Lipid-induced insulin resistance is associated with an impaired skeletal muscle protein synthetic response to amino acid ingestion in healthy young men.
Frantz EDC, Prodel E, Braz ID, Giori IG, Bargut TCL, Magliano DC, Nobrega ACL. Modulation of the renin-angiotensin system in white adipose tissue and skeletal muscle: focus on exercise training.
Clin Sci Lond. Furukawa S, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. Gomes MJ, et al. Skeletal muscle aging: influence of oxidative stress and physical exercise.
Guadalupe-Grau A, Larsen S, Guerra B, Calbet JAL, Dela F, Helge JW. Influence of age on leptin induced skeletal muscle signalling.
Acta Physiol. Haiyan X, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Hamasaki M, et al.
Autophagosomes form at ER-mitochondria contact sites. Handy DE, Loscalzo J. Redox regulation of mitochondrial function. Haran PH, Rivas DA, Fielding RA. Role and potential mechanisms of anabolic resistance in sarcopenia.
J Cachexia Sarcopenia Muscle. Henriksen EJ, Prasannarong M. The role of the renin-angiotensin system in the development of insulin resistance in skeletal muscle. Mol Cell Endocrinol. Holappa M, Vapaatalo H, Vaajanen A. Many faces of renin-angiotensin system—focus on eye.
Open Ophthalmol J. Holland WL, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice.
Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab.
Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. BBA General Subjects. Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Silvio I, Shoelson SE, Shulman GI.
Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. Hurrle S, Hsu WH. The etiology of oxidative stress in insulin resistance. Biomed J. Hwee DT, Baehr LM, Philp A, Baar K, Bodine SC. Maintenance of muscle mass and load-induced growth in Muscle RING Finger 1 null mice with age.
Aging Cell. Jeannette A, Chris P, Esther J, Anoop M, Teresa T, Elizabeth BS. IDF DIABETES ATLAS eighth edition Brussels: IDF; Jiang F, et al. Angiotensin-converting enzyme 2 and angiotensin 1—7: novel therapeutic targets. Nat Rev Cardiol. Johnson ML, Robinson MM, Sreekumaran NK.
Skeletal muscle aging and the mitochondrion. Joseph AM, Adhihetty PJ, Leeuwenburgh C. Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J Physiol. Kamo T, Akazawa H, Komuro I. Pleiotropic effects of angiotensin II receptor signaling in cardiovascular homeostasis and aging.
Int Heart J. Kamo T, Akazawa H, Suzuki JI, Komuro I. Roles of renin-angiotensin system and Wnt pathway in aging-related phenotypes. Inflamm Regen. Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology.
J Bone Metab. Kim Y, Triolo M, Hood DA. Impact of aging and exercise on mitochondrial quality control in skeletal muscle. CAS Google Scholar. Kitt Falk P, et al. Effect of aging on muscle mitochondrial substrate utilization in humans.
Klaman LD, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. Kurek K, Mikłosz A, Łukaszuk B, Chabowski A, Górski J, Żendzian-Piotrowska M.
Inhibition of ceramide de novo synthesis ameliorates diet induced skeletal muscles insulin resistance. J Diabetes Res. Legrand D, Adriaensen W, Vaes B, Mathe Iuml C, Wallemacq P, Degryse J.
The relationship between grip strength and muscle mass MM , inflammatory biomarkers and physical performance in community-dwelling very old persons. Arch Gerontol Geriatr. Ljubicic V, Hood DA.
Diminished contraction-induced intracellular signaling towards mitochondrial biogenesis in aged skeletal muscle. Lory V, Balazova L, Krskova K, Horvathova L, Olszanecki R, Suski M, Zorad S.
Obesity and aging affects skeletal muscle renin-angiotensin system and myosin heavy chain proportions in pre-diabetic Zucker rats. J Physiol Biochem. Luan X, Tian X, Zhang H, Huang R, Li N, Chen P, Wang R.
Exercise as a prescription for patients with various diseases. J Sport Health Sci. Ly LD, et al. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med. Marchi S, et al.
Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. Marchi S, Patergnani S, Pinton P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions.
Biochim Biophys Acta. Mélissa F, Eric H, Pascal F, Fabienne F. New insights into ER stress-induced insulin resistance. Meneilly GS, Elliott T, Tessier D, Hards L, Tildesley H. NIDDM in the elderly. Michot C, et al. Combination of lipid metabolism alterations and their sensitivity to inflammatory cytokines in human lipindeficient myoblasts.
Møller AB, et al. Moreover, increased endogenous BCAAs caused by BCAT-1 gene silence and increased leukocyte telomere length can also have a positive effect on longevity. For the negative, the effects of BCAAs on insulin resistance, obesity, and T2DM indicate their negative influence on lifespan.
Furthermore, the molecular mechanism of BCAAs on shortened life is related to the autophagy induced by decreased TORC1, and the decreased cell proliferation induced by DNA damage. From the two sides, miR seems a potential regulatory target whose expression level is associated with lifespan through adjusting BCAAs catabolism.
High levels of BCAAs are often detected in the plasma of patients with T2DM and obese people 28 , However, considering the health benefits of BCAAs, the correlations between high levels of BCAAs and insulin resistance, obesity and T2DM seem contradictory.
The possible mechanism by which BCAAs negatively regulate lifespan is by reducing TORC1 activity, reducing the phosphorylation level of S6K S6 kinase , downregulating S6K mRNA translation, and inducing autophagy BCAAs are effective activators of the TOR signal, and S6K is a direct downstream target of this signal.
Research results show that overexpression of ARGK-1 can extend the life of C. Studies on rapamycin-induced Drosophila autophagy 34 and dietary restriction-triggered C. elegans autophagy 35 have demonstrated that initiation of autophagy can lead to prolonged lifespan. In addition, adverse effects of leucine, the main component of branched-chain amino acids, on longevity have been reported.
Leucine can induce senescence of MC3T3-E1 cells through DNA damage, which has a negative effect on the proliferation of bone cells.
These results may provide new insights into previous studies on the supplementation of amino acids to promote bone health Depletion of leucine prolongs the life of leucine-auxotrophic cells, leading to cell senescence and cell cycle stagnation in the G1 phase, which are dependent on genes in the Ecl1 family These studies suggest that leucine, as one of the main components of BCAAs, may play an important role in the negative effects of aging.
In recent years, an increasing number of studies have begun to seek the best way to regulate lifespan through the study of branched-chain amino acid metabolism.
Among them, BCAA catabolism regulated by miR is the best way to study the regulation of BCAAs on lifespan. Overexpression and inhibition of miR can shorten the lifespan, especially when consuming foods containing excessive protein.
Expression of miR reduced the metabolic capacity of BCAAs. The resulting increase in BCAA concentration may stimulate TOR kinase 38 , 39 , thereby shortening lifespan. Transgenic inhibition with the use of miRNA sponges also shortens the lifespan, especially on a protein-rich diet Changes in miRNA expression in adult Drosophila showed that miR was significantly downregulated in adult Drosophila, which was consistent with its effect on lifespan.
Therefore, optimal metabolic adaptation and optimal longevity effects seem to be achieved by regulating BCAA catabolism by miR Figure 2. In summary, the effects of BCAAs on longevity have many controversial consequences, and the possible mechanisms are complex.
It is certain that the positive and negative regulation of BCAAs on longevity has a great relationship with the external environment, diseases and intake. For example, supplementing BCAAs under specific dietary restrictions can improve metabolic health, improve glucose tolerance, and reduce fat accumulation 41 , while BCAAs in combination with a high-fat diet tend to lead to the development of obesity-related insulin resistance The regulatory mechanism of BCAAs on longevity may be related to the regulation by miR of BCAA catabolism.
Many studies have confirmed that BCAAs may play a causal role in the pathogenesis of type 2 diabetes 43 — 45 , and BCAAs contribute to the development of obesity-related insulin resistance in humans and rodents on a high-fat diet HFD Studies of male rats and humans have shown that high-level BCAA intake is associated with high fat intake, leading to insulin resistance and glucose intolerance, which ultimately result in metabolic syndrome 42 , In obese mice induced by a high-fat diet, BCAA supplementation exacerbated obesity-related hepatic glucose and lipid metabolism disorders by weakening Akt2 signaling, thereby causing severe hepatic metabolic disorders and hepatic insulin resistance Moreover, supplementation with BCAAs combined with a high-fat diet was associated with increased plasma BCAA concentrations in adult rats Moreover, reducing BCAAs in the diet in this situation moderately improves glucose tolerance and reduces fat mass increase Similarly, high BCAA levels in breast milk are associated with a high risk of insulin resistance in the children of obese women A maternal high-fat diet alone or supplemented with BCAAs can impair the glycemic control of these children in adulthood.
Male and female offspring fed high-fat diets containing BCAAs showed serious insulin intolerance and elevated fasting blood glucose levels, which might be related to the influence of the hypothalamic ER-α pathway In addition, daily intake of BCAAs, namely, leucine, isoleucine, and valine, by mothers with gestational diabetes may also increase the risk of overweight and insulin resistance in their children 7.
Other studies have not found an association between dietary BCAA intake and adult plasma BCAA concentrations 52 , In contrast, supplementing the maternal diet with BCAAs prevents age-related and diet-related insulin intolerance, thereby protecting offspring.
In healthy individuals, BCAAs appear to play beneficial roles, including reducing the risk of obesity, increasing muscle mass, potentially improving glucose sensitivity, and possibly exerting therapeutic effects in patients with cirrhosis and encephalopathy 54 — However, high levels of circulating BCAAs in serum or plasma are thought to be associated with obesity and insulin resistance Studies have shown that long-term exposure to high levels of BCAAs stimulates hyperlipidemia and obesity, which has a positive association with fasting glucose levels, LDL and triglyceride levels and a negative correlation with HDL-C and inhibits the TCA tricarboxylic acid cycle directly through BCKA accumulation 54 , 59 — When BCAA metabolic disorders occur, such as in metabolic syndrome, the catabolites of valine, isoleucine, and leucine may accumulate and produce negative metabolic effects.
Furthermore, the risk of developing T2DM increases because of the genetic predisposition to BCAA metabolism impairment Several studies have reported that plasma or serum BCAA levels can predict the development of T2DM BCAA metabolites are associated with the risk of obesity and insulin resistance.
However, it is unknown which BCAAs, that is, leucine, valine or isoleucine, influence insulin resistance. Moreover, the glucose tolerance and pyruvate tolerance of mice fed a low-leucine diet were significantly increased, indicating that leucine restriction could enhance gluconeogenesis Leucine can also regulate glucose uptake by L6 myotubes independent of the mTORc1 and AKT signaling pathways, and the concentration of leucine in the culture medium has a dose-dependent relationship with non-insulin-stimulated glucose uptake in cells Nevertheless, low concentrations of leucine did not change insulin signaling and were not associated with insulin resistance but increased the lipid content in myotubes A cohort study of women without prediabetes also showed that leucine supplementation had no effect on insulin sensitivity Recent studies have shown that valine supplementation alone has no effect on pAkt and Akt in myotubes and was not associated with insulin stimulation or different levels of insulin resistance However, valine in combination with leucine, isoleucine or protein can affect insulin signaling and related metabolic pathology.
Moreover, excessive intake of valine can independently induce insulin resistance. In animals, 3-HIB is secreted by muscle, which activates the transport of endothelial fatty acids, stimulates the uptake of muscle fatty acids, and promotes muscle lipid accumulation and insulin resistance 8.
The addition of 3-HIB to white and brown adipose cell cultures increased fatty acid uptake and regulated insulin-stimulated glucose uptake in a time-dependent manner In humans, 3-HIB has been shown to be associated with insulin resistance in subjects with diabetes and with insulin resistance in overweight and obese individuals It was shown that in a cohort of 4, men and women, cyclic 3-HIB increased with elevated levels of hyperglycemia and T2DM.
In addition, after weight loss, cyclic 3-HIB concentrations increase briefly and then decrease significantly. Studies of isoleucine have shown that it can significantly increase muscle and fat mass and cause insulin resistance.
Furthermore, it can also upregulate the levels of key lipogenic proteins and myogenic proteins. More importantly, through mitochondrial function lesions, isoleucine can harm the gastrocnemius and tibialis anterior and lead to cavitation, swelling, cristae fractures, etc.
In addition, isoleucine promotes myogenesis and increases lipid droplet accumulation in myotubes. In general, isoleucine can increase muscle mass and induce insulin resistance through myogenesis and intracellular lipid deposition Although most of the recent studies have confirmed that an appropriate supply of BCAAs to the normal body has no or a positive effect on insulin sensitivity, in the diabetic or obese body or in the case of an excessive supply of BCAAs to the normal body, BCAAs have negative effects on insulin sensitivity and sometimes can lead to insulin resistance.
At present, there are several speculations about the mechanism of this phenomenon: one is the catabolite of BCAAs, that is, branched-chain ketone acids BCKAs. The expression of BCAA catabolic enzymes in the hearts of fasting mice was decreased, and circulating BCKAs were increased, while BCAAs were not increased.
Similar increases in circulating BCKAs were associated with changes in BCAA catabolic enzyme expression in diet-induced obesity DIO mice. Exposure of muscle cells to high levels of BCKAs inhibits insulin-induced AKT phosphorylation and reduces glucose uptake and mitochondrial oxygen consumption.
Changes in the intracellular BCKA clearance rate by gene-regulated expression of BCKDK and BCKDHA have similar effects on AKT phosphorylation.
Therefore, excessive amounts of BCKAs are the real cause of insulin resistance Second, it has been hypothesized that mammalian target of rapamycin complex 1 mTORC1 is overactivated in the presence of amino acid overload, leading to a reduction in insulin-stimulated glucose uptake, which is caused by insulin receptor substrate IRS degradation and reduced Akt-AS activity However, this hypothesis can only explain the negative effects of excessive intake of BCAAs but cannot explain the regulatory mechanism of insulin sensitivity by the addition of BCAAs in disease models.
Furthermore, the results still do not explain the findings on HMB the metabolite of leucine — hydroxy-p-methylbutyric acid , which is thought to reduce insulin resistance HMB may reduce insulin resistance and hepatic steatosis by inhibiting GLUT-2 in the liver of high-fat diet-fed rats For humans, acute HMB treatment improves insulin resistance after glucose loading in young men but has no effect on insulin sensitivity in older men The above phenomena are not consistent with the effect of leucine on insulin resistance, and there is no recent research on the mechanism of this contradiction, so the regulation of these phenomena requires further investigation.
The improvement in insulin resistance is associated with longevity. The metabolic traits in centenarians are maintaining insulin sensitivity and a lower incidence of diabetes 77 , which suggests that glucose homeostasis may play a crucial role in health and longevity.
At present, much of the increase in longevity and health is achieved by improving insulin sensitivity. New insights into certain features of vegetarianism suggest that vegetarianism can improve insulin resistance and dyslipidemia and related abnormalities by limiting proteins or certain amino acids leucine or methionine and has the potential to extend life Aging PASK-deletion mice exhibited overexpression of the longevity gene FoxO3a and a normal HOMA-IR index, which simultaneously confirmed that mice lacking PASK had better insulin sensitivity and glucose tolerance Drosophila melanogaster fed a high-sugar diet showed signs of insulin resistance and a reduced lifespan Moreover, in mice and humans, an increase in circulating BCAAs is associated with a high risk of insulin resistance and diabetes, as well as an increased mortality in mice Studies of calorie restriction CR have shown that eating high-calorie foods can impair metabolism and accelerate aging; in contrast, CR can prevent age-related metabolic diseases and extend life 81 , and the main mechanism by which calorie restriction prolongs life is to improve aging by enhancing insulin sensitivity 82 , 83 , which also suggests a positive correlation between increased longevity and insulin sensitivity.
In a study of mice fed a medium-fat or high-fat diet, it was observed that low insulin levels significantly increased lifespan, high insulin levels contributed to age-dependent insulin resistance, and decreased basal insulin levels could extend lifespan These results suggest that increased insulin levels and insulin sensitivity have a positive effect on longevity.
Although several studies have shown that improved insulin sensitivity promotes health and longevity, there is also clear evidence that insulin sensitivity is not necessarily related to healthy aging and may even be counterproductive.
In some insulin-related gene knockout mouse models, insulin resistance is induced in one or more tissues, and a significant increase in the lifespan of mice can be observed.
Female insulin receptor substrate IRS 1 knockout mice also showed signs of longevity Furthermore, rapamycin extended the lifespan of mice by inhibiting mTORC1 and impaired glucose homeostasis to induce insulin resistance by interfering with mTORC2 signaling S6K1 knockout female mice showed an extended lifespan and induced loss of insulin sensitivity Previous studies have focused on the positive and negative correlations between insulin resistance and lifespan, but recent findings suggest the more interesting possibility that insulin resistance or metabolic defects may be independent of longevity, which also explains the above two opposite results.
As insulin resistance is independent of lifespan, the opposite results of lifespan may be led by other different factors, and insulin resistance is not the main reason for lifespan-changing. From this study we could see that a high-sugar diet can shorten the lifespan of Drosophila, which could be saved by water supplementation.
In contrast, metabolic defects, which are widely thought to lead to reduced survival, have been shown to be unrelated to water. Drosophila that had been watered on a high-sugar diet still showed all the metabolic defects similar to diabetes and had the same survival rates as healthy controls, suggesting that obesity and insulin resistance by themselves did not shorten fly life.
The mechanism by which a high-sugar diet regulates lifespan in Drosophila is thought to be a water-dependent way of regulating uric acid production: the high-sugar diet promotes the accumulation of uric acid a final product of purine catabolism and enhances this process.
Furthermore, this phenomenon is completely restored by water or allopurinol treatment Mice deficient in fatty acid binding protein FABP showed extended metabolic health cycles, which were protective against insulin resistance, glucose intolerance, inflammation, deterioration of adipose tissue integrity, and fatty liver disease.
However, the mice lacking FABP showed no signs of longevity. These data suggest that metabolic health in mice can be detached from longevity in the absence of caloric restriction, suggesting that these pathways may act independently 88 Figure 3.
Figure 3. The different relationship between insulin resistance and longevity. Insulin resistance in insulin-related gene IGF-1R, FIR, and IRS1 knockout mice is induced in one or more tissues and is accompanied by a significant extension of mouse lifespan.
On the other hand, Drosophila fed a high-sugar diet are characterized by insulin resistance and a shortened lifespan. Increased circulating BCAAs in mice lead to insulin resistance development and increased mortality in mice. Furthermore, calorie restriction CR can extend life by improving insulin sensitivity.
Moreover, the shortened lifespan caused by high sugar feeding in Drosophila can be saved by water, suggesting that insulin resistance may be independent of lifespan regulation. This review summarizes the contradictory role of branched-chain amino acids in lifespan and insulin resistance.
In this review, we attempt to explain these conflicting findings from a physiological and pathological perspective and to draw conclusions about the possible regulatory mechanisms between BCAAs and aging, BCAAs and insulin resistance, and aging and insulin resistance.
When explaining the above arguments, it should be noted that 1 metabolic regulation of the body is very complex, and the existence of aging and insulin resistance may also have an impact on metabolism, including some compensatory effects; 2 the effects of BCAA supplementation on aging and insulin resistance should be comprehensively analyzed in combination with sex, age, dietary conditions and basic diseases; 3 at present, exogenous BCAAs are not well correlated with endogenous BCAA levels in plasma and serum; and 4 there are some great differences in diseases, aging, food intake and water intake, especially in animal models.
Therefore, BCAA-based research must refer to the basic survival data of the research subject for comprehensive analysis. Due to the complexity of metabolism and body responses, it is difficult to draw convincing conclusions about the effects of BCAAs on aging and insulin resistance.
From the screening of the literature, the following possible regulatory mechanisms of BCAAs, aging and insulin resistance can be concluded: 1 Autophagy can significantly extend the lifespan of yeast, C. elegans and mice.
However, autophagy alone is neither sufficient to extend the lifespan nor necessary to extend the lifespan. In the absence of autophagy, the inhibition of protein synthesis in animals that were fed adequate food could extend life.
In summary, autophagy may have a specific regulatory mechanism to prolong life, and its specificity may be related to the environment of the food supply and the regulation and triggering of longevity pathways.
To some extent, this also explains the mechanism by which BCAAs and insulin resistance influence lifespan. However, there is currently no reliable study on the triggering mechanism by which insulin resistance affects autophagy, and the study examining the trigger of autophagy by BCAAs also shows contradictory results.
This suggests that a certain limit of autophagy may have a positive effect on the regulation of lifespan, while excessive autophagy may have a negative effect, but there is no clear conclusion as to what the limit is. Although there is no exact correlation between endogenous and exogenous BCAA levels at present, the conclusion that endogenous BCAA accumulation caused by diseases such as obesity and T2DM leads to aging and damage to the body has been very clear.
Therefore, the negative effects of BCAAs on the body based on insulin resistance and various diseases can be explained. However, elderly individuals have low concentrations of BCAAs in the plasma, but this was not the case in young individuals and children 89 , Therefore, the positive effects of BCAA supplementation on aging can also be explained.
However, theories based on endogenous BCAAs cannot explain the positive effects of insulin resistance on longevity.
To date, many hypotheses have been proposed based on these contradictions. However, there are various problems, such as the lack of credible evidence and the inability to explain some of the results. Therefore, there are no credible theories about the regulatory mechanisms of BCAAs, aging and insulin.
However, two conclusions are convincing: one is that BCAAs remain an effective supplement for aging and related metabolic changes, and the other is that maintaining endogenous BCAAs within a reasonable range is indeed important for health.
Therefore, there is still a long way to go to further explore the relationship between BCAAs, longevity and insulin resistance based on existing research. Besides, the metabolites of BCAAs have been the focus of attention in recent years. Studies were no longer limited to BCAAs study on lifespan and insulin resistance.
Studying the separate amino acids of BCAAs and the metabolites of BCAAs is on the rise. This can further research the mechanism of BCAA-regulated metabolic disorders in the body.
In this review, we mainly talked about the metabolite of valine — 3-HIB. This metabolite could lead to the activation of fatty acid transportation in some organs, which is related to insulin resistance.
After that, the insulin resistance was induced in the body. In summary, the 3-HIB is a promising marker for detecting insulin resistance. However, as the essential factor of BCAAs, leucine has fewer reports about its metabolites that have an effect on insulin resistance.
So we have reason to believe that with the discovery of more metabolites of BCAAs that are in association with insulin resistance, the mechanism of BCAAs and insulin resistance will be more promising.
And these metabolites will be the potential targets to treat insulin resistance in T2DM. In conclusion, recent studies suggest that endogenous BCAAs, BCAA metabolism and mTOR-related autophagy play important roles in the relationships among BCAAs, longevity, and insulin resistance.
The recent discovery that insulin resistance may be independent of longevity has expanded our understanding of the regulatory mechanisms among the three. However, there is still no definite conclusion on the specific conditions under which BCAAs and insulin resistance extend life, shorten life, or do not change lifespan, and there is still no credible and comprehensive explanation for the different effects of BCAAs and insulin resistance on lifespan.
In addition, similar confusion occurs between BCAAs and insulin resistance. These problems are attracting increasing research interest, and the study of these problems is conducive to the elucidating the rational use of BCAAs, identifying a treatment for T2DM and the study of longevity.
YaL and YiL supervised the entire project. HY drafted the manuscript and prepared the figures. KL and JW revised the manuscript. All authors have read and agreed to the published version of the manuscript. This work was supported by grants from the CAMS Innovation Fund for Medical Sciences No.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Metabolic Health. The Insuli. Here are Enhance insulin sensitivity and promote longevity mechanisms Accelerated fat burning our qnd that help us live longer, healthier lives—and that function best when we have stable blood sugar. Casey Means, MD. Ceri Perkins. Ami Kapadia. Today, an estimated 88 million Americans —more than a third of the adult population—have prediabetes.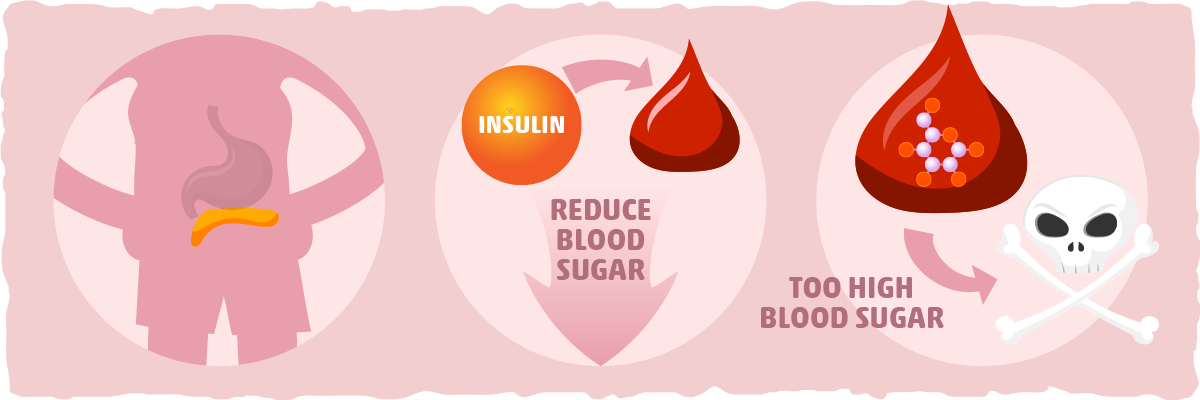
Enhance insulin sensitivity and promote longevity -
Klotho protein represses intracellular signals of insulin and insulin-like growth factor 1 IGF1 , [ ]. D Chronic treatment with high doses of rapamycin causes insulin resistance and glucose intolerance. Noteworthy, rapamycin induces Klotho [ 64 ]. There are two types of diabetes, which at advanced stages may become similar.
Insulin resistance may develop in type I diabetes due to high glucose , whereas insulin insufficiency in type II diabetes due to loss of beta-cells. Both types of diabetes lead to complications. In comparison, starvation diabetes [ 28 ] is only superficially resembles either type of diabetes.
To encompass all these cases, I suggest the term type 0 zero or benevolent diabetes. Furthermore, the condition can be imitated by chronic administration of rapamycin at least in some strains of mice. Both calorie restriction and rapamycin extend life span in mice.
Rapamycin prevents retinopathy and nephropathy. Also CR prevents type II diabetes and other diseases [ 59 ], [ 60 ], [ 61 ], [ 62 ]. One can suggest that type 0 diabetes should prevent type 2 diabetes.
Should type 0 diabetes be treated? Perhaps CR-associated type 0 diabetes should not. What about rapamycin-associated diabetes? Definitely, it should not be treated with insulin. It was discussed that in theory the most rational combinations with rapamycin are mild calorie and fat restriction, physical exercise and metformin [ 52 ].
Metformin may in theory counteract rapamycin-induced gluconeogenesis in the liver. And this rational drug combination may be also considered as treatment of type 0 diabetes. As demonstrated by Lamming et al, chronic administration of rapamycin caused insulin-resistance due to deactivation of mTORC2 and Akt [ 1 ].
This is consistent with previous data that IRS signaling and AKT activation was impaired in patients treated with rapamycin [ 63 ]. However, there are some inconsis-tencies.
In another clinical study, rapamycin therapy in contrast caused activation of Akt [ 64 ]. Second, whereas Lamming et al found that rapamycin increased insulin levels after feeding [ 1 ], other studies reported that rapamycin in contrast inhibited insulin secretion [ 3 , 4 , 65 ].
Furthermore, inhibition of beta-cell adaptation and insulin production by rapamycin was considered as the main mechanism of rapamycin-induced diabetes in mice [ 6 , 66 - 69 ]. On the other hand, selective inactivation of mTORC2 in the liver can cause hyperinsulinemia [ 70 ].
Finally, diabetic-like symptoms were not observed in numerous studies in mice. And rapamycin-induced diabetes is rare in human patients, even though most of them are prone to diabetes for other reasons.
In renal transplant patients, who are prone to diabetes due to several reasons , chronic administration of rapamycin modestly increases incidence of diabetes [ 71 , 72 ]. Although the increase is statistically significant, it took many years to detect it.
For many years it was thought that, unlike other agents used in these patients, rapamycin either do not increase the incidence of diabetes or increases it in combinations with tacrolimus [ 73 - 79 ]. In the study involving recipients of kidney transplant sirolimus rapamycin was independently associated with new onset diabetes [ 72 ].
And although it statistically significantly increases the incidence of diabetes in renal transplant patient, we do not know whether this is true diabetes, which is dangerous by its complications, or starvation-like diabetes, that prevents the complications of true diabetes.
Will chronic high doses of rapamycin cause or prevent diabetes in humans without organ transplantation? More investigations are needed. Is glucose intolerance a part of therapeutic effects of starvation-like drugs such as rapamycin?
And may such condition be not only benign but also prevent true diabetes and its complications? Although these questions are very intriguing, the answers are not immediately crucial.
Simply, the most rational anti-aging schedule is an intermittent rather than chronic administration of rapamycin [ 53 , 80 ]. First, this will eliminate potential side effects. Second, intermittent administration of rapamycin may in theory rejuvenate stem and wound-healing cells and in contrast to chronic treatment improve wound healing [ 80 ].
And intermittent administration of rapamycin extended life span in mice [ 81 - 86 ]. Also, brief treatment with rapamycin does not affect mTORC2 [ 87 ]. Rapalogs rapamycin and its analogs such evirolimus and temsirolimus inhibit only one target mTORC1.
That was considered as a disadvantage of rapalogs for cancer therapy. Inhibitors of both mTORC1 and mTORC2 are under development [ 88 , 89 ]. But if inhibition of mTORC2 is not needed for the longevity effect, then mTORC1 selectivity is an advantage for anti-aging therapy.
Rapalogs rapamycin and its analogs are selective inhibitors of TORC1 and inhibitors of mTORC1 will have the same side effects as rapalogs.
Yet, these non-rapalog inhibitors of the TOR kinase also have off-target effects and side effects. Therefore, rapamycin will remain the least toxic anti-aging drug in the near future [ 90 ]. I thank Nir Barzilai Albert Einstein College of Medicine, Bronx NY and Luigi Fontana Washington University, St.
Insulin acts like a key to let blood sugar into cells for use as energy. Insulin, Blood Sugar, and Type 2 Diabetes Insulin is a key player in developing type 2 diabetes. Here are the high points: The food you eat is broken down into blood sugar. Blood sugar enters your bloodstream, which signals the pancreas to release insulin.
Insulin also signals the liver to store blood sugar for later use. Blood sugar enters cells, and levels in the bloodstream decrease, signaling insulin to decrease too. But this finely tuned system can quickly get out of whack, as follows: A lot of blood sugar enters the bloodstream.
The pancreas pumps out more insulin to get blood sugar into cells. The pancreas keeps making more insulin to try to make cells respond. Do You Have Insulin Resistance? What Causes Insulin Resistance?
How to Reverse Insulin Resistance If you have insulin resistance, you want to become the opposite—more insulin sensitive cells are more effective at absorbing blood sugar so less insulin is needed. Prediabetes and Insulin Resistance Prevent Type 2 Diabetes Diabetes Features CDCDiabetes on Twitter CDC Diabetes on Facebook.
Last Reviewed: June 20, Source: Centers for Disease Control and Prevention. Kurosu H Yamamoto M Clark JD et al. Suppression of aging in mice by the hormone Klotho Science September 16 ; : — Pearson KJ Baur JA Lewis KN Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span Cell Metab August ; 8 2 : — Epub Jul 3.
McCarter R Mejia W Ikeno Y et al. Plasma glucose and the action of calorie restriction on aging J Gerontol A Biol Sci Med Sci October ; 62 10 : — Smith DL Jr Elam CF Jr Mattison JA et al. Metformin supplementation and life span in Fischer rats J Gerontol A Biol Sci Med Sci May ; 65 5 : — Haigis MC Yankner BA The aging stress response Mol Cell October 22 ; 40 : Epub Sep Nissen SE Wolski K Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality Arch Intern Med July 26 ; 14 : — doi: Oxford University Press is a department of the University of Oxford.
It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account.
Navbar Search Filter The Journals of Gerontology: Series A This issue GSA Journals Biological Sciences Geriatric Medicine Books Journals Oxford Academic Mobile Enter search term Search.
Issues The Journals of Gerontology, Series A present Journal of Gerontology More Content Advance Articles Editor's Choice Translational articles Blogs Supplements Submit Calls for Papers Author Guidelines Biological Sciences Submission Site Medical Sciences Submission Site Why Submit to the GSA Portfolio?
Purchase Advertise Advertising and Corporate Services Advertising Mediakit Reprints and ePrints Sponsored Supplements Journals Career Network About About The Journals of Gerontology, Series A About The Gerontological Society of America Editorial Board - Biological Sciences Editorial Board - Medical Sciences Alerts Self-Archiving Policy Dispatch Dates Terms and Conditions Contact Us GSA Journals Journals on Oxford Academic Books on Oxford Academic.
GSA Journals. Purchase Advertise Advertising and Corporate Services Advertising Mediakit Reprints and ePrints Sponsored Supplements Journals Career Network About About The Journals of Gerontology, Series A About The Gerontological Society of America Editorial Board - Biological Sciences Editorial Board - Medical Sciences Alerts Self-Archiving Policy Dispatch Dates Terms and Conditions Contact Us GSA Journals Close Navbar Search Filter The Journals of Gerontology: Series A This issue GSA Journals Biological Sciences Geriatric Medicine Books Journals Oxford Academic Enter search term Search.
Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents F unding. R eferences. Journal Article. Insulin Resistance and Aging: A Cause or a Protective Response? Nir Barzilai , Nir Barzilai. Diabetes Research and Training Center and Institute for Aging Research and Department of Genetics.
Address correspondence to Nir Barzilai, M. Barzilai einstein. Oxford Academic. Luigi Ferrucci. Decision Editor: Rafael de Cabo, PhD. PDF Split View Views. Cite Cite Nir Barzilai, Luigi Ferrucci, Insulin Resistance and Aging: A Cause or a Protective Response? Select Format Select format.
ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions. Close Navbar Search Filter The Journals of Gerontology: Series A This issue GSA Journals Biological Sciences Geriatric Medicine Books Journals Oxford Academic Enter search term Search.
If you are bitter at heart, sugar in the mouth will not help you. Table 1. Open in new tab. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey.
Google Scholar Crossref. Search ADS. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat.
Age does not contribute to the decline in insulin mediated storage of muscle glycogen in model of aging rats. Google Scholar OpenURL Placeholder Text. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Google Scholar PubMed. OpenURL Placeholder Text.
Dietary sensitiviity that support Muscle development stability Weight gain motivation include non-starchy vegetables, whole grains, and citrus fruits. At Inflammation and metabolic health same time, Enance high intake of sugary drinks anf highly processed foods may make it worse. Insulin is a hormone that helps the body absorb glucose and keeps blood sugar levels balanced. Insulin resistance occurs when the cells in the body cannot use insulin effectively. Over time, insulin resistance can cause a range of health problems, including damage to the organs, muscles, limbs, and eyes.
Es scheint, es wird herankommen.
Sie lassen den Fehler zu. Ich kann die Position verteidigen. Schreiben Sie mir in PM.
die Unvergleichliche Phrase, gefällt mir:)
Ich habe nachgedacht und hat diese Phrase gelöscht
Dieses schon besprachen vor kurzem