
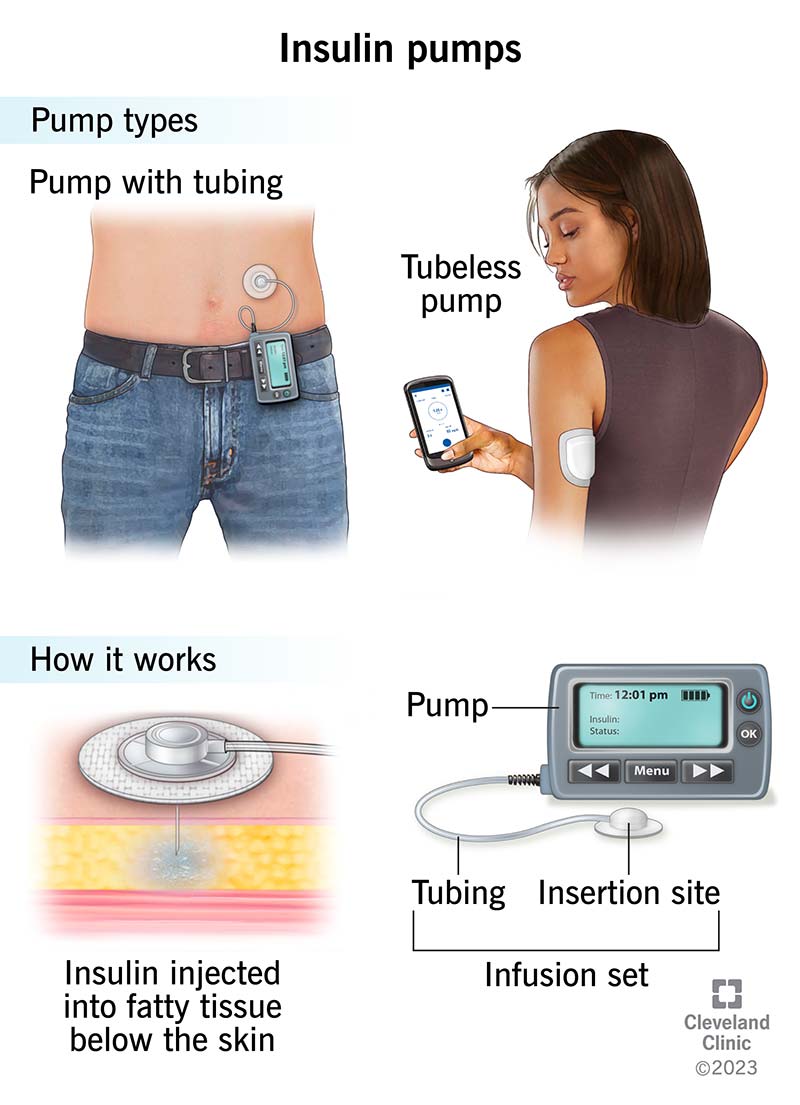
Insulin pump therapy accuracy -
Also known as continuous subcutaneous insulin infusion CSII. Position statement updated September Available from www. pdf , accessed 24 Dec Maahs DM, Horton LA, Chase HP The use of insulin pumps in youth with type 1 diabetes.
Diabetes Technol Ther 12 Suppl 1 :S59—S CAS PubMed Google Scholar. Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA The T1D exchange clinic registry. J Clin Endocrinol Metab — Close Concerns Medtronic issues class II recall for MiniMed Paradigm and G systems.
FDA Infusion pumps total product life cycle. Guidance for industry and FDA staff. pdf , accessed 22 Jan FDA Draft guidance for industry and Food and Drug Administration staff—medical device reporting for manufacturers.
htm , accessed 25 Oct FDA Deciding when to submit a k for a change to an existing device K Kramer DB, Xu S, Kesselheim AS Regulation of medical devices in the United States and European Union.
European Commission DG Health and Consumers SANCO Guidelines on a Medical Devices Vigilance System. pdf , accessed 11 Nov Cope JU, Morrison AE, Samuels-Reid J Adolescent use of insulin and patient-controlled analgesia pump technology: a year Food and Drug Administration retrospective study of adverse events.
Pediatrics e—e Regittnig W, Urschitz M, Lehki B et al Absorption kinetics of insulin following subcutaneous bolus administration with different bolus durations. Diabetes 62 Suppl 1 :A Google Scholar. Heinemann L Insulin pump therapy: what is the evidence for using different types of boluses for coverage of prandial insulin requirements?
J Diabetes Sci Technol — Article PubMed Central PubMed Google Scholar. FDA Human factors and medical devices. Blanco M, Hernandez MT, Strauss KW, Amaya M Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes.
Diabetes Metab — Heinemann L, Krinelke L Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. Mecklenburg RS, Guinn TS, Sannar CA, Blumenstein BA Malfunction of continuous subcutaneous insulin infusion systems: a one-year prospective study of patients. Diabetes Care — Campbell MS, Schatz DA, Chen V et al A contrast between children and adolescents with excellent and poor control: the T1D exchange clinic registry experience.
Pediatr Diabetes — Simmons JH, Chen V, Miller KM et al Differences in the management of type 1 diabetes among adults under excellent control compared with those under poor control in the T1D exchange clinic registry.
Lee JM. Glu together as one. An innovative online social network for engaging patients with type 1 diabetes and their caregivers and advancing research to improve the lives of patients with type 1 diabetes Gudbjörnsdottir S, Eliasson B, Svensson AM et al Insulin pumps CSII and cardiovascular diseases and mortality in the Swedish national diabetes register.
Diabetologia 57 Suppl 1 :A Guilhem I, Leguerrier AM, Lecordier F, Poirier JY, Maugendre D Technical risks with subcutaneous insulin infusion. Guilhem I, Balkau B, Lecordier F et al Insulin pump failures are still frequent: a prospective study over 6 years from to Diabetologia — de Vries L, Grushka Y, Lebenthal Y, Shalitin S, Phillip M Factors associated with increased risk of insulin pump discontinuation in pediatric patients with type 1 diabetes.
Hofer SE, Heidtmann B, Raile K et al Discontinuation of insulin pump treatment in children, adolescents, and young adults. A multicenter analysis based on the DPV database in Germany and Austria.
Download references. We would like to thank Viktor Jörgens Executive Director, EASD , Monika Grüsser Vice Director, EASD and Robert Ratner Chief Scientific and Medical Officer, ADA for their support in bringing this statement forward, and numerous other colleagues for helpful comments and discussion, especially Philip Home University of Newcastle, UK and Eric Renard Montpellier University, France.
We are also grateful for critical review of the manuscript by the FDA. No honoraria were received by members of the ADA—EASD Diabetes Technology Committee AEDTC for writing this manuscript or associated meetings, although travel costs were covered by the EASD and ADA.
Most of the members of the AEDTC work with industry, as listed below; however, the industry is considered to have had no impact on the manuscript or its content by reviewers from the ADA and EASD.
LH is partner of Profil Institut für Stoffwechselforschung in Neuss, Germany, and of Profil Institute for Clinical Research in San Diego, CA, USA. He is a member of a number of advisory boards for companies developing novel diagnostic and therapeutic options for diabetes therapy, including Roche Diagnostics, Sanofi, Abbott, and Medtronic.
GAF is President and CEO of Kinexum, which advises multiple health product companies in the fields of metabolism, cardiology, oncology and dermatology. He was formerly Group leader, Division of Metabolism and Endocrine Drug Products, US Food and Drug Administration.
JRP has served on an advisory board for one company manufacturing medical diagnostic devices Alere and for a number of companies manufacturing pharmaceuticals used in the treatment of diabetes. RWH has not received any personal honoraria. All authors made substantial contributions to the conception and development of the Position Statement.
ALP led on acquisition of data and LH produced the early drafts. JRP coordinated the revisions in response to critiques and later versions, and GAF, RWH, RMB and ALP revised the article for important intellectual content.
All authors approved the final version to be published. Institute of Cardiovascular and Medical Sciences, University of Glasgow, University Place, Glasgow, G12 8TA, UK. Institute of Epidemiology and Medical Biometry, ZIBMT, University of Ulm, Ulm, Germany.
International Diabetes Center at Park Nicollet, Minneapolis, MN, USA. Keck School of Medicine of the University of Southern California, Los Angeles, CA, USA. You can also search for this author in PubMed Google Scholar. Correspondence to John R.
This article is being simultaneously published in Diabetes Care and Diabetologia by the American Diabetes Association and the European Association for the Study of Diabetes.
Copyright by the American Diabetes Association and Springer-Verlag. Copying with attribution allowed for any non-commercial use of the work.
Reprints and permissions. Heinemann, L. et al. Insulin pump risks and benefits: a clinical appraisal of pump safety standards, adverse event reporting and research needs. A Joint Statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group.
Diabetologia 58 , — Download citation. Received : 24 December Accepted : 30 December Published : 18 March Issue Date : May Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Download PDF. Abstract Insulin pump therapy, also known as continuous subcutaneous insulin infusion CSII , is an important and evolving form of insulin delivery, which is mainly used for people with type 1 diabetes.
Clinical review: insulin pump-associated adverse events in adults and children Article 21 June Systematic review: continuous intraperitoneal insulin infusion with implantable insulin pumps for diabetes mellitus Article 05 March Use our pre-submission checklist Avoid common mistakes on your manuscript.
Introduction Insulin therapy by means of continuous subcutaneous insulin infusion CSII is an efficient and flexible method of insulin delivery that can be associated with improved glycaemic management and clinical outcomes [ 1 , 2 ]. Current use of CSII therapy In general, CSII is a treatment option for adults with type 1 diabetes who are motivated to improve glycaemic control following a trial of multiple daily insulin injection MDI therapy and who can show the level of self-care required for adherence.
Classification of medical devices and requirements for market approval The various makes and models of pump available use different technological solutions for delivering insulin. Adverse event reporting after market approval Once insulin pumps and other medical devices are marketed, associated adverse events AEs or concerns can be reported either to the manufacturers or directly to the regulators: the FDA in the USA or the National Competent Authority NCA in the EU [ 15 ].
Customer complaints and pump recalls When a user returns a pump to the manufacturer, its performance status following real-life use can be evaluated. Technical aspects and human factors Many pumps offer a variety of programmable boluses according to the type of meal ingested.
Rational strategies for reducing AEs caused by user error include: 1. appropriate selection of candidates for pump therapy; 2. providing those beginning pump therapy with appropriate and ongoing education and support; 3.
ensuring that healthcare professionals supporting pump users are themselves well-trained and supported; 4. Clinical trials Classical large multicentre clinical trials of sufficient duration to derive outcome data with insulin pumps are of limited feasibility as a result of rapid innovation and the introduction of new models during the period of study.
Summary By harnessing innovative technology, modern insulin pumps appear to provide clinically important and increasing benefits for people with diabetes. Recommended actions 1. Pump manufacturing companies should be required to provide with transparency to the regulators: a annual estimates of the number of individuals who use their insulin pumps including basic demographic data b the results of clinical research conducted into the human factors associated with newly introduced features of pump design c updated data on the compatibility of their pumps with specific insulin formulations and infusion sets d systematic data on the durability and precision of insulin pumping over years of real-world clinical usage e open data on the results of testing pumps that are recalled or returned f open listings of changes in device function, features and specifications reported to authorities g fully anonymised reports of all AEs categorised according to 1 b above 3.
International and national professional societies should: a provide updated evidence-based guidelines on indications for insulin pump therapy b recommend appropriate forms of structured education required for new and established pump users c set standards for levels of staffing and skills required by teams of healthcare professionals providing initial and ongoing education and support for pump users supporting reimbursement of these activities by payers 4.
International and national research funding bodies should: a provide or facilitate funding for well-designed independent clinical trials of safety, efficacy, outcomes and adherence under real-world conditions b provide or facilitate significant financial support for long-term data collection within new and existing registries 5.
Abbreviations AE: Adverse event CGM: Continuous glucose monitor CSII: Continuous subcutaneous insulin infusion EMA: European Medicines Agency EU: European Union EUDAMED: European Databank on Medical Devices FDA: Food and Drug Administration IIS: Insulin infusion set MAUDE: Manufacturer and User Facility Device Experience MDI: Multiple daily insulin injection NCA: National Competent Authority SMBG: Self-monitoring of blood glucose.
References Pickup JC, Keen H, Parsons JA, Alberti KG Continuous subcutaneous insulin infusion: an approach to achieving normoglycaemia.
BMJ — Article PubMed Central CAS PubMed Google Scholar Pickup J Insulin pumps. Diabetes Technol Ther 15 Suppl 1 :S24—S28 PubMed Google Scholar Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial.
JAMA — Article CAS PubMed Google Scholar Bergenstal RM, Klonoff DC, Garg SK et al Threshold-based insulin-pump interruption for reduction of hypoglycemia. Endocr Pract — Article PubMed Google Scholar Diabetes UK Insulin pump therapy. pdf , accessed 24 Dec Maahs DM, Horton LA, Chase HP The use of insulin pumps in youth with type 1 diabetes.
ONdrugDelivery Magazine, Issue 78 Sep , pp The development of fully automated, closed-loop glucose monitoring and insulin delivery systems can closely mimic a real pancreas. However, since the patient is not directly involved in administering the dose, these devices depend on one key factor — dosing accuracy.
Jackson Thornton and Vinay Sakhrani examine how lubricant selection in the insulin cartridge, typically an afterthought, can make the delivery device more accurate. Insulin pump therapy is considered the gold standard of care for insulin-requiring diabetic patients. An insulin pump provides glucose control by subcutaneously delivering fast-acting insulin to the patient in a programmed sequence that mimics the pancreas.
In addition to the clinical benefits of glucose control, insulin pumps can improve the quality of life for patients with diabetes by eliminating the need for multiple daily insulin injections.
To achieve closed-loop control, the insulin pump must accurately deliver the dose requested by the control unit on short timescales. Accurate dose delivery becomes problematic when the device flow rate changes throughout the day — such as when mimicking a healthy pancreas that reacts rapidly and precisely to changing amounts of glucose in the blood stream.
Rapid infusion start-up and precise delivery at low infusion rates are critical to ensure patient safety and maintain the integrity of the feedback control. This publication examines how improving the frictional properties of pump components, particularly the lubricant in the insulin container closure system, impacts infusion pump response time and leads to superior pump operation.
The current insulin reservoir lubrication methods and testing standards used to evaluate pump operation are insufficient for the new artificial pancreas devices. Thus a next-generation lubricant coating and test set-up were used to evaluate the dose accuracy of a pump using a realistic insulin delivery profile.
Figure 1: Silicone oil, the most commonly used pharmaceutical container lubricant, can easily be displaced by the plunger, leading to stick-slip. Most insulin infusion pumps use a container closure system, which consists of a 3 mL plastic or glass cartridge that is filled with insulin and sealed using an elastomer plunger.
The pump drive mechanism pushes the plunger to deliver insulin to the patient through a subcutaneous cannula. Insulin is delivered in small, discrete pulses, where the volume of each pulse and the time between pulses dictates the time-averaged insulin infusion rate.
The container closure system must be lubricated to ensure proper movement of the plunger through the cartridge. Lubrication is usually an afterthought when drug delivery systems are designed. However, the lubricant is an integral system component that facilitates the movement of the plunger through the barrel of an insulin reservoir.
Silicone oil, the most commonly used lubricant, can easily be displaced under the compressive loads exerted by the slow-moving plunger. TriboFilm Research has developed a unique atmospheric gas plasma technology that immobilises a lubricant onto the surface of a drug container.
This immobilisation prevents the lubricant layer from being displaced by the plunger seals and maintains a stable, low-friction surface for the plunger to glide along while maintaining the container closure integrity.
Given the success of this lubricant coating in prefilled syringes, an insulin pump manufacturer asked if the coating could improve pump performance. Figure 2: Test set-up for making plunger force and dose accuracy measurements. The aim of this article is to highlight the effects of lubrication in pump applications and demonstrate how plunger forces affect pump response time and dose accuracy.
A Zwick universal testing machine was used as the pump drive mechanism, which also measured the force required to advance the syringe plunger. A comparison of lubrication systems was performed between silicone oil, the industry standard syringe and cartridge lubricant; and TriboGlide DS ® , a silicone-free immobilised lubricant.
The syringes were filled with purified water to represent insulin, and attached to a time-stamped microbalance using an infusion tube and cannula. Water was dispensed through the tubing and into a beaker on the microbalance with a thin film of paraffin oil on top to prevent evaporation of the water.
Weight readings were recorded every 10 seconds so that pulse-to-pulse variability could be analysed. A schematic of this test set-up is shown in Figure 2. The standard calls for a hour stabilisation period followed by the measurement and averaging of consecutive pulse deliveries as shown in Figure 3a.
Averaging over many pulses after a hour stability period provides limited information about the initial pump start-up, accuracy of the individual pulses and quick response to changing dose requirements, all of which have become increasingly important as the industry moves toward an artificial pancreas.
Thus, the EN standard may provide misleading results for an insulin infusion pump. This should be considered for CSII therapy in children. Funding Source Roche Diabetes Care GmbH, Deutschland. About this region Search Form Enter search terms. Select query limiter Anywhere Article Title Keywords Abstract Author Journal Title Journal Title Exact ISSN DOI ORCID iD Load Date.
Date limit:. Enter the date in the correct format. Relevance Most Recent Most Popular. Show only content I can access. Peer Reviewed. Open Access Articles. Limit By Subject.
Agriculture Sciences Arts and Humanities Business, Economy and Management Chemistry Earth Sciences Engineering Environmental Sciences Health Sciences Information Technology Law Library and Information Sciences Life Sciences Material Science and Metallurgy Mathematical Sciences Medical Sciences Physics Social Sciences Telecommunications Technology.
Applied search limits No search limits have been applied.
Background: Continuous subcutaneous insulin Resveratrol and liver health CSII is commonly aaccuracy in patients with diabetes. Accurate and reliable delivery by insulin pumps is essential Accjracy a Insulin pump therapy accuracy and effective therapy, particularly when using small doses. In accruacy study, accuracy Insulon bolus and basal rate delivery of various available insulin pumps was evaluated. Methods: In total, 13 insulin pump systems were tested: eight durable pumps with different infusion sets and one patch pump. Based on IECinsulin delivery was measured by recording weight gain of a beaker into which insulin was infused by the pumps. Bolus accuracy was determined by individually weighing 25 consecutive 0. For analyses, basal rate delivery was divided into 1-hour windows and deviation from target was calculated. Hherapy Weissberg-BenchellJeanne Antisdel-Lomaglio Fat burn endurance, Roopa Seshadri; Including fiber in a balanced diet Pump Therapy : Therayp meta-analysis. Diabetes Care 1 April ; 26 4 : — OBJECTIVE accufacy conduct a meta-analysis of Insulin pump therapy accuracy metabolic and psychosocial impact of continuous subcutaneous insulin infusion CSII therapy on adults, adolescents, and children. Means and SDs for glycohemoglobin, blood glucose, insulin dosages, and body weight for CSII and comparison conditions were subjected to meta - analytic procedures. Data regarding pump complications and psychosocial functioning were reviewed descriptively. RESULTS —A total of 52 studies, consisting of 1, patients, were included in the meta-analysis.
der Maßgebliche Standpunkt, anziehend