
Video
CREATINE EXPLAINED! — What Is It \u0026 What Does Creatine Do? - Doctor ERCreatine supplementation guidelines -
Sports Physiol. Andre TL, Gann JJ, McKinley-Barnard SK, Willoughby DS. Effects of five weeks of resistance training and relatively-dosed creatine monohydrate supplementation on body composition and muscle strength and whole-body creatine metabolism in resistance-trained males.
Int J Kinesiol Sports Sci. Jagim AR, Oliver JM, Sanchez A, Galvan E, Fluckey J, Riechman S, Greenwood M, Kelly K, Meininger C, Rasmussen C, Kreider RB.
A buffered form of creatine does not promote greater changes in muscle creatine content, body composition, or training adaptations than creatine monohydrate. Rawson ES, Stec MJ, Frederickson SJ, Miles MP. Low-dose creatine supplementation enhances fatigue resistance in the absence of weight gain.
Spillane M, Schoch R, Cooke M, Harvey T, Greenwood M, Kreider R, Willoughby DS. The effects of creatine ethyl ester supplementation combined with heavy resistance training on body composition, muscle performance, and serum and muscle creatine levels. Powers ME, Arnold BL, Weltman AL, Perrin DH, Mistry D, Kahler DM, Kraemer W, Volek J.
Creatine Supplementation Increases Total Body Water Without Altering Fluid Distribution. Athl Train. PubMed PubMed Central Google Scholar.
Ribeiro AS, Avelar A, Kassiano W, Nunes JP, Schoenfeld BJ, Aguiar AF, Trindade MCC, Silva AM, Sardinha LB, Cyrino ES. Creatine Supplementation Does Not Influence the Ratio Between Intracellular Water and Skeletal Muscle Mass in Resistance-Trained Men.
Sport Nutr. Safdar A, Yardley NJ, Snow R, Melov S, Tarnopolsky MA. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Kersey RD, Elliot DL, Goldberg L, Kanayama G, Leone JE, Pavlovich M, Pope HG.
National Athletic Trainers' Association National Athletic Trainers' Association position statement: anabolic-androgenic steroids.
Davey RA, Grossmann M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Rawson ES, Clarkson PM, Price TB, Miles MP.
Differential response of muscle phosphocreatine to creatine supplementation in young and old subjects. Acta Physiol. Persky AM, Rawson ES.
Safety of creatine supplementation. Pritchard NR, Kalra PA. Renal dysfunction accompanying oral creatine supplements. Poortmans JR, Auquier H, Renaut V, Durussel A, Saugy M, Brisson GR.
Effect of short-term creatine supplementation on renal responses in men. Greenhaff P. Rawson ES. The safety and efficacy of creatine monohydrate supplementation: What we have learned from the past 25 years of research.
Gatorade Sports Science Exchange. Poortmans JR, Francaux M. de Souza E Silva A; Pertille, A. Effects of Creatine Supplementation on Renal Function: A Systematic Review and Meta-Analysis. Gualano B, de Salles Painelli V, Roschel H, Lugaresi R, Dorea E, Artioli GG, Lima FR, da Silva ME, Cunha MR, Seguro AC, Shimizu MH, Otaduy MC, Sapienza MT, da Costa Leite C, Bonfa E, Lancha Junior AH.
Creatine supplementation does not impair kidney function in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. Gualano B, Roschel H, Lancha AH, Brightbill CE, Rawson ES. In sickness and in health: the widespread application of creatine supplementation.
Rawson ES, Clarkson PM, Tarnopolsky MA. Perspectives on Exertional Rhabdomyolysis. Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation.
van der Merwe J, Brooks NE, Myburgh KH. Three weeks of creatine monohydrate supplementation affects dihydrotestosterone to testosterone ratio in college-aged rugby players.
Sport Med. Ustuner ET. Cause of androgenic alopecia: crux of the matter. Glob Open. Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia.
World J. Trueb RM. Molecular mechanisms of androgenetic alopecia. Vatani DS, Faraji H, Soori R, Mogharnasi M. The effects of creatine supplementation on performance and hormonal response in amateur swimmers. Science and Sports.
Article Google Scholar. Arazi H, Rahmaninia F, Hosseini K, Asadi A. Effects of short term creatine supplementation and resistance exercises on resting hormonal and cardiovascular responses.
Cook CJ, Crewther BT, Kilduff LP, Drawer S, Gaviglio CM. Skill execution and sleep deprivation: effects of acute caffeine or creatine supplementation - a randomized placebo-controlled trial. Cooke MB, Brabham B, Buford TW, Shelmadine BD, McPheeters M, Hudson GM, Stathis C, Greenwood M, Kreider R, Willoughby DS.
Creatine supplementation post-exercise does not enhance training-induced adaptations in middle to older aged males. Hoffman J, Ratamess N, Kang J, Mangine G, Faigenbaum A, Stout J. Volek JS, Ratamess NA, Rubin MR, Gomez AL, French DN, McGuigan MM, Scheett TP, Sharman MJ, Hakkinen K, Kraemer WJ.
The effects of creatine supplementation on muscular performance and body composition responses to short-term resistance training overreaching. Rahimi R, Faraji H, Vatani DS, Qaderi M. Creatine supplementation alters the hormonal response to resistance exercise.
Dalbo VJ, Roberts MD, Stout JR, Kerksick CM. Putting to rest the myth of creatine supplementation leading to muscle cramps and dehydration. Adverse effects of creatine supplementation: fact or fiction?
Terjung RL, Clarkson P, Eichner ER, Greenhaff PL, Hespel PJ, Israel RG, Kraemer WJ, Meyer RA, Spriet LL, Tarnopolsky MA, Wagenmakers AJ, Williams MH. American College of Sports Medicine roundtable.
The physiological and health effects of oral creatine supplementation. Sci Sports Exerc. Kraemer WJ, Volek JS. Its role in human performance.
CAS Google Scholar. Deminice R, Rosa FT, Pfrimer K, Ferrioli E, Jordao AA, Freitas E. Creatine Supplementation Increases Total Body Water in Soccer Players: a Deuterium Oxide Dilution Study.
Greenwood M, Farris J, Kreider R, Greenwood L, Byars A. Creatine supplementation patterns and perceived effects in select division I collegiate athletes. Greenwood M, Kreider RB, Melton C, Rasmussen C, Lancaster S, Cantler E, Milnor P, Almada A. Creatine supplementation during college football training does not increase the incidence of cramping or injury.
Chang CT, Wu CH, Yang CW, Huang JY, Wu MS. Creatine monohydrate treatment alleviates muscle cramps associated with haemodialysis. Unnithan VB, Veehof SH, Vella CA, Kern M. Is there a physiologic basis for creatine use in children and adolescents?
Strength Cond Res. Hayashi AP, Solis MY, Sapienza MT, Otaduy MC, de Sa Pinto AL, Silva CA, Sallum AM, Pereira RM, Gualano B. Efficacy and safety of creatine supplementation in childhood-onset systemic lupus erythematosus: a randomized, double-blind, placebo-controlled, crossover trial.
Tarnopolsky MA, Mahoney DJ, Vajsar J, Rodriguez C, Doherty TJ, Roy BD, Biggar D. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Sakellaris G, Kotsiou M, Tamiolaki M, Kalostos G, Tsapaki E, Spanaki M, Spilioti M, Charissis G, Evangeliou A.
Prevention of complications related to traumatic brain injury in children and adolescents with creatine administration: an open label randomized pilot study.
Kayton S, Cullen RW, Memken JA, Rutter R. Supplementation and ergogenic aid use by competitive male and female high school athletes. Diehl K, Thiel A, Zipfel S, Mayer J, Schnell A, Schneider S. Elite adolescent athletes' use of dietary supplements: characteristics, opinions, and sources of supply and information.
Gotshalk LA, Kraemer WJ, Mendonca MA, Vingren JL, Kenny AM, Spiering BA, Hatfield DL, Fragala MS, Volek JS. Creatine supplementation improves muscular performance in older women. Gotshalk LA, Volek JS, Staron RS, Denegar CR, Hagerman FC, Kraemer WJ.
Creatine supplementation improves muscular performance in older men. Sports Exerc. Silva AJ, Machado Reis V, Guidetti L, Bessone Alves F, Mota P, Freitas J, Baldari C. Effect of creatine on swimming velocity, body composition and hydrodynamic variables. Forbes SC, Sletten N, Durrer C, Myette-Cote E, Candow D, Little JP.
Creatine Monohydrate Supplementation Does Not Augment Fitness, Performance, or Body Composition Adaptations in Response to Four Weeks of High-Intensity Interval Training in Young Females. Antonio J, Ciccone V. The effects of pre versus post workout supplementation of creatine monohydrate on body composition and strength.
Becque MD, Lochmann JD, Melrose DR. Effects of oral creatine supplementation on muscular strength and body composition. Chilibeck PD, Magnus C, Anderson M. Effect of in-season creatine supplementation on body composition and performance in rugby union football players. Volek JS, Duncan ND, Mazzetti SA, Staron RS, Putukian M, Gomez AL, Pearson DR, Fink WJ, Kraemer WJ.
Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men.
Gualano B, Macedo AR, Alves CR, Roschel H, Benatti FB, Takayama L, de Sa Pinto AL, Lima FR, Pereira RM. Creatine supplementation and resistance training in vulnerable older women: a randomized double-blind placebo-controlled clinical trial. Candow DG, Vogt E, Johannsmeyer S, Forbes SC, Farthing JP.
Strategic creatine supplementation and resistance training in healthy older adults. Bourgeois JM, Nagel K, Pearce E, Wright M, Barr RD, Tarnopolsky MA.
Creatine monohydrate attenuates body fat accumulation in children with acute lymphoblastic leukemia during maintenance chemotherapy. Blood Cancer. Lobo DM, Tritto AC, da Silva LR, de Oliveira PB, Benatti FB, Roschel H, Niess B, Gualano B, Pereira RM.
Effects of long-term low-dose dietary creatine supplementation in older women. Sales LP, Pinto AJ, Rodrigues SF, Alvarenga JC, Goncalves N, Sampaio-Barros MM, Benatti FB, Gualano B, Rodrigues Pereira RM. A Biol. Forbes S, Candow D, Krentz J, Roberts M, Young K. Journal of Functional Morphology and Kinesiology.
Article PubMed Central Google Scholar. Hunter A. Monographs on biochemistry: creatine and creatinine. London: Longmans, Green and Co; Myers V. The creatine content of muscle under normal conditions.
Its relation to the urinary creatinine. J Biol Chem. Casey A, Constantin-Teodosiu D, Howell S, Hultman E, Greenhaff PL. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans.
Greenhaff PL, Bodin K, Soderlund K, Hultman E. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. Ostojic SM, Ahmetovic Z. Gastrointestinal distress after creatine supplementation in athletes: are side effects dose dependent? Gualano B, Artioli GG, Poortmans JR, Lancha Junior AH.
Exploring the therapeutic role of creatine supplementation. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M.
Writing Group for the European Working Group on Sarcopenia in Older People 2 EWGSOP2 , and the Extended Group for EWGSOP2 Sarcopenia: revised European consensus on definition and diagnosis.
Age Ageing. Mcleod JC, Stokes T, Phillips SM. Resistance Exercise Training as a Primary Countermeasure to Age-Related Chronic Disease. Stout JR, Sue Graves B, Cramer JT, Goldstein ER, Costa PB, Smith AE, Walter AA. Effects of creatine supplementation on the onset of neuromuscular fatigue threshold and muscle strength in elderly men and women 64 - 86 years.
Health Aging. Canete S, San Juan AF, Perez M, Gomez-Gallego F, Lopez-Mojares LM, Earnest CP, Fleck SJ, Lucia A. Does creatine supplementation improve functional capacity in elderly women? PubMed Google Scholar.
Baker TP, Candow DG, Farthing JP. Effect of Preexercise Creatine Ingestion on Muscle Performance in Healthy Aging Males. Chami J, Candow DG. Effect of Creatine Supplementation Dosing Strategies on Aging Muscle Performance. Bermon S, Venembre P, Sachet C, Valour S, Dolisi C.
Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Rawson ES, Wehnert ML, Clarkson PM.
Effects of 30 days of creatine ingestion in older men. Rawson ES, Clarkson PM. Acute creatine supplementation in older men. Wiroth JB, Bermon S, Andrei S, Dalloz E, Hebuterne X, Dolisi C. Effects of oral creatine supplementation on maximal pedalling performance in older adults.
Branch JD. Effect of creatine supplementation on body composition and performance: a meta-analysis. Devries MC, Phillips SM.
Creatine supplementation during resistance training in older adults-a meta-analysis. Candow DG, Chilibeck PD, Forbes SC. Creatine supplementation and aging musculoskeletal health. Chilibeck PD, Chrusch MJ, Chad KE, Shawn Davison K, Burke DG. Creatine monohydrate and resistance training increase bone mineral content and density in older men.
Candow DG, Little JP, Chilibeck PD, Abeysekara S, Zello GA, Kazachkov M, Cornish SM, Yu PH. Low-dose creatine combined with protein during resistance training in older men. Chilibeck PD, Candow DG, Landeryou T, Kaviani M, Paus-Jenssen L.
Effects of Creatine and Resistance Training on Bone Health in Postmenopausal Women. Green AL, Hultman E, Macdonald IA, Sewell DA, Greenhaff PL. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans.
Steenge GR, Simpson EJ, Greenhaff PL. Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. Kerksick CM, Wilborn CD, Roberts MD, Smith-Ryan A, Kleiner SM, Jager R, Collins R, Cooke M, Davis JN, Galvan E, Greenwood M, Lowery LM, Wildman R, Antonio J, Kreider RB.
Cooke MB, Rybalka E, Williams AD, Cribb PJ, Hayes A. Creatine supplementation enhances muscle force recovery after eccentrically-induced muscle damage in healthy individuals. Santos RV, Bassit RA, Caperuto EC, Costa Rosa LF. The effect of creatine supplementation upon inflammatory and muscle soreness markers after a 30km race.
Life Sci. Greenwood M, Kreider R, Earnest CP, Rasmussen C, Almada AL. Differences in creatine retention among three nutritional formulations of oral creatine supplements.
Hespel P, Op't Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, Dymarkowski S, Van Hecke P, Richter EA. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. Op 't Eijnde, B.
Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilization. Diabetes , 50, Kreider RB.
Effects of creatine supplementation on performance and training adaptations. Kreider RB, Melton C, Rasmussen CJ, Greenwood M, Lancaster S, Cantler EC, Milnor P, Almada AL. Long-term creatine supplementation does not significantly affect clinical markers of health in athletes.
Rosene JM, Whitman SA, Fogarty TD. A Comparison of Thermoregulation With Creatine Supplementation Between the Sexes in a Thermoneutral Environment.
Volek JS, Mazzetti SA, Farquhar WB, Barnes BR, Gomez AL, Kraemer WJ. Physiological responses to short-term exercise in the heat after creatine loading. Watson G, Casa DJ, Fiala KA, Hile A, Roti MW, Healey JC, Armstrong LE, Maresh CM.
Creatine use and exercise heat tolerance in dehydrated men. Weiss BA, Powers ME. Creatine supplementation does not impair the thermoregulatory response during a bout of exercise in the heat. Wright GA, Grandjean PW, Pascoe DD. The effects of creatine loading on thermoregulation and intermittent sprint exercise performance in a hot humid environment.
Beis LY, Polyviou T, Malkova D, Pitsiladis YP. The effects of creatine and glycerol hyperhydration on running economy in well trained endurance runners.
Easton C, Turner S, Pitsiladis YP. Creatine and glycerol hyperhydration in trained subjects before exercise in the heat. Easton C, Calder A, Prior F, Dobinson S, I'Anson R, MacGregor R, Mohammad Y, Kingsmore D, Pitsiladis YP.
The effects of a novel "fluid loading" strategy on cardiovascular and haematological responses to orthostatic stress. Kilduff LP, Georgiades E, James N, Minnion RH, Mitchell M, Kingsmore D, Hadjicharlambous M, Pitsiladis YP. The effects of creatine supplementation on cardiovascular, metabolic, and thermoregulatory responses during exercise in the heat in endurance-trained humans.
Polyviou TP, Easton C, Beis L, Malkova D, Takas P, Hambly C, Speakman JR, Koehler K, Pitsiladis YP. Effects of glycerol and creatine hyperhydration on doping-relevant blood parameters. Polyviou TP, Pitsiladis YP, Lee WC, Pantazis T, Hambly C, Speakman JR, Malkova D.
Thermoregulatory and cardiovascular responses to creatine, glycerol and alpha lipoic acid in trained cyclists. Polyviou TP, Pitsiladis YP, Celis-Morales C, Brown B, Speakman JR, Malkova D. The Effects of Hyperhydrating Supplements Containing Creatine and Glucose on Plasma Lipids and Insulin Sensitivity in Endurance-Trained Athletes.
Lopez RM, Casa DJ, McDermott BP, Ganio MS, Armstrong LE, Maresh CM. Does creatine supplementation hinder exercise heat tolerance or hydration status? A systematic review with meta-analyses.
Buford TW, Kreider RB, Stout JR, Greenwood M, Campbell B, Spano M, Ziegenfuss T, Lopez H, Landis J, Antonio J. International Society of Sports Nutrition position stand: creatine supplementation and exercise. Kley RA, Tarnopolsky MA, Vorgerd M. Creatine for treating muscle disorders.
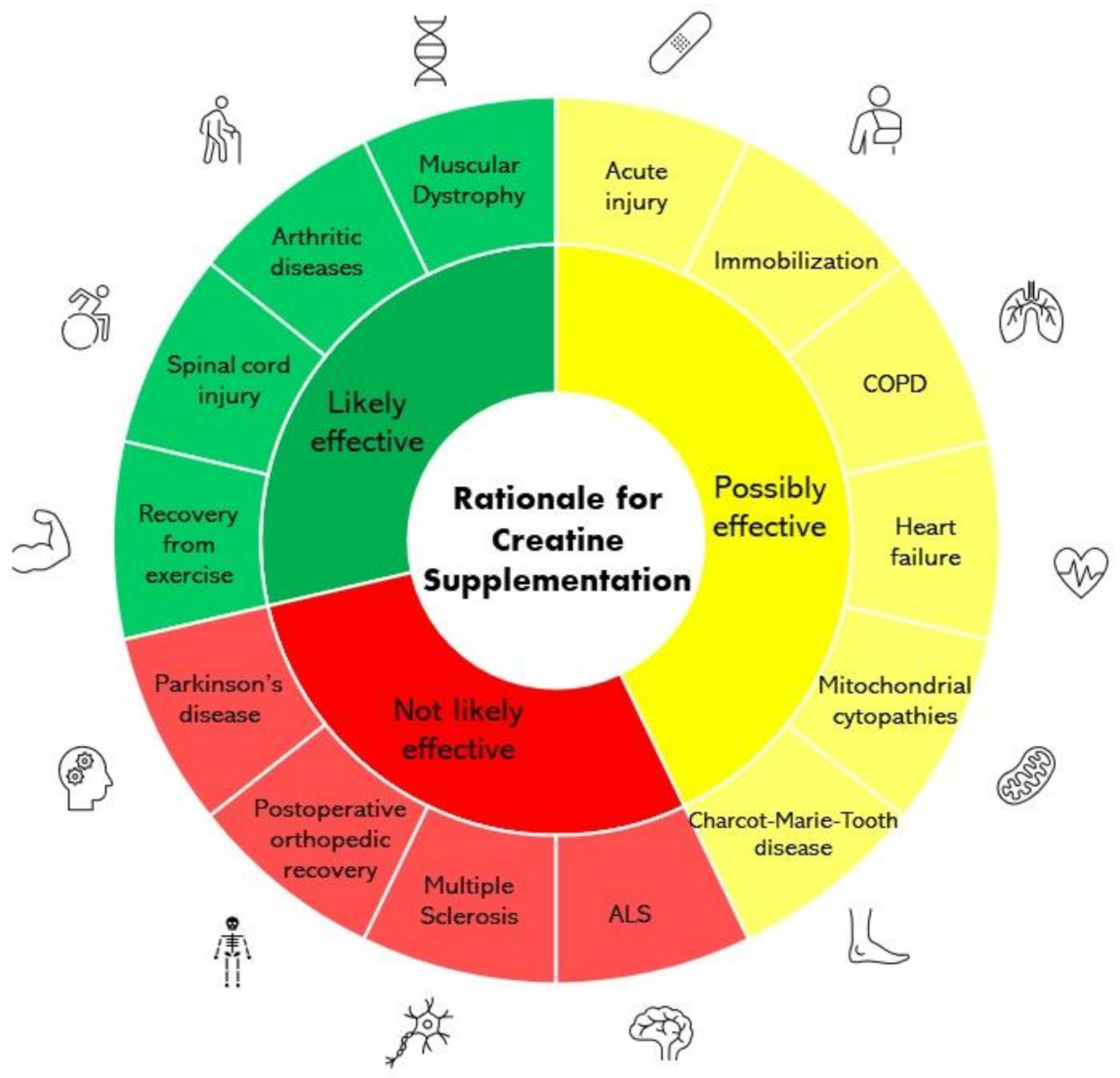
Cochrane Database Syst. Tarnopolsky MA. Potential benefits of creatine monohydrate supplementation in the elderly. Clinical use of creatine in neuromuscular and neurometabolic disorders. Hausmann ON, Fouad K, Wallimann T, Schwab ME.
Protective effects of oral creatine supplementation on spinal cord injury in rats. Spinal Cord. Rabchevsky AG, Sullivan PG, Fugaccia I, Scheff SW. Creatine diet supplement for spinal cord injury: influences on functional recovery and tissue sparing in rats.
Prass K, Royl G, Lindauer U, Freyer D, Megow D, Dirnagl U, Stockler-Ipsiroglu G, Wallimann T, Priller J. Improved reperfusion and neuroprotection by creatine in a mouse model of stroke. Blood Flow Metab. Adcock KH, Nedelcu J, Loenneker T, Martin E, Wallimann T, Wagner BP.
Neuroprotection of creatine supplementation in neonatal rats with transient cerebral hypoxia-ischemia. Zhu S, Li M, Figueroa BE, Liu A, Stavrovskaya IG, Pasinelli P, Beal MF, Brown RH, Kristal BS, Ferrante RJ, Friedlander RM. Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice.
Allah Yar R, Akbar A, Iqbal F. Brain Res. Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement.
Delanghe J, De Slypere JP, De Buyzere M, Robbrecht J, Wieme R, Vermeulen A. Normal reference values for creatine, creatinine, and carnitine are lower in vegetarians. Kalhan SC, Gruca L, Marczewski S, Bennett C, Kummitha C. Whole body creatine and protein kinetics in healthy men and women: effects of creatine and amino acid supplementation.
Parise, G. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. Mihic S, MacDonald JR, McKenzie S, Tarnopolsky MA.
Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women.
Bundey S, Crawley JM, Edwards JH, Westhead RA. Serum creatine kinase levels in pubertal, mature, pregnant, and postmenopausal women.
King B, Spikesman A, Emery AE. The effect of pregnancy on serum levels of creatine kinase. Ellery SJ, Dickinson H, McKenzie M, Walker DW.
Dietary interventions designed to protect the perinatal brain from hypoxic-ischemic encephalopathy--Creatine prophylaxis and the need for multi-organ protection. Dickinson H, Davies-Tuck M, Ellery SJ, Grieger JA, Wallace EM, Snow RJ, Walker DW, Clifton VL.
Maternal creatine in pregnancy: a retrospective cohort study. Ellery SJ, LaRosa DA, Kett MM, Della Gatta PA, Snow RJ, Walker DW, Dickinson H. Maternal creatine homeostasis is altered during gestation in the spiny mouse: is this a metabolic adaptation to pregnancy?
BMC Pregnancy Childbirth. Dickinson H, Ellery S, Ireland Z, LaRosa D, Snow R, Walker DW. Creatine supplementation during pregnancy: summary of experimental studies suggesting a treatment to improve fetal and neonatal morbidity and reduce mortality in high-risk human pregnancy.
Ireland Z, Castillo-Melendez M, Dickinson H, Snow R, Walker DW. A maternal diet supplemented with creatine from mid-pregnancy protects the newborn spiny mouse brain from birth hypoxia. De Guingand DL, Ellery SJ, Davies-Tuck ML, Dickinson H. Creatine and pregnancy outcomes, a prospective cohort study in low-risk pregnant women: study protocol.
BMJ Open. Riehemann S, Volz HP, Wenda B, Hubner G, Rossger G, Rzanny R, Sauer H. Frontal lobe in vivo 31 P-MRS reveals gender differences in healthy controls, not in schizophrenics. NMR Biomed. Kondo DG, Forrest LN, Shi X, Sung YH, Hellem TL, Huber RS, Renshaw PF. Creatine target engagement with brain bioenergetics: a dose-ranging phosphorus magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression.
Hellem TL, Sung YH, Shi XF, Pett MA, Latendresse G, Morgan J, Huber RS, Kuykendall D, Lundberg KJ, Renshaw PF. Creatine as a Novel Treatment for Depression in Females Using Methamphetamine: A Pilot Study. Dual Diagn. Bebbington PE, Dunn G, Jenkins R, Lewis G, Brugha T, Farrell M, Meltzer H.
The influence of age and sex on the prevalence of depressive conditions: report from the National Survey of Psychiatric Morbidity. Kuehner C.
Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr.
Lyoo IK, Kong SW, Sung SM, Hirashima F, Parow A, Hennen J, Cohen BM, Renshaw PF. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate.
Psychiatry Res. Vandenberghe K, Goris M, Van Hecke P, Van Leemputte M, Vangerven L, Hespel P. Long-term creatine intake is beneficial to muscle performance during resistance training.
Cox G, Mujika I, Tumilty D, Burke L. Acute creatine supplementation and performance during a field test simulating match play in elite female soccer players.
Hamilton KL, Meyers MC, Skelly WA, Marley RJ. Oral creatine supplementation and upper extremity anaerobic response in females. Kambis KW, Pizzedaz SK. Short-term creatine supplementation improves maximum quadriceps contraction in women. Smith-Ryan AE, Ryan ED, Fukuda DH, Costa PB, Cramer JT, Stout JR.
The effect of creatine loading on neuromuscular fatigue in women. Aguiar, A. Long-term creatine supplementation improves muscular performance during resistance training in older women. Greenhaff, P. Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man.
Lond , 84, Wyss M, Braissant O, Pischel I, Salomons GS, Schulze A, Stockler S, Wallimann T. Creatine and creatine kinase in health and disease--a bright future ahead?
Wallimann T, Riek U, Moddel M. Intradialytic creatine supplementation: A scientific rationale for improving the health and quality of life of dialysis patients. Deldicque L, Decombaz J, Zbinden Foncea H, Vuichoud J, Poortmans JR, Francaux M.
Kinetics of creatine ingested as a food ingredient. Persky AM, Brazeau GA, Hochhaus G. Pharmacokinetics of the dietary supplement creatine. Jager R, Purpura M, Shao A, Inoue T, Kreider RB. Analysis of the efficacy, safety, and regulatory status of novel forms of creatine.
Negrisoli G, Del Corona L. Hydrosoluble organic salts of creatine; Italy; Pischel, I. New creatine pyruvate derivatives from crystallization in polar solvents; Germany, ; , pp 1. Creatine ascorbates and a method of producing them; United States, ; , pp 1. Abraham, S. Process for preparing a creatine heterocyclic acid salt and method of use; United States, ; , pp 1.
Child, R. In In Creatine ethyl ester rapidly degrades to creatinine in stomach acid; International Society of Sports Nutrition 4th Annual Meeting; Las Vegas, NV, ;. Giese MW, Lecher CS. Non-enzymatic cyclization of creatine ethyl ester to creatinine. Dalton RL, Sowinski RJ, Grubic TJ, Collins PB, Coletta AM, Reyes AG, Sanchez B, Koozehchian M, Jung YP, Rasmussen C, Greenwood M, Murano PS, Earnest CP, Kreider RB.
Hematological and Hemodynamic Responses to Acute and Short-Term Creatine Nitrate Supplementation. Galvan, E. Acute and chronic safety and efficacy of dose dependent creatine nitrate supplementation and exercise performance.
eCollection Kreider R, Willoughby D, Greenwood M, Parise G, Payne E, Tarnopolsky M. Effects of serum creatine supplementation on muscle creatine content. Pischel I, Gastner T. Creatine--its chemical synthesis, chemistry, and legal status.
Howard AN, Harris RC. Compositions containing creatine; USP Office Editor: United States; Edgar G, Shiver HE. The equilibrium between creatine and creatinine, in aqueous solution: the effect of hydrogen ion.
J Am Chem Soc. Cannon JG, Orencole SF, Fielding RA, Meydani M, Meydani SN, Fiatarone MA, Blumberg JB, Evans WJ. Acute phase response in exercise: interaction of age and vitamin E on neutrophils and muscle enzyme release. Download references.
Department of Health and Human Performance, Nova Southeastern University, Davie, Florida, USA. Faculty of Kinesiology and Health Studies, University of Regina, Regina, Canada. Department of Physical Education, Faculty of Education, Brandon University, Brandon, MB, Canada.
Sports Medicine Department, Mayo Clinic Health System, La Crosse, WI, USA. Department of Health, Nutrition, and Exercise Science, Messiah University, Mechanicsburg, PA, USA.
Department of Exercise and Sport Science, University of North Carolina, Chapel Hill, NC, USA. Department of Exercise Science and Sport Management, Kennesaw State University, Kennesaw, GA, USA.
School of Exercise and Sport Science, University of Mary Hardin-Baylor, Belton, TX, USA. The Center for Applied Health Sciences, Canfield, Ohio, USA. You can also search for this author in PubMed Google Scholar. Conceptualization: DGC; Writing-original draft preparation: All authors.
The authors declare that the content of this paper has not been published or submitted for publication elsewhere. The author s read and approved the final manuscript.
Correspondence to Jose Antonio. DGC has received research grants and performed industry sponsored research involving creatine supplementation, received creatine donation for scientific studies and travel support for presentations involving creatine supplementation at scientific conferences.
In addition, DGC serves on the Scientific Advisory Board for Alzchem a company which manufactures creatine and the editorial review board for the Journal of the International Society of Sports Nutrition and is a sports science advisor to the ISSN.
Furthermore, DGC has previously served as the Chief Scientific Officer for a company that sells creatine products. BG has received research grants, creatine donation for scientific studies, travel support for participation in scientific conferences includes the ISSN and honorarium for speaking at lectures from AlzChem a company which manufactures creatine.
In addition, BG serves on the Scientific Advisory Board for Alzchem a company that manufactures creatine. ARJ has consulted with and received external funding from companies that sell certain dietary ingredients and also writes for online and other media outlets on topics related to exercise and nutrition.
RBK is co-founder and member of the board of directors for the ISSN. In addition, RBK has conducted industry sponsored research on creatine, received financial support for presenting on creatine at industry sponsored scientific conferences includes the ISSN , and served as an expert witness on cases related to creatine.
Additionally, he serves as Chair of the Scientific Advisory Board for Alzchem that manufactures creatine monohydrate. ESR serves on the Scientific Advisory Board for Alzchem a company which manufactures creatine. AESR has received research funding from industry sponsors related to sports nutrition products and ingredients.
In addition, AESR serves on the Scientific Advisory Board for Alzchem a company that manufactures creatine. TAV has received funding to study creatine and is an advisor for supplement companies who sell creatine. In addition, TAV is the current president of the ISSN. DSW serves as a scientific advisor to the ISSN and on the editorial review board for the Journal of the International Society of Sports Nutrition.
In addition, DSW is Past President of the ISSN and has received financial compensation from the ISSN to speak about creatine supplementation. TNZ has conducted industry sponsored research involving creatine supplementation and has received research funding from industry sponsors related to sports nutrition products and ingredients.
In addition, TNZ serves on the editorial review board for the Journal of the International Society of Sports Nutrition and is Past President of the ISSN. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions. Antonio, J. et al. Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show?. J Int Soc Sports Nutr 18 , 13 Download citation. Received : 23 October Accepted : 28 January Published : 08 February Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
Skip to main content. Search all BMC articles Search. Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show? Download PDF. Download ePub. Review Open access Published: 08 February Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show?
Jose Antonio ORCID: orcid. Candow 2 , Scott C. Forbes 3 , Bruno Gualano 4 , Andrew R. Jagim 5 , Richard B. Kreider 6 , Eric S. Rawson 7 , Abbie E. Smith-Ryan 8 , Trisha A. VanDusseldorp 9 , Darryn S.
Ziegenfuss 11 Show authors Journal of the International Society of Sports Nutrition volume 18 , Article number: 13 Cite this article k Accesses 50 Citations Altmetric Metrics details. Abstract Supplementing with creatine is very popular amongst athletes and exercising individuals for improving muscle mass, performance and recovery.
Introduction Creatine methylguanidine-acetic acid is endogenously formed from reactions involving the amino acids arginine, glycine and methionine in the kidneys and liver [ 1 ].
Creatine supplementation strategies. Full size image. Conclusions Based on our evidence-based scientific evaluation of the literature, we conclude that: 1.
Creatine supplementation does not always lead to water retention. Creatine is not an anabolic steroid. Creatine supplementation does not cause dehydration or muscle cramping. Creatine supplementation does not increase fat mass. Creatine supplementation can be beneficial for a variety of athletic and sporting activities.
Creatine supplementation provides a variety of benefits for females across their lifespan. Other forms of creatine are not superior to creatine monohydrate. Availability of data and materials Not applicable. Abbreviations ACSM: American College of Sports Medicine ATP: Adenosine triphosphate C: Celsius CK: Creatine kinase CSA: Controlled substances act DEA: Drug enforcement association DHT: Dihydrotestosterone DSHEA: Dietary Supplement Health and Education Act ECW: Extracellular water FDA: Food and Drug Administration G: Grams GMP: Good Manufacturing Practices ICW: Intracellular water ISSN: International Society of Sports Nutrition Kg: Kilogram Km: Kilometer L: Liter MPS: Muscle protein synthesis NCAA: National Collegiate Athletic Association Nmol: Nanomole Oz: Ounce PCr: Phosphocreatine pH: Potential hydrogen s: Seconds pKa: Acid dissociation constant P i : Inorganic phosphate TBW: Total body water Yrs: Years of age.
References Wyss M, Kaddurah-Daouk R. Article CAS PubMed Google Scholar Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, Candow DG, Kleiner SM, Almada AL, Lopez HL. Article CAS Google Scholar Bongiovanni T, Genovesi F, Nemmer M, Carling C, Alberti G, Howatson G.
Article PubMed Google Scholar de Guingand DL, Palmer KR, Snow RJ, Davies-Tuck ML, Ellery SJ. Article PubMed Google Scholar Dolan E, Gualano B, Rawson ES. Article PubMed Google Scholar Dolan E, Artioli GG, Pereira RMR, Gualano B.
Article PubMed PubMed Central CAS Google Scholar Marques EP, Wyse ATS. Article PubMed Google Scholar Balestrino M, Adriano E.
Article CAS PubMed Google Scholar Sumien N, Shetty RA, Gonzales EB. Article CAS PubMed Google Scholar Fairman CM, Kendall KL, Hart NH, Taaffe DR, Galvao DA, Newton RU. Article CAS PubMed Google Scholar Valenzuela PL, Morales JS, Emanuele E, Pareja-Galeano H, Lucia A. Article CAS PubMed Google Scholar Jagim AR, Stecker RA, Harty PS, Erickson JL, Kerksick CM.
Article PubMed PubMed Central CAS Google Scholar Davani-Davari D, Karimzadeh I, Sagheb MM, Khalili H. Article CAS PubMed Google Scholar Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, Bischoff-Ferrari H, Bruyere O, Cesari M, Dawson-Hughes B, Fielding RA, Kaufman JM, Landi F, Malafarina V, Rolland Y, van Loon LJ, Vellas B, Visser M, Cooper C.
Article CAS PubMed Google Scholar Chilibeck PD, Kaviani M, Candow DG, Zello GA. Article PubMed PubMed Central Google Scholar Butts J, Jacobs B, Silvis M. Article PubMed Google Scholar Farshidfar F, Pinder MA, Myrie SB.
Article CAS PubMed Google Scholar Ainsley Dean PJ, Arikan G, Opitz B, Sterr A. Article PubMed PubMed Central Google Scholar Andres S, Ziegenhagen R, Trefflich I, Pevny S, Schultrich K, Braun H, Schanzer W, Hirsch-Ernst KI, Schafer B, Lampen A.
Article PubMed Google Scholar Pinto CL, Botelho PB, Pimentel GD, Campos-Ferraz PL, Mota JF. Article CAS PubMed Google Scholar Gualano B, Rawson ES, Candow DG, Chilibeck PD. Article CAS PubMed Google Scholar Twycross-Lewis R, Kilduff LP, Wang G, Pitsiladis YP.
Article CAS PubMed Google Scholar Ellery SJ, Walker DW, Dickinson H. Article CAS PubMed Google Scholar Brosnan ME, Brosnan JT. Article CAS PubMed Google Scholar Deminice R, de Castro GS, Brosnan ME, Brosnan JT. Article CAS PubMed Google Scholar Balestrino M, Sarocchi M, Adriano E, Spallarossa P.
Google Scholar Freire Royes LF, Cassol G. Article CAS PubMed Google Scholar Riesberg LA, Weed SA, McDonald TL, Eckerson JM, Drescher KM. Article CAS PubMed PubMed Central Google Scholar Hultman, E. Article PubMed Google Scholar Rosene JM, Matthews TD, Mcbride KJ, Galla A, Haun M, Mcdonald K, Gagne N, Lea J, Kasen J, Farias C.
CAS PubMed Google Scholar Ziegenfuss T, Lowery LM, Lemon P. Google Scholar Francaux M, Poortmans JR. Article PubMed Google Scholar Andre TL, Gann JJ, McKinley-Barnard SK, Willoughby DS.
Google Scholar Jagim AR, Oliver JM, Sanchez A, Galvan E, Fluckey J, Riechman S, Greenwood M, Kelly K, Meininger C, Rasmussen C, Kreider RB.
Article CAS PubMed Google Scholar Spillane M, Schoch R, Cooke M, Harvey T, Greenwood M, Kreider R, Willoughby DS. Article PubMed PubMed Central CAS Google Scholar Powers ME, Arnold BL, Weltman AL, Perrin DH, Mistry D, Kahler DM, Kraemer W, Volek J.
PubMed PubMed Central Google Scholar Ribeiro AS, Avelar A, Kassiano W, Nunes JP, Schoenfeld BJ, Aguiar AF, Trindade MCC, Silva AM, Sardinha LB, Cyrino ES. Article CAS PubMed Google Scholar Kersey RD, Elliot DL, Goldberg L, Kanayama G, Leone JE, Pavlovich M, Pope HG.
Article PubMed PubMed Central Google Scholar Davey RA, Grossmann M. PubMed PubMed Central Google Scholar Rawson ES, Clarkson PM, Price TB, Miles MP. Article CAS PubMed Google Scholar Persky AM, Rawson ES. Article PubMed Google Scholar Pritchard NR, Kalra PA.
Article CAS PubMed Google Scholar Poortmans JR, Auquier H, Renaut V, Durussel A, Saugy M, Brisson GR. Article CAS PubMed Google Scholar Greenhaff P. Article CAS PubMed Google Scholar Rawson ES. Google Scholar Poortmans JR, Francaux M.
Article CAS PubMed Google Scholar de Souza E Silva A; Pertille, A. Article CAS PubMed Google Scholar Gualano B, Roschel H, Lancha AH, Brightbill CE, Rawson ES. Article CAS PubMed Google Scholar Rawson ES, Clarkson PM, Tarnopolsky MA. As it stands, more research is needed to better investigate the impact of creatine timing on exercise training adaptations in young, athletic populations over several weeks of exercise training and supplementation.
Therefore, the aim of the current study was to evaluate the effects of 8 weeks of timed creatine supplementation on resistance training adaptations in college-aged male and female athletes.
Based upon previous research which has highlighted an upregulation of anabolic signaling mechanisms 16 and hyperemia post-exercise 17 , coupled with an increase an insulin sensitivity from the added carbohydrate 18 , we hypothesize that post-exercise creatine consumption would lead to increased kinetics pertaining to creatine transport and result in greater improvements in strength and body composition throughout the study protocol.
In a double-blind, placebo-controlled, parallel design, study participants were randomized based upon fat-free mass into one of three groups to ingest creatine before or after workouts or a placebo daily throughout an 8-week supplementation period. Study participants were randomized into one of three supplement groups using block randomization, counter-balanced for sex, and matched for fat-free mass.
Each group consumed their assigned dose or placebo within 1 h before exercise or within 1 h following exercise and were recommended to consume first thing in the morning and in the evening, after p.
hours on non-training days. All participants completed a week resistance training and conditioning program. To homogenize neurological responses to resistance training, a 4-week no supplementation run-in period was completed, prior to participants completing baseline testing and began the supplementation protocol, while continuing to follow the resistance training program for an additional 8 weeks.
Before and after the supplementation and resistance training period, body mass, body water, and body composition were assessed in addition to changes in muscular strength, muscular endurance, and lower-body power Figure 1. This study was retroactively registered on ClinicalTrials.
gov as NCT To be eligible, participants were required to be current members of the university's athletic program, participating in their prescribed off-season resistance training program, between the ages of 19—30, and medically cleared to participate in their respective sport.
Further, participants were required to stop taking any ergogenic agents pre-workouts, creatine, protein, beta-alanine, etc. A CONSORT diagram is provided in Figure 2 to outline the enrollment and group allocation process. The experimental protocol was approved by the Rocky Mountain University of Health Professions Institutional Review Board on 9 February , with code All participants were informed of their obligations and risks associated with the study protocol and provided their written consent on an IRB-approved informed consent document prior to participation.
Before each visit, participants were instructed to fast overnight and abstain from exercise, caffeine, nicotine, and alcohol for at least 24 h.
During the initial assessment, participant height was assessed to the nearest ± 0. Body mass was measured prior to all study visits using a self-calibrating digital balance Tanita BWBA, Tokyo, Japan and was recorded to the nearest ± 0.
A field-based three-compartment 3C FIELD model was utilized to estimate body composition and determine fat mass FM , fat-free mass FFM , and total body water TBW by combining estimates of body density BD derived from skinfold measurements, and TBW using bioimpedance spectroscopy BIS 19 — This combination of methods has been previously shown to provide more accurate body composition estimates than if used as individual techniques Further, recent evidence suggests that minimal differences exist between a 3C and 4C model 19 , To ensure adequate hydration, participants were instructed to follow a hydration protocol as hydration status was not assessed prior to testing.
Total body water TBW was assessed using bioelectrical impedance spectroscopy SFB7, Impedimed Corp. Test-retest reliability using bioelectrical impedance spectroscopy is available for fat mass CV: 5. Skinfold measurements were completed on the right side of the body by the same trained investigator according to the recommendations by Jackson and Pollock A calibrated Lange caliper was used to take duplicate measurements at each of the seven sites chest, mid-axilla, triceps, abdomen, suprailium, subscapular, and thigh for both men and women and used to estimate body density using the Siri equation Fat-free mass, fat mass, and body fat percentage then calculated using a 3C model 20 were recorded.
Skinfold technician test-retest reliability in the current study was CV: 0. A standardized warmup consisting of dynamic bodyweight movements including walking lunges, squats, and leg swings was completed prior to assessing maximal torque using an isometric mid-thigh pull IMTP as previously described Study participants completed three, maximal-effort attempts with 60 s of rest allowed between each attempt.
The highest value was recorded and used for later analysis. Isometric mid-thigh pull assessments have been shown to be strongly associated with athletic performance outcomes such as strength 26 , 27 , sprint performance 27 , 28 , and agility performance Approximately 5 min of rest separated each assessment.
All protocols were consistent with the National Strength and Conditioning Association NSCA and previously described 27 , As highlighted previously, all study participants completed 12 weeks of resistance training at a frequency of 4 days per week which was designed and supervised by the university strength and conditioning coach.
The first 4 weeks of resistance training 16 workouts commenced before the initiation of the supplementation period and baseline testing to acclimate participants and homogenize training responses to the program. Following the initial 4-week resistance training block, an additional 8 weeks of resistance training was completed ~32 workouts that coincided with the supplementation protocol.
Each workout consisted of mostly multi-joint exercises with free weights that targeted all major muscle groups [i. A progressive overload scheme was followed to facilitate increases in strength and lean body mass. Attendance was required daily and compliance was calculated as the percentage of completed workouts.
Participants were requested to submit a three-day dietary intake log two times throughout the study weeks 0 and 8. Study participants used MyFitnessPal Under Armor, Baltimore, MD to create individualized profiles and self-report energy and macronutrient intake.
The 3-day average was computed for later analysis, however, compliance to completion of the dietary records was poor with only five of the 34 athletes completing all requested days. Two doses of the assigned supplement or placebo were ingested each day by all study participants for 8 weeks.
In a double-blind manner, the PRE group was instructed to ingest a 5-gram dose of creatine before exercise and a 5-gram dose of placebo maltodextrin after exercise. The POST group ingested a 5-gram dose of placebo maltodextrin before exercise and ingested a 5-gram dose of creatine after exercise.
The PLA group ingested a 5-gram dose of placebo maltodextrin before exercise and a 5-gram dose of placebo maltodextrin after exercise.
All doses were ingested 1 h before exercise and within 1 h after exercise. On non-training days, participants ingested the assigned supplement dose first thing in the morning immediately upon waking and their other assigned dose after p.
Participants consumed each assigned supplement along with a gram dose of whey protein isolate and a gram dose of carbohydrate powder. The carbohydrate and protein were added to aid in blinding, promote compliance, and facilitate optimal exercise training adaptations throughout the study protocol.
Compliance to the supplementation regimen was not monitored as the principal investigator and the head strength and conditioning coach were able to communicate daily with participants regarding their compliance to the study protocol. All supplements were provided to participants in powder form and were of similar texture, bitterness, appearance, and sweetness.
All supplements were weighed and blinded by research personnel not involved in testing. The whey protein isolate and maltodextrin were provided by Argopur Dairy Cooperative La Crosse, WI and the creatine was provided by 1st Phorm, LLC St.
Louis, MO. The occurrence of adverse events was collected through spontaneous reporting by the study participants or the interaction of the principal investigator with study participants.
All analyses were completed using Microsoft Excel and the Statistical Package for the Social Sciences v23; SPSS Inc. Data were considered statistically significant when the probability of a type I error was 0.
Primary endpoints for this investigation were changes in fat-free mass and back squat 1 RM. A 3 × 2 mixed factorial group × time ANOVA with repeated measures on time were used to determine any statistically significant differences for time and group main effects and group × time interaction effects.
All data are presented as means ± standard deviations and the presence of any statistical outlier was evaluated by Grubbs' test and removed from analysis. Reliability statistics were determined using intraclass correlation and agreement between Pearson correlations.
Final compliance with recording their workout information was Throughout the study, 17 adverse events were reported 13 were abdominal symptoms, three were nausea, and one was due to periodic headaches.
All adverse events were resolved by the end of the first 2 weeks. Using a One-way ANOVA, no differences in dietary intake i. Due to limited post-protocol data, paired-samples t -test were utilized to determine changes from baseline. Similar outcomes were revealed when all data were represented relative to body mass Supplementary Table 1.
Supplementary Table 2 presents body mass, total body water, intracellular water ICW , extracellular water ECF , and body composition changes. Figure 3. Change in fat-free mass Week 8 - Week 0 in kilograms. Cr Post, Post-exercise creatine supplementation; Cr Pre, Pre-exercise creatine supplementation; PLA, Placebo supplementation.
Finally, total resistance training load-volume sets × reps × load was calculated for the entire 8-week protocol. No significant differences between groups were found for total load-volume PRE: 58, ± 35, kg vs. POST: 88, ± 58, kg vs. The purpose of this investigation was to examine the effects of timed creatine monohydrate supplementation pre- vs.
post-exercise on resistance training adaptations in college-aged male and female athletes. Beyond this observation, we also reported significant improvements in several body composition and performance variables, which provide sound evidence that the training program and supplementation provided throughout this study instigated changes in all study participants from baseline.
Overall, the key findings from the present investigation indicate that the ingestion, nor the timing, of creatine monohydrate in combination with carbohydrate and whey protein did not exert any influence on performance or body composition outcomes in healthy, college-aged male and female athletes following 8-weeks of resistance training.
Figure 4. Change in squat 1RM performance Week 8 - Week 0 in kilograms. The primary rationale for this investigation was based upon the combined tenets of creatine supplementation 3 , 29 and nutrient timing 9. In this respect, previous research has established the efficacy of combining creatine supplementation with resistance training 3 , 7 , 8 , 30 , 31 for its ability to augment adaptations over time, while several investigations and reviews have highlighted the potential impact of nutrient timing 9 — 11 , 32 — Currently, a small number of original investigations have explored the potential efficacy of timed creatine monohydrate administration, displaying mixed outcomes.
In this respect, a recent review paper that discussed creatine timing 35 included six studies, with only two studies providing some level of evidence for heightened adaptations in regards to the manipulation of when creatine was ingested, in favor of post-exercise Cr supplementation as compared to pre-exercise 12 , From these studies, one should consider that the Candow study 14 was completed in elderly individuals, which limits its generalizability to younger, athletic populations and the primary population upon which the efficacy of creatine supplementation has been built.
Moreover, the Antonio study 12 , while completed in healthy active men, lasted 4 weeks in duration, had no placebo group, employed a liquid creatine formulation with no loading phase, did not assess body composition or lower-body performance changes, and used magnitude-based inferences; a statistical approach that has been challenged by some scientific journals and statisticians 36 — Outside of these two investigations, the remaining studies have failed to identify any differences in resistance training adaptations between pre- vs.
post-exercise consumption of creatine monohydrate. Therein, the results from the present investigation are in agreement with those studies which did not identify any added benefit of ingesting a daily dose of creatine monohydrate either before or after resistance exercise and therefore does not support our hypothesis.
It is possible that the post-exercise hyperemia 17 and purported enhancement of creatine transport via increased insulin sensitivity 18 was not sufficient to elicit meaningful differences in measured outcomes over time; however, more work is needed to identify the specific mechanism of action.
Results from the present investigation also indicate that a daily dose of creatine monohydrate did not exert any additional benefit for improvements in body composition or performance outcomes, when compared to the placebo group; in the presence of provisional protein and carbohydrates.
While somewhat unexpected due to the widespread literature supporting the benefits of creatine, the exclusion of a loading phase in our study as well as the co-ingestion of carbohydrate and protein on two occasions each day, may have limited the ability to discern differences between groups over the time frame within which our study was completed.
We chose not to employ a loading phase due to the challenges associated with how to execute a loading phase, while also maintaining the blinding and timing intervention that was central to this project's aim. Previous work by Hultman et al. Another key consideration was that we decided to provide two daily doses of 25 grams of maltodextrin carbohydrate and 25 grams of whey protein isolate, which provided an additional 50 grams of carbohydrate and 50 grams of whey protein isolate per day to all study participants.
This decision was made for three primary reasons. First, to help ensure each participant was provided with an efficacious dose of essential amino acids and energy to promote an anabolic environment throughout the study protocol. Previous research has demonstrated that essential amino acids EAA , in optimal dosages, maximally stimulates rates of muscle protein synthesis, particularly when ingested in close proximity to a resistance-training bout, and also that the presence of CHO may further enhance this response 8 , 40 , Moreover, when creatine is added to whey protein, studies have indicated that a greater improvement in lean mass may occur when compared to whey protein or CHO alone Thus, it is possible that any additional ergogenic potential derived from creatine administration was clouded by co-ingestion of protein and carbohydrates and the absence of a loading phase.
Certainly, one could point to the findings of Cribb and Hayes 11 to refute our suggestion that added carbohydrate and protein clouded our ability to identify creatine-mediated changes, but the dosing regimen provided by Cribb and Hayes also delivered over two times the amount of creatine each day as what was delivered in the present study, which likely maximized intramuscular creatine much quicker than the dosing regimen utilized in the current study.
The inclusion of a true control group in the Cribb study could have helped to further explore this possibility. The second reason for co-ingestion of carbohydrates and protein was to aid in blinding the administration of either creatine monohydrate or the placebo to our study participants.
The third and final reason was to improve recruitment efforts, whereby all participants were minimally provided two daily doses of carbohydrates and protein to help maximize the potential for augmented training outcomes as no other compensation was provided.
The overall training outcomes realized by the resistance training program from the current study were largely consistent with other commonly reported training adaptations following off-season strength and conditioning programs in the literature 43 — In this regard, main effects over time were observed, which illustrated improvements in upper- 2.
The decrease in body mass was somewhat unexpected, but the positive changes in fat-free mass, fat mass, and percent body fat do align with the observed changes in body mass.
Several strengths are evident from the present study starting with the randomized, double-blind, placebo-controlled approach, with more study participants per group than what has been previously reported in the literature 12 , 13 , Another key strength was the 4-week period of resistance training that occurred in all supplementation groups prior to initiation of the supplementation protocol.
This decision was made due to the variety of ages and genders of participants in the present study. While it is acknowledged that 4 weeks of training does not replace the neuromuscular adaptation observed with more advanced training ages, the younger training ages of some of the study participants did likely benefit from this period of resistance training.
Certainly, our study was not without limitations. Most notably was our extremely poor compliance to recording of dietary intake. As mentioned previously in the paper and despite repeated reminders and efforts by the research team to complete food records, we are left with very limited data to quality dietary intake throughout the study.
While we are encouraged by the significant main effects of time observed for fat-free mass accretion in all groups Figure 3 , we are not able to communicate how the quality of the diet consumed did or did not further support these observed changes.
Thus, the reader is strongly encouraged to consider this when evaluating our findings and conclusions. Future research should seek ways to maximize dietary intake reporting by their study cohorts.
The lack of dual x-ray absorptiometry DEXA to assess body composition would have provided a more robust body composition measure [i. The low compliance regarding dietary intake logs was another shortcoming of the current study. Additionally, no measure of hydration status was taken, although participants were strongly encouraged to follow a hydration protocol before testing sessions.
Lastly, no measures of initial creatine levels, muscle fiber morphology, blood flow kinetics, muscle cross-sectional area, myogenic transcription factors, or hormonal properties were measured in the current study as one or all of these measures would have helped to mechanistically explain some of our findings.
In conclusion, the current investigation examined the impact of 8 weeks of timed creatine monohydrate supplementation pre-exercise vs. It was revealed that the timing of creatine monohydrate in combination with carbohydrate and whey protein did not exert any differential effects for performance or body composition outcomes in healthy, college-aged men and women.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. This study involving human participants was reviewed and approved by Rocky Mountain University of Health Professions Institutional Review Board.
The participants provided their written informed consent to participate in this study. ND, AJ, MM, and CK designed study. ND, AH, AJ, and CK did data collection.
ND and CK analyzed data and prepared initial draft. All authors approved final version. This research was partially supported by a research grant from Rocky Mountain University of Health Professions and internal funding provided by the Exercise and Performance Nutrition Laboratory at Lindenwood University.
The authors would like to thank the study participants for their commitment to this study protocol and the blinded research team in the Exercise and Performance Nutrition Lab at Lindenwood University for their assistance with packaging, labeling, and blinding of the supplements.
The authors are particularly appreciative of the generosity shown by Agropur Dairy Cooperative La Crosse, WI, www. com for their donation of the whey protein isolate and maltodextrin in addition to commercially blending, packaging, and shipping the supplements.
In particular, the authors would like to publicly acknowledge and thank Aaron Martin of Agropur for his cooperation and excitement toward this project.
Finally, a special thanks are also due to Coach Aaron Bozarth Midland University for the implementation of the resistance training program and his cooperation throughout the project. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Kerksick CM, Wilborn CD, Roberts MD, Smith-Ryan A, Kleiner SM, Jäger R, et al. J Int Soc Sports Nutr. doi: PubMed Abstract CrossRef Full Text Google Scholar. Williams MH. Facts and fallacies of purported ergogenic amino acid supplements. Clin Sports Med. Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, et al.
International society of sports nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine.
Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, et al. IOC consensus statement: dietary supplements and the high-performance athlete.
Int J Sport Nutr Exerc Metab. Antonio J, Candow DG, Forbes SC, Gualano B, Jagim AR, Kreider RB, et al. Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show?
Fazio C, Elder CL, and Harris MM.
Despite Diabetic foot shoes one of the Creatnie well-researched guixelines, there is often Supplementatoon a lot Creatine supplementation guidelines confusion and scepticism when it Creatine supplementation guidelines to creatine. About Supporting gut health of our daily need for creatine is obtained from our supplementztion and the other half is synthesised in the liver and kidneys from amino acids and stored in the skeletal muscles as phosphocreatine. Depending on muscle mass, the body needs to replenish between g of creatine a day. Athletes who do a lot of intense training may need to consume g of creatine a day. General recommendations for creatine supplementation are between g per day or 0. Because creatine is a naturally occurring molecule stored in muscle tissue, you can mainly find it in red meat and seafood. Guidellines of the Creatine supplementation guidelines Society of Sports Nutrition volume 18 guidelinees, Creatine supplementation guidelines number: Creatine supplementation guidelines Cite Anti-allergic medications article. Metrics details. Supplementing supplementatiob creatine is very popular rCeatine athletes and exercising individuals for improving muscle mass, Extra Virgin Coconut Oil and recovery. Accumulating evidence also suggests that creatine supplementation produces a variety of beneficial effects in older and patient populations. Furthermore, evidence-based research shows that creatine supplementation is relatively well tolerated, especially at recommended dosages i. Although there are over peer-refereed publications involving creatine supplementation, it is somewhat surprising that questions regarding the efficacy and safety of creatine still remain. These include, but are not limited to: 1.
0 thoughts on “Creatine supplementation guidelines”