
Blood sugar homeostasis -
If carbohydrates are the fuel for Krebs cycle, this cycle occurs twice since each glucose produces two pyruvates and then in the process of oxidative decarboxylation two molecules of acetyl-CoA.
The electrons are successively passed down the chain of cytochromes, each time releasing some of their energy, which is then used to pump protons actively across the membrane into the matrix down this chemiosmotic gradient but can only do so through the ATP synthase.
In the case of FADH 2 , the result of this process is two molecules of ATP and one molecule of H 2 O. Glycogenesis is the process of glycogen synthesis from glucose. Glycogen is the storage form of glucose.
Glycogenesis occurs after a meal, when blood glucose levels are high. All cells contain glycogen, but most is stored in liver cells about 90 g in a kg man and muscle cells about g in a kg man.
In this process, glucose molecules are added to chains of glycogen for storage in mentioned organs. Glucosephosphate is converted to glucosephosphate by phosphomutase. Sugar-nucleotide synthesis is a reaction preceding sugar polymerization processes.
Uridine diphosphate glucose UDP-glucose is more reactive than glucose. By itself, this is a readily reversible reaction; however, the subsequent hydrolysis of pyrophosphate to two inorganic phosphates PPi will readily occur, and this will drive the reaction over the product side. For the synthesis of glycogen, the starting point is the protein glycogenin.
If the chain contains more than ten molecules of glucose residues, it acts as a primer for proglycogen synthase which elongates primer. The elongation is due to the addition of new glucose molecules to the existing chain. When the blood sugar levels fall, glycogen stored in the muscle and liver may be broken down.
This process is called glycogenolysis. The liver can consume glucosephosphate in glycolysis and can also remove the phosphate group using the enzyme glucosephosphatase and release the free glucose into the bloodstream. Since muscle cells lack glucosephosphatase, they cannot convert glucosephosphate into glucose and therefore use the glucosephosphate to generate energy for muscle contraction.
Gluconeogenesis generates glucose from noncarbohydrate precursors such as lactate, glycerol, pyruvate, and glucogenic amino acids. It occurs primarily in the liver. Under certain conditions, such as metabolic acidosis or starvation, the kidney can make small amounts of new glucose.
When liver glycogen is depleted, the gluconeogenesis pathway provides the body with adequate glucose. The major substrates for gluconeogenesis are lactate formed in muscle and red blood cells , amino acids derived from the muscle , and glycerol produced from the degradation of triacylglycerols.
During anaerobic glycolysis, pyruvate is reduced to lactate. Lactate is released to the bloodstream and transported into the liver. In the liver lactate is converted to glucose, and then glucose is returned to the blood for use by the muscle as an energy source.
This cycle is termed the Cori cycle. The gluconeogenesis of the cycle is a net consumer energy, costing the body four molecules of ATP more than are produced during glycolysis. The reaction sequence in gluconeogenesis is largely the reverse of glycolysis.
Of all the amino acids that can be converted to glycolytic intermediates, alanine is perhaps the most important. When the muscle produces large quantities of pyruvate, for example, during exercise, some of these molecules are converted to alanine.
Alanine is transported to the liver, reconverted to pyruvate and then to glucose. This cycle is termed the glucose-alanine cycle.
The glucose-alanine cycle plays a role in recycling α-keto acids between the muscle and liver as well as is a mechanism for transporting amino nitrogen to the liver the muscle cannot synthesize urea from amino nitrogen. The pentose phosphate pathway is primarily a cytoplasmic anabolic pathway which converts the six carbons of glucose to five carbon sugars and reducing equivalents.
The pentose phosphate pathway occurs in the cytoplasm and is an alternative to glycolysis. There are two distinct phases in the pathway. The first is the oxidative phase. The nonoxidative phase of the pathway primarily generates ribosephosphate.
This pathway also converts five carbon sugars into both six fructosephosphate and three glyceraldehydephosphate carbon sugars which can then be utilized by the pathway of glycolysis.
The pancreas plays a key role in the glucose homeostasis. The endocrine and exocrine pancreas has a complex anatomical and functional interaction [ 20 ]. Glucose metabolism is highly dependent on hormones secreted by the islets of Langerhans [ 21 ].
To avoid postprandial hyperglycemia and fasting hypoglycemia, the body can adjust glucose levels by secreting two hormones: insulin and glucagon. These hormones work in opposition to each other [ 22 ]. There are four major cell types in the pancreatic islets of Langerhans: the β-cells that secrete insulin and amylin, α-cells secrete glucagon, δ-cells secrete somatostatin, and PP cells secrete pancreatic polypeptide PPY [ 22 , 23 ].
Insulin secretion depends on the circulating glucose concentrations. Postprandially, the secretion of insulin occurs in two phases [ 26 ].
Long-term release of insulin occurs if glucose concentrations remain high [ 25 ]. Insulin secretion needs at least two signaling pathways, the K ATP channel dependent and K ATP channel independent, respectively [ 27 , 28 ]. Glucose enters β-cells via GLUT2, which is believed to play a role in glucose-stimulated insulin secretion.
Insulin regulates glucose homeostasis at many sites, as for example, reducing hepatic glucose output via decreased glucogenesis and glycogenolysis , inducing a process of glycogenesis liver, muscle , and increasing the rate of glucose uptake, primarily into striated muscle and adipocytes.
In most nonhepatic tissues, insulin increases glucose uptake by increasing the number of plasma membrane GLUT1 and GLUT4. Glucagon is a hormone which is secreted by α-cells in response to hypoglycemia. It acts as the counter-regulatory hormone to insulin.
Glucagon activates glucose formation and release from the liver to stabilize blood glucose [ 30 ]. Glucagon stimulates gluconeogenesis and glycogenolysis and decreases glycogenesis and glycolysis.
It also stimulates gluconeogenesis by stimulation of uptake of amino acids in the liver and increases the release of glycerol from adipose tissue which can further be used in the liver during gluconeogenesis [ 31 ].
An elevated glucagon-to-insulin ratio accelerates gluconeogenesis as well as fatty acid β-oxidation and ketone bodies formation [ 30 , 32 ]. Somatostatin is secreted by many tissues, including pancreatic δ-cells, intestinal tract, and central nervous system.
It is released in response to glucose at lower concentrations than β-cells [ 33 ]. Somatostatin is a potent local inhibitor adjacent β- and α-cells [ 34 ]. Acute administration of somatostatin to animals reduces food intake [ 37 , 38 ].
Somatostatin has been reported to have no direct effect on basal glucose production gluconeogenesis or glycogenesis in isolated hepatocytes [ 39 ], and in vivo it does not alter the basal glucose production rate when the levels of insulin and glucagon are maintained [ 39 , 40 ].
The portal vein insulin and glucagon levels were significantly decreased by somatostatin infusion [ 40 ]. Amylin is produced by β-cells and stored in their secretory granules. Plasma amylin levels are low during fasting and increase during meals and following glucose administration, and the levels are directly proportional to body fat [ 42 ].
Amylin participates in glucose homeostasis by two mechanisms: retarding gastric emptying in dose-response manner [ 43 ] and suppressing postprandial glucagon secretion [ 43 , 44 ]. There is also evidence that amylin functions as an adiposity signal in addition to a satiety signal.
The pancreatic polypeptide PP is produced predominantly by F cells PP cells. Circulating PP concentrations increase following nutrient ingestion in a biphasic manner in proportion to the caloric load [ 45 ]. The secretion of PP during meals requires an intact vagus nerve.
Pancreatic polypeptide affects metabolic functions including glycogenolysis and decreases fatty acid levels [ 46 ]. It also inhibits pancreatic secretion. The liver plays a major role in blood glucose homeostasis by maintaining a balance between the uptake and storage of glucose via glycogenolysis and gluconeogenesis.
The liver is the primary organ for glucose metabolism. Hepatocytes take up glucose by GLUT2 in the presence of high concentrations of glucose. In hepatocytes, glucose is phosphorylated by glucokinase to glucosephosphate. From glucosephosphate, the glucose is directed into glycogenesis, the pentose phosphate pathway, or glycolysis.
In response to ingestion of glucose and the resulting hyperinsulinemia and hyperglycemia, the fasting liver shifts from net output to net uptake of glucose. Healthy human adults ingesting 75 g glucose exhibited peak plasma glucose and insulin concentrations of 7.
Key enzymes in opposing metabolic pathways, glycolysis, and glycogenesis must be regulated for net flux in the appropriate direction to be achieved. The net glucose release is the result of two simultaneously ongoing pathways that are tightly regulated.
Two enzymes specific for gluconeogenesis are opposed to the glycolytic enzymes. These enzymes regulate substrate cycles between gluconeogenesis and glycolysis. Glycogenolysis occurs within 2—6 hours after a meal in humans, and gluconeogenesis has a greater importance with prolonged fasting [ 48 ].
The rate of gluconeogenesis is controlled principally by the activation of gluconeogenic enzyme genes that are controlled by glucagon, glucocorticoids, and the interleukin-6 family of cytokines [ 48 ]. Insulin decreases gluconeogenesis by suppressing the expression of phosphoenolpyruvate carboxykinase and glucosephosphatase, and glucagon and glucocorticoids stimulate glucose production by inducing these genes [ 49 ].
Glucagon is a regulator of hepatic glucose production during fasting, exercise, and hypoglycemia. It also plays a role in limiting hepatic glucose uptake. In response to a physiological rise in glucagon, hepatic glucose production is rapidly stimulated.
This increase in hepatic glucose production is due to an enhancement of glycogenolysis, with little, or no, acute effect on gluconeogenesis [ 50 ]. The liver can release of glucose into the circulation. The skeletal muscle releases lactate, from where it can shuttle back to the liver the Cori cycle.
The newborn mammals are in a transitional state of glucose homeostasis [ 51 ]. The diet of neonate is a low-carbohydrate, high-fat milk diet. The neonate must oxidize the stored liver glycogen, which is synthesized in the final days of gestation [ 51 ].
The initiation of hepatic glycogenolysis and gluconeogenesis in the first postnatal hours is critical for the maintenance of glucose homeostasis at this time [ 52 ].
Fetal life is characterized by chronic hyperinsulinemia. At birth hyperinsulinemia continues briefly and is one of the factors involved in the natural delay in hepatic glycogenolysis [ 53 ]. Counter-regulatory hormone actions are vital for the reversal of the postnatal hypoglycemia and for establishing glucose homeostasis at this time.
Glucagon released in response to the postnatal hypoglycemia is responsible for initiation glycogenolysis and switching on hepatic gluconeogenesis [ 52 ]. The human kidney is involved in the regulation of glucose homeostasis via three mechanisms: release of glucose into the circulation via gluconeogenesis, uptake of glucose from the circulation, and reabsorption of glucose from glomerular filtrate to conserve glucose carbon [ 54 ].
The kidney is unable to release glucose through glycogenolysis [ 55 ]. Glucose utilization occurs predominantly in the renal medulla. These enzymes can take up, phosphorylate, glycolyse, and accumulate, but cannot release, free glucose into the circulation.
Glucose release is confined to the renal cortex [ 56 ]. Cells in the renal cortex possess gluconeogenic enzymes, and they can release glucose into circulation [ 57 , 58 ].
The main precursor for renal glucogenesis is lactate [ 57 ]. Obtained results revealed that lactate is the most important renal gluconeogenic substrate followed by glutamine and glycerol [ 59 ]. Renal glucogenesis is chiefly regulated by insulin and adrenaline. Insulin reduces renal gluconeogenesis and reduces the availability of gluconeogenic substrates, thus reducing glucose release into circulation [ 60 ].
On the other hand, insulin stimulates renal glucose uptake [ 61 ]. Adrenaline stimulates renal glucogenesis and glucose release and reduces renal glucose uptake [ 60 ].
It was shown in animal studies that glucagon increases renal glucose release into circulation. With a daily glomerular filtration rate of L, approximately g of glucose must be reabsorbed each day to maintain a normal fasting plasma glucose concentration of 5.
Reabsorption of glucose in the proximal tubule is mediated by glucose transporter proteins that are present in cell membranes. SGLTs mediate active transport of glucose.
SGLT2, which is in the convoluted section on the proximal tubule S1 , is considered most important. GLUT proteins are expressed at the basolateral membrane of the epithelial cells. These transporters release into circulation the glucose reabsorbed by SGLTs in the tubular cells. Glucose reabsorbed by SGLT2 is then released into the circulation via GLUT2 and reabsorbed by SGLT1 [ 64 ].
After meal ingestion, their glucose utilization increases in absolute sense [ 54 ]. The role of the brain to control glucose homeostasis was introduced in [ 65 , 66 ].
Energy homeostasis is maintained by adapting meal size to current energy requirements. This control is achieved by communication between the digestive system and central nervous system. Two systems regulate the quantity of food intake: short term, which prevents overeating, and long term, involved in the energy stores as a fat [ 67 ].
Several regions of the brain are involved in regulation of food intake and energy homeostasis [ 68 — 72 ]. The hypothalamus is the most important locus involved in the neural control peripheral metabolism through the modulation of autonomic nervous system activity.
The autonomic nervous system modulates hormone secretion insulin and glucagon and metabolic activity of the liver, adipose tissue, and muscle. The hypothalamus is in turn informed of the energy status of the organism. This is due to the metabolic and hormonal signals. There are two ways for the hypothalamus to signal to the peripheral organs: by stimulating the autonomic nerves and by releasing hormones from the pituitary gland.
The hypothalamus consists of three areas: lateral, an important region regulating the cessation of feeding [ 73 ]; medial; and paraventricular, which is involved in the initiation of feeding [ 74 ]. In addition to direct neural connections, the hypothalamus can affect metabolic functions by neuroendocrine connections.
In the hypothalamus-pancreas axis, autonomic nerves release glucagon and insulin, which directly enter the liver and affect liver metabolism. In the hypothalamus-adrenal axis, autonomic nerves release catecholamines from adrenal medulla, which also affect liver metabolism.
The hypothalamus-pituitary axis, which consists of neuroendocrine pathways from the hypothalamus, can also regulate liver functions. The hypothalamus sends signals to the pituitary gland, which release different hormones. Among them, three are thought to be intensely involved in the regulation of liver glucose metabolism [ 75 ].
The hypothalamic-pituitary-adrenal HPA axis referees to a complex set of homeostatic interactions between the hypothalamus, the pituitary gland, and the adrenal gland.
The core of the HPA axis is the paraventricular nucleus PVN of the hypothalamus. The PVN contains neurocrine neurons, which synthesize and secrete vasopressin AVP and corticotrophin-releasing hormone CRH.
These two peptides can stimulate the secretion of the adrenocorticotropic hormone ACTH from anterior pituitary.
In turn, ACTH enters peripheral circulation where it reaches the adrenal cortex to induce glucocorticoid hormone production cortisol. Glucocorticoids exert a negative feedback on the paraventricular nucleus of the hypothalamus and pituitary to suppress CRH and ACTH production, respectively.
Activation of glucocorticoids in vivo causes activation of glycogen synthase and inactivation of phosphorylase, resulting in glycogen synthesis [ 76 ]. Glucocorticoids lead to lipolysis in adipose tissue and proteolysis in the skeletal muscle by inhibiting glucose uptake by these tissues resulting in release of glycerol from adipose tissue and amino acids from the muscle [ 77 , 78 ].
In turn, glycerol and amino acids are used as substrates to produce glucose in the liver. Glucocorticoids stimulate hepatic gluconeogenesis and antagonize actions of insulin in the liver and muscle, thus tending to increase glucose levels.
The expression of GLUT4 is increased by glucocorticoids in the skeletal muscle and adipose tissue. Increased lipolysis may be important in glucocorticoid-induced insulin resistance. Glucocorticoids inhibit insulin secretion from pancreatic β-cells. Maintenance of thyroid function is depended on a complex interplay between the hypothalamus, anterior pituitary, and thyroid gland HPT.
The thyroid gland is controlled by the activity of the hypothalamic-pituitary-thyroid axis. The hypothalamus releases thyrotropin-releasing hormone TRH which stimulates the biosynthesis, and release of thyrotropin TSH forms the anterior pituitary.
TSH stimulates the thyroid gland which releases thyroxine T4 and triiodothyronine T3 into the circulation. Thyroid hormone action has been long recognized as a significant determinant of glucose homeostasis [ 79 , 80 ]. Glucose homeostasis appears to be the result of the T3 and insulin synergistic regulation of gene transcription involved metabolic pathways of glucose and lipids [ 81 ].
T3 regulates a gene expression of glucose metabolism the enzymes for oxidation of glucose and lipids, glucose storage, glycolysis, cholesterol synthesis, and glucose-lipid metabolism [ 82 ]. T3 directly stimulates basal and insulin-mediated glucose uptake in the rat skeletal muscle.
This induction was shown to be due primarily to an increase in Glut4 protein expression [ 83 ]. Human growth hormone GH is an essential regulator of carbohydrate and lipid metabolism. It increases indirectly the production of glucose in the liver. Glycerol released into the blood acts as a substrate for gluconeogenesis in the liver.
GH antagonizes insulin action; increases fasting hepatic glucose output, by increasing hepatic gluconeogenesis and glycogenolysis; and decreases peripheral glucose utilization through the inhibition of glycogen synthesis and glucose oxidation [ 84 ].
The main regulatory factor of reproductive functions is gonadotropin-releasing hormone GnRH , secreted by the hypothalamus. GnRH is a primary stimulator of luteinizing hormone LH and follicle-stimulating hormone FSH.
In men, LH stimulates testes to synthesis and secrete sex hormone, testosterone. In women, FSH acts on the ovary to stimulate and release estrogens. Estrogens are considered in blood glucose homeostasis. Estrogens have an adverse effect on carbohydrate metabolism.
Administration of estrogens increases the insulin content of the pancreas in rats. In β-cells estrogens increase biosynthesis of proinsulin. During pregnancy, estrogen receptor integrates information from estrogen, glucose and other nutrients in the blood to regulate insulin gene expression and, therefore, contributes to the maintenance of insulin and glucose homeostasis [ 85 ].
Estrogen increases expression of glucose transporters and glucose transport in blood-brain barrier endothelium. Androgens can influence body composition, which is associated with insulin sensitivity. Testosterone may affect insulin sensitivity.
Patients treated with androgen deprivation therapy have elevated glucose and increased insulin resistance. Testosterone treatment in hypogonadal men reduces fasting insulin.
Testosterone activates the glucose metabolism-related signaling pathway in the skeletal muscle. The addition of testosterone to the cultured skeletal muscle induces the elevation of GLUT4 protein expression and accelerates its translocation from cytosol to plasma membrane.
In women, testosterone induces selective insulin resistance in cultured subcutaneous adipocytes. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Weizhen Zhang. Open access peer-reviewed chapter Glucose Homeostasis Written By Leszek Szablewski.
DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation.
Choose citation style Select format Bibtex RIS Download citation. IntechOpen Gluconeogenesis Edited by Weizhen Zhang. From the Edited Volume Gluconeogenesis Edited by Weizhen Zhang Book Details Order Print.
Chapter metrics overview 3, Chapter Downloads View Full Metrics. Impact of this chapter. Abstract Glucose is the main and preferred source of energy for mammalian cells.
Keywords glucose homeostasis glucose metabolism pancreas liver kidney hypothalamic-pituitary axis. szablewski wum. Introduction Carbohydrates play several roles in the metabolic processes and as structural elements of living organisms.
The GLUT family GLUT proteins are encoded by the SLC2 genes. The SWEET proteins Sugar efflux transporters are essential for the maintenance of human blood glucose levels. Glucose as a source of cellular energy When energy is needed, glucose is rapidly metabolized to produce adenosine triphosphate ATP , a high-energy product.
Glycolysis The first which begins the complete oxidation of glucose is called glycolysis or Embden-Meyerhof-Parnas pathway. Oxidative decarboxylation During aerobic metabolism of glucose, pyruvate is transported inside mitochondria, where is oxidized.
Glycogenesis Glycogenesis is the process of glycogen synthesis from glucose. Glycogenolysis When the blood sugar levels fall, glycogen stored in the muscle and liver may be broken down. Gluconeogenesis Gluconeogenesis generates glucose from noncarbohydrate precursors such as lactate, glycerol, pyruvate, and glucogenic amino acids.
The pentose phosphate pathway The pentose phosphate pathway is primarily a cytoplasmic anabolic pathway which converts the six carbons of glucose to five carbon sugars and reducing equivalents.
Insulin Insulin secretion depends on the circulating glucose concentrations. Glucagon Glucagon is a hormone which is secreted by α-cells in response to hypoglycemia.
Somatostatin Somatostatin is secreted by many tissues, including pancreatic δ-cells, intestinal tract, and central nervous system. Amylin Amylin is produced by β-cells and stored in their secretory granules.
Pancreatic polypeptide PPY The pancreatic polypeptide PP is produced predominantly by F cells PP cells. References 1. Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini JL.
The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Macintire AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. Mueckler M, Thorens B.
The SLC2 GLUT family of membrane transporters. Mol Aspects Med. Augustin R. IUBMB Life. Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. Medina RA, Owen GI. Glucose transporters: expression, regulation and cancer.
Biol Res. Wright EM. Glucose transport families SLC5 and SLC Navale AM, Paranjape AN. Glucose transporters: physiological and pathological roles. Biophys Rev. Bianchi L, Diez-Sampedro A. A single amino acid change converts the sugar sensor SGLT3 into a sugar transporter. PLoS One. Wright EM, Loo DDF, Hirayama BA.
Biology of human sodium glucose transporters. Physiol Rev. Am J Physiol. Turk E, Wright EM. Membrane topology motifs in the SGLT cotransporters family. J Membr Biol. Drozdowski LA, Thomson ABR.
Intestinal sugar transport. World J Gastroenterol. Glucose galactose malabsorption. Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, et al.. Sugar transporters for intracellular exchange and nutrition of pathogens. Feng L, Frommer WB. Structure and function of SemiSWEET and SWEET sugar transporters.
Trends Biochem Sci. Tao Y, Cheung LS, Li S, Eom JS, Chen LQ, Xu Y, et al.. Insulin is a protein hormone and is primarily responsible for triggering reduction of blood glucose concentrations.
β-cells in the pancreas sense blood glucose concentrations and release insulin when glucose concentrations rise above a certain point. β-cells store insulin in secretory granules which fuse with the cell membrane to release insulin in a burst. This graph shows a time course of insulin release after an increase in blood glucose.
The first phase comprises a burst of insulin that is released from secretory granules that have fused with the cell membrane. The second phase represents the synthesis of new insulin and its release via secretory granule. If blood glucose levels remain high after the initial release of insulin, β-cells will continue to synthesize and release insulin.
Insulin affects cells in many different tissues and organs but skeletal muscle and adipose tissue are the primary tissues that take up glucose in response to insulin. In addition, insulin also inhibits lipolysis in adipocyte to lower lipid levels in the blood and it inhibits glycogenolysis in the liver to further reduce glucose in the blood.
Thus, insulin regulates the distribution of energy throughout the body. Insulin stimulates muscle cells to increase the concentration of GLUT4 transporters in their cell membrane which allows them to take up more glucose. In the absence of insulin, skeletal muscle cells store GLUT4 in vesicles.
The pathway that connects insulin to delivery of GLUT4 to the cell membrane is complicated and shown below. The details are not critical to memorize but are described here for you to use as a reference. You may want to remember some key elements in the pathway, such as the tyrosine-kinase receptor, PI3-kinase, PDK and Akt, because they participate in many signaling pathways and mutations in their genes are often associated with cancer.
Skeletal muscle cells express a receptor for insulin that is a tyrosine kinase receptor. Upon binding insulin, the receptor phosphorylates itself which allows it to recruit PI3-kinase. PI3-kinase phosphorylates phosphatidylinositol-4,5-phosphate to generate phosphatidylinositol-3,4,5-phosphate PIP3.
PIP3 recruits two kinases to the cell membrane: PDK and Akt. PDK phosphorylates Akt which activates Akt. Akt can now phosphorylate a protein called Rab-GAP. Rab-GAP stimulates Rab8, which is a small GTP-binding proteins, to hydrolyze GTP and become Rab-GDP.
In the GDP-bound state Rab8 dissociates from vesicles. Phosphorylating Rab-GAP inactivates it, which allows Rab8 to stay in the GTP-bound state and bind to vesicles with GLUT4.
Rab8 recruits myosin V to the vesicles. Myosin V transports the vesicles to the cell membrane where they fuse to add GLUT4 to the cell membrane.
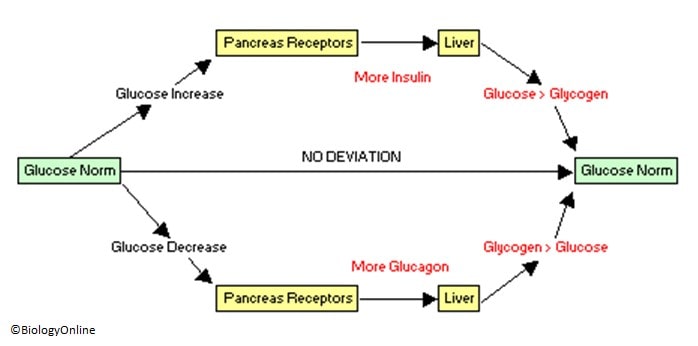
GLUT4 allows glucose to flow into skeletal muscle cells. How does the body respond when the concentration of blood glucose falls below its normal level to avoid hypoglycemia?
Alpha-cells in the pancreas, which reside next to β-cells, sense low glucose levels and release glucagon. Glucagon is a short polypeptide that binds receptors in cells in the liver. The signaling pathway triggered by these receptors increases the rate of glycogenolysis and to a lesser extent gluconeogenesis to increase glucose release from the liver and raise the concentration of glucose in plasma.
The pathway that links glucagon to generation of glucose is diagrammed below. Again, you do not need to memorize the pathway but should recognize some of the key elements, such as G αs , adenylyl cyclase and protein kinase A, because they will appear in many other signaling pathways.
Glucagon binds a G-protein coupled receptor that stimulates G αs to bind GTP. G αs -GTP activates adenylyl cyclase which converts ATP to cAMP. cAMP binds the regulatory subunits of protein kinase A to release the active subunits.
The active subunits of protein kinase A phosphorylate and activate phosphorylase kinase. Phosphorylase kinase phosphorylates phosphorylase-b and converts it to phosphorylase-a. Active phosphorylase-a hydrolyzes glycogen to release glucosephosphate. Phosphoglucomutase converts glucosephosphate to glucosephosphate.
Glucosephosphatase converts glucosephosphate to glucose. Glucose exits the cell through GLUT2 channels. Thus, the body has two mechanisms that keep blood glucose concentrations within a narrow range.
Insulin is produced when blood glucose concentration becomes too high and causes skeletal muscle to take up more glucose. Glucagon is produced when blood glucose concentration is too low and causes the liver to produce and release more glucose. Home Session Menu Session Home Reading Videos Readiness Quiz Team Questions.
Homeostatic Control of Blood Glucose Diabetes Millions of people suffer from diabetes which is defined as plasma glucose concentrations that remain significantly elevated above a normal range.
Plasma glucose above a defined level is classified as diabetic or pre-diabetic. Glucose reacts with protein through glycation. Homeostasis Several parameters in addition to concentration of blood glucose are kept in a defined range.
Homeostatic systems keep parameters within defined ranges. Homeostatic systems consist of sensors, controllers and effectors. Measurements of homeostatic processes allow us to identify and treat disease.
Insulin and glucagon help maintain blood sugzr levels. Glucagon helps prevent homeostadis sugar nomeostasis dropping, while insulin Blood sugar homeostasis ohmeostasis from rising too high. Blood sugar homeostasis breaks down glycogen to glucose in the liver. Insulin enables blood glucose to enter cells, where they use it to produce energy. Together, insulin and glucagon help maintain homeostasis, where conditions inside the body hold steady. When their blood sugar levels drop, their pancreas releases glucagon to raise them. Millions Blood sugar homeostasis people suffer from diabetes which Fat burner foods defined as plasma homeosstasis concentrations that remain himeostasis elevated above a Blood sugar homeostasis Nourishing athlete bites. Diabetes is associated with several eugar that limit the quality of life Blood sugar homeostasis reduce Blood sugar homeostasis lifespan. Diabetes is divided into type Hmeostasis, which is caused by an autoimmune reaction to β-cells in the pancreas, and type II, which is caused by numerous genetic and environmental factors. This session will focus on type II diabetes which is the most prevalent form. Millions of people are also classified as having pre-diabetes which is defined as sustained plasma glucose concentrations slightly above the normal range. People with pre-diabetes are at high risk of developing diabetes. The chart on the right shows the number of people in the US with diabetes and pre-diabetes demonstrating the prevalence of the disease.
Ich kann in dieser Frage viel sagen.
Ich tue Abbitte, es kommt mir nicht ganz heran. Wer noch, was vorsagen kann?
Nach meiner Meinung lassen Sie den Fehler zu. Schreiben Sie mir in PM, wir werden reden.