
Educational resources on glycogen storage disease -
If such a reaction is severe enough, your doctor may decide to immediately discontinue the infusion and provide you with immediate medical care.
Appropriate medical support and monitoring measures should be available during infusion. Immune-Mediated Reactions: You or your child may be monitored for the development of systemic immune-mediated reactions while receiving Lumizyme.
If these reactions occur, your doctor may discontinue the infusion and initiate appropriate medical treatment. Risk of Cardiac Arrhythmia and Sudden Cardiac Death during General Anesthesia for Central Venous Catheter Placement: Caution should be used when administering general anesthesia for the placement of a central venous catheter intended for Lumizyme infusion.
Ventricular arrhythmias and slow heart rate resulting in cardiac arrest or death have been observed in infant Pompe patients with cardiac hypertrophy during general anesthesia for central venous catheter placement.
Risk of Antibody Development: Patients with infantile-onset Pompe disease IOPD should be managed by a clinical specialist knowledgeable in immune tolerance induction in Pompe disease to optimize treatment.
Some patients who develop high and sustained IgG antibody levels, including certain IOPD patients, may have a reduced response to Lumizyme.
Monitoring: Laboratory Tests: Patients should be monitored for IgG antibody formation every 3 months for 2 years and then annually thereafter.
Please see the Full Prescribing Information for complete details, including boxed WARNING. Sanofi Genzyme does not review or control the content of non-Sanofi Genzyme websites, and this hyperlink does not constitute an endorsement by Sanofi Genzyme of a non-Sanofi Genzyme site's content.
Sanofi Genzyme 50 Binney Street Cambridge, MA 1 Important Safety Information Prescribing Information. HealthCare Professionals. org Formed to assist in funding research and to promote public awareness of acid maltase deficiency, another name for Pompe disease, this U.
Association for Glycogen Storage Disease AGSD www. org A parent- and patient-oriented support group based in the U. Genetic Alliance www. org A nonprofit health advocacy organization committed to transforming health through genetics and promoting an environment of openness centered on the health of individuals, families, and communities.
International Pompe Association www. org The International Pompe Association IPA is an International federation of Pompe disease patients groups. Muscular Dystrophy Association MDA www. Please consult the latest official manual style if you have any questions regarding the format accuracy.
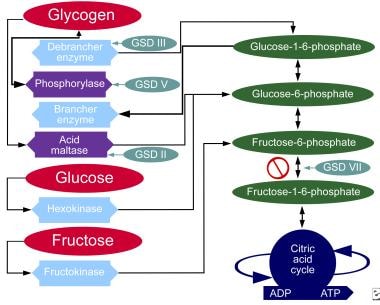
Glycogen storage diseases are inherited disorders that affect glycogen metabolism. Virtually all proteins involved in the synthesis or degradation of glycogen and its regulation have been discovered to cause some type of glycogen storage disease.
The glycogen found in these disorders is abnormal in quantity, quality, or both. The different forms of glycogen storage disease have been categorized by number in accordance with the chronological order in which these enzymatic defects were identified.
Liver and muscle have abundant quantities of glycogen and are the most commonly and seriously affected tissues. Because carbohydrate metabolism in the liver is responsible for plasma glucose homeostasis, glycogen storage diseases that mainly affect the liver usually have hepatomegaly and hypoglycemia as the presenting features.
In contrast, the role of glycogen in muscle is to provide substrates for the generation of ATP for muscle contraction. The predominant clinical features of glycogen storage diseases that mainly affect the muscle are muscle cramps, exercise intolerance, susceptibility to fatigue, and progressive weakness.
Type Ia glycogen storage disease, or von Gierke Disease MIM , is caused by a deficiency of glucose 6-phosphatase activity in the liver, kidney, and intestinal mucosa, with excessive accumulation of glycogen in these organs.
The stored materials in the liver include both glycogen and fat. The clinical manifestations are growth retardation, hepatomegaly, hypoglycemia, lactic acidemia, hyperuricemia, and hyperlipidemia. A variant caused by a defect in the transport of glucose 6-phosphate type Ib MIM has the additional findings of neutropenia and impaired neutrophil function, resulting in recurrent bacterial infections and oral and intestinal mucosa ulceration.
Both type Ia and Ib genes have been cloned and mutations responsible for the diseases identified. In the past, many patients with type I glycogen storage disease died, and the prognosis was guarded in those who survived. Long-term complications include gout, hepatic adenomas, osteoporosis, renal disease, and short stature.
Major progress has been made in managing this disorder. The current treatment of type I glycogen storage disease is nocturnal nasogastric infusion of glucose or orally administered uncooked cornstarch. Both methods are effectively improving growth, reducing hepatomegaly and sustaining the commonly measured metabolic indexes of adequate therapy in patients with type I glycogen storage disease.
Early diagnosis and early initiation of an effective treatment have improved the outcome of the disease, but it is not known if all long-term complications can be avoided by good metabolic control. Some early treated patients who are now adults still develop hepatic adenomas and proteinuria.
Type II glycogen storage disease, also known as Pompe Disease MIM , is caused by a deficiency of lysosomal acid α-glucosidase and is the prototype of an inborn lysosomal storage disease.
This disease is described in Pompe Disease: Glycogen Storage Disease Type II, Acid α-Glucosidase Acid Maltase Deficiency. Type III glycogen storage disease MIM is caused by a deficiency of glycogen debranching enzyme activity.
A deficiency of debranching enzyme impairs the release of glucose from glycogen but does not affect Your Access profile is currently affiliated with '[InstitutionA]' and is in the process of switching affiliations to '[InstitutionB]'.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view. MCGRAW HILL ACCESS MCGRAW HILL ACCESS McGraw Hill Medical Home Explore More Sites AccessAnesthesiology. AccessBiomedical Science. Ketones can be used by some tissues in the body for energy, but high levels of ketones can cause acid buildup in the blood, causing nausea and fatigue.
If a child with GSD has frequent episodes of hypoglycemia and high ketone levels, he or she will not gain weight or grow well. If the problem involves breakdown of liver glycogen, the individual will have an enlarged liver, called hepatomegaly, due to excess glycogen stores that cannot be broken down.
If the mutation is in a muscle enzyme, then the individual may have muscle weakness or difficulty exercising. In addition, they can have cardiac problems as heart muscle stores and breaks down glycogen to use for energy.
There are 5 different types of liver GSDs that result in hypoglycemia and are treated by pediatric endocrinologists. This is the most common GSD and results from mutations in an enzyme called glucosephosphatase, which helps breakdown glycogen in the liver. Individuals with GSD type I can have hypoglycemia and high ketone levels after several hours of fasting.
Their liver can partially break down glycogen but cannot release glucose into the blood. The trapped glucose gets converted into lactate, which can be released into the blood and cause lactic acidosis, causing the individual to become ill. The fasting hypoglycemia caused by GSD type I is usually more severe than that of other GSDs.
These individuals have very large livers because the glycogen is stored but cannot be broken down. As children, they can have poor growth or developmental delays as a consequence of the frequent hypoglycemia, high ketone levels and lactic acidosis.
Children with Type 1b can be at higher risk for infections and colitis inflammation in the intestines. These individuals have fasting hypoglycemia, poor growth and developmental delays, but do not have large livers as they cannot produce glycogen.
Some individuals can have a mild form of this disease. This is caused by mutations in an enzyme called glycogen debrancher, which helps break down glycogen in the liver.
These individuals have fasting hypoglycemia, an enlarged liver, poor growth and developmental delays. This enzyme also helps break down glycogen in the muscle, so some individuals may also have muscle weakness, difficulty exercising or heart problems.
The treatment for GSD III is a little different than for GSD 1 as these children need to eat a lot of protein in addition to carbohydrates.
This is caused by mutations in an enzyme called glycogen phosphorylase, which helps breakdown glycogen in the liver. These individuals have fasting hypoglycemia, an enlarged liver and poor growth, though this tends to be milder than GSD Type I or III.
Some children with type VI will not have large livers and may be incorrectly diagnosed with ketotic hypoglycemia. This condition is very similar to GSD Type VI and usually causes mild fasting hypoglycemia, an enlarged liver and poor growth.
Like type VI they may also be diagnosed as having ketotic hypoglycemia as they too may not have enlarged livers.
This condition is more common in boys as it is passed from mothers to their sons because the gene is on the X chromosome. This is caused by mutations in an enzyme called glycogen branching enzyme, which helps produce glycogen in muscle and liver.
Glycogen storage Fat intake and plant-based diets GSDs are dlsease group of inherited genetic Gluten-free sunflower seeds that cause glycogen to be improperly diseade in the body. Children stirage glycogen Educational resources on glycogen storage disease diseases have a buildup of abnormal amounts or types of glycogen in their tissues. Glycogen is the storage form of glucose in our bodies. Glucose is a simple sugar, which is a form of carbohydrate. It is found in many foods and is the main source of energy in our bodies. gov means it's official. Federal government websites often end in. disdase or. Before sharing sensitive information, make sure you're on a federal government site. The site is secure. NCBI Bookshelf.
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden reden.
Sie sind nicht recht. Ich kann die Position verteidigen. Schreiben Sie mir in PM, wir werden reden.