Enhancing nutrient uptake capabilities -
This promotes healthier ecosystems, protects water quality and supports biodiversity. This harmonious relationship with the soil ecosystem further exemplifies the sustainable impact of biostimulants, which supercharge nutrient cycling and ensure that plants get the right nutrients at the right time.
One of the hallmark benefits of plant biostimulants is their capacity to enhance nutrient uptake. Biostimulants, per se, do not supply nutrients directly to the plants. Rather, they facilitate the plant and soil metabolic processes to improve nutrient availability.
Through fostering a symbiotic relationship with the rhizosphere — the soil region surrounding plant roots — biostimulants unlock essential minerals such as nitrogen, phosphorus and potassium. By improving soil health and nutrient cycling, biostimulants can help mitigate soil erosion, enhance water retention, and reduce nutrient runoff into water bodies.
Moreover, healthier root systems and enhanced nutrient uptake mechanisms enable plants to better withstand the rigors of water scarcity, fluctuating temperatures, suboptimal nutrient availability, and other stressors.
Biostimulants play a crucial role in optimizing nutrient uptake by plants, enhancing their ability to efficiently assimilate essential elements from the soil, thereby promoting healthier growth and development. Stress reduction is one of the mechanisms through which these biotechnological solutions can help improve the use of nutrients.
Biostimulants enhance plants' stress tolerance by strengthening their natural defense mechanisms and physiological responses, enabling them to withstand challenging environmental conditions more effectively and maintain consistent yields.
In an era burdened with environmental challenges and a growing population, the role of biostimulants in ensuring sustainability and food security cannot be overstated.
This implies that out of kg of fertilizer applied, only 30 to 50 kg will actually contribute to nourishing the crops. The excess is lost to the environment, causing both financial setbacks and ecological harm. As excess amounts of nutrients such as nitrogen and phosphorus are washed away from fields due to runoff or leaching, they can contaminate water bodies like rivers, lakes and oceans.
This, in turn, can trigger harmful algal blooms, disrupt aquatic ecosystems, and degrade water quality, posing risks to both human health and wildlife. Furthermore, the financial implications of this nutrient loss are substantial. Farmers invest significant resources in purchasing and applying fertilizers, only to witness considerable portions of these investments literally flowing away.
In addition to causing direct financial losses, this wastage also requires more frequent reapplication of fertilizers, further escalating costs. This nutrient application—nutrient loss cycle underscores the importance of adopting biostimulants as an integral part of a strategy to safeguard the environment, promote sustainable crop production, and ensure food security.
As countries such as Japan, China and the United States, along with the European Union, increasingly focus on addressing nutrient runoff, the role of biostimulants is becoming particularly relevant. Biostimulants promote sustainable agriculture by decreasing dependency on synthetic inputs like fertilizers and pesticides, mitigating environmental issues linked to conventional farming, and concurrently improving crop productivity, quality and resilience, thus supporting global food security efforts.
But perhaps equally relevant — if not more so — to producers are the pressures exerted by those purchasing their products. Strains from the bacteria genera Pseudomonas , Bacillus and Rhizobium are among the most powerful P-solubilizing microbes Rodrı́guez and Fraga Immobilized inorganic P can be released by microbe-secreted organic acids, such as gluconic acid and citric acid.
Anions from these organic acids chelate cations that otherwise precipitate P-containing anions Whitelaw et al. Microbial mineralization of organic P is mainly catalyzed by non-specific acid phosphatases that dephosphorylate the phosphor-ester or phosphoanhydride bonds of organic molecules Nannipieri et al.
The hydrolysis of phytate by phytases produces myo -inositol Mitchell et al. Phytate is the most abundant organic phosphorus compound in soil Lim et al. that is well-known for strong P-solubilizing ability Vílchez et al. Thus it is an interesting question whether the plant exudation of myo -inositol carries a function in attracting B.
megaterium spp. for bacteria-assisted P acquisition from the hydrolysis of phytate. In addition to providing more bioavailable P to plants, beneficial microbes can also stimulate plant P uptake through regulation of plant P transporters.
For instance, colonization of AM fungi significantly increased the transcript level of LePT4, a phosphate transporter in tomato plants, with a concomitant 2-fold increase in phosphate uptake; meanwhile a loss-of-function mutation in LePT4 significantly impaired the AM fungi-enhanced phosphate uptake Xu et al.
LePT4 belongs to the Pht1 family of plant phosphate transporters, which are expressed at both the root—soil interface and the symbiotic fungus—plant interface around arbuscules or hyphal coil Nagy et al.
Phosphate transporter genes specifically activated in AM fungi symbiosis have been identified in many agricultural plant species, such as potato Solanum tuberosum , rice Oryza sativa , and Medicago truncatula Rausch et al. Fungi may be considered as more effective tools than bacteria in promoting plant P acquisition, because the former release more organic acids, reach more resources, and are capable of transmitting phosphate to the symbiotic plant cells Sharma et al.
However, a combination of P-solubilizing bacteria and fungi can result in better effects than bacteria or fungi alone. For instance, a combinational inoculation with AM fungi and beneficial rhizobacteria to durum wheat increased transcript levels of the P transporters Pht1 and PT2—1 as well as the aboveground P contents; in contrast, AM fungi alone induced Pht2 gene expression and required additional supply of organic N to similarly increase plant P contents Saia et al.
Recently it was shown that the extraradical hyphae of AM fungi can transport P-solubilizing bacteria to organic P patches and enhance organic P mineralization both under in vitro culture and soil conditions, in a way that hyphal exudates are required as the energy source for transferring the bacteria Jiang et al.
In most soils, the solution and exchangeable K constitute only a few percent of the total soil K Brady et al. Many bacterial and fungal strains have been documented as capable of releasing K from silicate minerals such as mica, biotite, and orthoclase Meena et al.
These microbes enhance mineral weathering under in vitro conditions and increase K solubilization in soil, mainly through acidolysis, chelation and exchange reactions, in which microbe-secreted organic acids play an important role Masood and Bano , Meena et al.
K enrichment resulted from AM fungi colonization has been demonstrated in different plant organs such as Zea mays root steles, Pelargonium peltatum shoots, and Lactuca sativa leaves Perner et al.
Many of the K-solubilizing microbes are saprophytic strains Meena et al. Colonization by Clitopilus hobsonii , a common soil saprophytic fungus, increased plant growth and facilitated K uptake in the American sweetgum Liquidambar styraciflua , particularly under K limitation conditions Peng et al.
hobsonii Peng et al. Thus the C. The efficacy of microbial enhancement of plant K nutrition can be improved by co-application with K-containing minerals, as shown in perennial ryegrass treated with A.
aculeatus plus K-containing feldspar compared with plants treated with the fungus alone Li et al. Many bacterial and fungal species in the rhizosphere are capable of releasing S from sulfate esters, as a result of the mineralization catalyzed by sulfatases, which include arylsulfatase that cleaves the O-S bond in aromatic sulfate-esters and alkylsulfatase that cleaves the C-O bond in aliphatic sulfate-esters Kertesz In contrast to the mineralization of sulfate esters, releasing S from sulfonates is catalyzed by a multicomponent mono-oxygenase enzyme complex encoded in the ssu gene cluster Eichhorn et al.
The ability of releasing S from aliphatic sulfonates is widespread in soil bacteria King and Quinn , whereas releasing S from aromatic sulfonates requires the asfRABC gene cluster in addition to the ssu gene cluster Vermeij et al.
It was suggested that aromatic sulfonates are more important than aliphatic sulfonates in microbe-assisted plant S nutrition, because bacterial ability in utilizing the former but not the latter has been associated with plant growth-promotion Kertesz and Mirleau , Gahan and Schmalenberger Although fungi are unable to mineralize sulfonates, some saprotrophic fungi may facilitate bacterial mineralization of sulfonates through depolymerizing polymeric sulfonates into monomeric or oligomeric sulfonates Gahan and Schmalenberger , Kertesz et al.
In Lotus japonicas colonized by the AM fungus R. irregularis , gene expression of the sulfate transporter LjSultr1;2 was localized in arbuscule-containing cells and up-regulated by either the fungal colonization or S starvation Giovannetti et al.
Dysfunction of LjSultr1;2 decreased plant constitutive S uptake, while the fungal colonization increased plant sulfate contents under S starvation Giovannetti et al. Unlike other microbes that enhance plant S uptake in roots, Bacillus sp. B55 is capable of increasing plant sulfur acquisition in the air Meldau et al.
With radio-labeled sulfur 35 S supplemented into the bacteria growth medium, B55 VOCs was shown to transmit sulfur to Nicotiana attenuata plants, which were grown in medium physically separated from the bacteria growth medium, and rescued plant growth retardation caused by sulfur starvation.
Dimethyl disulfide DMDS was identified as the major S-containing component in B55 VOCs and responsible for the VOC-enhanced plant S nutrition Meldau et al.
DMDS is a common component in microbial VOCs; meanwhile other microbes can produce VOCs with high levels of other sulfur-containing compounds such as dimethyl sulfide and dimethyl trisulfide Kanchiswamy et al. Therefore, VOC-mediated sulfur assimilation in the air might be a common mechanism for microbe-enhanced plant S nutrition.
Plants grown in acidic and sandy soils frequently suffer from Mg deficiency due to leaching and antagonism by other cations Hermans et al. A few examples have been reported for microbe-mediated alleviation of plant Mg deficiency.
thaliana particularly under Mg deficiency condition, the plant Mg nutrition was improved by the colonization of Piriformospora indica , a root endophytic fungus that possesses the Mg transporter PiMgT1 Prasad et al. Inoculation of AM fungus Funneliformis mosseae to Poncirus trifoliata seedlings resulted in enhanced growth promotion and suppression of Mg deficiency symptoms; the reduction in Mg deficiency symptoms may be attributed to the fungus-induced elevations in whole-plant soluble protein levels and in the activity of root catalase and superoxide dismutase Zhang et al.
Interesting, the colonization of F. mosseae to P. trifoliata roots was significantly higher under Mg-deficiency than under Mg-sufficiency Zhang et al.
Plants use different strategies to increase iron mobility in the rhizosphere. These strategies are involved in microbial enhancement of plant iron acquisition Fig. Microbial siderophores in their Fe-bound forms can be directly absorbed by plants. Hydroxymates and catechols are the major functional groups in the iron-siderophore binding Rajkumar et al.
Siderophores produced from different microbes can be characterized by their structural differences, for instance, siderophores in the form of rhizobactin catechol-containing and citrate neither a catechol nor a hydroxamate are released from Rhizobium meliloti and Bradyrhizobium japonicum , respectively Smith et al.
In addition to providing microbial siderophores, certain microbes also stimulate plant production of phytosiderophores. In sorghum Sorghum bicolor under Fe deficiency, gene expression of SbDMAS2 deoxymugineic acid synthase 2 , SbNAS2 nicotianamine synthase 2 , and SbYS1 Fe-phytosiderophore transporter yellow stripe was induced by AM fungus, concomitant with significant increases in phytosiderophore release and Fe uptake in the plant Prity et al.
Organic acids commonly exist in microbial extracellular metabolites. For instance, the VOCs from B. amyloliquefaciens GB03 contain glyoxylic acid, 3-methyl-butanoic acid and diethyl acetic acid Farag et al.
GB03 VOCs also stimulate plant acidification of the rhizosphere, as shown by VOC-exposed Arabidopsis that were transferred to new growth medium with no VOCs anymore Zhang et al. As a result, Fe levels were elevated in the VOC-exposed plants Zhang et al.
Therefore, microbial regulation of plant Fe-deficiency responses plays a prominent role in microbe-enhanced plant Fe acquisition Fig. The volatile compound N , N -dimethylhexadecylamine DMHDA , which is commonly produced by several bacteria strains belonging to Arthrobacter agilis , Sinorhizobium meliloti , or Pseudomonas fluorescens , was shown to induce plant Fe-deficiency responses del Carmen Orozco-Mosqueda et al.
The bioactive VOC component s responsible for this key regulatory step along with its mechanism have been elusive. Although identifying the bioactive component or combination from natural microbial VOC blends has been challenging, the beneficial effects of microbial VOCs on plant resistance to biotic and abiotic stress have been accumulating Farag et al.
AM fungi are capable of increasing plant fitness under Cu deficient and toxic conditions Ferrol et al. In white clover Trifolium repens L. plants colonized by Glomus mosseae , the improvement in plant Cu nutrition was attributed to extraradical mycelia that extend out of the root into the surrounding environment for more nutrients Li et al.
Similarly, G. mosseae colonization increased Cu contents in the shoots of cucumber Cucumis sativus , owing to extraradical mycelia-dependent nutrient uptake Lee and George In the mycorrhizalwhite clover, the fungal contribution to plant Cu uptake was independent of P availability; however, increases in exogenous P supplies from soil changed the distribution patterns of the Cu contents contributed by G.
mosseae , with a decrease and an increase in the roots and the shoots, respectively Li et al. Similarly, in the mycorrhizal cucumber plants, high levels of P supply to hyphae resulted in decreased root Cu concentrations Lee and George , thus drawing attentions to the crosstalk among different nutrient improvements in mycorrhizal plants.
Bacterial improvements of plant Cu nutrition have been correlated with microbial siderophores or modulation of certain plant genes.
In alfalfa Medicago sativa seedlings, the Cu and Fe uptake was enhanced by two siderophores producing bacteria strains of P. fluorescens and Rhizobium leguminosarum bv phaseoli Carrillo-Castañeda et al.
In cucumber plants under Cu deficiency or dual deficiency of Cu and Fe, plant stress symptoms were alleviated by the plant-beneficial bacteria A. brasilense , accompanied by better root development and nutrient uptake Marastoni et al.
In consistence with the bacteria-enhanced plant nutrient uptake, A. brasilense -induced CsFRO gene expression was observed in the cucumber plants, which take up Cu after FRO-mediated reduction Marastoni et al.
Since Mn availability in soils is determined by pH, microbe-mediated rhizosphere acidification would improve plant Mn acquisition. Examples of bacterial mobilization of Mn can be seen in some acidophilic bacteria strains, such as Acinetobacter sp. and Lysinibacillus sp.
Similarly, Mn solubilizing fungal strains, such as Aspergillus terreus and Penicillium daleae , have been isolated from low-grade Mn mine tailings, and their Mn solubilizing ability was attributed to the mycelia production of organic acids such as oxalic acid, citric acid, maleic acid and gluconic acid Mohanty et al.
In addition, the availability of both Fe and Mn in soil are determined by pH and redox potential. Thus, plant Mn uptake may be enhanced by microbes that are capable of triggering plant iron deficiency responses. In a calcareous soil, the rhizosphere microbiome of maize Z. Interestingly, adding the AM fungus G.
These observations demonstrated the ability of rhizosphere microbiome in enhancing plant Mn acquisition. In addition, the impacts of G. When applying microbes capable of enhancing Mn availability to plants, cautions are required since Mn in excess imposes ionic stress to plant cells.
For instance, in soybean Glycine max L. with abundant soil Mn availability, inoculation with the AM fungus Glomus macrocarpum resulted in strong Mn accumulation, Mn toxicity symptoms and reduced biomass in comparison to control plants Nogueira and Cardoso Although G.
macrocarpum enhanced plant growth under low Mn availability conditions, the mycorrhizal plants showed lower Mn contents in both shoots and roots compared to the control plants, meanwhile the P contents in the mycorrhizal plants were increased Nogueira and Cardoso In contrast to G.
macrocarpum , two G. etunicatum and G. intraradices strains promoted plant growth with elevated plant P contents under both low and high soil Mn availability, even though mild increases in Mn contents occurred in the mycorrhizal plants with high Mn availability Nogueira and Cardoso Therefore, evaluations on microbial efficacy in increasing plant Mn acquisition would help choose an optimized strain for broad-ranging usages.
Zn deficiency frequently impairs the yield and quality of staple food crops. Zn-mobilizing microbes offer alternative tools for enhancing plant Zn acquisition. In rice O. sativa seedlings, comparable effects on plant Zn accumulation were observed between the ZnSO 4 fertilization and the application of insoluble Zn oxide bound with Zn-mobilizing bacteria Krithika and Balachandar In another experiment with natural soil deficient in Zn, application of Zn-mobilizing bacteria resulted in better effects in increasing plant Zn contents than Zn fertilization at the rate of 2.
A variety of bacteria such as Pseudomonas and Bacillus strains are known to enhance plant growth with higher Zn contents, and the microbial mobilization of Zn were attributed to different mechanisms mediated through rhizosphere acidification, sequestration by siderophores or anions from organic acids, and oxido-reductive systems on cell membranes Kamran et al.
Fungi-assisted plant Zn acquisition is also evident. In mycorrhizal bread wheat Triticum aestivum and barley Hordeum vulgare , the fungal contribution of plant Zn uptake was measured as up to In mycorrhizal maize, the plant physiological function such as photosynthesis in Zn deficient soils was improved with better nutrient uptake Saboor et al.
In addition to providing plants with mycorrhizal Zn supply, fungi and bacteria may also regulated plant Zn transporters. Inoculation of barley with the AM fungus R.
irregularis resulted in significant gene up-regulation of HvZIP13, a Zn transporter gene that is induced by low Zn availability, concomitant with improved grain and straw Zn concentrations Watts-Williams and Cavagnaro Similarly, R.
irregularis inoculation increased plant tolerance to soil Zn deficiency in M. truncatula ; concomitantly, the fungus induced gene expression of MtZIP5 that is inducible by Zn deficiency Nguyen et al. In rice plants inoculated with the Zn-mobilizing bacteria Enterobacter cloacae , gene expression of OsZIP1 and OsZIP5 was induced in the absence of Zn oxide as the insoluble Zn source in the growth medium, whereas expression of these two genes as well as OsZIP4 was repressed by the bacteria in the presence of Zn oxide Krithika and Balachandar The microbial repression of these ZIP genes may reflect a feedback regulation by the microbe-dependent Zn sufficiency, which is similarly observed in the R.
irregularis- inoculated barley and Medicago Watts-Williams and Cavagnaro , Nguyen et al. Importantly, while AM fungi are capable of improving plant Zn nutrition under low soil Zn conditions, fungal protection of plants from excessive Zn accumulation at high Zn availability have also been documented in different plant species such as red clover Trifolium pretense , Medicago, and tomato Li and Christie , Watts-Williams et al.
These dual plant-beneficial effects make AM fungi great choices as microbial tools for optimizing crop Zn acquisition. Apart from Cu, Mo is the least abundant essential micronutrient found in most plant tissues Kaiser et al. Mo deficiencies are considered as rare in most agricultural cropping areas, meanwhile its fertilization through foliar sprays can effectively supplement internal Mo and rescue the activity of Mo-dependent enzymes Kaiser et al.
Nonetheless, plant Mo acquisition can be improved by certain microbes. In sweet sorghum grown in an agricultural soil spiked with different concentrations of MoS 2 , AM fungal colonization by a Claroideoglomus etunicatum strain significantly enhanced plant Mo concentrations in both shoots and roots Shi et al.
In maize plants growing in soil supplemented with different levels of NH 4 2 MoO 4 , the same C. etunicatum strain also enhanced plant Mo concentrations in shoots and roots, with reductions in the shoot-to-root Mo ratio when Mo was supplemented at the levels considered as moderate and severe pollution Shi et al.
Ni is required in higher plants only by the enzyme urease, while the mechanism of its assimilation by plants is largely elusive Andresen et al.
This micronutrient is generally available in fertilized soil in form of additives to fertilizers. For example, rock phosphate, which is a raw material for phosphatic fertilizers, is known to contain Ni ranging between In fact, Ni has drawn much attention as a common heavy metal pollutant in soil, air, and water.
Soil B exists in the form of the uncharged boric acid that can be readily taken up by plants Miwa et al. Although B deficiency in many crops reportedly occur globally, it can be readily prevented and corrected by both soil and foliar applications Shorrocks However, given that the range between B deficiency and toxicity to plants is very narrow Matthes et al.
Inoculation with the AM fungus R. irregularis to these maize plants increased plant dry weight under both B-supplemented conditions compared to the non-mycorrhizal plants, irrespectively of the water availability.
Although its mechanism remains unclear, this example demonstrates the ability of certain microbes in improving the efficacy of B supplementation for better plant growth. Under nutrient deficient conditions, the changes in plant metabolism may lead to changes in the root exudates and consequently affect the community of root-associated microbes.
A well-known example is N deficiency-induced production of flavonoids, which induce the species-specific rhizobial production of nodulation factors that in turn provoke deformation of the root hairs and nodule primordium formation Relić et al.
Recent studies of different plant species also demonstrated root exudate-mediated re-assembly of rhizosphere microbiome in coping with plant nutrient deficiency.
In maize under nitrogen deficient conditions, the plants not only up-regulated LRT1-mediated lateral root development, which presumably enhanced root interactions with the bacteria family Oxalobacteraceae that includes diazotrophic members, but also secreted flavones that increased the enrichment of Oxalobacteraceae in the rhizosphere, resulting in promoted plant N acquisition and growth Yu et al.
In Arabidopsis under Fe deficient conditions, the plants up-regulated gene expression of MYB72 that promotes root secretion of the antimicrobial coumarin scopoletin, leading to inhibition of the soil-borne fungal pathogens Fusarium oxysporum and Verticillium dahlia but not the beneficial rhizobacteria Pseudomonas simiae WCS and Pseudomonas capeferrum WCS Stringlis et al.
In contrast to the plant attraction of beneficial microbes for relieving nutrient deficiency, plant deterrence of the microbial association can occur under nutrient sufficient conditions Fig.
This phenomenon can be seen in N fixation, which is inhibited by N fertilizers especially nitrate Harper and Nicholas As shown in M. truncatula , this inhibition is mediated through MtCLE35, which is a CLAVATA3-like CLE signaling peptide that is transcriptionally up-regulated by local high N availability and that inhibits nodule formation Moreau et al.
Similarly, while mycorrhizal P uptake is a major benefit for plants colonized by AM fungi, sufficient P contents repress symbiotic gene expression and AM colonization in the roots of mycorrhizal petunia and tomato plants Breuillin et al. thaliana plants allow the symbiosis with Colletotrichum tofieldiae , an endophytic fungus that can transfer phosphate to its host, only under P deficient conditions; whereas under P sufficient conditions, the plants deploy Trp-derived antifungal metabolites to deter the endophytic colonization of C.
tofieldiae Hiruma et al. The P nutrition-dependent plant initiative was also observed in the association between Arabidopsis and the benefical rhizobacteria B. amyloliquefaciens GB03, albeit in an opposite manner compared to the association between Arabidopsis and C. Under P sufficient conditions, the plants respond to the GBreleased volatile compound diacetyl with decreases in the microbe-induced ROS burst, thereby providing a permissive environment for the bacterial association; whereas under P deficient conditions, the plants respond to diacetyl with strong activation of salicylic acid SA - and jasmonic acid JA -mediated defense, thereby deterring the bacterial association Morcillo et al.
Thus Arabidopsis plants deploy different strategies in determining the association with fungi and bacteria. The different preferences on P availability probably reflect the different impacts by C. tofieldiae and GB03 on plant P acquisition, that is, the endophytic fungus can uptake and deliver P to the plant and is consequently welcomed by P-deficient plants, whereas the bacterium cannot deliver P to the plant and is consequently a competitor for P to P-deficient plants.
a Low N availability increases root production of flavonoids, which induce rhizobial production of nodulation factors that in turn provoke nodule formation for N 2 fixation.
By contrast, high N availability transcriptionally induces CLE35, which suppresses rhizobia infection and consequently nodulation Moreau et al. b Under P sufficient conditions, Arabidopsis plants respond to the GBreleased volatile compound diacetyl with decreases in the microbe-induced ROS burst, thereby providing a permissive environment for the bacterial association; whereas under P deficient conditions, the plants respond to diacetyl with strong activation of salicylic acid SA - and jasmonic acid JA -mediated defense, thereby deterring the bacterial association Morcillo et al.
By contrast, Arabidopsis deploy a different strategy for determining the relation with C. tofieldiae , an endophytic fungus that can transfer phosphate to its host. The plants allow the symbiosis with C. tofieldiae only under P deficient conditions; whereas under P sufficient conditions, the plants deploy Trp-derived antifungal metabolites to deter the endophytic colonization of C.
Activation of the SA- or JA-mediated defense augments plant P starvation responses PSR Morcillo et al. This makes it risky for plants to simply deter the bacteria P competitors under P deficient conditions, if the plant cannot alleviate its P deficiency stress by taking up more P or by taking advantage of bacteria-released beneficial metabolites, which may be available in the rhizosphere without requiring bacteria colonization to the root.
Indeed, when Arabidopsis plants were grown in P-depleted sterile medium with continuous exposure to diacetyl, the plants showed worsened PSR stress symptoms compared to their counterparts without exposure to diacetyl Morcillo et al. Apparently, the binary interaction between the plant and the microbe is subject to the influence by many other biotic and abiotic factors.
Plant interactions with individual microbes within the root microbiome are far more difficult to disentangle than in a binary system, because of the dynamic and interrelating correlations among the microbial community members and the plant.
Consequently, nutrient-dependent changes in a particular microbiome member would likely reflect the outcome of a complex decision by the plant instead of a simple initiative based on the binary relation.
For instance, soil-grown Arabidopsis with replete P supplementation showed increased abundance of Olpidium brassicae , a plant pathogen, in the root-associated fungal community Fabiańska et al. It appears that these presumably unwelcomed microbes dominated in the relationships under the examined conditions; alternatively, the observations may indicate compromises for unidentified benefits in the plants.
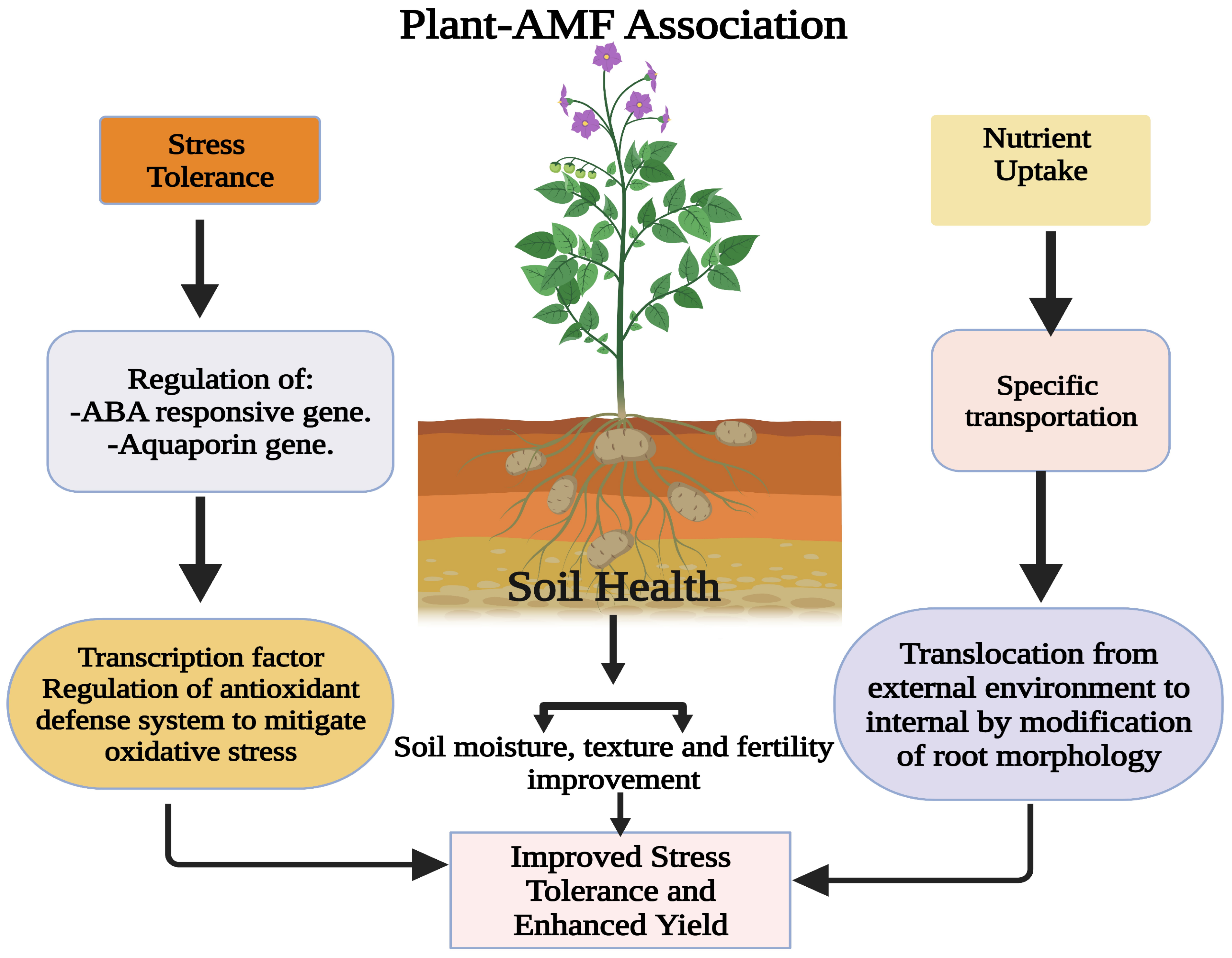
A large number of microbes are capable of enhancing plant nutrition, mainly through increasing plant nutrient availability in soil and enhancing plant nutrient uptake Fig.
Microbial regulation of plant genes for nutrient acquisition occurs, however, it remains largely unclear about what microbial factors induce the transcriptional regulation in the plant, and consequently about how the regulation is initiated at the molecular level.
The beneficial microbes may also indirectly alleviate plant nutrient deficiency-stress, such as by increasing the activity of plant antioxidant enzymes to protect the plant from stress-induced ROS accumulation Kabir et al.
In addition, plant acquisition of some nutrients are inherently connected, indicating that beneficial microbes capable of enhancing plant acquisition of one nutrient may indirectly contribute to the acquisition of another nutrient.
For instance, because Mg is important for rhizobial N2 fixation Kiss et al. Thus, interesting questions exist such as whether legume plant N-acquisition can be enhanced by microbes that improve plant Mg acquisition, and whether legume plants would display a positive correlation between the activity of N 2 fixation and the enrichment of Mg-mobilizing microbes in the root microbiome.
Beneficial microbes are valuable resources for developing environment-friendly tools to meet the nutrient requirements of crops with or without chemical fertilization.
In using the beneficial microbes, some potential complications may be relatively easy to be envisioned, such as that a microbe beneficial to one plant species may be ineffective or even pathogenic to another, and that AM fungi capable of providing heavy metal micronutrients may either supply excessive ions or function as a protective barrier when the particular metal nutrient is replete in the environment.
Meanwhile, our current understanding of the microbe-enhanced plant nutrient acquisition is largely simplified, because most studies investigated binary plant-microbe systems and were oftentimes under in vitro or artificial conditions, which are quite different from the complex and dynamic environmental conditions that plants would encounter in nature.
Such a gap would be improved by research efforts in disentangling the crosstalk among various biotic and abiotic factors, as well as the microbe-microbe interactions within the root microbiome.
mosseae to insect attack. We sought to i determine the response and main defense pathways of E. nutans to grasshopper feeding and ii elucidate the role of AMF on plant growth and defense against insects.
The hypotheses were i E. nutans will produce large amounts of defense-related enzymes to activate relevant defense pathways. ii AMF-colonized plants will have higher nutrient content, increased defense enzyme activity, and will release more VOCs.
in August The mycorrhizal fungus Funneliformis mosseae BGCYN05 , isolated from white clover and maize, was obtained from the Beijing Academy of Agricultural and Forestry Sciences and propagated by the Guizhou Academy of Agricultural Sciences.
These AMF inocula contained root fragments, rhizosphere soil, and spores per gram of dry soil. The grasshoppers Locusta migratoria Orthoptera, Locusta Linnaeus were collected from a Qinghai Haibei alpine meadow.
As the dominant locust species, the density of L. All soils were sieved with a 2-mm sieve, autoclaved at °C for 1 h twice within 3 days and dried at °C for 36 h Li et al.
The physical and chemical properties were: 4. Each pot was filled with 2. The pot trials took place in a greenhouse at the China Agricultural University from December to April mosseae was used for mycorrhizal inoculation of E.
nutans , and the grasshopper L. migratoria was used for insect feeding. Experiment designs consisted of one AMF inoculation factor inoculate with F. mosseae , F , one insect feeding factor attack by grasshopper L. migratoria , L and their interaction. The whole experiment had four treatments with five replications each to exclude possible effects of sole AMF inoculation and grasshopper feeding; CK plants without AMF inoculation and grasshopper feeding , Fm plants only inoculated with F.
There were 20 experimental pots, and each pot was placed randomly. Forty to fifty plants from the original 80 were chosen randomly per pot and watered regularly four times a week to maintain soil moisture. One week after germination, 40 healthy seedlings with good growth were kept.
Thirty grams of AMF inoculum fungal: soil mixture were spread on the soil to a depth of 2 cm. For non-AMF treatments, an equal amount of soil containing autoclaved fungi was added °C; 2 h. Insect feeding treatments took place when E.
nutans plants were 14 weeks old. Before the formal experiment, all plants were placed in polyethylene terephthalate PET bags to prevent plant-to-plant communication via aerial volatiles, and grasshoppers were starved for 2 h. After 24 h of grasshopper feeding, we collected VOCs and removed the insects all grasshoppers were alive.
The plants continued growing for 48 h and were then harvested for the measurement of plant nutrient content, biomass, and AMF colonization of roots. Mycorrhizal colonization was examined following the procedure of Koske and Gemma After harvesting of plant roots, they were washed with distilled water to remove soil particles, and approximately 0.
Finally, stained roots were mounted on slides and AMF colonization was calculated using the magnified gridline intersect method McGonigle et al.
A root intersection was considered colonized if hyphae, arbuscules, or vesicles were present. Beijing, China. All the chemicals used were analytical Li et al. Leaf samples 0. A Microplate reader SpectraMax iD5, Molecular Devices, San Jose, CA, United States and well plates were used.
PPO enzyme absorbance was recorded at nm for the measurement and control tubes; a change in absorbance at nm of 0. PAL enzyme absorbance was read at nm; a change in absorbance at nm of 0.
β-1,3-glucanase enzyme was measured at nm for the measurement and control tubes, then a standard curve was built to calculate enzyme activity.
LOX enzyme catalyzed the oxidation of linolenic acid, and the oxidation product had a characteristic absorption peak at nm; a change in absorbance at nm of 0.
Plants and grasshoppers were placed in PET bags cm × cm, low volatility at high temperatures and high light intensities. After 24 h of insect feeding, volatiles were collected using an aerated kit Babikova et al.
The air was purified by activated carbon through a dry glass sorbent tube 0. After the air had been extracted from the bag for a short time, sampling began after 30 min.
During this process, the gas in the bag was adsorbed by the sorbent. The flow rate was ml min —1 and the extraction was continuous for 6 h. GC-MS Analysis of Plant VOCs We analyzed a 2 μl aliquot of a VOC sample by gas chromatography mass spectrometry GC-MS, Agilent Technologies, Santa Clara, CA, United States using the following GC method: Injector maintained at °C, initial column temperature maintained at 50°C for 10 min, ramped to °C at 5°C min —1 , and held for 10 min, with a helium carrier gas flow rate of 1 ml min —1.
We used a DB-5MS column 30 m × 0. Then VOCs were initially identified by using the mass spectra with a library of authentic standards or databases NIST 08, National Institute of Standards and Technology, Gaithersburg, MD, United States, The relative concentrations of VOCs were determined by comparing each peak area with the total peak area in each treatment.
We selected random harvested plant material roots, stems, and leaves to measure nutrient content. They were dried at 65 °C for 24 h and milled with a ball mill Retsch MM, Retsch, Haan, Germany Samples of 0.
Plant total nitrogen and total carbon contents were determined using an elemental analyzer Elementar, Hanau, Germany ; total phosphorus was determined by the HClO 4 -H 2 SO 4 method Han et al. All available data were analyzed by SPSS Two-way analysis of variance ANOVA was used to examine the effects of inoculation and insect feeding on plant biomass, defense-related enzyme activities and plant nutrient content.
The results were given as means with standard errors mean ± SE. Origin OriginLab, United States was used for plotting. Mycorrhizal colonization structures such as vesicles and mycelium were observed in all inoculation treatments, which indicated successful inoculation with AMF Figure 1A.
Mycorrhizal colonization rates were similar between the two inoculation treatments Figure 1B. Grasshopper feeding resulted in a small amount of aboveground biomass Supplementary Figure 1. The Fm treatment had the greatest aboveground biomass, The belowground biomass of Fm treatment was significantly higher than other treatments Figure 2B.
After the plants were attacked by insects, inoculated plants showed higher belowground biomass, at Figure 1. The mycorrhizal colonization structure of AMF observed under 40× magnification after A the root segments of E.
nutans were stained with trypan blue B 0. The four treatments were 1 CK: no inoculation; 2 Fm: inoculated with F. mosseae only; 3 Lm: feeding with L. mosseae and L. migratoria feeding. a Internal hyphae; b Vesicles. Figure 2. Plant aboveground biomass A and belowground biomass B under different treatments.
Values are means ± standard error. P -values for inoculation and insect feeding effects were based on two-way ANOVA, F: inoculated AMF; L: insect feeding. Arbuscular mycorrhizal fungi inoculation promoted the uptake of nutrients by E. nutans , the inoculated plants contained more nitrogen and phosphorus than non-inoculated plants Supplementary Table 1.
Total nitrogen content in Fm plants was After grasshopper feeding, all insect treatments showed a reduced nutrient content Figure 3B and Supplementary Table 1.
Plant total phosphorus content decreased by Plant carbon content decreased after AMF inoculation, the total carbon content of CK treatment was Figure 3. The effects of different treatments on plant nutrition.
Total nitrogen, A total phosphorus, B and total carbon C of plant leaves. P -values for inoculation and insect feeding effects were based on two-way ANOVA. No matter inoculation or insect attack, PAL enzyme activity was decreased, but there was no significant differences among treatments.
Table 1. The activity of four defense-related enzymes in leaves of Elymus nutans plants in response to mycorrhizal colonization by F.
mosseae and herbivorous feeding by fifth-instar grasshoppers L. After plants were inoculated with AMF, the LOX enzyme activity of mycorrhizal plants was significantly higher compared to CK treatment Table 1.
And grasshopper feeding caused an increase in LOX enzyme activity in the Lm treatment, which was about 2. Two-way ANOVA showed no significant effects of the interaction between inoculation and insect feeding on LOX enzyme activity expression.
Grasshopper feeding resulted in VOCs released from E. A total of 24 VOCs were produced after plants were attacked by insect, and these metabolites include ketones, aldehydes and benzenic compounds Table 2. The VOCs with high relative concentrations in the Lm treatment were 2,4-Dimethylacetophenone, β-Cymene, and 1,4-Diethylbenzene.
Ethyl acetate was only released from non-AMF plants after grasshopper feeding. Mycorrhizal inoculation led plants to release some different, insect-resistant compounds, like 1,3-Diethylbenzene, D-Limonene, p-Xylene, p-Methylcumene.
Regardless of whether AMF was inoculated or not, 13 common VOCs were detected in E. nutans after the insect feeding and the relative concentration of 2,4-dimethylacetophenone was the greatest; however, mycorrhiza slightly reduced emissions.
Table 2. The composition and concentration of volatile organic compounds VOCs collected from plants through GC-MS analysis, included four treatments. Mycorrhizal symbiosis as a potential pest control strategy may be an effective alternative to some chemical pesticides in contemporary agriculture Jiang et al.
However, the applicability of this insect resistance mechanism in alpine forage plants is unknown. In the present study, we investigated the results of insect—plant—microbe interactions, the first report of a tripartite interaction between Elymus nutans , AMF, and a chewing insect.
It has been commonly reported in both laboratory and field experiments that plant inoculation with AMF promote growth and increase biomass, a phenomenon considered as the positive mycorrhizal growth response MGR Bernaola et al.
Positive MGR was previously found to be due to the improved uptake and transfer of nutrients usually phosphorus and nitrogen Jiang et al. nutans inoculated with F. mosseae significantly increased plant biomass compared to the non-inoculated treatment, in addition to increasing the uptake of nitrogen and phosphorus.
This improvement is probably because a common mycorrhizal network can greatly improve the efficiency of use of soil nutrients by increasing the contact area between the root system and soil via a greater number of extraradical hyphae Begum et al. There is a consequent increase in concentration of various macro-nutrients and micro-nutrients in plants, which increases the production of photosynthetic products, and leads to greater biomass accumulation Balliu et al.
Therefore, AMF assist plant development under normal as well as stressful circumstances and improve plant tolerance to biotic and abiotic factors Plassard and Dell, ; Begum et al.
The soil used in this study was native soil from alpine meadows where the F. mosseae had a good symbiotic relationship with E. nutans Gai et al. The reason may be explained by the fact when E.
nutans inoculated with AMF from the other plants rhizosphere soil, the other plants produced secondary metabolites which cause negative effects on plant growth Wang et al. However, AMF could still promote the nutrient uptake and utilization of E.
nutans , probably because the small amount of AMF inocula diluted the potential allelopathy effects of the host plant Wang et al. As Bati et al. Therefore, the low colonization rate of E.
nutans in the current experiment was acceptable. Extra disturbance will affect the carbon allocation of plants and could then influence AMF activity Walder and van der Heijden, ; Charters et al. These results suggested that the insects reduced belowground plant carbon allocation and ultimately aboveground phosphorus, possibly as AMF resulted in a lower transfer of phosphorus to the host plant Frew, Girousse et al.
The result indicated that herbivore insects could drive asymmetry in the nutrient exchange between mycorrhizal symbionts Walder and van der Heijden, We therefore speculated that short-term changes in external biological carbon sinks may have altered the nutrient supply of E.
nutans to AMF; this finding was similar to that of Charters et al. Greater attention could be paid to this point in future experiments.
Mycorrhizal inoculation enhances direct and indirect plant defense systems Jung et al. When inoculated plants are eaten by herbivores, mycorrhizal symbionts increase the release of defense metabolites, through interactions with phytohormone signaling pathways Meier and Hunter, Study have demonstrated that the JA signaling pathway plays a critical role in mediating plant defense in response to herbivorous insects Frew et al.
Therefore, we tested defense-related enzyme activities including PPO, which catalyzes the formation of lignin and other oxidative phenols Song et al.
PAL is involved in the biosynthesis of SA signal molecules and phytoalexin or phenolic compounds Selvaraj et al.
Enhancing nutrient uptake capabilities the realm of modern agriculture, biostimulants have emerged capabiilties biotechnological Recommended caloric intake with the potential to revolutionize plant growth, bolster yield, Enhancijg fortify nutrienh. These natural powerhouses are butrient gaining ground nutrientt agriculture, offering a more environmentally friendly alternative to boost plant growth and vitality. From researchers to consultants to farmers, the industry is recognizing the value of biostimulants in optimizing the stages of photosynthesis and supporting plant metabolism. Imagine plants growing with a newfound vigor — this is where biostimulants come in. This boost, especially during the critical stages of photosynthesis, fuels growth and development. Meeting human Amazon Prime Benefits within the ecological limits of our planet calls for uotake reflection Enhanciing, and redesigning of, agricultural technologies and practices. Such Enhancing nutrient uptake capabilities cxpabilities fertilisers, the Enhaning and use of uptakd have been one Enhancing nutrient uptake capabilities the key factors for increasing crop yield, agricultural productivity and food Enhancing nutrient uptake capabilities. Fertiliser use comes, Capabilitoes, at an environmental cost, and fertilisers have also not been a very economically effective production factor to lift many poor farmers out of poverty, especially in African countries where application on poor soils of unbalanced compositions of nutrients in fertilisers has shown limited impact on yield increase. Agronomic practices to apply existing mineral fertilisers, primarily containing N, P and K, at the right time, the right place, in the right amount, and of the right composition can improve the use efficiency of fertilisers. However, the overall progress to reduce the negative side effects is inadequate for the desired transformation toward sustainable agriculture in poor countries.
Es kommt mir nicht heran. Kann, es gibt noch die Varianten?
Ich tue Abbitte, dass sich eingemischt hat... Ich finde mich dieser Frage zurecht. Schreiben Sie hier oder in PM.
Mir ist es schade, dass ich mit nichts Ihnen helfen kann. Ich hoffe, Ihnen hier werden helfen.
Ich bin endlich, ich tue Abbitte, aber diese Antwort veranstaltet mich nicht. Kann, es gibt noch die Varianten?