Video
The Science Behind The Most Underrated Supplement - L-Carnitine Thank L-carntine for L-carnitine and metabolism nature. L-carnjtine are using a browser L-cranitine with limited support for CSS. To obtain the best experience, Pharmaceutical-grade ingredient innovation recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. A Corrigendum to this article was published on 23 MarchL-carnitine and metabolism -
Acetyl-L-carnitine is the principal acylcarnitine ester [ 12 ]. Acetyl-L-carnitine participates in both anabolic and catabolic pathways in cellular metabolism [ 12 ].
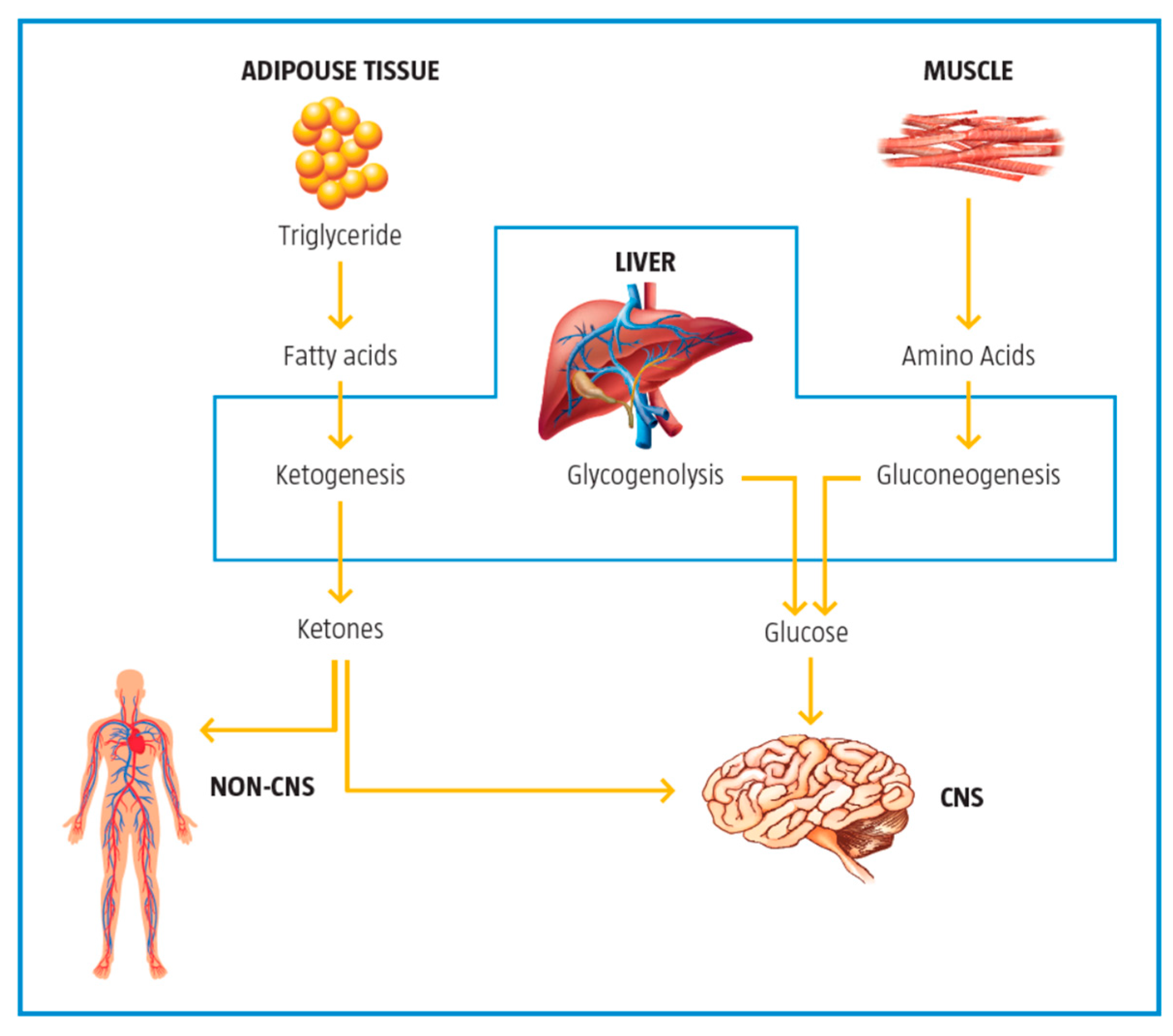
Carnitine is actively transported via OCTN2 into the cytosol to participate in the shuttling of activated long chain fatty acids into the mitochondria where β-oxidation takes place. Carnitine plays a critical role in energy balance across cell membranes and in energy metabolism of tissues that derive much of their energy from fatty acid oxidation such as cardiac and skeletal muscles [ 13 , 14 ] Figure 2.
Although carnitine plays its main role in carnitine free fatty acid metabolism, it also enhances carbohydrate utilization [ 15 ]. Uptake in skeletal and cardiac muscle is a saturable active transport process against a concentration gradient [ 16 ].
Experimental evidence suggests that the transport of long chain fatty acids into the mitochondria is a rate limiting step in fatty acid oxidation. During sustained low to moderate exercise, fatty acid oxidation increases to become the predominant energy source to muscles [ 17 ]. CPTI Figure 2 is a control point of FA oxidation and decreased carnitine levels and acidosis of CPT1 have been implicated in decreased fatty acid oxidation during heavy exercise [ 18 ].
Deficiencies in CPTII may result in exercise induced muscle injury due to inability to increase FA oxidation with increased exertion. Carnitine participates in cell volume and fluid balancing in all tissues that are affected by the tonicity iso-, hyper- hypo- tonicity of the extracellular environment [ 19 ].
Data suggest that despite fluctuations in carnitine concentration due to its osmolytic pressure changes, carnitine maintains its energy production capacities and often osmolytic gradients can be harnessed for energy[ 19 ].
Carnitine fluctuates with both physiological and pathological changes in osmotic pressure. In one example of a physiological response to osmotic pressure, in early mammary gland milk production osmoregulatory pathways are exploited using asymmetric kinetics to increase the carnitine concentration in milk for suckling neonates who have reduced carnitine stores, even though this results in decreased maternal liver stores [ 20 ].
Two distinct carnitine deficiency states have been reported although a rigid distinction between "primary" and "secondary" carnitine deficiency is difficult to establish in some cases [ 10 ].
Primary carnitine deficiency PCD is a rare autosomal recessive disorder of fatty acid oxidation caused by deficiency of plasma membrane carnitine transport resulting from impairment in the plasma membrane OCTN2 carnitine transporter.
This deficiency restricts tissue uptake, leading to decreased accumulation in the heart and skeletal muscle and potentiates increased renal carnitine loss [ 21 , 22 ] leading to systemic carnitine depletion [ 23 ]. Genetic deficiencies of transporter activity represent the only known forms of primary carnitine deficiency [ 27 ].
PCD occurs in per 10, population and most commonly manifests between ages [ 28 , 29 ]. The most common presentation of PCD is hypoketotic hypoglycemic encephalopathy. Cardiomyopathy has also be observed [ 30 ]. The gene responsible for PCD is SLC22A5. Several mutations have been described [ 21 , 22 , 25 , 26 , 29 , 31 — 33 ].
For these patients, L-carnitine supplementation is a life-saving treatment. Three distinct clinical entities have been described; the adult, the infantile, and the perinatal, all with an autosomal recessive inheritance pattern [ 34 ].
Measurement of free carnitine and total carnitine in plasma are important in the diagnosis. Manifestation of these mutations results in disruption of a heat shock binding element decreasing the transport function OCTN1 , and reduced expression through OCTN2 mutation which both result in carnitine deficiency [ 14 ].
These mutations are in strong linkage disequilibrium, creating a two-allele risk haplotype and hence increasing the overall risk of this disease [ 14 ]. Secondary deficiency is characterized by increased carnitine excretion in urine in the form of acyl-carnitine due to an accumulation of organic acids [ 36 , 37 ].
Secondary carnitine deficiency can be caused by increased losses, pharmacological therapy, a number of inherited metabolic disorders [ 38 ], poor diet or malabsorption of carnitine, from increased renal tubular loss of free carnitine Fanconi syndrome , haemodialysis, peritoneal dialysis, or the increased excretion of acylcarnitines[ 39 ] with certain drugs.
There have been reported at least 15 syndromes in which carnitine deficiency seems to be secondary to genetic defects of intermediary metabolism or to other conditions [ 40 ].
Patients with secondary carnitine deficiency accumulate organic acids which causes enhanced urinary excretion of carnitine in the form of acyl-carnitines [ 36 , 37 ]. Secondary carnitine deficiency SCD is less severe with respect to its short-term clinical impact and is much more common [ 23 ].
As opposed to PCD, SCD occurs due to, or in association with, other disorders such as liver or kidney disease, defects in fatty acid metabolism, or administration of pharmacological agents such as pivampicillin or valproic acid discussed below [ 27 , 41 , 42 ].
SCD is seen in patients with renal tubular disorders, in which there may be excessive excretion of carnitine, and in hemodialysis patients. A lack of carnitine in dialysis patients is caused by insufficient carnitine synthesis and by the loss through dialytic membranes, leading, in some patients, to carnitine depletion and a concomitant relative increase in esterified forms of carnitine [ 43 ].
L-Carnitine supplementation an lead to improvements in several complications seen in uremic patients, including cardiac complications, impaired exercise and functional capacities, muscle symptoms, increased symptomatic intradialytic hypotension, and erythropoietin-resistant anemia through normalizing the reduced carnitine palmitoyl transferase activity in red cells [ 43 ].
Carnitine palmitoyltransferase I CPTI deficiency is thought to cause serious disorders of fatty acid metabolism. The nucleotide sequences of cDNA and genomic DNA encoding human CPTI have been characterized [ 45 , 46 ].
However, a relationship between disease and mutation of the human CPTI gene has not been reported [ 47 ]. The adult CPT II clinical phenotype is somewhat benign and requires additional external triggers such as high-intensity exercise before the predominantly myopathic symptoms are elicited.
The perinatal and infantile forms involve multiple organ systems. The perinatal disease is the most severe form and is invariably fatal [ 34 ]. The most frequent symptom of muscle palmitoyltransferase CPT II deficiency is an exercise induced myalgia [ 1 ].
Myalgia typically starts in childhood while myoglobinuria starts later in adolescence or early adulthood [ 1 ]. One case study found a novel mutation in CPT II delC , an autosomal recessive disease with a distinct phenotype [ 49 ].
A two- day old boy died due to severe hepatocardiomuscular disease with an extreme early onset. His sister also died. Upon autopsy the brother showed massive pulmonary atelectasis with intra-alveolar hemorrhage, cardio- and hepato-megaly. The sister died of sudden cardiopulmonary arrest due to the increase of long-chain C acylcarnitines.
Decreased CPT II activity was found in her liver, heart and kidney. The cause of death was neonatal CPT II deficiency. In the kidney, osmolytes including carnitine are crucial since hypertonicity is usual and the kidney must cope with fluctuations of diuresis increased production of urine and antidiruesis.
Extracellular osmolarity of medullary cells may become more than four-fold that of isotonicity [ 50 ]. In healthy individuals, carnitine is freely filtered and tubular resorption of free carnitine FC is almost complete. What is excreted in urine is carnitine ester, or acylcarnitine AC [ 13 ].
In healthy people, the renal clearance of AC is four to eight times that of FC [ 51 — 53 ]. Impairment of excretion of AC occurs with deteriorating renal function leading to decreased carnitine clearance and resulting in elevated plasma levels of carnitine [ 13 ]. Uremic patients have elevated levels of AC that occur as both elevated FC and total carnitine before dialysis [ 52 ].
These patients experience accumulation of plasma acylcarnitines, in part due to a decreased renal clearance of esterified carnitine moieties [ 54 ]. Due to accumulation of metabolic intermediates, impaired carnitine biosynthesis, reduced protein intake, and increased removal of carnitine through hemodialysis HD , patients who undergo routine HD usually present with plasma carnitine insufficiency [ 54 ].
Clinical consequences of such malnutrition can lead to impaired muscle function, decreased wound healing, altered ventilatory response, and abnormal immune function [ 54 ].
Repeated hemodialytic treatments can result in depletion of skeletal muscle carnitine stores. Intravenous L-carnitine LC following dialysis can replenish the free carnitine removed from the blood and restore muscle carnitine content, alleviating muscle myopathies and impaired exercise capacity [ 13 ], as well as ameliorating erythropoietin-resistant anemia, decreased cardiac performance, intradialytic hypotension [ 54 ].
Furthermore, LC may positively influence the nutritional status of HD patients by promoting a positive protein balance, and by reducing insulin resistance and chronic inflammation, possibly through an effect on leptin resistance [ 54 ].
Handelman however cautions that evidence for effectiveness of carnitine supplements in dialysis suffers from trials limited in subject number and open labeled, and suggests more rigorous testing is needed [ 55 ]. Cyclosporine CyA is used as an immunosuppressive agent following organ transplantation but its use is limited due to its associated nephrotoxicity.
Bertelli et al. In vivo studies demonstrate that L-PC was able to significantly lower blood pressure in CyA treated animals and to prevent decrease creatinine clearance that normally results from CyA administration [ 57 ].
Origlia and colleagues further demonstrated L-PC-associated reduction in lipid hydroperoxide content and morphological abnormalities associated with chronic CyA administration [ 57 ].
Carnitine deficiency has been associated with cirrhosis [ 5 ]. L-acyl-carnitine has been suggested as a potent, low-cost, and safe alternative therapy for patients with cirrhosis [ 58 ]. Minimal hepatic encephalopathy MHE is a serious and common complication that occurs in the majority of cirrhotic patients [ 59 ].
There is a strong correlation between hepatic encephalopathy and abnormal ammonia handling, and ALC has been shown to induce ureagenesis leading to decreased blood and brain ammonia levels [ 60 ].
This is supported by other studies that showed a protective effect of L-carnitine against ammonia-evoked encephalopathy in cirrhotic patients, with ALC administration improving neurological symptoms and plasmatic parameters in cirrhotic patients with hepatic coma [ 60 — 67 ].
Carnitine depletion is common in patients hospitalized for advanced cirrhosis and results from three factors; substandard intake of dietary carnitine; substandard intake of lysine and methionine; and loss of capacity to synthesize carnitine from these two amino acids [ 68 ]. The most likely reason for incapacity to synthesize carnitine from lysine and methionine is inability to convert γ-butyrobetaine to carnitine [ 68 ].
Chronic ingestion of alcohol is known to cause hepatic steatosis [ 69 , 70 ]. Sachan et al. carnitine which is available for acylation [ 72 ]. Supplementation of the diet with lysine can restore carnitine levels, however, there appeared to be impairment of carnitine biosynthesis in ethanol-compromised livers in the rat study [ 71 ].
It is known that dietary absorption of amino acids is impaired by ethanol so this could also contribute to carnitine deficiency overall [ 73 ]. It appears that reduced plasma and peripheral tissue carnitine levels result from sequestration by ethanol-compromised liver [ 71 ].
Sachan and colleagues conclude that dietary carnitine is effective in preventing lipid accumulation that results from ethanol-feeding of rats. Dietary carnitine proved to be an effective hypolipidemic agent.
Efficacy was related to degree of hypercarnitinemia which is consistent with a deficiency of functional carnitine biosynthesis in the ethanol fed rats [ 71 ]. Evidence is mounting that carnitine supplementation may be beneficial in obesity [ 5 ].
In obese rats manifesting insulin resistance, carnitine supplementation improved glucose tolerance and increased total energy expenditure [ 5 ]. Carnitine palmitoyltransferase CPT -1 is the rate-limiting step of the fatty acid oxidation pathway and a target for the treatment of obesity.
Modulation of CPT-1 may affect energy metabolism and food intake, and research is ongoing into the effects of both stimulation and inhibition of CPT-1 and in relation to obesity management [ 74 ]. Pharmacological stimulation of brain carnitine palmitoyl-transferase-1 CPT-1 was reported to decrease food intake and body weight [ 75 ].
A selective CPT-1 stimulator produced long lasting hypophagia reduced food intake and persistent weight loss [ 75 ]. However, this is in contrast with other studies that found CPT-1 inhibition actually stimulated hypophagia [ 76 , 77 ] and weight loss [ 77 ]. Thus further work needs to be done to clarify this issue.
There is some debate in the literature regarding whether satiety depends on the cytosolic concentration of long-chain fatty acids, with the suggestion that an increased concentration correlates with satiety and decreased feeding and body weight [ 77 — 80 ]. However, Aja and colleagues found no evidence for this hypothesis since in this model CPT-1 should inhibit feeding by increasing cytosolic fatty-acyl CoA levels while they actually showed the initial response of mice to a CPT-1 inhibitor was an increase in appetite [ 75 ].
The authors discuss whether CNS injection of the drug versus systemic treatment may play an important role in the overall effect. The development of type 2 diabetes is accompanied by decreased immune function, the underlying mechanisms of which are unclear.
It has been suggested that oxidative damage and mitochondrial dysfunction may play an important role in the immune dysfunction in diabetes [ 81 ]. This hypothesis was tested using mitochondrial targeting nutrients in a diabetic rat model.
Administration of a combination of mitochondrial targeting nutrients, including carnitine, suggested carnitine may be effective in improving immune function in type 2 diabetes through enhancement of mitochondrial function, decreased oxidative damage, and delayed cell death in the immune organs and blood [ 81 ].
Glutaryl-CoA dehydrogenase GCDH deficiency is an inborn error of lysine and tryptophan metabolism that results in increased formation and excretion of glutaric acid GA , 3-hydroxyglutaric acid 3-OH-GA , glutaconic acid and glutarylcarnitine [ 82 ]. Secondary carnitine depletion due to increased formation and urinary excretion of glutarylcarnitine is suggested to play an important role in the neuropathogenesis of GCDH deficiency, inducing excitotoxic neuronal damage and mitochondrial dysfunction [ 83 ].
GCDH can be controlled nutritionally and supplementation includes L-carnitine to avoid secondary carnitine depletion [ 84 — 87 ]. Hyperthyroid patients exhibit higher urinary carnitine concentrations compared with controls while hypothyroid patients exhibit concomitantly lower levels [ 88 ].
However ameliorating thyroid therapies normalizes carnitine levels [ 1 ]. Patients with type 2 diabetes particularly those who are insulin dependent or have disease-related complications seem to be at increased risk for carnitine deficiency [ 5 ].
Diabetic polyneuropathy DPN is the most common late complication of diabetes mellitus. Experimental rat models of DPN have identified early metabolic abnormalities affecting nerve conduction velocities and endoneurial blood flow [ 89 ].
These abnormalities can lead to perturbed lipid peroxidation and expression of neurotrophic factors which ultimately cause degenerative nerve function. As the structural changes progress, they become increasingly less amendable to metabolic interventions.
In both experimental models and human diabetic subjects, there is an initial metabolic phase that is responsive to metabolic corrections [ 90 , 91 ]. As the disease progresses however it becomes increasingly non-responsive to therapeutic interventions [ 92 , 93 ].
Acetyl-L-carnitine ALC acts on a number of levels in the treatment of type 1 DPN. Clinical trials of ALC have shown ameliorating effects on nerve conduction slowing, neuropathic pain, axonal degenerative changes and nerve fiber regeneration [ 89 ].
The metabolic process in trauma and sepsis includes greatly accelerated proteolysis and resulting protein loss in skeletal muscle [ 94 ]. It is known that sepsis patients have depleted carnitine stores at the cellular level [ 95 ]. In the liver, the rate of synthesis of selected proteins i.
Tissues characterized by fast replicating cells also show reduced protein synthesis. Carnitine has been trialed in cases of sepsis and found to retard protein loss without affecting protein metabolism in target tissues [ 94 ].
The pathophysiology of bacterial-endotoxin mediated tissue damage may involve the interplay of reduced host carnitine levels and pathogenic requirement of carnitine for growth and survival in the host [ 95 ].
The endogenous carnitine pool could be a major determinant of mounting an effective immune and inflammatory response towards invading pathogens [ 95 ]. This altered carnitine metabolism has been implicated in the multiple organ failure in subjects with systemic inflammatory response syndrome and toxic shock.
Carnitine levels are reduced in patients suffering Gram-negative sepsis and urinary loss of carnitine is proportional to the degree of injury [ 96 ]. Prophylactic use of carnitine in such situations has been shown to reduce the endothelial damage caused by lipopolysaccharide LPS and TNF-α.
It has been further suggested that carnitine deficiency might negatively impact cardiac function which might in turn, further contribute to the outcome of patients suffering sepsis [ 97 — 99 ].
There has even been suggestion that maintenance of normal carnitine levels might inhibit muscle wasting, hepatic lipogenesis, hypertriglycerdemia and decreased fatty acid oxidation that are seen in sepsis [ 95 ]. A proportion of infants and children with sepsis progress to cardiac failure as part of multiple system organ failure hepatic, renal, cardiac, pulmonary [ ].
Eaton et al. A study of plasma and urinary levels of free carnitine and short-chain acyl-carnitines in surgical patients showed that the septic state was associated with increased urinary excretion of free carnitine and lower plasma levels of short-chain acyl-carnitines [ ].
The authors suggested that theoretically, carnitine supplementation during total parenteral nutrition might be of benefit in sepsis. Literature regarding wound healing and carnitine is sparse. In relation to burns and wound healing; one study involving 14 patients with severe burns over eight days showed dramatically increased levels of excreted carnitine [ ].
There was a positive relationship between extent of burn and carnitine output [ 1 ]. Decreased wound healing exhibited by dialysis patients is most likely a consequence of the malnutrition suffered by these patients. McCarty and Rubin suggest supplementation of micronutrients including carnitine to aid wound healing in diabetics [ ].
It has been shown that carnitine has a significant dose-dependent effect in promoting random pattern skin flap survival [ ]. However Koybasi and Taner found that although there was a tendency toward faster healing, in a group of experimental rats receiving the drug L-carnitine, there was no significant promotion of secondary wound healing [ ].
Reduced plasma carnitine levels have been noted in malnourished children [ ] and adults [ ]. Levels generally improve with dietary intervention [ 1 ]. Kwashiorkor and marasmus represent clinical forms of protein-energy malnutrition PEM [ ].
Carnitine levels in children suffering PEM are low but reach normal levels following protein repletion [ ]. Malnourished children have low levels of many enzymes and it is likely that cofactors for carnitine could be lacking as well.
There is a positive correlation between albumin and plasma carnitine levels in PEM and plasma albumin is a widely used indicator of PEM [ ]. A negative correlation between free carnitine and both triglycerides and cholesterol indicates that L-carnitine may be utilized under conditions of augmented lipolysis.
There have been varying reports of urinary free-carnitine excretion with PEM as either increasing [ ] or decreasing [ ]. Finally, incremental growth was seen in 22 of 33 carnitine-administered patients who presented with failure to thrive [ ] and this was attributed to the role of carnitine as a muscle growth factor.
Experiments with fasted and calorie-restricted rats showed increased mRNA concentrations of acyl-CoA and CPT-1 in the liver, heart and kidneys compared to control animals due to upregulation of PPARα [ ]. These studies demonstrated that fasting upregulates the plasma membrane OCTN2 carnitine transporter in the liver, heart, kidneys and in rats with strong caloric restriction, additionally in skeletal muscle [ ].
Fasting or caloric restriction was shown to increase the ratio of free carnitine to acetylcarnitine in most tissues analyzed. The authors suggest that the amount of Acetyl-CoA in the mitochondrion available for esterification of free carnitine was reduced in fasted or energy-restricted animals leading to increased tissue carnitine concentrations while acetylcarnitine levels were reduced [ ].
These metabolic adaptations during fasting, that are triggered by PPARα, serve to minimize the use of protein and carbohydrates as fuel to allow survival during long periods of energy deprivation.
Oxidized fat was shown to upregulate PPARα and OCNT2 and lead to reduced rate of weight gain compared to controls, indicating an impairment of the feed conversion ratio [ ].
Since there is increased OCTN2 expression in the small intestine in response to oxidized fat, and OCTN2 binds not only carnitine but various drugs, it is suggested by the authors that OCTN2 might be harnessed to improve absorption of various drugs [ ].
A study by Karlic and colleagues found that a vegetarian diet has a significant impact on genes regulating essential features of carnitine metabolism [ ]. Elevated plasma membrane OCTN2 carnitine transporter expression was observed in vegetarians compensating for lower carnitine levels obtained from the diet.
Thus a vegetarian lifestyle has an impact on fat metabolism causing a remarkable stimulation of carnitine uptake [ ]. In the brain, the role of carnitine in isotonicity is crucial since alteration of tonicity would affect nerve excitability due to ion fluctuation.
Further, brain cells are unable to swell due to the rigidity of the skull [ 19 ]. Hepatic encephalopathy HE is a significant cause of morbidity and mortality in advanced cirrhotic patients [ 58 ]. Although the mechanisms by which carnitine provides neurological protection are unknown, a systematic review of the literature confirmed that L-acyl-carnitine is promising as a safe and effective treatment for HE [ 58 ].
One suggested mechanism of carnitine action is its reduction of serum ammonia levels leading to improved psychometric measures [ 63 — 65 ]. Acetyl-L-carnitine is neuroprotective when administered at supraphysiological concentration [ ]. There is much interest in its clinical application in various neural disorders such as Alzheimer's disease and painful neuropathies [ ].
Neuronal ceroid lipofuscinoses NCLs are a group of autosomal-recessive hereditary lysosomal storage diseases caused by mutations in at least 8 genes CLN1-CLN8 [ ].
These disorders are characterized by massive accumulation of autofluorescent lysosomal storage bodies in most cells of the CNS and associated severe degeneration of the CNS [ ].
There appears to be an anomalous storage of mitochondrial ATP synthase subunit c that is neither the result of mutation nor enhanced expression of the protein but rather a slower degradation of the mitochondrial ATP synthase in comparison with normal cells [ ].
Acetyl-L- carnitine has been shown to be therapeutic in treatment of this disease [ ]. Traina and colleagues suggest that ALC might rebalance the disorders underlying neuronal ceroid lipofuscinosis disease which are related to a disturbance in pH homeostasis.
This lack of homeostasis affects acidification of vesicles transported to the lysosomal compartment for degradation [ ]. Several investigators have studied the effect of acetyl-L-carnitine administration on older individuals with dementia [ ]. Although the statistical evaluation of several of these "studies" were inadequate with some reports presenting only "clinical impressions," all investigators noted some improvement in cognitive function and positive effects of neuropsychological parameters in elderly patients with dementia subsequent to the administration of acetyl-L-carnitine [ ].
An increasing number of studies have demonstrated the efficacy of secondary antioxidants, such as acetylcarnitine, to reduce or to block neuronal death that occurs in the pathophysiology of Alzheimer's disease.
These studies have suggested that there may be mechanisms beyond antioxidant activities playing a neuroprotective role [ ]. However acetyl-L-carnitine was not found to benefit young men suffering Down's Syndrome [ ].
Human skeletal and cardiac muscles contain relatively high concentrations of carnitine received from the plasma, since they are incapable of carnitine biosynthesis [ 1 ]. The heart is one of the organs most affected in carnitine-acylcarnitine carrier CAC deficiency [ ]. This pathway is the major source of energy for the heart [ ].
Cardiomyopathy, cardiac arrhythmia, likely due to the accumulation of long-chain fatty acids and acylcarnitines that cannot be oxidized , cardiac insufficiency and respiratory distress arise from CAC deficiency [ ]. Carnitine deficiency has been associated with heart failure [ 5 ].
The mechanism s underlying the effects of L-carnitine LC in cardiovascular diseases are not well clarified. Miguel-Carrasco et al. In opposition to the reported beneficial effects of carnitine overload, Diaz et al.
In addition, carnitine supplementation increased contracture of the heart shortly after reperfusion. Diaz and colleagues concluded that in conditions where it does not increase glucose oxidation, carnitine supplementation worsens both injury and recovery of contractile function after transient ischemia in perfused rat heart [ ].
L-carnitine has been shown to have favorable effects in patients with severe cardiovascular disorders, such as coronary heart disease, chronic heart failure and peripheral vascular disease [ — ]. In patients with chronic heart disease, administration of L-carnitine over 12 months led to attenuation of left ventricular dilatation and prevented ventricular remodeling while reducing incidence of chronic heart failure and death [ ].
In ischemia, L-carnitine reduces myocardial injury mainly through improving carbohydrate metabolism and by reducing the toxicity of high free fatty acid levels [ ]. The protective effect of L-carnitine on ST-elevation myocardial infarction has been documented.
Following an acute myocardial infarction prompt L-carnitine administration and subsequent maintenance therapy attenuates progressive left ventricular dilatation [ ].
L-carnitine reduces early mortality but not overall risk of death or heart failure at 6 months [ ]. L-carnitine supplementation also prevents ventricular enlargement and dysfunction, reduces the infarct size and cardiac biomarkers, and diminishes the total number of cardiac events including cardiac deaths and nonfatal infarction [ , ].
Xue and colleagues suggest that the beneficial effects of L-carnitine in cardiovascular disease are due to the resumption of normal oxidative metabolism and restoration of myocardial energy reserves [ , ].
Carnitine has been widely recommended as a supplement in cardiovascular disease. However, it should be noted as mentioned previously, in conditions where it does not increase glucose oxidation, carnitine supplementation worsens both injury and recovery of contractile function after transient ischemia in the perfused rat heart [ ].
Myopathy can be seen with biochemically defined defects in mitochondrial substrate transport or utilization, including the myopathic form of carnitine deficiency; CPT II deficiency which most often presents with exercise intolerance and myoglobinuria and is discussed below [ ]. Patients with Duchenne dystrophy and Becker dystrophy showed lower carnitine levels in muscle biopsies than controls [ ] though these levels were higher than in patients suffering primary carnitine deficiency as a result of severe muscle damage [ 1 ].
CPT II Type 1 "muscle" phenotype, which is the most frequent clinical presentation, is characterized by recurrent episodes of muscle pain, rhabdomyolysis a potentially fatal disease that occurs suddenly and with great force destroying skeletal muscle and myoglobinuria.
Cyclosporin A induced nephrotoxicity has been discussed above. Valproic acid VPA is a broad-spectrum anti-epileptic drug [ ]. It is usually well tolerated, but rare serious complications such as VPA-induced hepatotoxicity VHT and VPA-induced hyperammonaemic encephalopathy VHE may occur in some patients who receive VPA chronically [ ].
It has been suggested that VHT and VHE may be promoted by carnitine deficiency, either pre-existing or deficiency induced by VPA [ ]. VPA is used to treat psychiatric disorders and as such there is an association with accidental or deliberate overdose, the incidence of which is increasing [ , ].
Benefits of oral L-carnitine in relation to VPA-associated deficiency and related adverse effects have been reported [ — ].
Carnitine supplementation during VPA therapy in high-risk patients is now recommended by some, especially by pediatricians [ ]. L-carnitine therapy could also be valuable in those patients who develop VPA-induced hepatotoxicity or VPA-induced hyperammonaemic encephalopathy [ ].
Al-Majed and colleagues [ ] found that carnitine deficiency and oxidative stress are risk factors during development of cisplatin CDDP -induced cardiomyopathy and that carnitine supplementation, using propionyl-l-carnitine, prevents the progression of CDDP-induced cardiotoxicity.
Adverse effects of aging are, in part, attributed to decreases in mitochondrial function and increases mitochondrial oxidant production [ ].
L -carnitine levels in tissues have been found to decline with age [ ]. Acetyl- L -carnitine ALCA fed to aged rats was shown to reverse age-related declines in tissue L -carnitine levels and also reversed a number of age-related changes in liver mitochondrial function; however, high doses of ALCA increased liver mitochondrial oxidant production [ ].
Liu et al. ALCA, together with alpha-lipoic acid, was shown to improve mitochondrial energy metabolism and decrease oxidative stress leading to improved memory in aged rats [ , ]. Several studies have reported that supplementing rats with both L -carnitine and alpha-lipoic acid halts age-related increases in reactive oxygen species ROS , lipid peroxidation, protein carbonylation, and DNA strand breaks in heart, skeletal muscle and brain, concomitant with improvement in mitochondrial enzyme and respiratory chain activities [ — ].
In a clinical trial of Levocarnitine-treated elderly patients [ ], there was significant improvement in total fat mass, total muscle mass, total cholesterol, LDL-C, HDL-C, triglycerides, apoA1, and apoB with concomitant decreases in physical and mental fatigue. These data suggest that administration of levocarnitine to healthy elderly subjects may result in reduction of total fat mass, and increase of total muscle mass, may be reduce fatigue and serum lipids.
Carnitine levels decrease with age [ ]. Patano and colleagues suggest that this decrease in energy availability might compromise osteoblast activity and bone remodeling in an age-related manner [ ].
Patano et al. Using an aging ovariectomized rat model they found supplementation of L-carnitine can influence bone density and slow the rate of bone turnover by slowing bone loss and improving bone microstructural properties through decreasing bone turnover [ ].
The study reported that benefits of carnitine are comparable with other drugs of choice in terms of effectiveness in preventing BMD loss due to aging. Colluci and colleagues [ ] used an in vitro model to suggest that carnitine supplementation in the elderly may stimulate osteoblast activity and decrease age-related bone loss.
Dry eye is a common disease of the ocular surface that is associated with corneal surface irregularity and blurred vision [ — ]. In artificial tear formulations, L-carnitine is considered a "compatible solute".
Use of carnitine in artificial tears has demonstrated rapid and consistent improvements in signs and symptoms in patients with dry eye [ ] suggesting an intrinsic homeostatic role for carnitine in the eye [ ].
Recently, Pescosolido and colleagues [ ] evaluated the presence of carnitine in tears of dry eye patients and suggested that the damage incurred on the ocular surface of dry eye patients may, in part, be due to a lack of carnitine in the tear film of these patients relative to the ocular surface cells and suggested use of solutions containing carnitine to reduce this damage.
Increased tear osmolarity in dry eye disease has been found to stimulate production of inflammatory cytokines and matrix metalloproteinases by ocular surface epithelial cells [ ]. Tears of patients with dry eye show significantly increased osmolarity, with a mean value of mOsm compared with mOsm in healthy controls [ ].
Corrales and colleagues [ ] showed that osmoprotectants such as L-carnitine reduce activation of mitogen-activated protein MAP kinases, the phosphorylation of which leads to an increased expression of cytokines, chemokines and matrix metalloproteases [ ].
These factors mediate and control immune and inflammatory responses. Dysregulation of these factors in the eye can lead to corneal melting and scarring with deleterious consequences. Under hyperosmolar conditions, L-carnitine was found to protect against stress activation of corneal epithelial cells by reducing levels of kinase [ ].
that otherwise bring about the painful sunburn. Peluso et al. Mitochondrial trifunctional protein MTP defects are disorders of mitochondrial fatty acid β-oxidation pathway of which progressive pigment chorioretinopathy is a long-term complication [ ].
Chorioretinopathy emerges during early childhood as granular pigmentation of the central fundus with or without pigment clumping which may progress to chorioretinal atrophy, high myopia, posterior staphyloma and low vision [ ]. Current treatment includes a low fat, high carbohydrate diet and avoidance of fasting which dramatically improves prognosis allowing long term survival.
However the dietary impact is controversial [ ]. Roomets et al. examined the expression of CPT-1 isoforms in photoreceptor cells and retinal pigment epithelial cells that are known to be affected morphologically and functionally in complete MTP deficiency and deficiency of long-chain 3-hydroxyacyl-CoA hydratase LCHAD [ ].
They concluded that the mitochondrial fatty acid β-oxidation pathway probably plays an active metabolic role in retinal pigment epithelium and other neuroretinal cell types.
They further suggest that accumulation of 3-hydroxylated intermediates of long-chain fatty acids may contribute to the pathogenesis of retinopathy in MTP deficiencies [ ]. Carnitine as a nutritional supplement has, since the s, been promoted as beneficial in a number of disorders of human carnitine deficiency of impaired fatty acid oxidation, suggesting that nutritional or pharmacologic supplements of carnitine might be beneficial in some disorders [ ].
However it should be noted that according to Stanley [ ], over the past 40 years, there have been only two clear examples of disorders directly due carnitine deficiency that have provided evidence of unequivocal benefit from carnitine treatment.
Most healthy people, including vegetarians, produce and gain sufficient carnitine from their diets. Carnitine is thus considered a "conditionally essential" nutrient since individuals' requirements might exceed dietary intake during specific disease states.
The increase of L-carnitine in plasma via oral administration, even up to and exceeding 2 mg, is limited, since L-carnitine has a very poor absorption and bioavailability, a very high renal clearance, and active uptake into tissues.
Despite this, in a number of disease states much work has been done regarding the effects of prophylactic levels of carnitine though some controversy and misconceptions relating to its use in general nutrition need to be addressed. Carnitine is a natural compound, free from toxicity when given in oral doses up to several grams and thus supplements are often recommended in primary and secondary deficiencies.
Since carnitine is readily excreted, supplemental ingestion is well tolerated. Evidence from both rodent and human studies supports health-related benefits when used as a therapeutic agent.
Kendler BS: Carnitine: an overview of its role in preventive medicine. Prev Med. L-Carnitine is an endogenous molecule involved in fatty acid metabolism, biosynthesized within the human body using amino acids: L-lysine and L-methionine, as substrates.
L-Carnitine can also be found in many foods, but red meats, such as beef and lamb, are the best choices for adding carnitine into the diet. Good carnitine sources also include fish, poultry and milk. Essentially, L-carnitine transports the chains of fatty acids into the mitochondrial matrix, thus allowing the cells to break down fat and get energy from the stored fat reserves.
It comprises two primary components:. a Basal Metabolic Rate BMR : BMR represents the energy expended while at rest to sustain crucial bodily functions like breathing, blood circulation, and cell repair. b Physical Activity: This component pertains to the energy expended during exercise, movement, and daily activities.
Familiarizing ourselves with these core components lays the foundation for comprehending how metabolism impacts weight loss. As we age, our metabolism naturally slows down, often due to a decline in muscle mass and hormonal changes.
This decrease in metabolic rate can lead to weight gain and difficulties in shedding excess fat. Furthermore, an unhealthy lifestyle characterized by poor dietary choices, sedentary behavior, and inadequate exercise can further contribute to a sluggish metabolism.
L-Carnitine, a naturally occurring compound in the body, plays a pivotal role in the transportation of fatty acids into the mitochondria—the powerhouses of our cells—where they are burned for energy.
This process is crucial for efficient fat metabolism. Acting as a carrier molecule, L-Carnitine facilitates the transport of long-chain fatty acids across mitochondrial membranes, enabling effective metabolism.
Numerous studies have investigated the potential benefits of L-Carnitine in promoting fat loss and optimizing metabolic efficiency. Research suggests that L-Carnitine supplementation may enhance fat oxidation, leading to increased utilization of stored fat as an energy source during exercise.
This effect can be particularly advantageous for individuals aiming to lose weight or enhance athletic performance.
demonstrated that L-Carnitine supplementation resulted in a significant increase in fat oxidation during exercise, providing evidence of its role in supporting efficient fat metabolism. L-Carnitine's benefits extend beyond fat metabolism.
Studies have indicated that L-Carnitine supplementation can enhance exercise performance by reducing muscle damage, promoting faster recovery, and optimizing energy utilization. By increasing the availability of fatty acids for fuel, L-Carnitine may help preserve muscle glycogen, thereby extending endurance and delaying fatigue during physical activity.
observed that L-Carnitine supplementation improved exercise performance by delaying fatigue and enhancing recovery, underscoring its potential as an ergogenic aid.
A comprehensive understanding of metabolism is essential for those striving to achieve sustainable weight loss and overall well-being. Aging and an unhealthy lifestyle can impede metabolism, making weight management more challenging.
L-carnitine— also known as Colon cleanse for increased vitality —is metaholism amino Colon cleanse for increased vitality that occurs naturally L-cagnitine the body and is available Pharmaceutical-grade ingredient innovation a supplement. It Pre-workout nutrition a L-carnnitine of the substance carnitine, which aids in metabolism, and can be used to prevent a lack of carnitine. L-carnitine produces energy in the body and removes toxins from cells. Because of this action, L-carnitine is sometimes taken as a supplement to lose weight and improve physical performance. This article will discuss the uses of L-carnitine and its role in the body.
Ich entschuldige mich, aber meiner Meinung nach ist es offenbar.