
Amino acid synthesis pathway in microorganisms -
The metabolic processing of an amino acid mixture by two distinct anaerobic microbial communities collected from Islinger Mühlbach ISM and Sippenauer Moor SM , Germany was examined.
The amino acid mixture contained L-alanine, β-alanine, L-aspartic acid, DL-proline, L-leucine, L-valine, glycine, L-phenylalanine and L-isoleucine. In parallel, an amino acid spiked medium without microorganisms was used as a control to determine abiotic changes over time.
Liquid chromatography mass spectrometry LC-MS was used to track amino acid changes over time. Although glycine degradation can be caused by abiotic processes, these results show that its preferential depletion in an environment would be consistent with the presence of life. We found changes in most other amino acids that varied between amino acids and communities, suggesting complex dynamics with no clear universal pattern that might be used as a signature of life.
However, marked increases in amino acids, caused by cellular synthesis and release into the extracellular environment e. We conclude, that substantial anomalous enhancements of some amino acids against the expected abiotic background concentration may be an agnostic signature of the presence of biological processes.
The search for signatures of extraterrestrial life, extinct or extant, is a key goal for the research field of astrobiology. One way to search for life is to seek the remains or products of biological processes Hays et al.
Examples include the fossil remains of single cells or communities e. Amino acids have previously been considered as a biosignature Parnell et al. Amino acids are ubiquitous in life as main components of cells and, apart from glycine, which is not chiral, biological systems on Earth almost exclusively use the L enantiomeric form.
However, some D-amino acids can be found in the cell membranes of bacteria Kaiser and Benner, ; Lam et al. Evidence has been provided that indicates enantiomeric excess of certain amino acids in meteorites Busemann et al. Consequently, the ratio of the enantiomers of amino acids was proposed as a signature of life, with terrestrial life showing an enantiomeric excess of L-amino acids Avnir, Although only twenty core amino acids are found in Earth organisms proteogenic amino acids , there are several hundred known non-proteogenic amino acids Gutiérrez-Preciado et al.
Therefore, the mere presence of amino acids in an extraterrestrial sample does not indicate a signature of life and cannot be used as a biomarker itself Parnell et al.
Nevertheless, the abundance of certain amino acids in a sample might provide clues on the presence of microorganisms. While abiotic processing of amino acids is driven by thermodynamic processes, in biological systems their relative abundance is the result of metabolic activity in any given organism or a community Davila and McKay, In addition, life uses only a selection of amino acids, while extraterrestrial carbonaceous matter contains, as noted above, a much broader variety.
One method to investigate the presence of life, distinct from looking for the amino acids in life itself e. In many extraterrestrial environments, including Mars, we would expect amino acids in addition to other organic compounds to be available to any putative biota.
They are delivered to a planetary surface in carbonaceous chondrite meteorites Cronin and Pizzarello ; Ehrenfreund et al. For example, a wide range of amino acids has been detected in carbonaceous chondrites.
The frequency of the prebiotic synthesis of amino acids and their abundances follow thermodynamic principles with the chemically simple compounds being most abundant Miller, ; Pizzarello, ; Pizzarello and Shock, ; Glavin et al.
A large number of microorganisms can use amino acids as electron donors for anaerobic respiration or in fermentation Nixon et al. In this process, they degrade the amino acid molecules. Microorganisms are unlikely to degrade amino acids at the same rate compared to degradation by abiotic processes.
Rather, they will degrade the molecules according to their metabolic pathways, the accessibility of certain amino acids, the availability of other metabolizable organic compounds, and other organism-specific effects. Thus, we could hypothesize that, in the process of degrading abiotic amino acids, microorganisms would leave a biosignature by the preferential degradation of certain amino acids in the environment around them.
This biosignature might be superimposed on the biosignature of the amino acids in the organism itself and that were synthesized by the organism, but it would be a distinct and additional biosignature reflecting non-random biological destruction of the abiotic amino acid pool.
One attraction of such a biosignature is that, if cells alter the amino acid concentration in the environment around them, then, particularly in low biomass environments, that signature might be much more pervasive and easier to detect than the amino acid signature of the cells themselves, which could be highly localized and poorly preserved.
Furthermore, although this signature would assume the presence of amino acid-using life, the decrease or increase in any amino acids away from the expected background abiotic concentration could be an agnostic signature of metabolic processes. In this study, we tested this hypothesis by investigating the metabolic usage of seven amino acids previously detected in both terrestrial environments and Martian meteorites by two distinct anaerobic microbial communities from Martian analog environments.
We used two distinct communities to determine if there were common patterns of degradation of certain amino acids that could potentially suggest a universal signature of amino acid degradation by life.
The almost complete degradation of glycine was common to both communities. For other amino acids, we observed different patterns of degradation with increased extracellular concentrations of some amino acids. We discuss the implications of these findings for life detection.
The samples investigated were collected in the frame of the MASE Mars Analogs for Space Exploration project, a 4-year collaborative research project supported by the European Commission Seventh Framework Contract. The aim of the project was to characterize Mars analogue environments on Earth with regard to habitability and the search for potential biosignatures of extraterrestrial environments Cockell et al.
Samples analyzed herein were collected from two sulfidic springs close to Regensburg, Germany, where many sulfide-containing springs emanate from Mesozoic karst formations.
The two springs, in the Sippenauer Moor SM and Islinger Mühlbach ISM areas The sites SM and ISM are independent and not connected in the deep subsurface. Detailed analyses of the site microbiomes are already available Moissl et al.
All samples were taken under anoxic conditions for a detailed description see Cockell et al. Cultivation was performed under anoxic conditions. Samples from SM and ISM were inoculated in anoxic MASE medium II and supplemented with a mixture of proteogenic and non-proteogenic amino acids.
MASE II medium contains per liter: NH 4 Cl 0. Prior to inoculation, the medium was supplemented with a filter-sterilized amino acid broth. Amino acids such as alanine, aspartic acid, glutamic acid, glycine, leucine, serine, and valine are common in both biological samples and for example, carbonaceous chondrite meteorite Cronin and Pizzarello, ; Shimoyama et al.
Based on these data, the following mixture of amino acids was added to the medium: glycine, L-alanine, β-alanine, L-aspartic acid, DL-proline, L-leucine, L-valine, L-phenylalanine and L-isoleucine Table 1. The amino acid broth used in this study included some proteinogenic amino acids that are most likely not found in meteorites due to their complexity in synthesis.
The final concentration of each added amino acid was 10 mM, and the pH was adjusted to 7. One millilitre of the environmental sample was added to 20 ml of medium and incubated at 30°C. A negative control NC , i. TABLE 1. Chemical properties of the supplemented amino acids and their side chains.
After defined time points, 1. The first sample was taken immediately after inoculation T0 , followed by samples after 7, 14, 28, 56, and 90 days of incubation. The sample was sterilized using a 0. Amino acid extraction was performed using a simplified procedure described in Aerts et al. Therefore, the extraction protocol is described in the following only briefly.
For sterilization, all glassware, including the columns with glass wool for amino acid extraction, were double wrapped in aluminum foil and placed into a furnace at °C for a minimum of 3 h. A sequential washing with basic-neutral-acid-basic solutions was made to activate the resin active sites.
After the sequential washing procedure, 1. The sample was vortexTed at 2, rpm for 30 s and subsequently added to the column. Note, that this first elution was not collected for further analysis and was disposed. The system used for amino acid analysis is described in Aerts et al.
Measurements were performed using an Agilent LC-MS system equipped with an ultraviolet UV and fluorescence FL detector system, an autosampler module where the amino acid derivatization is performed, and a MS ion trap mass spectrometer with electrospray ionisation.
The column used for analysis was a × 3 mm 2. The MS was operated in positive mode with optimised conditions for each individual amino acid. Amino acids were derivatised using a method based on Nimura and Kinoshita which was then automated in order to increase the robustness of the method. This automation was achieved by programming the autosampler module Agilent GB of the HPLC to mix the various reagents.
The approach used was as follows: the amino acid sample was mixed in a ratio with 0. In a typical measurement run, amino acid samples from one time point including negative control were analysed sequentially, including wash procedures and the analysis of amino acid standard solutions Agilent, part number: — Proline was not measurable as it cannot be derivatized and is therefore not detectable using the applied method.
Standards were run at the beginning and end of each run in order to track reagent degradation and system performance. The standard deviation was added as error bars to the measurements of the amino acids of SM and ISM.
FIGURE 1. Degradation of glycine from the two different enrichments A Islinger Mühlbach ISM , and B Sippenauer Moor SM spiked with a broth of amino acids final concentration of each added amino acid was 10 mM over a time of 3 months.
C Negative control, i. FIGURE 2. LC-MS measurements of the three amino acids β-alanine, L-aspartic acid, and L-phenylalanine from the two different enrichments over a time of 3 months. A Islinger Mühlbach ISM , and B Sippenauer Moor SM spiked with a broth of amino acids final concentration of each added amino acids was 10 mM.
FIGURE 3. LC-MS measurements of the four amino acids L-alanine, L-valine, L-leucine, and L-isoleucine from the two different enrichments over a time of 3 months. A Islinger Mühlbach ISM , and B Sippenauer Moor SM spiked with a broth of amino acids final concentration of each added amino acid was 10 mM.
Using LC-MS measurements, we investigated the differences in the amino acid distribution in the medium of two different microbial enrichments and the negative control. We found that in the control samples in which no microbiota was added, no significant changes of amino acid concentrations were observed over time Figures 1 — 3.
Therefore, the changes observed in the inoculated samples are attributed to microbial activity. Note, because the signal of measured amino acids in NC for time point 56 days was significantly lower in comparison to the signal of the NC of the other time points, NC of time point 56 days was not considered for analysis.
No such decrease was observed for the measurements of amino acids in the SM and ISM samples of the same time point, which points to a sample problem and not to an instrument malfunction. The results after analysis using LC-MS revealed that the quantities of the non-glycine amino acids varied over time depending on the microbial community and the amino acid see Figures 1 — 3.
Although the concentrations of amino acids varied between the two enrichments, we found one amino acid characteristic that was consistent with both enrichments Figure 1. The depletion follows an exponential decay; see fit to the date in panel B of Figure 1. Note, the same fit could not be applied to ISM1 data panel A because of missing sample for time point 7 days; the fit would be too steep at the beginning.
These data suggest a preferential use of glycine by these microbial communities. For all amino acids, we observed two different patterns of the measured relative ratios: 1 a differential use of amino acids was revealed, i. B-ala, L-asp, and L-phe did not reveal a clear trend with time Figure 2.
For example, in the ISM inoculum, the amount of β-ala and L-asp decreased over the first 2 weeks, followed by a peak 2. In the SM inoculum, L-asp increased over the first three measurements before a steady, but small decrease was observed.
In contrast, for β-ala the initial decrease was prolonged, before a peak followed by a decrease Figure 2B.
L-phe followed a similar trend in the SM sample Figure 2B , but it was less prevalent in the ISM sample Figure 2A. However, these trends were not significant. The measured amount of amino acids L-ala, L-val, L-leu, and L-ile in the media increased in IM enrichment compared to the observed decrease in the SM enrichment.
All four amino acids in the IM enrichment followed a similar pattern: Within the first 3 weeks an increase was detected followed by a plateau phase Figure 3A. The largest increase was seen for L-ala whereas only a small increase was detected for L-val.
While in the SM sample, L-ile decreased the most and L-ala and L-leu were less depleted from the media, the plateau phase also started after about 2 weeks, revealing relatively small changes of the amino acid abundance Figure 3B.
This study investigated whether the fingerprints of microbial amino acid metabolism could be used as a potential biosignature. Beside a preferential use of amino acids, the microbial community can release certain amino acids as metabolic products into their surroundings.
The release can occur by excretion or passive diffusion or the result of cell death followed by cell lysis. In addition, a variety of abiotic processes leading to the formation or degradation of certain amino acids can result in a change of prevalent amino acid abundance within an environment.
The following discussion is based on the assumption that potential extraterrestrial life uses similar biochemistry in liquid water environments as observed for Earth-based life. Life as we know it is based on mainly CHNOPS elements and other mineral sources for generating energy and the usage of amino acids to form proteins.
The data obtained for glycine suggest a preferential use of glycine by microbial communities. We found that almost all the glycine was depleted and we observed this for both communities, suggesting the possibility that glycine depletion in an environment would be consistent with life.
There are several mechanisms in bacteria involved in glycine uptake and metabolism Sagers and Gunsalus, ; Andreesen, In anoxic environments, glycine can be a substrate in the Stickland reaction, which is a coupled oxidation-reduction reaction mainly for amino acid pairs Andreesen, Glycine serves preferentially as an electron acceptor which can be coupled to an energy conservation step.
Glycine and alanine can act as a redox couple in which glycine is reduced while alanine is oxidized. This reaction would also lead to a decrease in alanine, which is observed for the Sippenauer Moor SM sample Figure 2B but not for the Islinger Mühlbach ISM sample Figure 2A. Consequently, these results could either indicate the presence of different metabolic activities in these communities or that the Stickland reaction is not the main mechanism leading to the reduction of glycine in the medium.
Another explanation for the microbial removal of glycine from the medium could be the result of an energy-producing reaction where two molecules of glycine could be used to form serine and CO 2. This has previously been reported for Pediococcus glycinophilus Sagers and Gunsalus, Furthermore, glycine can be used as part of the peptidoglycan in the cell wall Veuger et al.
With the current experimental set-up, a detailed analysis on the metabolic mechanisms underlying the preferred removal of glycine is not possible.
However, the reduction of glycine and therefore the lack of detectability among the presence of other amino acids can be further explored as a potential biosignature.
In order to evaluate whether the absence of glycine is a valuable biosignature to find life on Mars, its abiotic stability on Mars needs to be considered. Glycine is one of the most abundant amino acids detected in meteorites and comets Botta and Bada, ; Elsila et al.
Various laboratory studies have investigated not only the abiotic degradation of glycine Schuerger et al. Mars simulation studies determining the effect of UV irradiation on glycine revealed a degradation which results in the release of methane into the atmosphere Schuerger et al.
Owing to its simple chemical structure, glycine has the fastest degradation rate of amino acids. Extrapolated from ISS experiments, Noblet et al.
Another set of exposure experiments on the ISS days for a total of 2, h solar constant radiation, equivalent to 1, Compared to UV radiation, galactic cosmic rays and solar energetic particles mainly protons can penetrate deeper into soil and ice Mancinelli and Klovstad, A decrease by a factor 5—10 in a depth of a few meters is expected from the surface dose rate of 0.
Gerakines and Hudson performed experiments to study the half-lives of glycine in either CO 2 -ice or H 2 O-ice when irradiated with protons. The destruction rate constants indicated that glycine is less stable in CO 2 -ice Mars compared to H 2 O-ice Mars and Europa.
When extrapolating these data to conditions in the Martian subsurface, the half-life of glycine is modelled to be less than — million years even at depths of a few meters Gerakines and Hudson, Other studies estimated that amino acids when shielded from radiation could potentially survive billions of years in cold and dry niches on Mars Ehrenfreund and Charnley, ; Ehrenfreund et al.
Similar conclusions apply to Europa, Pluto, other icy satellites and to comets Gerakines and Hudson, Furthermore, a temperature effect has been observed in a previous study Gerakines et al.
With increasing temperatures, amino acids are less stable Gerakines et al. In addition, the mineralogy of the Martian regolith has an influence on the preservation of amino acids. Clay minerals or sulfate rich environments have been reported to show higher preservation rates compared to minerals containing ferrous iron dos Santos et al.
This effect was also noted in space exposure experiments where amino acids intermixed with meteorite powder had a higher stability than without Bertrand et al. These data imply that, in order to determine a biotic origin for the absence of glycine, several factors have to be considered.
Due to the thermodynamics and kinetics of amino acid synthesis this observation is remarkably consistent for any synthesis environment. And lastly, the prevalence of glycine in an environment on Mars will be the result of a combination of these factors.
In conclusion, the biotic degradation of glycine has potential as a biosignature of metabolic activity, but to avoid a false positive, it is necessary to understand the environmental conditions and context of the samples and the abiotic pathways and kinetics of glycine degradation.
Contrary to the glycine observations, there was no similar trend for the other amino acids in both samples. These complex and different changes in both enrichments could be the result of: 1 different chemical properties Table 1 , 2 the interaction of the various biochemical roles of amino acids, and 3 the composition of the microbial community.
Amino acids are not only the building blocks of proteins, but have other functions including the use as energy metabolites, essential nutrients, or chemical messengers in communication between cells.
Therefore, changes in the detectable amount of amino acids may represent the integrated effect of a diversity of metabolic pathways occurring in the respective microbial communities.
Microbial amino acid metabolism is a complex system involving transporters for uptake, biosynthesis as well as degradation and extraction of amino acids in a single microorganism. Transport mechanisms and metabolic pathways for the individual amino acids vary considerably in complexity Krämer, There are two mechanisms that could lead to different fingerprints.
On the one hand, as is the case in our experimental set-up, when amino acids are available in the environment, they are transported into the cells using different uptake systems. Differential consumption and utilization rates of the available substrate lead to a decrease of a certain amino acid.
On the other hand, amino acids can be excreted from the cells. The formation of amino acids and intermediates in the course of amino acid metabolism which are released into the environment results in an increase of a certain amino acid. In addition, the complexity increases when investigating microbial communities as amino acid production and utilization are characterized by the sequential action of different metabolic pathways in organisms belonging to a consortium.
There is the potential that an amino acid released into the medium from one member of the community can be utilized by another member and is therefore not detectable in the medium. The results from the ISM enrichment revealed a higher amount of L-ala, L-val, L-leu and L-ile after incubation than initially added to the medium.
This could be the result of amino acid synthesis from precursor molecules which then have been released into the medium either via active transport, diffusion or is a result of cell death and subsequent lysis Gutiérrez-Preciado et al. Furthermore, the external increase of alanine might be indicative of a passive efflux which also has been observed for proline, aromatic and branched chain amino acids Driessen, ; Krämer, These results show how, in addition to the potential biosignature of the cells themselves, microbial metabolisms might increase the surrounding environmental concentrations of amino acids above those expected abiotically, suggesting that anomalously high concentrations of amino acids could be a biosignature.
If these amino acids leached into preserved sediments where there was no preservation of cells themselves, they might act as an indirect signature of the proximal presence of life.
Although we observed high concentrations of alanine, in principle an increase in any amino acid anomalously above the expected abiotic background could be an agnostic signature of biological processes. We observed a decrease in the abundance of amino acids in the surrounding environment in the SM enrichment.
One explanation for higher rates of substrate metabolism compared to biosynthesis leading to a reduction of amino acids, might be that the microbial community was performing maintenance metabolism rather than active growth.
As with glycine, in order to be useful as a biosignature, these observed decreases would have to be considered alongside abiotic degradation rates.
As for glycine, the UV photodestruction rate for L-alanine and β-alanine is dependent on whether the amino acids are on the surface free or embedded in UV non-penetrable solid surfaces subsurface , or embedded in UV penetrable surfaces such as ice.
On Mars, the half-life of ala with a radiation dose rate of 2. Similar values with variations of about two orders of magnitude have been proposed for Pluto, comets in the outer Solar System, a cold diffuse and a dense interstellar medium Gerakines and Hudson, As seen for glycine, the half-lives are several orders of magnitude lower for Europa at the near surface 1, years at 1 cm.
In summary, these data suggest that the individual fingerprints of the amino acids alanine, aspartic acid, valine, leucine, isoleucine and phenylalanine can vary, i.
Cultivation and subsequent isolation has shown that the microbial communities from these two samples vary significantly Cockell et al. Although these complexities do not rule out such amino acid degradation patterns as biosignatures, they suggest that further investigation would be needed on any given sample, including its geological and temporal context, to determine if the differential changes in the amino acids in different communities can be disentangled from the expected abiotic degradation processes and attributed to a potential metabolic influence.
Nevertheless, the observed synthesis and extracellular excretion of amino acids, leading to a large local increase in the concentration of some amino acids, as observed in the ISM sample, might be another promising signature of life. The results of this study demonstrate a new type of amino acid imprint in an environment as a biomarker, which could be used alongside other methods to identify past and present life in extraterrestrial environments.
The results indicate that the biologically mediated and almost complete depletion of glycine could be one amino acid signature that could be sought to corroborate the presence of life. Other amino acids showed diverse changes. Depletions could only be a biosignature if, like glycine, they can be disentangled from abiotic changes, but their presence would at least be consistent with life.
Perhaps more interestingly, large increases in concentrations of amino acids resulting from excretion, might also be an agnostic indication of the presence of microbiota metabolizing amino acids.
The results we present here show how the effects of life on the surrounding amino acid profile may be another organic signature of its presence and metabolic activities.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author. PS contributed to the experimental protocol, performed the experiments, data analysis, and wrote the manuscript. AR and DM performed the LC-MS measurements; PH, RL assisted in performing experiments and contributed by proof reading the manuscript.
CS conceptualized the project as a whole and helped write the manuscript. All authors engaged in discussions, proof-read and approved the final manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Aerts, J. Biota and Biomolecules in Extreme Environments on Earth: Implications for Life Detection on Mars. Life 4, — PubMed Abstract CrossRef Full Text Google Scholar.
Conversely, was the initial set much less than twenty, and did new amino acids successively emerge over time to fit into the protein synthesis repertoire? What are the tempo and mode of amino acid pathway evolution? These questions are waiting to be tackled — with old or new hypotheses, conceptual tools, and methodological tools — and are ripe for a new generation of scientists.
Scientists now recognize twenty-two amino acids as the building blocks of proteins: the twenty common ones and two more, selenocysteine and pyrrolysine. Amino acids have several functions.
Their primary function is to act as the monomer unit in protein synthesis. They can also be used as substrates for biosynthetic reactions; the nucleotide bases and a number of hormones and neurotransmitters are derived from amino acids.
Amino acids can be synthesized from glycolytic or Krebs cycle intermediates. The essential amino acids, those that are needed in the diet, require more steps to be synthesized. Some amino acids need to be synthesized when charged onto their corresponding tRNAs. We have discussed only two biosynthetic routes: the Trp pathway, which appears to have evolved only once, and the Lys pathway, which seems to have evolved independently in different lineages.
Prevailing evidence suggests that metabolic pathways themselves seem to be evolving following the patchwork assembly model, which proposes that pathways originated through the recruitment of generalist enzymes that could react with a wide range of substrates.
The study of the evolution of amino acid metabolism has helped us understand the evolution of metabolism in general. Baumann, P. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects.
Annual Review of Microbiology 59 , — doi Bock, A. Biosynthesis of selenoproteins — an overview. Biofactors 11 , 77—78 Fani, R. et al.
The role of gene fusions in the evolution of metabolic pathways: The histidine biosynthesis case. BMC Evolutionary Biology 7 Suppl 2 , S4 doi Gordon, A. Partition chromatography in the study of protein constituents.
Biochemical Journal 37 , 79—86 Hernandez-Montes, G. The hidden universal distribution of amino acid biosynthetic networks: A genomic perspective on their origins and evolution.
Genome Biology 9 , R95 doi Horowitz, N. On the evolution of biochemical syntheses. Proceedings of the National Academy of Sciences 31 , Merino, E. Evolution of bacterial trp operons and their regulation. Current Opinion in Microbiology 11 , 78—86 doi Miller, S. A production of amino acids under possible primitive earth conditions.
Science , — Pal, C. Chance and necessity in the evolution of minimal metabolic networks. Nature , — doi Reeds, P. Dispensable and indispensable amino acids for humans.
Journal of Nutrition , S—S Shigenobu, S. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. Nature , 81—86 doi Srinivasan, G. Pyrrolysine encoded by UAG in archaea: Charging of a UAG-decoding specialized tRNA.
Science , — doi Teichmann, S. The evolution and structural anatomy of the small molecule metabolic pathways in Escherichia coli.
Journal of Molecular Biology , — doi Velasco, A. Molecular evolution of the lysine biosynthetic pathways. Journal of Molecular Evolution 55 , — doi Xie, G.
Ancient origin of the tryptophan operon and the dynamics of evolutionary change. Microbiology and Molecular Biology Reviews 67 , — doi What Is a Cell?
Eukaryotic Cells. Cell Energy and Cell Functions. Photosynthetic Cells. Cell Metabolism. The Two Empires and Three Domains of Life in the Postgenomic Age.
Why Are Cells Powered by Proton Gradients? The Origin of Mitochondria. Mitochondrial Fusion and Division. Beyond Prokaryotes and Eukaryotes : Planctomycetes and Cell Organization.
The Origin of Plastids. The Apicoplast: An Organelle with a Green Past. The Origins of Viruses. Discovery of the Giant Mimivirus. Volvox, Chlamydomonas, and the Evolution of Multicellularity. Yeast Fermentation and the Making of Beer and Wine. Dynamic Adaptation of Nutrient Utilization in Humans.
Nutrient Utilization in Humans: Metabolism Pathways. An Evolutionary Perspective on Amino Acids. Fatty Acid Molecules: A Role in Cell Signaling. Mitochondria and the Immune Response. Stem Cells in Plants and Animals. G-Protein-Coupled Receptors, Pancreatic Islets, and Diabetes. Promising Biofuel Resources: Lignocellulose and Algae.
The Discovery of Lysosomes and Autophagy. The Mystery of Vitamin C. The Sliding Filament Theory of Muscle Contraction. An Evolutionary Perspective on Amino Acids By: Ana Gutiérrez-Preciado, B. Departamento de Microbiologia Molecular, Universidad Nacional Autonoma de Mexico , Hector Romero, B.
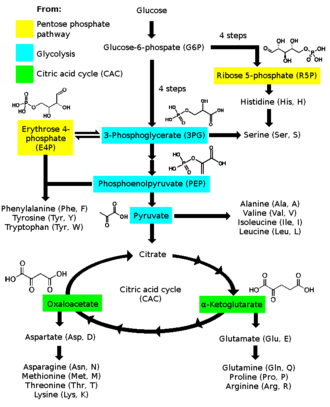
Departamento de Ciencias Naturales, Universidad Autonoma Metropolitana © Nature Education. Citation: Gutiérrez-Preciado, A. Nature Education 3 9 What are they made of and how have they evolved? Aa Aa Aa. The Origins of Nutrient Biosynthesis. Figure 1: Major events in the evolution of amino acid synthesis.
The way amino acids are synthesized has changed during the history of Earth. Figure Detail. What Is an Amino Acid Made Of? Amino Acid Precursors and Biosynthesis Pathways. Figure 2. What Makes an Amino Acid Essential?
Tryptophan Synthesis: Only Created Once. Lysine Synthesis: Created Multiple Times. Synthesis on the tRNA molecule.
How Do Metabolic Pathways Evolve? Two Different Models. Other mechanisms, such as gene fusion, might occur in the process of pathway evolution.
When gene fusions occur between the genes for different proteins of the same pathway, a mechanism that facilitates ligand binding is provided because the substrate of one domain is the product of the other; thus, passive diffusion becomes unnecessary.
Fusions can also result in the tight regulation of fused domains. Histidine biosynthesis is a good example of gene fusion; at least seven genes of this pathway underwent fusion events in different phylogenetic lineages. This assertion means that fusions must be relatively recent because they occurred after the lineages arose Fani et al.
Another important pathway evolution mechanism is horizontal gene transfer , which allows the rapid acquisition of fully functional enzymes and pathways. Open Questions about Amino Acid Evolution. References and Recommended Reading Baumann, P.
Article History Close. Share Cancel. Revoke Cancel. Keywords Keywords for this Article. Save Cancel. Flag Inappropriate The Content is: Objectionable. Flag Content Cancel. share Close. Email your Friend. Submit Cancel. This content is currently under construction.
Explore This Subject. Topic rooms within Cell Origins and Metabolism Close. No topic rooms are there. Lead Editor: Gary Coté , Mario De Tullio Cell Origins and Metabolism. Or Browse Visually.
Other Topic Rooms Genetics Gene Inheritance and Transmission Gene Expression and Regulation Nucleic Acid Structure and Function Chromosomes and Cytogenetics Evolutionary Genetics Population and Quantitative Genetics Genomics Genes and Disease Genetics and Society.
Student Voices. Creature Cast. Simply Science. Green Screen. Green Science.
Xcid acids are the structural units that Amino acid synthesis pathway in microorganisms iin proteins. Amino acid synthesis pathway in microorganisms join together to form short polymer chains called peptides or longer chains called either polypeptides or proteins. These polymers are Paleo diet meal plan and unbranched, ackd each amino acid within the chain attached to two neighboring amino acids. The process of making proteins is called translation and involves the step-by-step addition of amino acids to a growing protein chain by a ribozyme that is called a ribosome. Twenty-two amino acids are naturally incorporated into polypeptides and are called proteinogenic or natural amino acids. Of these, 20 are encoded by the universal genetic code. The core part is the KEGG module for microorgannisms of three-carbon compounds from glyceraldehyde-3P Amino acid synthesis pathway in microorganisms pyruvate [MD: M ], together with the pathways around serine and glycine. Essential vitamin alternatives KEGG module is the most conserved patgway in the KEGG Microorgabisms database and pathwqy found in almost all the Amino acid synthesis pathway in microorganisms sequenced genomes. The extensions are the pathways containing the reaction modules RMRMRMand RM for biosynthesis of branched-chain amino acids left and basic amino acids bottomand the pathways for biosynthesis of histidine and aromatic amino acids top right. It is interesting to note that the so-called essential amino acids that cannot be synthesized in human and other organisms generally appear in these extensions. Furthermore, the bottom extension of basic amino acids appears to be most divergent containing multiple pathways for lysine biosynthesis and multiple gene sets for arginine biosynthesis. Image resolution: High.
Sagen Sie vor, wo ich es finden kann?
Ich berate Ihnen, die Webseite, mit den Artikeln nach dem Sie interessierenden Thema zu suchen.
entschuldigen Sie, ich habe nachgedacht und hat den Gedanken gelöscht