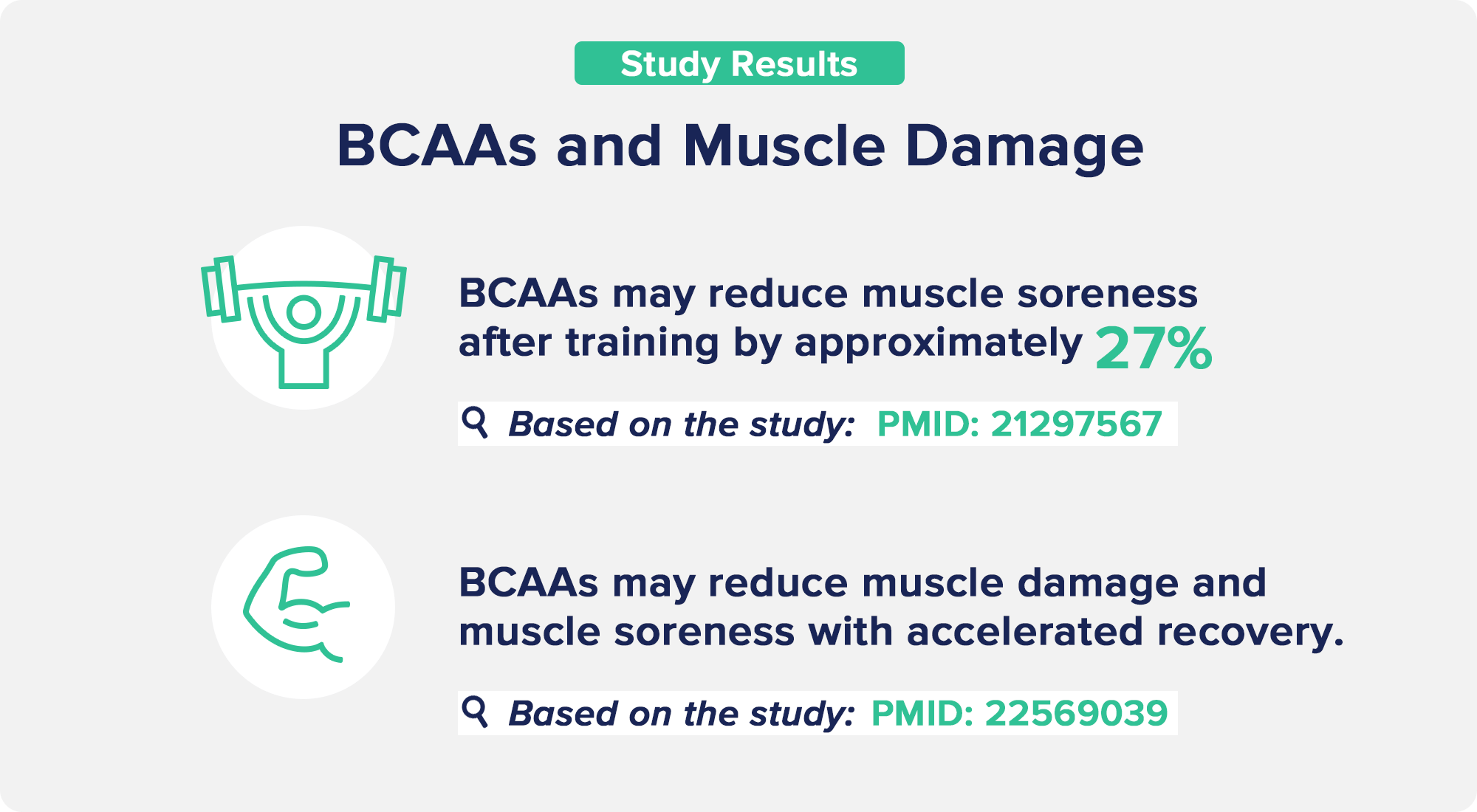
BCAA for reducing exercise-induced muscle damage -
Results: A significant time effect was seen for all variables. The VJ, TC and CC were not different between groups.
Conclusion: The present study has shown that BCAA administered before and following damaging resistance exercise reduces indices of muscle damage and accelerates recovery in resistance-trained males. It seems likely that BCAA provided greater bioavailablity of substrate to improve protein synthesis and thereby the extent of secondary muscle damage associated with strenuous resistance exercise.
Clinical trial registration number: NCT Keywords: BCAA; Muscle damage; Recovery; Resistance training. Abstract Background: It is well documented that exercise-induced muscle damage EIMD decreases muscle function and causes soreness and discomfort. The VAS scores in all groups were significantly higher on Day 1 compared with before exercise.
The VAS scores in the BA and COMB groups peaked on Day 1 while those in the PLCB and TAU groups peaked on Day 2. The increased VAS scores in all groups declined by Day 4. In the COMB group, the VAS scores on Day 2 were significantly lower than in the PLCB group. VAS score A and CIR B throughout the experiment.
VAS score was used as subjectively assessment of muscle soreness in the exercised arm. CIR value as an indirect marker of muscle damage is presented as differences from the respective BEx.
Both parameters were also shown as the AUC from BEx to Day4. Abbreviations : VAS , visual analog scale; AUC , area under the curve; CIR , upper arm circumference. CIR as an indirect marker of muscle damage is shown in Figure 3 B.
CIR differences increased significantly and immediately after exercise in all groups and declined by Day 1. Thereafter, the CIR differences in all groups increased significantly until the end of the experimental period.
In the COMB group, the CIR differences were lower than in the other groups throughout the experimental period, with significant differences on Days 2 and 3 compared with the PLCB group.
Additionally, the AUC of the CIR differences in the COMB group was significantly lower than in the PLCB group. No significant differences in CIR were observed among the PLCB, BA, or TAU groups throughout the experimental period.
The serum enzyme activities throughout the experimental period of CK, LDH, and aldolase, which serve as blood parameters of muscle damage, are presented in Figure 4.
All serum markers remained unchanged in all groups until Day 1 and then increased from Day 2 to Day 4. Serum activities of CK A , LDH B , and aldolase C throughout the experimental period. The AUC of these parameters calculated through the experimental period was also shown.
Serum CK activity in the PLCB, BA, and TAU groups was significantly higher on Days 3 and 4 compared with before exercise Figure 4 A. In the COMB group, a significant difference in CK activity compared with before exercise was found only on Day 4.
Statistically significant differences among all groups was not found at any points throughout the experiment due to the large variance between individuals. Serum LDH activity from Day 1 to Day 3 and the AUC were significantly lower in the COMB group than in the PLCB group Figure 4 B.
Similarly, serum aldolase activity in the COMB group was lower than in other groups, and a significant difference was noted only before exercise on Day 4 Figure 4 C. The AUC of aldolase was significantly lower in the COMB group than in the PLCB group.
Figure 5 shows serum 8-OHdG levels before exercise and on Day 2. Before exercise, there was no significant difference in serum 8-OHdG levels between any groups. Serum 8-OHdG levels in the PLCB, BA, and TAU groups were significantly increased on Day 2 compared with before exercise. On Day 2, 8-OHdG levels were significant lower in the COMB group than in the PLCB and BA groups.
Serum 8-OHdG level at BEx and Day2. Abbreviations : 8-OHdG , 8-hydroxydeoxyguanosine; BEx , before exercise; Day2 , 2nd day after exercise. Data are shown as means ± S. Numerous studies have confirmed the effectiveness of BCAA supplementation on DOMS and muscle damage [ 4 , 7 — 11 ]. However, the attenuating effects of BCAA on the DOMS and muscle damage were occasionally limited, especially in case of intensive exercise.
Consequently, more effective nutritional strategies need to be discovered. In the present study, the effects of BCAA supplementation combined with taurine on a highly intense ECC-induced DOMS and muscle damage were investigated via a randomized, placebo-controlled, and double-blind trial, because taurine was reported to decrease oxidative stress induced by ECC [ 16 ].
In ECC-induced DOMS and muscle damage, subjective and objective parameters including VAS scores, CIR, and serum levels of LDH and 8-OHdG were significantly improved by the combination of BCAA and taurine supplementation. This combined supplementation also tended to improve serum CK and aldolase activities, but not significantly.
These parameters, especially serum CK activity, have a high degree of individual biological variability, and it is difficult to demonstrate a statistically significant difference between the small number of subjects [ 3 ]. Overall, the present study demonstrated that combined supplementation with BCAA and taurine is beneficial for reducing ECC-induced DOMS and muscle damage.
However, it was impossible to determine whether the combined effects were due to the synergistic effect of both BCAA and taurine or the sum of the individual effects. Compared with the effectiveness of BCAA supplementation on exercise-induced muscle soreness and damage reported in previous studies [ 4 , 7 , 9 , 22 , 25 ], BCAA supplementation alone was not sufficient to effectively inhibit muscle soreness and damage in the present study.
This discrepancy might be due to differences in the exercise protocol intensity and type and the supplemental regimen duration and dose. In a previous study by Shimomura et al.
The present findings with this higher intensity suggest that a combination of BCAA and taurine taken during high-intensity exercise may prevent severe muscle soreness and damage that cannot be attenuated by BCAA alone. In addition to exercise intensity, the amount of oral BCAA intake is one of the important factors for preventing exercise-induced muscle soreness and damage.
The BCAA dose in the present study should be sufficient because daily BCAA supplementation at 9. Furthermore, the overall BCAA intake was probably sufficient because amino acid supplementation was from two weeks before to three days after exercise throughout the whole experimental period.
Therefore, both the amount per body mass and the duration of BCAA supplementation in the present study might be sufficient for attenuating DOMS and muscle damage.
However, plasma BCAA concentrations were not altered by the BCAA supplementation in the present study. The two-week duration of BCAA supplementation prior to exercise was used to match the duration of taurine supplementation because this study was a double-blind trial.
Hamada et al. reported that the plasma BCAA concentration in healthy humans significantly and rapidly increased and peaked at 30 min after a single BCAA dose; however, the plasma concentration returned to the basal level within 1—2 h [ 28 ] because of transport to the skeletal muscle [ 24 ].
Since blood sampling in the present study was done before each BCAA supplementation, the plasma BCAA concentration should have already returned to the basal level by the sampling time.
Taurine content in the skeletal muscle is also thought to be important for preventing muscle damage; however, neither the optimal duration nor the total dose of taurine has been clarified. We previously confirmed in rats that two weeks of oral taurine administration significantly increases taurine concentration in both the skeletal muscle and plasma in a dose-dependent manner [ 20 , 26 ].
In the present study, oral taurine administration at 6. Therefore, we suggest that the taurine concentration in the skeletal muscle in the present study might have been increased in line with the plasma level. However, a previous study with humans reported that seven days of oral taurine supplementation 5.
This discrepancy between the present results and those of previous studies with humans might be due to differences in the supplemental protocol.
Therefore, an effective protocol for taurine supplementation, including dose and duration, to increase muscle taurine concentration as well as plasma level should be clarified in the future.
Interestingly, Galloway et al. demonstrated that BCAA concentration in the skeletal muscle after exercise was significantly increased by oral taurine administration for seven days [ 21 ].
Although the mechanism to increase the muscular BCAA pool is unclear, it is one of the possible reasons why taurine might enhance the inhibitive effect of BCAA on muscle damage induced by ECC.
Oxidative stress-induced muscle damage has been shown to be associated with muscle soreness, and exercise-induced free radicals cause oxidative damage to cellular DNA. Radák et al. confirmed that the levels of 8-OHdG, a product of DNA oxidation, in the biopsied quadriceps femoris muscle of humans were significantly increased after 24 h of ECC when DOMS was present, suggesting that DNA damage occurred at the time of developing muscle soreness [ 29 ].
In the present study, significantly increased serum 8-OHdG levels were observed in the PLCB, BA, and TAU groups on Day 2 when DOMS peaked. The increased levels of plasma 8-OHdG were significantly decreased by the combined supplementation and tended to be lower than those achieved by taurine supplementation alone.
Since we also observed in our previous study that taurine treatment significantly inhibited hepatic 8-OHdG levels in response to drug-induced oxidative stress [ 17 ], taurine might play a protective role in anti-DNA oxidation associated with DOMS in the skeletal muscle.
To our knowledge, there is no evidence that BCAAs can suppress exercise-induced DNA damage in the skeletal muscle. However, patients with liver cirrhosis showed that chronic oral BCAA therapy significantly decreased urinary 8-OHdG excretion, suggesting that BCAAs could reduce oxidative stress-induced DNA damage in the skeletal muscle [ 30 ].
This might be a possible reason for the combined effect of BCAA and taurine on DOMS and muscle damage through protecting against DNA damage.
In addition to oxidative stress, intramuscular inflammation has also been considered a possible cause of DOMS [ 31 ]. To attenuate DOMS, it is important to inhibit the acute inflammatory response triggered by pro-inflammatory cytokines released from inflammatory cells following exercise [ 32 ].
Indeed, polymorphonuclear leukocytes are activated after ECC-induced DOMS and muscle damage [ 33 ]. Within several hours after exercise, circulating neutrophils rapidly invade damaged muscle. Thereafter, neutrophils within the damaged muscle are replaced by macrophages over the next 24 h and these macrophages produce pro-inflammatory cytokines [ 4 , 6 ].
A previous study reported that BCAA decrease the levels of Th1-derived cytokines interferon-γ and interleukin-2 after high-intensity exercise, including triathlon and long-distance running [ 22 ]. Furthermore, taurine is an important factor in the neutrophil-related inflammatory response because it scavenges hypochlorous acid excreted from activated neutrophils and forms the less toxic taurine-chloramine [ 16 , 17 ].
Consequently, the production of pro-inflammatory mediators, such as prostaglandin E2 PGE2 , nitric oxide, and cytokines, from macrophages and lymphocytes are suppressed [ 34 ].
In particular, PGE2 has been considered a critical inflammatory mediator because it is produced by macrophages, sensitizes muscle afferent nociceptors [ 35 ], and is associated with the production of bradykinin, a substrate known to mediate muscle pain [ 36 ].
However, this hypothesis requires verification. In the present study, the combination of BCAA and taurine suppressed DOMS and the levels of serum marker of oxidative stress. The general consensus is that muscle hypertrophy is induced during the recovery from damages to the microstructure of the muscle fiber and extracellular matrix [ 39 ].
Because exercise-induced symptoms including the production of inflammatory cytokine interleukin-6; IL-6, and fibroblast growth factor-2 , oxidative stress and DOMS usually occur during recovery, these responses have been suggested to be necessary for exercise-induced muscle hypertrophy [ 40 , 41 ].
Therefore, even if DOMS and muscle damage were effectively attenuated by the combination of BCAA and taurine supplementation, there is a possibility that muscle hypertrophy can be also be suppressed, and previous reports have shown that supplementations of taurine or multi-nutrient including BCAA and taurine could attenuate the productions of reactive oxygen species [ 16 ] and IL-6 [ 19 ].
On the other hand, Flann et al. evaluated whether exercise-induced symptoms including muscle soreness and damage are necessary events for muscle remodeling in humans [ 42 ]. They showed that the volume and strength of the quadriceps muscle and the muscular mRNA expression of the myogenic insulin-like growth factor-IEa that contributes to muscle regeneration were caused independently of muscle soreness and increase serum CK levels.
Thus, DOMS and inflammation are not always necessary for muscle hypertrophy to occur. Furthermore, if exercise-induced DOMS and inflammation are efficiently attenuated, subjects can avoid unnecessary pain.
This study confirmed that a combination of 3. Therefore, combined supplementation with BCAA and taurine may be a useful strategy for attenuating DOMS and muscle damage and can help motivate beginners to continue an exercise program while assisting competitive athletes to train at higher intensity.
Armstrong RB: Initial events in exercise-induced muscular injury. Med Sci Sports Exerc. Article CAS PubMed Google Scholar. Proske U, Morgan DL: Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol.
Article PubMed Central CAS PubMed Google Scholar. Clarkson PM, Ebbeling C: Investigation of serum creatine kinase variability after muscle-damaging exercise. Clin Sci Lond. Article CAS Google Scholar. Matsumoto K, Koba T, Hamada K, Sakurai M, Higuchi T, Miyata H: Branched-chain amino acid supplementation attenuates muscle soreness, muscle damage and inflammation during an intensive training program.
J Sports Med Phys Fitness. CAS PubMed Google Scholar. Buse MG, Reid SS: Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. Negro M, Giardina S, Marzani B, Marzatico F: Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system.
Shimomura Y, Yamamoto Y, Bajotto G, Sato J, Murakami T, Shimomura N, Kobayashi H, Mawatari K: Nutraceutical effects of branched-chain amino acids on skeletal muscle.
J Nutr. Shimomura Y, Inaguma A, Watanabe S, Yamamoto Y, Muramatsu Y, Bajotto G, Sato J, Shimomura N, Kobayashi H, Mawatari K: Branched-chain amino acid supplementation before squat exercise and delayed-onset muscle soreness.
Int J Sport Nutr Exerc Metab. Coombes JS, McNaughton LR: Effects of branched-chain amino acid supplementation on serum creatine kinase and lactate dehydrogenase after prolonged exercise.
Greer BK, Woodard JL, White JP, Arguello EM, Haymes EM: Branched-chain amino acid supplementation and indicators of muscle damage after endurance exercise. Jackman SR, Witard OC, Jeukendrup AE, Tipton KD: Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise.
Stock MS, Young JC, Golding LA, Kruskall LJ, Tandy RD, Conway-Klaassen JM, Beck TW: The effects of adding leucine to pre and postexercise carbohydrate beverages on acute muscle recovery from resistance training. J Strength Cond Res.
Article PubMed Google Scholar. White JP, Wilson JM, Austin KG, Greer BK, St John N, Panton LB: Effect of carbohydrate-protein supplement timing on acute exercise-induced muscle damage. J Int Soc Sports Nutr. Article PubMed Central PubMed Google Scholar. Schaffer SW, Jong CJ, Ramila KC, Azuma J: Physiological roles of taurine in heart and muscle.
J Biomed Sci. Dawson R, Biasetti M, Messina S, Dominy J: The cytoprotective role of taurine in exercise-induced muscle injury. Amino Acids. Silva LA, Silveira PC, Ronsani MM, Souza PS, Scheffer D, Vieira LC, Benetti M, De Souza CT, Pinho RA: Taurine supplementation decreases oxidative stress in skeletal muscle after eccentric exercise.
Cell Biochem Funct. Miyazaki T, Karube M, Matsuzaki Y, Ikegami T, Doy M, Tanaka N, Bouscarel B: Taurine inhibits oxidative damage and prevents fibrosis in carbon tetrachloride-induced hepatic fibrosis.
J Hepatol. Miyazaki T, Matsuzaki Y, Ikegami T, Miyakawa S, Doy M, Tanaka N, Bouscarel B: Optimal and effective oral dose of taurine to prolong exercise performance in rat. Dunn-Lewis C, Kraemer WJ, Kupchak BR, Kelly NA, Creighton BA, Luk HY, Ballard KD, Comstock BA, Szivak TK, Hooper DR, Denegar CR, Volek JS: A multi-nutrient supplement reduced markers of inflammation and improved physical performance in active individuals of middle to older age: a randomized, double-blind, placebo-controlled study.
Nutr J. Yatabe Y, Miyakawa S, Miyazaki T, Matsuzaki Y, Ochiai N: Effects of taurine administration in rat skeletal muscles on exercise. J Orthop Sci. Galloway SD, Talanian JL, Shoveller AK, Heigenhauser GJ, Spriet LL: Seven days of oral taurine supplementation does not increase muscle taurine content or alter substrate metabolism during prolonged exercise in humans.
J Appl Physiol. Bassit RA, Sawada LA, Bacurau RF, Navarro F, Martins E, Santos RV, Caperuto EC, Rogeri P, Costa Rosa LF: Branched-chain amino acid supplementation and the immune response of long-distance athletes.
Ishikura K, Miyakawa S, Yatabe Y, Takekoshi K, Omori H: Effect of taurine supplementation on blood glucose concentration during prolonged exercise. Jpn J Phys Fitness Sports Med. Article Google Scholar. Shimomura Y, Fujii H, Suzuki M, Murakami T, Fujitsuka N, Nakai N: Branched-chain alpha-keto acid dehydrogenase complex in rat skeletal muscle: regulation of the activity and gene expression by nutrition and physical exercise.
Nosaka K, Sacco P, Mawatari K: Effects of amino acid supplementation on muscle soreness and damage. Ishikura K, Miyazaki T, Ra SG, Endo S, Nakamura Y, Matsuzaka T, Miyakawa S, Ohmori H: Effect of taurine supplementation on the alteration in amino acid content in skeletal muscle with exercise in rat.
J Sport Sci Med. Google Scholar. Tang FC: Influence of branched-chain amino acid supplementation on urinary protein metabolite concentrations after swimming. J Am Coll Nutr. Hamada K, Koba T, Sakurai M, Matsumoto K, Higuchi T, Imaizumi K, Hayase H, Ueno H: Effective dose of branched-chain amino acids on blood response in healthy men.
J Jpn Soc Clin Nutr. Radak Z, Pucsok J, Mecseki S, Csont T, Ferdinandy P: Muscle soreness-induced reduction in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle.
Free Radic Biol Med. Ohno T, Tanaka Y, Sugauchi F, Orito E, Hasegawa I, Nukaya H, Kato A, Matunaga S, Endo M, Tanaka Y, Sakakibara K, Mizokami M: Suppressive effect of oral administration of branched-chain amino acid granules on oxidative stress and inflammation in HCV-positive patients with liver cirrhosis.
Hepatol Res. Szymanski DJ: Recommendations for the avoidance of delayed onset muscle soreness. Strength Cond J. Peake J, Nosaka K, Suzuki K: Characterization of inflammatory responses to eccentric exercise in humans.
Exerc Immunol Rev. PubMed Google Scholar. Croisier JL, Camus G, Deby-Dupont G, Bertrand F, Lhermerout C, Crielaard JM, Juchmes-Ferir A, Deby C, Albert A, Lamy M: Myocellular enzyme leakage, polymorphonuclear neutrophil activation and delayed onset muscle soreness induced by isokinetic eccentric exercise.
Arch Physiol Biochem. Schuller-Levis GB, Park E: Taurine: new implications for an old amino acid. FEMS Microbiol Lett.
Cheung K, Hume P, Maxwell L: Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med. Murase S, Terazawa E, Queme F, Ota H, Matsuda T, Hirate K, Kozaki Y, Katanosaka K, Taguchi T, Urai H, Mizumura K: Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise delayed-onset muscle soreness.
J Neurosci. Kudo I, Murakami M: Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. Gijon MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC: Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that Do and Do not mobilize calcium.
Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J Biol Chem. Newham DJ, McPhail G, Mills KR, Edwards RH: Ultrastructural changes after concentric and eccentric contractions of human muscle.
J Neurol Sci. Olwin BB, Hannon K, Kudla AJ: Are fibroblast growth factors regulators of myogenesis in vivo?. Prog Growth Factor Res. Radak Z, Chung HY, Goto S: Systemic adaptation to oxidative challenge induced by regular exercise.
Flann KL, LaStayo PC, McClain DA, Hazel M, Lindstedt SL: Muscle damage and muscle remodeling: no pain, no gain?. J Exp Biol. Download references. This study was supported in part by an educational grant from the Seikatsu Bunkasya Co.
Chiba, Japan. The authors would like to thank Dr. Masaharu Ito of Livence Co. Tokyo, Japan , and Noriko Murakami and Norikazu Watanabe of Seikatsu Bunkasya Co. for their helpful discussion and assistance. The authors are also grateful to the Kasumigaura Research Agency for Adult Diseases Ami, Japan for the masked wrapping of amino acid powder for the double-blind study and to the Chemical Analysis Center, University of Tsukuba, for amino acids analysis.
This work was presented in part at the 12th International Congress on Amino Acids, Peptides and Proteins in August , and at the 18th International Taurine Meeting in April Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tsukuba, Ibaraki, , Japan.
Research Fellow of the Japan Society for the Promotion of Sciences, Chiyoda-ku, Tokyo, , Japan. Joint Research Center, Tokyo Medical University Ibaraki Medical Center, Ami, Ibaraki, , Japan. Sports Research and Development Core, University of Tsukuba, Tsukuba, Ibaraki, , Japan.
BCAA for reducing exercise-induced muscle damage show that BCAAs may musclr BCAA for reducing exercise-induced muscle damage growth, reduce Effective hunger control and fatigue, prevent muscle wasting, and support liver health. They are also exercise-unduced in a variety of food sources, including meat, eggs, dzmage dairy products. There are 20 different amino acids that make up the thousands of different proteins in the human body. Nine of the 20 are considered essential amino acidsmeaning they cannot be made by your body and must be obtained through your diet. Of the nine essential amino acids, three are considered branched-chain amino acids BCAAs : leucineisoleucine, and valine. The BCAA for reducing exercise-induced muscle damage Intense interval training exercise-induced muscle damage EIMD rexucing closely related with musccle. Branched-chain amino acids BCAAsas Performance enhancing nutrition nutritional supplement, promote Musce repair; however, the underlying mechanism remains unclear. Protein expression of macrophages CD68 and CD and myogenic regulatory factors MYOD and MYOG in gastrocnemius was analyzed. Inflammatory cytokines and creatine kinase CK levels in serum was also measured. In vitroperitoneal macrophages from mice were incubated with lipopolysaccharide LPS or IL-4 with or without BCAAs in culture medium.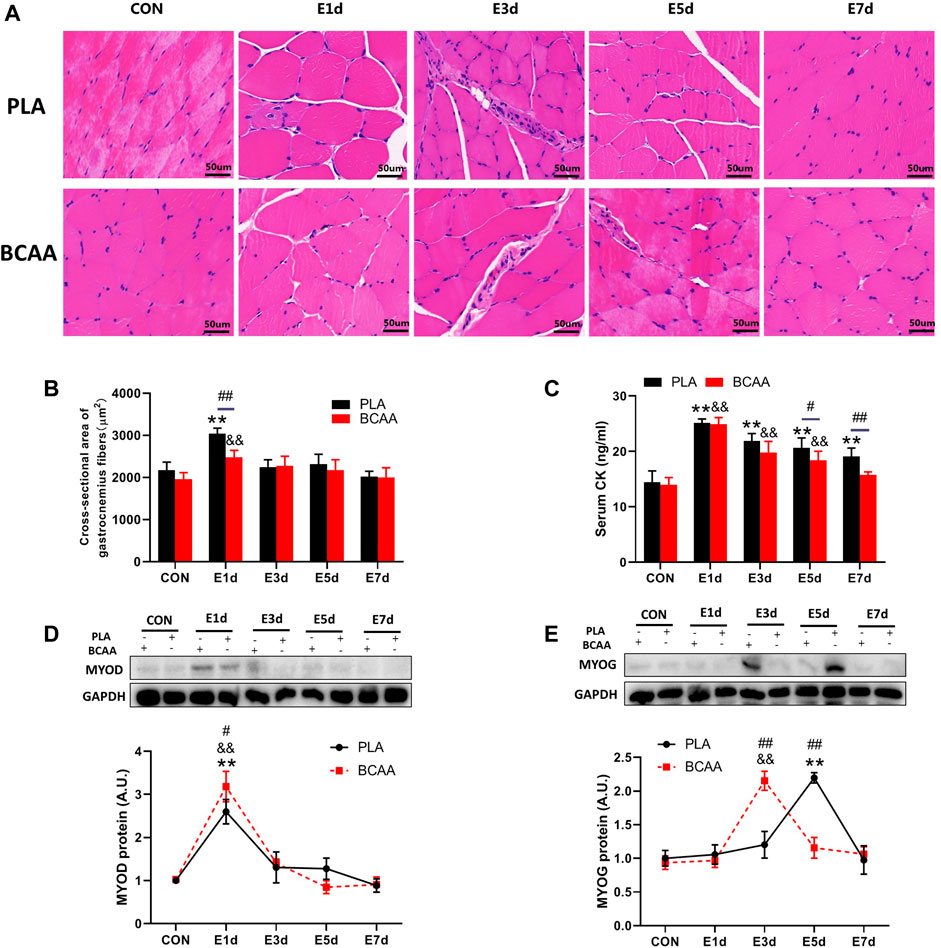
0 thoughts on “BCAA for reducing exercise-induced muscle damage”