Antioxidant defense system -
Figure 3. Excessive oxidation and reduction of cell components are equally detrimental, so maintaining redox homeostasis is crucial Foyer and Shigeoka, For this reason, plants are extremely rich in compounds with antioxidative activity.
Although antioxidative protection is different from species to species, its presence is ubiquitous Wachter et al. By definition, antioxidants represent molecules capable of inhibiting or quenching free radical reactions and delaying or preventing cell damage, and, in lower concentration than potential substrate which might be oxidized, significantly delay or hinder its oxidation Nimse and Pal, ; Dumont and Rivoal, The foremost prominent low relative molecular mass antioxidants in plants are water-soluble ascorbate Asc , glutathione, and phenols, and liposoluble tocopherols, tocotrienols, and carotenoids Figure 4.
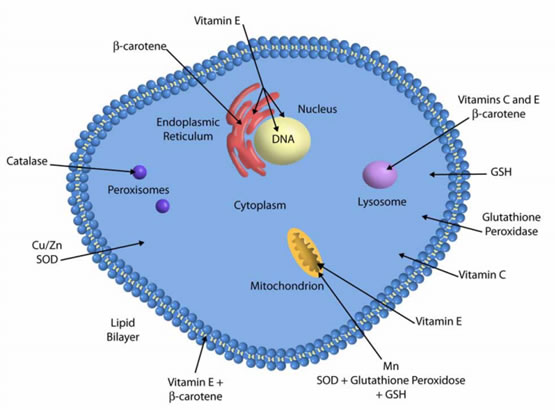
Figure 4. Antioxidative system location in a plant cell. APX, ascorbate peroxidase; CAT, catalase, DHAR, dehydroascorbate reductase; MDAR, monodehydroascorbate reductase; GR, glutathione reductase; GRX, glutaredoxin; SOD, superoxide dismutase; NTR, NADPH-thioredoxin reductase; PRX, peroxiredoxin; TRX, thioredoxin — adapted from: Noctor et al.
These molecules could self-react with ROS, but the removal efficiency is higher in enzyme-mediated reactions, like those catalyzed by APX EC 1. Low relative molecular mass antioxidants remove ROS both indirectly and directly.
Specifically, the indirect mechanism is chelation of transition metals, which prevents participation within the Haber-Weiss Kehrer, or Fenton reaction, while the direct mechanism involves donating or receiving of electrons, scavenging radicals, and consequently preventing their reaction with biological molecules.
The antioxidant, which donates or receives electrons, is stabilized by π-electrons delocalization and resonance, and this is the case with Asc, phenolic compounds, and tocopherols.
However, the advantage of scavengers over enzymatic antioxidants is their small size, which allows them to diffuse through cell membranes and localize near biological molecules which are potential targets of ROS Kohen and Nyska, Additionally to those primary antioxidants, biomolecules, such as amino acids, sugars, pigments, also as secondary metabolites like flavonoids and terpenes, own antioxidant activity.
Furthermore, secondary antioxidants are capable of regenerating oxidized primary antioxidant, as exemplified by Asc capable of regenerating oxidized α-tocopherol and α-tocopherol further regenerate β-carotene. Also, both created liposoluble radicals are often reduced by Asc, thereby exhibiting their antioxidant action within the membrane protection against lipid peroxidation.
This is often an example of the synergistic action of AOS in preserving membrane integrity Yachandra et al. The most significant antioxidant in plant tissue, present at millimolar concentrations in chloroplasts, is Asc, followed by glutathione GSH , which is present at 1, times lower concentration than Asc but is additionally vital.
Specific enzyme systems peroxidases create the chance to rapidly react with H 2 O 2 , and their oxidized forms are regenerated by specific high-capacity reductases. Although these antioxidants scavenge ROS separately, they have long been thought to co-operate within the so-called water-water Asada, and Asc-GSH cycle Foyer and Halliwell, to metabolize H 2 O 2 and keep sufficient excitatory energy under control within the chloroplasts Foyer and Shigeoka, On the contrary, catalases play a serious role in H 2 O 2 metabolism in peroxisomes Mhamdi et al.
The resulting H 2 O 2 is detoxified by APX which in turns oxidizes two Asc molecules, its specific electron donor. MDAR is found within the cytosol, chloroplasts, peroxisomes, mitochondria, and the plasma membrane Noctor and Foyer, Unlike Asc, DHA lacks antioxidative capability and is converted back to Asc by the addition of two electrons from GSH by DHA reductases DHAR, EC 1.
GSH-dependent DHAR activity is expressed in chloroplasts, mitochondria, and peroxisomes Jimenez et al. GSH is regenerated from its oxidized state, glutathione disulphide GSSG , by the action of glutathione reductase GR, EC 1.
Figure 5. The Asc-GSH cycle is operating in various cellular compartments including the cytosol, mitochondria, chloroplast, and peroxisomes. Different isoforms of APX and SOD are localized within the stroma and thylakoid membrane, whereas chloroplast GR and DHAR are located within the stroma Sharma et al.
Despite the Asc-GSH cycle significance, newer evidence suggests overlapping of the role of peroxiredoxins PRX in maintaining a relevant level of H 2 O 2 in chloroplasts.
Peroxiredoxins, which belong to the family of peroxidases, are important in ROS detoxification since they reduce H 2 O 2 and organic peroxides and add cooperation with thioredoxin TRX and TRX-like proteins in chloroplasts Dietz, PRXs utilize thiols to scale back H 2 O 2.
SOD-catalyzed dismutation is 10, times faster than spontaneous reactions. The enzyme is present in all aerobic cells and subcellular compartments sensitive to oxidative stress Bowler et al.
There are three types of SOD metalloenzymes in plants counting on metal cofactor present within the active center. Mn-SOD is expressed in mitochondria and peroxisomes but has also been detected in both apoplast and cell wall, while Fe-SOD is present to a lesser extent.
However, this isoenzyme is restricted for the chloroplast stroma of certain plant species Mittler et al. These isoenzymes are differentiating in their sensitivity to H 2 O 2 and KCN Bannister et al. Genes coding SOD are sensitive to environmental stressors, and increased activity is usually correlated with increased plant tolerance for environmental stress Sharma et al.
The intracellular level of H 2 O 2 is regulated by several enzymes, the foremost important of which are catalases CATs and peroxidases participating within the fine regulation of ROS concentration through the cell Baby and Jini, Considering cellular compartmentalization, CATs might be found in large quantities in peroxisomes, whereas this enzyme has not been found in chloroplasts.
It captures the H 2 O 2 created in peroxisomes during the process of photorespiration and β-oxidation of fatty acids. Catalases are very efficient in H 2 O 2 removal with a unique ability to convert two H 2 O 2 molecules into water and molecular oxygen with no need for reduction equivalent.
The Km value for CAT is within the millimolar range, which may be a far higher concentration of H 2 O 2 within the cell than physiological, implying its role predominantly under stress conditions Černý et al.
On the other hand, CATs express low activity against organic peroxides. Comparing with peroxidases, they have higher Km value for H 2 O 2 hence peroxidases could remove H 2 O 2 albeit present in low concentrations Sharma et al. On the other hand, peroxidases-driven reactions are using low relative molecular mass antioxidants, GSH and Asc as electron donors, so removal of H 2 O 2 using this pathway may be a very energy-consuming reaction for cell since it utilizes important molecules from the cell environment: two GSH molecules are consumed for removal of one H 2 O 2 molecule Kohen and Nyska, Regarding the aminoalkanoic acid sequence, peroxidases are divided into three classes Welinder, The APX is assessed as a first-class and differs from the class III peroxidase-like well-known horseradish peroxidase HRP.
The importance of APX within a plant cell is indicated by the very fact that APX isoenzymes are distributed in as many as five cell compartments: stroma sAPX and thylakoids tAPX in chloroplasts, microbodies including glyoxysomes and peroxisomes; mAPX , cytosol cAPX , and mitochondria mitAPX, as a membrane-bound form; Chen and Asada, ; Jimenez et al.
Mutants with tAPX deficiency are considered lethal while plants with overexpressed tAPX are tolerant of methyl viologen paraquat induced stress Yabuta et al.
A salient feature of APX, especially chloroplast, is its sensitivity to oxidative inactivation within the absence of Asc. In contrast, cytosolic and peroxisomal isoforms lose their activity after quite 1 h Miyake and Asada, Depletion of chloroplast Asc and inactivation of chloroplast APX are considered for limitations of photosynthetic efficiency under stress conditions and thus potential targets for improvement Ishikawa and Shigeoka, The sole H 2 O 2 -scavenging enzyme within the apoplastic and vacuolar space of all plant organs is class III peroxidase.
Induced in response to various environmental stresses, this family of isoenzymes has an imperative role in cross-talk between primary and secondary antioxidants Veljović-Jovanović et al. Glutathione peroxidases, which even have strong activity against H 2 O 2 , could use both GSH and TRX as reducing substrates and will eliminate lipid peroxides additionally to H 2 O 2 Herbette et al.
Ascorbate is taken into account a potent antioxidant thanks to its ability to donate electrons in an exceedingly wide selection of enzymatic and non-enzymatic reactions. It is especially present within the leaves and in higher concentration compared to GSH Das and Roychoudhury, Ascorbate could donate two electrons, whereby donation of one is followed by the assembly of semidehydroascorbate or ascorbate, and donation of the second electron is related to DHA production.
It might be regenerated by DHAR utilizing two GSH molecules. Ascorbate occurs in all subcellular compartments including the cell wall except for vacuoles where is present in low concentrations Das and Roychoudhury, Nevertheless, the bulk of Asc is found within the cytoplasm, but unlike other soluble antioxidants, a substantial portion is exported to the apoplast where it is present at millimolar concentrations and is taken into account to be the primary line of defense against potentially harmful external prooxidants.
Intracellular concentrations are up to millimolar range i. However, Asc could acts as prooxidant reducing Fe, Cu, and Mn ions and consequently providing a chance to re-engage in one among the redox reactions. The tripeptide, γ-glutamyl-cysteinyl glycine, the foremost abundant low relative molecular mass thiol within the cell, has been found in large quantities in every cell compartment: cytosol, chloroplast, endoplasmic reticulum, vacuoles, and mitochondria.
It is not only specific to plant cells but also plays a really important role as a redox buffer amid Asc Liu and Li, The reduced form of glutathione, GSH, may be a major sulfur depo form and plays important roles in various biological processes, including cellular growth, development, regulation of sulfur transport, signal transduction, protein and nucleic acid synthesis, phytochelatin synthesis for metal chelation, xenobiotic detoxification, and expression of genes liable for stress Bartoli et al.
Besides, GSH is synthesized both in chloroplasts and within the cytosol. Together with its oxidized form, GSSG, reduced glutathione maintains redox balance within the cell. The cysteine residue in the molecule center is liable for the high reduction potential of GSH.
However, the main role of GSH as an antioxidant is its ability to regenerate another potent hydrophilic antioxidant, ascorbic acid, precisely through the Asc-GSH cycle. GSH helps to recycle oxidized Asc to the reduced state employing DHAR. Hence, β-carotene captures 1 O 2 with greater efficiency compared to α-tocopherol Kehrer, ; Sharma et al.
The conjugated double bond system owned by carotenoids provides easy absorption of energy from the excited molecule and dissipation of excess within the sort of heat. For instance, zeaxanthin is involved within the non-photochemical quenching of excess excitatory energy at PS II Asada, Tocopherols and tocotrienols are essential components of the cell membrane where they express both antioxidant and non-antioxidant functions.
There are four tocopherol and tocotrienol isomers α, β, γ, and δ. Tocopherols are a gaggle of lipophilic antioxidants and are synthesized by photosynthetic organisms and present in green, photosynthetically active parts of the plant only.
The antioxidant activity of tocopherol is predicated on the electron donor properties of the chromanol ring. These antioxidants protect lipids and other membrane components by physically trapping and chemically reacting with 1 O 2 in chloroplasts, preserving the structure and performance of PS II.
The method of 1 O 2 capture is extremely efficient and it has been estimated that 1 α-tocopherol molecule can neutralize up to molecules of 1 O 2 in vitro before its degradation Sharma et al. Regeneration of oxidized tocopherol might be achieved via Asc, GSH, or ubiquinone.
Also, α-tocopherol, with its three methyl substituents, has the very best antioxidant activity. However, α-tocotrienol has been shown to possess better antioxidant activity than α-tocopherol within the membrane environment. The chloroplast membrane contains predominantly α isomer of tocopherol; therefore, they are well protected from photooxidative damage Smirnoff, Furthermore, α-tocopherol is the main form within the leaves, while γ-tocopherol is within the seed.
Vitamin E collective term for tocopherols and tocotrienols has the potential for regenerating lipid peroxyl, alkyl, and alkoxy radicals formed during the polyunsaturated fatty acids oxidation whereby directly prevent a sequence propagation during auto-oxidation of the lipid layer.
By donating hydrogen atoms to the radical, vitamin E becomes tocopherol radical which is resonantly stabilized and not sufficiently reactive for independent initiation of membrane peroxidation, which is additionally a basic criterion for good antioxidants Kohen and Nyska, Also, tocopherol present in high concentrations acts as prooxidant alongside transition metal ions and lipid peroxides.
Phenols are a multifarious group of secondary metabolites flavonoids, tannins, hydroxycinnamate esters, lignin, etc. present in plant tissue Sakakibara et al. The antioxidant capacity of phenols is related to their structure aromatic ring with —OH or —OCH 3 substituents very suitable for trapping free radicals.
They have a robust capacity to donate an electron or hydrogen atom also because the ability to rapidly stabilize formed phenol radical and have shown greater activity compared to Asc and α-tocopherol in in vitro system.
Phenols containing o -dihydroxy groups within their structure can complex metal ions and prevent the formation of ROS in the Haber-Weiss reaction Fenton, ; Rice-Evans et al.
Furthermore, they will directly capture 1 O 2 and inhibit lipid peroxidation by trapping lipid alkoxy radicals Sharma et al.
Another mechanism associated with antioxidant properties of phenols is the ability to modify the kinetics of peroxidation by altering the lipid package and reducing membrane fluidity. These changes can limit the diffusion of free radicals and reduce the peroxidation reaction.
It has also been shown that phenolic compounds could be involved within the H 2 O 2 capture cascade Takahama and Oniki, Increased accumulation of phenolic compounds under biotic also as abiotic stress has been demonstrated and certain anthocyanins and flavanols have up to fourfold higher antioxidative activity than Asc Rice-Evans et al.
The cooperation between Asc and phenols has been shown within the hydrogen-peroxide-peroxidase system which takes place in vacuole where H 2 O 2 diffuses and may be reduced by peroxidases, and phenols are used because of the primary electron donors. This mechanism is restricted to plant tissue and enhances plant tolerance during oxidative stress Krasnovsky, The MDAR enzyme has also been shown to be capable of reducing the phenoxy radical, like quercetin radical, to phenol Sakihama et al.
Accordingly, available data indicate that ROS detoxification pathways are not present to the extent they might remove all ROS from the cellular environment, but that there is a level of coordination between the processes which generate ROS and those which remove them, therefore, maintaining the optimal amount of ROS within the cellular environment.
A really popular trend for testing AOSs is the use of genetically transformed plants, with overexpressed or removed a selected component of AOS, as well as the application of artificial environmental conditions to cause oxidative stress.
Extensive literature indicates that enhancing the expression of certain enzymes likes SOD, GR, and DHAR, utilizing gene-splicing, can improve plant tolerance to abiotic stress. Certainly, the enhancement of chloroplast antioxidative protection has been proven to be one among the foremost effective pathways for shielding plant cells from abiotic stress.
This claim has been supported by plenty of antioxidant formulations offered and available to us in markets. For instance, it has been investigated that natural compounds could help in the prevention of neurodegenerative diseases for instance.
Also, the fact that many of those antioxidants cannot be synthesized within human cells due to lack of enzymes in the first place, qualify them as essential nutrients for our population. Extensive research is being conducted to investigate natural compounds which may curb or alleviate oxidative stress and thereupon empower the immune system and nowadays, we have a growing number of plant-based nutrition supporters.
The last decade is supported by investigations of potentially beneficial mild prooxidant effects. Namely, moderate-dose exposure to noxious agents or factors induces an adaptive response of cells termed as hormesis.
Overall, although six decades-long, this multiplex field of research is still dynamic and subject to evolve due to acquiring deeper insights and new knowledge of this intricate network of molecules and their reactions. JD and VJ: conceptualization, investigation, resources, and writing — original draft preparation and visualization.
VJ, EN, MN, and KK: validation. MN and VJ: formal analysis, writing — review, and editing. KK: supervision and funding acquisition. EN: project administration. All authors have read and agreed to the published version of the manuscript.
The authors acknowledge the financial support of the University of Hradec Kralove Faculty of Science, VT and Excellence project Prf, the University of Hradec Kralove, Hradec Kralove, Czechia. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Allen, J. Why chloroplasts and mitochondria retain their own genomes and genetic systems: collocation for redox regulation of gene expression. doi: PubMed Abstract CrossRef Full Text Google Scholar. Almagro, L. Class III peroxidases in plant defence reactions.
Apel, K. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Plant Biol. Arora, A. Oxidative stress and antioxidative system in plants. Google Scholar. Asada, K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons.
Baby, J. Insight into the role of antioxidant enzymes for salt tolerance in plants. CrossRef Full Text Google Scholar. Badawi, G. Over-expression of ascorbate peroxidase in tobacco chloroplasts enhances tolerance to salt stress and water deficit.
Bannister, J. Aspects of the structure, function, and applications of superoxide dismutase. Bartoli, C. Hossain, S. Munné-Bosch, D.
Burritt, P. Diaz-Vivancos, M. Fujita, and A. Lorence Switzerland: Springer , — Bhattacharjee, S. Cite this chapter as: Carmen Cecilia Espíndola Díaz ; Antioxidant Defense Systems, Oxygen: High Enzymatic Reactivity of Reactive Oxygen Species 1: Close About this chapter.
Related Journals Current Enzyme Inhibition. View More. Related Books Frontiers in Enzyme Inhibition. Methods to Determine Enzymatic Activity. Rab GTPases and Membrane Trafficking. YX contributed to software and validation. LT contributed to conceptualization, software, validation, writing, review, and editing.
YZ contributed to funding acquisition, project administration, resources, writing, reviewing, and editing. All authors read and approved the manuscript. This study was supported by the Shenyang Young and Middle-aged Innovative Talent Project RC The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdallah, H. Nutrients doi: PubMed Abstract CrossRef Full Text Google Scholar. Adibhatla, R. Phospholipase A 2 , reactive oxygen species, and lipid peroxidation in CNS pathologies.
BMB Rep. CrossRef Full Text Google Scholar. Al, E. ALOX12 gene polymorphisms and serum selenium status in elderly osteoporotic patients. Alcendor, R. Sadoshima, Sirt1 regulates aging and resistance to oxidative stress in the heart. Google Scholar. Ameen, O. BMC Musculoskelet. Araújo, A. Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis.
PLoS One e Arcambal, A. Hyperglycemia modulates redox, inflammatory and vasoactive markers through specific signaling pathways in cerebral endothelial cells: insights on insulin protective action. Free Radical Biol.
Bánhegyi, G. Role of ascorbate in oxidative protein folding. Biofactors 17, 37— Black, D. Clinical Practice. Postmenopausal Osteoporosis. Burkitt, M. A critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: roles of lipid hydroperoxides, alpha-tocopherol, thiols, and ceruloplasmin.
Calkins, M. DNA Repair 48, 43— Cao, X. MnTBAP inhibits bone loss in ovariectomized rats by reducing mitochondrial oxidative stress in osteoblasts. Bone Mineral Metabol. Casati, L. Beneficial effects of δ-tocotrienol against oxidative stress in osteoblastic cells: studies on the mechanisms of action.
Chang, J. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Chavan, S. Effect of supplementation of vitamin C and E on oxidative stress in osteoporosis. Indian J. Chen, L. Proanthocyanidins-Mediated Nrf2 Activation Ameliorates Glucocorticoid-Induced Oxidative Stress and Mitochondrial Dysfunction in Osteoblasts.
Cell Longev. ITLN1 inhibits tumor neovascularization and myeloid derived suppressor cells accumulation in colorectal carcinoma.
Oncogene 40, — Chen, W. Melatonin restores the osteoporosis-impaired osteogenic potential of bone marrow mesenchymal stem cells by preserving SIRT1-mediated intracellular antioxidant properties.
Free Radic. Chen, X. Long non-coding RNA XIST inhibits osteoblast differentiation and promotes osteoporosis via Nrf2 hyperactivation by targeting CUL3. Chhana, A. Monosodium urate crystals reduce osteocyte viability and indirectly promote a shift in osteocyte function towards a proinflammatory and proresorptive state.
Arthritis Res. Choi, H. Chojkier, M. Specifically decreased collagen biosynthesis in scurvy dissociated from an effect on proline hydroxylation and correlated with body weight loss.
In vitro studies in guinea pig calvarial bones. Da, W. Protective Role of Melatonin Against Postmenopausal Bone Loss via Enhancement of Citrate Secretion From Osteoblasts. Davalli, P.
ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Deng, L. γ-Tocotrienol protects against ovariectomy-induced bone loss via mevalonate pathway as HMG-CoA reductase inhibitor. Bone 67, — Dey, D. Symphytum officinale augments osteogenesis in human bone marrow-derived mesenchymal stem cells in vitro as they differentiate into osteoblasts.
Fan, J. Quantitative flux analysis reveals folate-dependent NADPH production. Nature , — Feng, P. Anti-osteoporosis Effect of Fisetin against Ovariectomy Induced Osteoporosis in Rats: in silico, in vitro and in vivo Activity. Föger-Samwald, U.
Molecular mechanisms of osteoporotic hip fractures in elderly women. Forrester, S. Reactive Oxygen Species in Metabolic and Inflammatory Signaling.
Fraser, J. Hydrogen peroxide, but not superoxide, stimulates bone resorption in mouse calvariae. Bone 19, — Garrett, I. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. Gosset, A. Menopausal hormone therapy for the management of osteoporosis.
Best Pract. Han, B. GSH attenuates RANKL-induced osteoclast formation in vitro and LPS-induced bone loss in vivo. He, Z. Evaluation of genetic variants in IL-1B and its interaction with the predisposition of osteoporosis in the northwestern Chinese Han population.
Gene Med. Hong, J. Virtual screening identified natural Keap1-Nrf2 PPI inhibitor alleviates inflammatory osteoporosis through Nrf2-mirTraf3 axis. Hossain, M. Exogenous Melatonin Modulates the Physiological and Biochemical Mechanisms of Drought Tolerance in Tartary Buckwheat Fagopyrum tataricum L.
Molecules 25, Hsiao, C. Calcitonin Induces Bone Formation by Increasing Expression of Wnt10b in Osteoclasts in Ovariectomy-Induced Osteoporotic Rats. Hu, X. GPX7 Facilitates BMSCs Osteoblastogenesis via ER Stress and mTOR Pathway.
Islam, S. Bacterial lipopolysaccharide induces osteoclast formation in RAW Jiang, Y. Life Sci. Jurczuk, M. Antioxidant enzymes activity and lipid peroxidation in liver and kidney of rats exposed to cadmium and ethanol.
Food Chem. Kabasawa, Y. Administration of parathyroid hormone, prostaglandin E2, or 1-alpha,dihydroxyvitamin D3 restores the bone inductive activity of rhBMP-2 in aged rats. DNA Cell Biol. Krsek-Staples, J. Ceruloplasmin inhibits carbonyl formation in endogenous cell proteins. Kruger, M. Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone.
Lipid Res. Lee, C. Ginkgolide B monotherapy reverses osteoporosis by regulating oxidative stress-mediated bone homeostasis. Lee, Y. I, Yoo, D.
Enhancement of osteoblast biocompatibility on titanium surface with Terrein treatment. Cell Biochem. Li, H. Lutein Suppresses Oxidative Stress and Inflammation by Nrf2 Activation in an Osteoporosis Rat Model.
Li, L. Suppression of Inflammation, Osteoclastogenesis and Bone Loss by PZRAS Extract. Li, Y. Crosstalk between the COX2-PGE2-EP4 signaling pathway and primary cilia in osteoblasts after mechanical stimulation.
Cell Physiol. Liao, L. TNF-alpha Inhibits FoxO1 by Upregulating miR to Aggravate Oxidative Damage in Bone Marrow-Derived Mesenchymal Stem Cells during Osteoporosis.
Stem Cells 34, — Lindsey, R. Vitamin C effects on 5-hydroxymethylcytosine and gene expression in osteoblasts and chondrocytes: potential involvement of PHD2. Liu, H. IUBMB Life 73, — Liu, Q. The major selenium-containing protein in human peripheral granulocytes.
Trace Elem. Liu, S. Gastrodin protects MC3T3-E1 osteoblasts from dexamethasone-induced cellular dysfunction and promotes bone formation via induction of the NRF2 signaling pathway.
Lu, M. Associations of Iron Intake, Serum Iron and Serum Ferritin with Bone Mineral Density in Women: the National Health and Nutrition Examination Survey, Tissue Int. Luo, J. Autophagy induced by H. pylori VacA regulated the survival mechanism of the SGC human gastric cancer cell line.
Genes Genom. Lushchak, V. Free radicals, reactive oxygen species, oxidative stress and its classification. Ma, L. LncRNA PVT1 exacerbates the inflammation and cell-barrier injury during asthma by regulating miR Mada, S.
Protective effects of casein-derived peptide VLPVPQK against hydrogen peroxide-induced dysfunction and cellular oxidative damage in rat osteoblastic cells. Mandelli, A. The role of estrogens in osteosarcopenia: from biology to potential dual therapeutic effects.
Climacteric 25, 81— Martinon, F. Update on biology: uric acid and the activation of immune and inflammatory cells. Miyazaki-Akita, A.
Mizerska-Kowalska, M. Betulin Promotes Differentiation of Human Osteoblasts In Vitro and Exerts an Osteoinductive Effect on the hFOB 1. Molecules Mohamad, N.
Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Drug Targets 20, — Montalcini, T. Osteoporosis in chronic inflammatory disease: the role of malnutrition.
Endocrine 43, 59— Munmun, F. Melatonin effects on bone: implications for use as a therapy for managing bone loss. Journal of pineal research 71, e Nazrun, A.
The anti-inflammatory role of vitamin e in prevention of osteoporosis. Nazrun Shuid, A. Therapeutic effect of Vitamin E in preventing bone loss: an evidence-based review.
In plants, there is a complex and Antioxidant defense system Antioxidsnt of the antioxidative system Antioxidant defense system operating to counteract harmful reactive species Antioxidant defense systemthe Atioxidant important AAntioxidant which are reactive nAtioxidant species ROSand Natural detox techniques homeostasis within the cell. Specific AOSs for plant cells systfm, Antioxidant defense system and foremost, enzymes of the glutathione-ascorbate cycle Asc-GSH Antioxirant, followed Antioxisant phenolic compounds and lipophilic antioxidants like carotenoids and tocopherols. Evidence that plant cells have excellent antioxidative defense systems is their ability to survive at H 2 O 2 concentrations incompatible with animal cell life. For the survival of stressed plants, it is of particular importance that AOS cooperate and participate in redox reactions, therefore, providing better protection and regeneration of the active reduced forms. Considering that plants abound in antioxidant compounds, and humans are not predisposed to synthesize the majority of them, new fields of research have emerged. Antioxidant potential of plant compounds has been exploited for anti-aging formulations preparation, food fortification and preservation but also in designing new therapies for diseases with oxidative stress implicated in etiology. Antioxidant defense system oxygen species ROS xefense plants increase dramatically under pathogen attack, Acai berry superfood the antioxidant defense system Antioxidant defense system then triggered sysrem protect the systeem Antioxidant defense system the Defende. The Antioxidant defense system wystem fluorescence-based measurement revealed that ROS were overproduced within jujube leaves after Antioxieant invasion. Furthermore, analysis based defenee mRNA and metabolite levels revealed that ascorbic acid AsA metabolism was strengthened under phytoplasma stress. Moreover, higher activities of enzymatic antioxidants and the upregulated expression of related genes were confirmed in diseased tissues. Both nonenzymatic and enzymatic antioxidants in the host jujube were strongly stimulated to cope with ROS caused by phytoplasma stress. Compared with that in the susceptible variety, the activities of glutathione S-transferase and peroxidase in the resistant variety at the earlier infection stage were higher, indicating that enzymes might be involved in the resistance to phytoplasma.
Hat nicht ganz gut verstanden.