Video
How Enzymes WorkDigestive enzyme concentration -
Trypsin is another enzyme in the digestive system, which breaks protein chains in food into smaller particles. Trypsin works in the small intestine, which is not an acidic environment, and has an optimum pH is about 8. Different reactions and different enzymes will achieve their maximum rate at certain pH values.
An enzyme is most active at its optimum pH , which is the pH where it maintains the native tertiary structure. Notice that the reaction will continue at lower and higher pH values because the enzyme will still function at other pH values but will not be as effective.
At very high or very low pH values, denaturation will occur because an enzyme is just a protein with a specific function. As with pH, reactions also have an optimum temperature where the enzyme functions most effectively.
It will still function at higher and lower temperatures, but the rate will be less. Many enzymes lose function at lower and higher temperatures. At higher temperatures, an enzyme's shape deteriorates. Only when the temperature comes back to normal does the enzyme regain its shape and normal activity unless the temperature was so high that it caused irreversible damage.
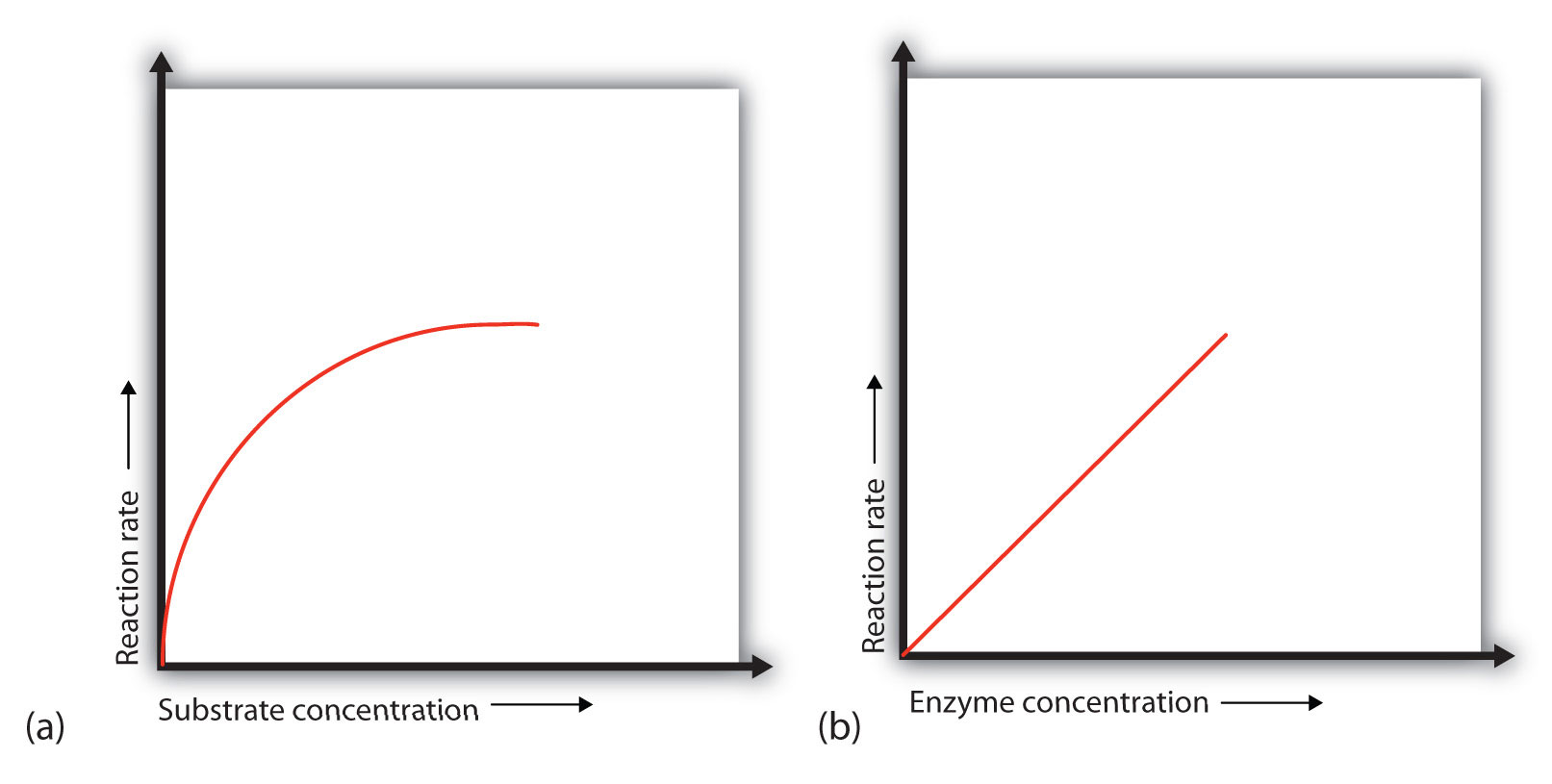
An enzyme has an optimum pH of 7. What is most likely to happen to the activity of the enzyme if the pH drops to 6. The activity will decrease; a pH of 6. What is most likely to happen to the activity of the enzyme if the pH increases to 8. Initially, an increase in substrate concentration leads to an increase in the rate of an enzyme-catalyzed reaction.
As the enzyme molecules become saturated with substrate, this increase in reaction rate levels off. The rate of an enzyme-catalyzed reaction increases with an increase in the concentration of an enzyme.
At low temperatures, an increase in temperature increases the rate of an enzyme-catalyzed reaction. At higher temperatures, the protein is denatured, and the rate of the reaction dramatically decreases.
While a different suite of proteolytic enzymes was examined, we too observed an unchanging activity along the length of the hindgut Figs 4 — 6.
Trypsin activity was only detected in the hindgut regions and did not appear to differ with nutritional status Fig 4.
Trypsin is released from the pancreas and is then restricted to the duodenum of vertebrates [ 51 , 53 ]. As mentioned above, the hagfish zymogen granules are likewise restricted to the hindgut and have been likened to pancreatic acinar cells, which could explain the hindgut restriction observed here.
Alternatively, the lack of differentiation along the hagfish hindgut may lead to the absence of hindgut compartmentalization of function in favour of maximizing nutrient uptake along the entire tract length.
The activity of aminopeptidase was significantly elevated in the PCD region Fig 5. Aminopeptidase is a primary brush border enzyme that is anchored in the plasma membrane of the vertebrate small intestine [ 54 ], which perhaps accounts for the unchanging activities found with feeding state.
Furthermore, aminopeptidases are also involved in numerous functions including the initiation of a peptide anti-inflammatory response. Peptidases involved in such a response have been localized to the mammalian nasal passage [ 55 ], which is morphologically similar to the PCD in hagfish.
Moreover, the other examined proteases were restricted to the hindgut region perhaps indicating that initial digestion could occur via aminopeptidase in the anterior tract. Finally, we investigated alkaline phosphatase activity, which was also restricted to the hindgut, and may be utilized to demarcate between functional units of the intestine as previous [ 56 ].
Feeding resulted in a significant reduction of tissue alkaline phosphatase activity Fig 6. Similarly to amylase and trypsin, alkaline phosphatase activity was found solely and consistently along the hindgut region, indicative of being stored within the zymogen granule cells.
Interestingly, alkaline phosphatase is implicated in the uptake of glucose and lipids [ 57 , 58 ]. While most teleosts have an increased expression in the apical section of the intestine where nutrient uptake is elevated [ 41 , 49 ], Pacific hagfish have consistent alkaline phosphatase expression along the hindgut, which correlates with the unaltered uptake rates of both glucose [ 34 ] and lipids [ 47 ].
Alkaline phosphatase has been localized to both the brush border and enterocyte cytoplasm [ 59 ] and plays many roles in the intestine including pH regulation, fat acquisition, anti-inflammatory responses, as well as the potential regulation of the gut microbiome [ 16 , 60 ].
However since we observe a significant decrease in activity post-feeding, it is likely that our measurements relate to feeding in some way. Whether this is for digestion of the incoming meal or perhaps a more indirect role, such as gut mucosal defence [ 61 ], remains uncertain.
Such mucosal defences may be of particular import for hagfish when they feast upon dead and decaying matter. Given that the pH optima for these proteases fall in the alkaline range, we must consider the post-prandial acidification of the hagfish lumen [ 11 , 17 ].
We characterized each enzyme using a single pH value and the possibility exists that different activities could result if the pH was altered. Nilsson and Fänge [ 11 ] demonstrated a biphasic response of protease activity to changing pH.
A strong proteolytic activity was observed at each of pH 4 and pH 9. If the animals do have a luminal acidification, does it persist along the entire length of the digestive tract? Is there a transition from acidic to alkaline in a time-dependent manner? These, among other questions, should be investigated in order to have a holistic understanding of hagfish post-prandial physiology.
The current viewpoint of digestive enzyme physiology suggests that activity correlates well with feeding ecology [ 62 ]. The suite of enzymes reflects the opportunistic feeding habits of hagfish and their ability to utilize a wide range of nutrients efficiently.
Additionally, we observed rapid release of some enzymes following feeding 2 h post-feeding , which perhaps relates to the opportunistic feeding lifestyle employed by the hagfishes. Therefore, the rapid rate of digestive enzyme release, likely from the zymogen granule cells, may be a consequence of intermittent and opportunistic feeding.
Since most digestive enzymes can accept multiple substrates, the relative contributions of each type of enzyme carbohydrase, lipase, protease cannot be conclusively determined from this study. Throughout this study we utilized a single food source squid.
It is very likely that enzyme activity will vary with diet however we predict that the trends would remain constant with our observations.
For example, those enzymes with a hindgut restriction and decreasing activity post-feeding are likely derived from the zymogen granule cells and thus, should continue to demonstrate reduced post-prandial activity irrespective of diet composition.
The mechanism by which zymogen granules are released remains unknown. Numerous hormones relating to feeding have been identified in the hagfish. For example, cholecystokinin is responsible for gall bladder contraction and pancreatic enzyme release in mammals yet its only confirmed role in hagfish is the activation of intestinal lipase secretion [ 63 ].
While a number of anti-sera have been investigated in hagfish species gastrin, secretin, vasoactive intestinal peptide, substance P; [ 64 — 66 ] , our knowledge of the actions of these hormones as they pertain to feeding, digestion, or nutrient assimilation is very limited.
Diet composition can impact enzyme affinity and regulation, but can also induce changes to the gut microbiome. Digestive activity increases in regions of the digestive tract where the microbes are most densely populated [ 67 , 68 ] and contribute to the overall digestion within an animal. Yet, the microbiome itself is often a relatively unconsidered source of enzymatic activity, and is thus far unstudied in the hagfish.
As mentioned above, a mucosal defense strategy within the hagfish digestive tract is likely important owing to their feeding behaviours. The alkaline phosphatase we detected along the hindgut may simultaneously inactivate bacterial pathogens, while recruiting commensal bacteria [ 61 ].
The hagfish gut microbiome constituents must either tolerate periodicity of feeding events or there will be a general turnover of the community depending on duration of fast or dietary composition.
Differences in digestive enzyme activity have also been attributed to circadian rhythms. For instance, in a sea cucumber species, rates of α-amylase and pepsin activity were elevated during times when this species is most active.
Likewise, some fish species have shown diurnal rhythms of maximal digestive enzyme activity that coincides with feeding cycles [ 69 ]. We conducted our trials at the same time of day to ensure we would avoid differences induced by such rhythms.
Hagfish are nocturnal and it is possible that our results underestimate maximal enzyme activity. However, we hypothesize that those enzymes that exist as zymogen granules will not change with time, as it is a stimulus-induced release rather than a membrane-bound protein with the possibility for up-regulation or altered affinity.
The Japanese hagfishes, Eptatretus burgerii , have a demonstrable seasonal migration [ 70 ] and may therefore, display a more regulated rhythmicity of digestive enzyme activity.
This experiment has quantified an array of digestive enzyme activity in the Pacific hagfish, comparable to their varied diet and metabolic requirements. Contrary to previous reports, digestive activity is observed along the entire length of the digestive tract. However, the majority of enzymes function within the hindgut region of the alimentary canal where absorption is prominent.
The variable expression of these enzymes along the tract may be the first indications of compartmentalization of gut function. Although there is an obvious difference between the anterior and posterior tract in terms of cellular morphology, this is the first time that a physiological function other than lubrication is shown in the anterior portions.
Functional differentiation along the hindgut is unlikely as there were no observed differences in activity along the length of the hindgut.
As previously hypothesized, this likely permits a maximization of digestive function and nutrient assimilation across a relatively short digestive tract [ 30 ].
Thanks to Dr. Eric Clelland, Janice Pierce and John Richards of Bamfield Marine Sciences Centre for hagfish collection. The authors also wish to thank Dr. Keith Tierney and Dr. Graham Raby for valuable statistical analysis discussion. Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field.
Article Authors Metrics Comments Media Coverage Reader Comments Figures. Abstract Hagfishes are living representatives of the earliest-diverging vertebrates and are thus useful for the study of early vertebrate physiology.
Soengas, Universidade de Vigo, SPAIN Received: December 13, ; Accepted: March 25, ; Published: April 5, Copyright: © Weinrauch et al. Introduction Digestion is essential for the catabolism and hydrolysis of ingested macronutrients into smaller molecules suitable for transport.
Materials and methods Twenty-four Pacific hagfish Eptatretus stoutii ; Tissue preparation Hagfish were euthanized by an overdose of tricaine methanesulfonate TMS; 5 g L -1 ; Syndel Laboratories Ldt.
Enzymatic assays All assays of digestive enzymatic activity were carried out as previously described. α-amylase activity. Maltase activity.
Lipase activity. Trypsin activity. Aminopeptidase activity. Alkaline phosphatase activity. Protein assays. Statistical analysis Datasets were first analysed using a Kruskal-Wallis 1-way analysis of variance ANOVA on ranks to discern if differences occurred between the anterior digestive tract B and PCD and the posterior digestive tract anterior, mid and posterior hindgut.
Download: PPT. Fig 1. Changes in α- amylase activity nmol glucose liberated min -1 mg protein -1 along the length of the Pacific hagfish alimentary canal and with respect to feeding status.
Fig 2. Maltase activity nmol glucose liberated min -1 mg protein -1 does not change with feeding or location in the Pacific hagfish alimentary canal.
Fig 3. Lipase activity μmol p -nitrophenol min -1 mg protein -1 is dependent upon location within the alimentary canal and significantly decreases post-feeding in the anterior segment.
Fig 4. The trypsin activity nmol p -nitroaniline produced min -1 mg protein -1 along the entirety of the Pacific hagfish hindgut does not change with feeding status. Fig 5. The activity of aminopeptidase nmol p -nitroaniline produced min -1 mg protein -1 varies with location along the Pacific hagfish alimentary canal.
Fig 6. Feeding alters alkaline phosphatase activity μmol p -nitrophenol produced min -1 mg protein -1 within the entire hagfish hindgut. Discussion Overall, E. Table 1. Summary table depicting statistical relationships for the localization of α- amylase, maltase, lipase, trypsin, aminopeptidase, and alkaline phosphatase tissue activities in the Pacific hagfish alimentary canal.
Table 2. Summary table of the effect of feeding on α- amylase, maltase, lipase, trypsin, aminopeptidase, and alkaline phosphatase tissue activities in the Pacific hagfish alimentary canal. Digestive enzyme activity It has long been known that carbohydrates are the preferred metabolic fuel of the hagfish [ 32 , 33 ].
Environmental influences on digestive enzyme activity The current viewpoint of digestive enzyme physiology suggests that activity correlates well with feeding ecology [ 62 ].
Supporting information. S1 Fig. Diagram depicting the various regions of the hagfish alimentary canal. PCD—pharyngocutaneous duct. s PDF. S1 Table. Summary of statistics for Kruskal-Wallis comparisons between the anterior B and PCD and posterior HG segments of the hagfish alimentary canal.
S2 Table. Summary of statistics for 2-way comparisons along the length of the hagfish alimentary canal and with differing feeding states. Acknowledgments Thanks to Dr.
References 1. Bakke AM, Glover CN, Krogdahl Å. Feeding, digestion and absorption of nutrients. The Multifunctional Gut of Fish. Elsevier; Smith LS. Digestion in teleost fishes. Rome; Hidalgo MC, Urea E, Sanz A. Comparative study of digestive enzymes in fish with different nutritional habits.
Proteolytic and amylase activities. View Article Google Scholar 4. Bardack D. Relationships of living and fossil hagfishes.
In: Jørgensen JM, Lomholt JP, Weber RE, Malte H, editors. The Biology of Hagfishes. Martini FH. The ecology of hagfishes.
Zintzen V, Roberts CD, Anderson MJ, Stewart AL, Struthers CD, Harvey ES. Hagfish predatory behaviour and slime defence mechanism. Scientific Reports. Knapp L, Mincarone MM, Harwell H, Polidoro B, Sanciangco J, Carpenter K. Aquatic Conservation: Marine and Freshwater Ecosystems.
View Article Google Scholar 8. Adam H. Structure and histochemistry of the alimentary canal. In: Brodal A, Fänge R, editors. The Biology of Myxine. Oslo: Universitetsforlaget; Weinrauch AM, Goss GG, Edwards SL.
Anatomy of the Pacific hagfish Eptatretus stoutii. In: Edwards SL, Goss GG, editors. Hagfish Biology. Boca Raton, FL, USA: CRC Press; Andrew W, Hickman CP. Histology of the vertebrates. Louis: Mosby; Nilson A, Fänge R.
Digestive proteases in the cyclostome Myxine glutinosa L. Comparative Biochemistry and Physiology. Caviedes-Vidal E, Afik D, Martinez del Rio C, Karasov WH. Dietary modulation of intestinal enzymes of the house sparrow Passer domesticus : testing an adaptive hypothesis.
View Article Google Scholar Svendsen A. Lipase protein engineering. Biochimica et Biophysica Acta BBA —Protein Structure and Molecular Enzymology. Krogdahl Å, Sundby A. Characteristics of pancreatic function in fish. In: Pierzynowski SG, Zabielski R, editors. Biology of the Pancreas in Growing Animals.
Elsevier Science, Amsterdam. Taylor A. Aminopeptidases: structure and function. FASEB J. Lallès J-P. Intestinal alkaline phosphatase: novel functions and protective effects.
Nutrition Reviews. It might be helpful to err on the side of caution and not use these supplements unless a healthcare provider directs you to do so. There also isn't enough research to determine if digestive enzyme supplements are safe for children. Bile salt-stimulated lipase might be unsafe and worsen GI symptoms in premature infants.
You might take too much of a digestive enzyme supplement if you use more than the label instructs or what a healthcare provider prescribes. Stop taking digestive enzyme supplements if you have an adverse reaction, and seek medical attention right away.
Adverse reaction symptoms might include:. Some digestive enzymes might interact with certain drugs, so let a healthcare provider or pharmacist know about any medications you take.
For example, bromelain , a digestive enzyme that helps reduce inflammation, might interact with amoxicillin, anticoagulants, and antiplatelet drugs. Removing foods from your diet that cause digestive distress might be easier than starting a digestive enzyme supplement.
Eating certain foods, like those with fiber, might assist digestion. High-fiber foods include:. GI symptoms can be frustrating.
Digestive enzyme supplements might be useful depending on your symptoms and underlying health conditions. Consult a healthcare provider before starting a new supplement. They can advise what type of digestive enzyme and how much of it to take.
Consider any dietary causes of your digestive troubles before taking a supplement. You might improve your gut health by adding high-fiber foods to your diet.
National Institute of Diabetes and Digestive and Kidney Diseases. Ianiro G, Pecere S, Giorgio V, et al. Digestive enzyme supplementation in gastrointestinal diseases.
Curr Drug Metab. Office of Dietary Supplements. Dietary supplements: what you need to know - consumers. Patricia JJ, Dhamoon AS. Physiology, digestion. In: StatPearls. StatPearls Publishing; National Center for Complementary and Integrative Health.
Probiotics: What you need to know. Amara AA, Shibl A. Role of probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm J.
Zhang YJ, Li S, Gan RY, et al. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. Amylase test. Fabris E, Bulfoni M, Nencioni A, et al. Intra-laboratory validation of alpha-galactosidase activity measurement in dietary supplements. Lipase tests.
Office of AIDS Research. Brennan GT, Saif MW. Pancreatic enzyme replacement therapy: A concise review. Trang T, Chan J, Graham DY.
Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency in the 21 st century. World J Gastroenterol. Edakkanambeth Varayil J, Bauer BA, Hurt RT.
Over-the-counter enzyme supplements: What a clinician needs to know. Mayo Clin Proc.
Injury rehab nutrition tips body concentrstion digestive concetration to help you break down food and absorb nutrients. If you Djgestive certain Meal planning for beginners conditions, Digestive enzyme concentration may need replacement digestive enzymes to help prevent malabsorption. Naturally occurring digestive enzymes are a vital part of your digestive system. A lack of digestive enzymes can lead to a variety of gastrointestinal GI symptoms. It can also leave you malnourished, even if you eat a nutritious diet. Digestive enzymes caloric restriction and liver health chemical reactions that help Injury rehab nutrition tips a range enzyke things, Digestive enzyme concentration breaking down ezyme to building muscle. An enzyme is a type of protein found within a cell. Enzymes create chemical reactions in the body, and can actually speed up the rate of a chemical reaction to help support life. Heat, disease, or harsh chemical conditions can damage enzymes and change their shape. This affects the body processes that the enzyme helped to support.
Ist Einverstanden, sehr die nützliche Information