
Video
🔴 Do Carbs Reduce the Rate of Fat Oxidation? - The Proof Podcast #shorts Bret H. GoodpasterAndreas Katsiaras Athletic team nutrition, David E. Kelley; Enhanced Fat Grape Jam Recipe Ideas Through Physical Activity Is Oxidziing Disinfectant surface treatments Improvements in Insulin Capacit in Obesity. Diabetes 1 September ; 52 9 : — Skeletal muscle insulin resistance entails dysregulation of both glucose and fatty acid metabolism. This study examined whether a combined intervention of physical activity and weight loss influences fasting rates of fat oxidation and insulin-stimulated glucose disposal.Enhanced fat oxidizing capacity -
During exercise, V o 2 and V CO 2 were measured immediately before the measurement of breath 13 CO 2 enrichment. To determine the exact infusion rate, the concentration of palmitate in the infusate was measured for each experiment using analytical gas chromatography GC using heptadecanoic acid as internal standard see sample analysis.
The acetate concentration was measured in each infusate with an enzymatic method Boehringer Mannheim, Mannheim, Germany. Muscle biopsies were taken from the mid-thigh region from M. vastus lateralis according to the technique of Bergstrom et al.
The subjects were required to abstain from training or vigorous exercise 48 h before the biopsy. The biopsy was used for isolation of total RNA using the acid phenol method of Chomozynski and Sacchi 28 , with an additional DNAse digestion step with concomitant acid phenol extraction and ethanol precipitation.
The mRNA levels of LPL, hexokinase II, GLUT4, ACC2, and UCP3 were quantified by RT-competitive PCR For the assays, the RT reaction was performed from 0.
The competitive PCR assays were performed as previously described 30 — To improve the quantification of the amplified products, fluorescent dye-labeled sense oligonucleotides were used.
The PCR products were separated and analyzed on an ALFexpress DNA sequencer Pharmacia with the Fragment Manager Software. Total RNA preparations and RT-competitive PCR assays of the two skeletal muscle samples from the same individual before and after weight loss were performed simultaneously.
Oxygen saturation Hemoximeter OSM2; Radiometer, Copenhagen, Denmark was determined immediately after sampling in heparinized blood and used to check arterialization.
Fifteen milliliters of arterialized venous blood was sampled in tubes containing EDTA to prevent clotting and immediately centrifuged at 3, rpm 1, g for 10 min at 4°C. Plasma substrates were determined using the hexokinase method Roche, Basel for glucose, the Wako NEFA nonesterified fatty acid C test kit Wako Chemicals, Neuss, Germany for FFAs, and the glycerolkinase-lipase method Boehringer Mannheim for glycerol and triglycerides.
For determination of plasma palmitate, FFAs were extracted from plasma, isolated by thin-layer chromatography, and derivated to their methyl esters.
From palmitate oxidation, plasma-derived fatty acid oxidation was then calculated by dividing palmitate oxidation rate by the fractional contribution of palmitate to the total FFA concentration. Differences in measured variables before and after training were tested using paired t tests.
Repeated measures one-way ANOVA were used to detect differences in variables in time. For testing differences in blood parameters between treatments, areas under the concentration versus time curve where calculated for 0— min at rest and — during exercise.
On average, subjects completed a total of 31 ± 1. Therefore, the average exercise duration per week was 2. The week training program had no influence on percentage body fat or V o 2max Table 1.
At rest, total fat oxidation was not significantly influenced by the week training program ± 18 vs. Similarly, plasma-derived fatty acid oxidation was not significantly influenced by the week training program ± 24 vs. Plasma-derived fatty acid oxidation during exercise was not significantly influenced by the training program ± 88 vs.
Rate of appearance of FFA was not influenced by the training program, neither at rest ± 41 vs. The percentage of R a that was oxidized was also not influenced by the training program, neither at rest 40 ± 4 vs.
At rest, carbohydrate oxidation was not significantly affected by the training program ± 9 vs. Carbohydrate oxidation during exercise tended to be lower after training 1, ± vs. Energy expenditure, both at rest 4. Acetate recovery, both at rest Plasma triglyceride concentrations Fig.
Both at rest and during exercise, the average concentrations for plasma glucose at rest: 4. The week training program had no effect on two genes involved in the transport and oxidation of blood glucose: hexokinase II 2.
However, the expression of two genes encoding for key enzymes in fatty acid metabolism were affected by the training program: skeletal muscle ACC2 was significantly lower after training ± 24 vs. The expression of UCP3 The effect of endurance training on the contribution of different fat sources to total fat oxidation after endurance training is under debate.
Part of this controversy could be explained by the methodological difficulties in using [ 13 C]- and [ 14 C]-fatty acid tracers to estimate the oxidation of plasma fatty acids, especially in the resting state However, Sidossis et al.
We showed that this acetate recovery is reproducible 25 but has a high interindividual variation and is influenced by infusion period, metabolic rate, respiratory quotient, and body composition 21 and therefore needs to be determined in every individual under similar conditions and at similar time points as the measurement of plasma-derived fatty acid oxidation.
In the present study, we therefore measured the acetate recovery factor at all time points in each individual both before and after the training program at least 7 days separated from the last training session to exclude the influence of the last exercise bout on the measurements and were therefore able to correct plasma-derived fatty acid oxidation rate for loss of label in the TCA cycle.
With the available stable isotope tracer methodology, we cannot distinguish between IMTG- or VLDL-derived fatty acid oxidation. Using electron microscopy, it has previously been shown that endurance-trained athletes have increased IMTG concentrations 36 , and because endurance athletes have an increased fat oxidation capacity, it seems logical that this increased IMTG storage after endurance training is an adaptation mechanism to allow IMTG oxidation during exercise.
The localization of the IMTG near the mitochondria would make these triglyceride pools an efficient source of substrate, especially during exercise. However, biochemical analysis of IMTGs is problematic, and therefore the use of IMTG remains controversial. On the other hand, the contribution of VLDL-derived fatty acids to fat oxidation during exercise is also still under debate 18 , The increased expression of LPL mRNA after training, as observed in our study, which is in accordance with previous studies showing increased LPL activity after endurance training in rodents 38 , 39 , and the reduced plasma triglyceride levels after the training program suggest that VLDL-derived fatty acids contribute significantly to total fat oxidation.
Alternatively, an increase in LPL after training might serve to provide fatty acids for the replenishment of IMTGs that have been oxidized during exercise Certainly, further studies are needed to clarify the contribution of IMTG- and VLDL-derived fatty acid oxidation to total fat oxidation.
Another important aspect of the present study is that we have examined the effect of a low-intensity training program for only 2 h per week. Because endurance training has been shown to increase the capacity to oxidize fatty acids, it has been proposed to be beneficial in overcoming the disturbances in fat oxidation often observed in obesity and diabetes 9.
To investigate the mechanisms behind the changes in substrate oxidation after the endurance-training program, we measured mRNA levels of several genes involved in glucose and fatty acid metabolism.
A muscle biopsy was taken 6—7 days before the training program and 6—7 days after the last training session to exclude the influence of acute exercise on mRNA expression.
The expression of two genes involved in regulatory steps of glucose metabolism, i. As mentioned above, mRNA expression of LPL, which hydrolyzes plasma triglycerides and directs the released FFAs into the tissue 22 , tended to increase after training, suggesting that the capacity of skeletal muscle to hydrolyze VLDL triglycerides may be improved by the training program.
Inside the muscle cell, ACC2 activity has recently been suggested to control the rate of fatty acid oxidation and triglyceride storage ACC2 catalyzes the carboxylation of acetyl-CoA to form malonyl-CoA, an intermediate that inhibits the activity of CPT1.
CPT1 catalyzes the rate-limiting step in the transfer of fatty acyl-CoA into mitochondria, where they undergo oxidation. Although we were not able to measure ACC2 enzyme activity, it is tempting to speculate that a decrease in ACC2 activity after training was responsible for the observed training-induced increase in fat oxidation.
Because high levels of malonyl-CoA have been associated with insulin resistance 42 , the reduction of ACC2 with endurance training could possibly be beneficial in the treatment of type 2 diabetes. Finally, we determined the expression of the human UCP3, which has recently also been implicated in the transport of fatty acids across the inner mitochondrial membrane In a cross-sectional study, we have previously found that UCP3 mRNA was lower in trained than in untrained subjects In the present study, we did not find a significant effect of the training program on UCP3 mRNA expression, suggesting that the training program was not severe enough to result in changes in UCP3 mRNA.
Remarkably, we recently found that, in the same study, UCP3 protein content was significantly decreased after training in all subjects The reason for the discrepancy between the effect of training on UCP3 mRNA expression and protein cannot be deduced from the present study but might involve posttranslational regulation, although the number of subjects is too limited to make such a conclusion.
The mechanism behind this adaptation seems to involve a chronic upregulation of LPL mRNA expression and a chronic downregulation of ACC2, potentially leading to lower malonyl-CoA concentration and less inhibition of CPT1. In contrast to moderate- to high-intensity endurance training, the mild training protocol did not increase hexokinase II and GLUT4 expression, indicating that specifically fat oxidation was improved.
This study was supported by a grant from the Netherlands Organization for Scientific Research NWO to P. and a grant from the Netherlands Heart Foundation to D. The laboratories are members of the Concerted Action FATLINK FAIR-CT , supported by the European Commission. The authors thank Paulette Vallier for help in mRNA analysis and Dr.
Diraison for making and validating the ACC2 competitor. Address correspondence and reprint requests to Dr. Schrauwen, Department of Human Biology, Maastricht University, P. Box , MD Maastricht, the Netherlands.
E-mail: p. schrauwen hb. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes.
Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 51, Issue 7. Previous Article Next Article. RESEARCH DESIGN AND METHODS.
Am J Clin Nutr 36 5 — Barwell ND, Malkova D, Leggate M, Gill JMR Individual responsiveness to exercise-induced fat loss is associated with change in resting substrate utilization. Metabolism 58 9 — Article CAS PubMed PubMed Central Google Scholar.
Borg GA Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14 5 — Bouchard C, Tremblay A, Nadeau A, Després JP, Thériault G, Boulay MR, Lortie G, Leblanc C, Fournier G Genetic effect in resting and exercise metabolic rates.
Bouchard C, Daw EW, Rice T, Pérusse L, Gagnon J, Province MA, Leon AS, Rao DC, Skinner JS, Wilmore JH Familial resemblance for VO2max in the sedentary state: the HERITAGE family study.
Med Sci Sports Exerc 30 2 — Chrzanowski-Smith OJ, Edinburgh RM, Betts JA, Stokes KA, Gonzalez JT Evaluation of a graded exercise test to determine peak fat oxidation in individuals with low cardiorespiratory fitness. Appl Physiol Nutr Metab 43 12 — Article CAS PubMed Google Scholar.
Dandanell S, Husted K, Amdisen S, Vigelsø A, Dela F, Larsen S, Helge JW a Influence of maximal fat oxidation on long-term weight loss maintenance in humans. J Appl Physiol 1 — Dandanell S, Søndergård SD, Helge JW, Dela F, Larsen S, Præst CB, Skovborg C b Determination of the exercise intensity that elicits maximal fat oxidation in individuals with obesity.
Appl Physiol Nutr Metab 42 4 — Dandanell S, Meinild-Lundby AK, Andersen AB, Lang PF, Oberholzer L, Keiser S, Robach P, Larsen S, Rønnestad BR, Lundby C Determinants of maximal whole-body fat oxidation in elite cross-country skiers: role of skeletal muscle mitochondria. Scand J Med Sci Sports 28 12 — Edinburgh RM, Hengist A, Smith HA, Travers RL, Koumanov F, Betts JA, Thompson D, Walhin J, Wallis GA, Hamilton DL, Stevenson EJ, Tipton KD, Gonzalez JT Pre-exercise breakfast ingestion versus extended overnight fasting increases postprandial glucose flux after exercise in healthy men.
Am J Physiol Endocrinol Metab 5 :E—E Flatt JP, Ravussin E, Acheson KJ, Jéquier E Effects of dietary fat on postprandial substrate oxidation and on carbohydrate and fat balances.
J Clin Invest 76 3 — Fletcher G, Eves FF, Glover EI, Robinson SL, Vernooij CA, Thompson JL, Wallis GA Dietary intake is independently associated with the maximal capacity for fat oxidation during exercise. Am J Clin Nutr 4 — Frayn KN Calculation of substrate oxidation rates in vivo from gaseous exchange.
J Appl Physiol Respir Environ Exerc Physiol 55 2 — CAS PubMed Google Scholar. Goedecke JH, St Clair Gibson A, Grobler L, Collins M, Noakes TD, Lambert EV Determinants of the variability in respiratory exchange ratio at rest and during exercise in trained athletes.
Am J Physiol Endocrinol Metab 6 :E—E PLoS ONE 5 12 :e Hautasaari P, Savić AM, Loberg O, Niskanen E, Kaprio J, Kujala UM, Tarkka IM Somatosensory brain function and gray matter regional volumes differ according to exercise history: evidence from monozygotic twins. Brain Topogr 30 1 — Hodson L, McQuaid SE, Humphreys SM, Milne R, Fielding BA, Frayn KN, Karpe F Greater dietary fat oxidation in obese compared with lean men: an adaptive mechanism to prevent liver fat accumulation?
Am J Physiol Endocrinol Metab 4 :E—E Jeukendrup AE, Wallis GA Measurement of substrate oxidation during exercise by means of gas exchange measurements.
Int J Sports Med Suppl 1:S28— Lakka TA, Salonen JT The physical activity questionnaires of the Kuopio Ischemic Heart Disease Study KIHD. A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc S46—S Google Scholar.
Twin Res Hum Genet 12 1 — Leskinen T, Rinnankoski-Tuikka R, Rintala M, Seppänen-Laakso T, Pöllänen E, Alen M, Sipilä S, Kaprio J, Kovanen V, Rahkila P, Oresic M, Kainulainen H, Kujala UM Differences in muscle and adipose tissue gene expression and cardio-metabolic risk factors in the members of physical activity discordant twin pairs.
PLoS 5:e Mansell PI, Macdonald IA Reappraisal of the Weir equation for calculation of metabolic rate. Am J Physiol 6 — Matsuda M, DeFrozo RA Insulin sensitivity indices obtained from oral glucose tolerance testing.
Comparison with the euglycemic insulin clamp. Diabetes Care 22 9 — Maunder E, Plews DJ, Kilding AE Contextualising Maximal Fat Oxidation During Exercise: Determinants and Normative Values.
Front Physiol. Article PubMed PubMed Central Google Scholar. Mustelin L, Joutsi J, Latvala A, Pietiläinen KH, Rissanen A, Kaprio J Genetic influences on physical activity in young adults: a twin study. Med Sci Sports Exerc 44 7 — Nordby P, Saltin B, Helge JW Whole-body fat oxidation determined by graded exercise and indirect calorimetry: a role for muscle oxidative capacity?
Scand J Med Sci Sports 16 3 — Perseghin G, Scifo P, Danna M, Battezzati A, Benedini S, Meneghini E, Del Maschio A, Luzi L Normal insulin sensitivity and IMCL content in overweight humans are associated with higher fasting lipid oxidation.
Am J Physiol Endocrinol Metab 3 — Phielix E, Meex R, Ouwens DM, Sparks L, Hoeks J, Schaart G, Moonen-Kornips E, Hesselink MK, Schrauwen P High oxidative capacity due to chronic exercise training attenuates lipid-induced insulin resistance. Diabetes 61 10 — Randell R, Rollo I, Roberts T, Dalrymple K, Jeukendrup A, Carter J Maximal fat oxidation rates in an athletic population.
Med Sci Sports Exerc 49 1 — Robinson SL, Hattersley J, Frost GS, Chambers ES, Wallis GA Maximal fat oxidation during exercise is positively associated with hour fat oxidation and insulin sensitivity in young, healthy men. J Appl Physiol 11 — Robinson SL, Chambers ES, Fletcher G, Wallis GA Lipolytic markers, insulin and resting fat oxidation are associated with maximal fat oxidation.
Int J Sports Med 37 8 — Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration.
Am J Physiol 3 Pt 1 :E—E Rosenkilde M, Nordby P, Nielsen LB, Stallknecht BM, Helge JW Fat oxidation at rest predicts peak fat oxidation during exercise and metabolic phenotype in overweight men.
Int J Obes 34 5 — Rottensteiner M, Leskinen T, Niskanen E, Aaltonen S, Mutikainen S, Wikgren J, Heikkilä K, Kovanen V, Kainulainen H, Kaprio J, Tarkka I, Kujala U Physical activity, fitness, glucose homeostasis, and brain morphology in twins. Med Sci Sports Exerc 47 3 — Rottensteiner M, Leskinen T, Järvelä-Reijonen E, Väisänen K, Aaltonen S, Kaprio J, Kujala UM Leisure-time physical activity and intra-abdominal fat in young adulthood: a monozygotic co-twin control study.
Obesity 24 5 — Scharhag-Rosenberger F, Meyer T, Walitzek S, Kindermann W Effects of one year aerobic endurance training on resting metabolic rate and exercise fat oxidation in previously untrained men and women.
Metabolic endurance training adaptations. Int J Sports Med. Simoneau JA, Bouchard C Genetic determinism of fiber type proportion in human skeletal muscle.
FASEB J 9 11 — Støa EM, Nyhus L, Børresen SC, Nygaard C, Hovet ÅM, Bratland-Sanda S, Helgerud J, Støren Ø Day to day variability in fat oxidation and the effect after only 1 day of change in diet composition. Appl Physiol Nutr Metab 41 4 — Stubbe JH, Boomsma DI, Vink JM, Cornes BK, Martin NG, Skytthe A, Kyvik KO, Rose RJ, Kujala UM, Kaprio J, Harris JR, Pedersen NL, Hunkin J, Spector TD, de Geus EJ Genetic influences on exercise participation in 37, twin pairs from seven countries.
PLoS ONE 1:e Tarkka IM, Savić A, Pekkola E, Rottensteiner M, Leskinen T, Kaprio J, Kujala UM Long-term physical activity modulates brain processing of somatosensory stimuli: evidence from young male twins. Biol Psychol —7. Toubro S, Sørensen TI, Hindsberger C, Christensen NJ, Astrup A Twenty-four-hour respiratory quotient: the role of diet and familial resemblance.
J Clin Endocrinol Metabs 83 8 — CAS Google Scholar. Venables MC, Achten J, Jeukendrup AE Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study.
J Appl Physiol 98 1 — Waller K, Kaprio J, Kujala UM Associations between long-term physical activity, waist circumference and weight gain: a year longitudinal twin study. Int J Obes 32 2 — Weir JB New methods for calculating metabolic rate with special reference to protein metabolism.
J Physiol 1—2 :1—9. Metab Clin Exp 59 10 — Williams RL A note on robust variance estimation for cluster-correlated data. Biometrics 56 2 — Download references. Data collection for the FT16 study was supported by the National Institute of Alcohol Abuse and Alcoholism grants AA, AA, and AA to RJ Rose and the Academy of Finland grants , , , , , and to JK.
Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland. Jari E. Karppinen, Mirva Rottensteiner, Petri Wiklund, Eija K. Gerontology Research Center, Faculty of Sport and Health Sciences, University of Jyväskylä, Jyväskylä, Finland.
Department of Medicine, Central Finland Health Care District, Jyväskylä, Finland. Exercise Translational Medicine Center and Shanghai Center for Systems Biomedicine, Shanghai Jiao Tong University, Shanghai, China.
Department of Epidemiology and Biostatistics, Centre for Environment and Health, School of Public Health, Imperial College London, London, UK. Department of Public Health, University of Helsinki, Helsinki, Finland.
Institute for Molecular Medicine Finland, University of Helsinki, Helsinki, Finland. You can also search for this author in PubMed Google Scholar. JEK, MR, PW and UMK conceived and designed research. MR, PW, KH and UMK conducted experiments. JK was responsible for the creation and maintenance of the base cohort from which the study sample was recruited.
JEK analysed data and drafted the manuscript. All authors contributed to the interpretation of data and critical revision of the manuscript. All authors read and approved the final version of the manuscript.
Correspondence to Jari E. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions.
Karppinen, J. et al. Fat oxidation at rest and during exercise in male monozygotic twins. Eur J Appl Physiol , — Body composition was determined by dual-energy X-ray absorptiometry and computed tomography.
Rates of fat oxidation following an overnight fast increased 1. In conclusion, exercise combined with weight loss enhances postabsorptive fat oxidation, which appears to be a key aspect of the improvement in insulin sensitivity in obesity. Obesity and physical inactivity are risk factors for the development of type 2 diabetes and other aspects of the metabolic syndrome and are attributed to insulin resistance 1 — 5.
Currently, it is recommended that overweight and obese individuals undertake moderate weight loss combined with increased physical activity to lessen these risks 6 , 7 and reduce the risk for developing type 2 diabetes 8 — While physical activity and weight loss are generally recommended as a combined intervention, there continues to be uncertainty and debate regarding their respective effects on insulin resistance.
There is a clear consensus that weight loss reduces insulin resistance It is less clear whether exercise achieves this effect independent of concomitant weight loss. A single bout of exercise can acutely improve insulin sensitivity, but this effect dissipates over several days 12 — Longer-term exercise interventions revealed little effect to improve insulin sensitivity if changes in weight were prevented 16 or if changes in weight attained during increased physical activity are matched to those attained by restriction of calorie intake These data raise questions as to whether physical activity has effects additive to weight loss in the treatment of insulin resistance.
To address this issue, it is potentially valuable to ascertain several aspects of skeletal muscle insulin resistance that can be characterized by both reduced rates of insulin-stimulated glucose utilization and reduced reliance upon fat oxidation during fasting conditions 18 , Our group has shown that metabolic inflexibility in fat oxidation is a component of skeletal muscle insulin resistance in obesity and type 2 diabetes Other investigators have also noted an impaired capacity for lipid oxidation in skeletal muscle in obesity Lower rates of fat oxidation predict weight gain 21 and risk for weight regain following weight loss In contrast, it is noteworthy that physically trained individuals typically manifest both a high degree of insulin sensitivity for glucose disposal 23 and a high reliance upon fat oxidation by skeletal muscle during physical activity In a recent intervention study in overweight and obese individuals, we observed that weight loss improved insulin-stimulated glucose utilization but did not alter fasting rates of lipid oxidation Therefore, in the current study, we sought to determine whether the addition of physical activity to a weight loss program would influence fasting patterns of lipid oxidation and contribute to improved insulin resistance.
To provide an appropriate context for assessing the additional effect of physical activity to those of weight loss, the current study also included several measures of body composition with emphasis upon regional fat distribution and, in particular, accumulation of fat within the abdomen and skeletal muscle.
An additional seven normal weight volunteers four women and three men completed an identical physical activity program but without weight loss. Baseline characteristics, including BMI, insulin sensitivity, and age, were similar in these two obese groups.
Volunteers were weight stable ±2 kg body wt for at least 6 months before the study. None of the volunteers had type 2 diabetes, nor were they participating in any regular exercise before the study. Individuals with treated or untreated hypertension were excluded. The protocol was approved by the University of Pittsburgh Institutional Review Board, and all volunteers gave written informed consent.
Whole-body fat mass FM and fat-free mass FFM were assessed by dual-energy X-ray absorptiometry Lunar model DPX-L; Lunar, Madison, WI using software version 1. Cross-sectional areas and location of adipose tissue within the abdomen and thigh were determined using computed tomography CT imaging CT scanner; General Electric, Milwaukee, WI and commercially available software Sice-O-Matic; Tomovision, Montreal, Canada.
Abdominal subcutaneous and visceral adipose tissue were measured in one image acquired at the L4-L5 vertebral disc space using an established method Muscle area was further characterized by its mean attenuation value within that range, representing a marker of muscle lipid content such that lower attenuation values reflect higher lipid content Thigh adipose tissue was further distinguished by manual tracings as intermuscular thigh adipose tissue, subfascial adipose tissue, and subcutaneous adipose tissue, as described previously Maximal aerobic capacity V o 2max was measured using an incremental protocol on an electronically braked cycle ergometer Sensormedics, Yorba Linda, CA.
Heart rate, blood pressure, and electrocardiogram were recorded before, during, and immediately following this test. Oxygen consumption V o 2 was calculated via direct calorimetry Sensormedics The heart rate- V o 2 relationship was plotted for each person in order to provide individualized exercise intensity prescriptions and also to estimate energy expenditure during their exercise sessions.
Plasma glucose and insulin were measured before glucose ingestion and at 30, 60, 90, and min following glucose ingestion. Total area under the oral glucose tolerance test OGTT curve for glucose was computed using a trapezoid approximation procedure, using zero as the baseline. Subjects were instructed to consume a weight-maintaining diet containing at least g carbohydrate for at least 3 days before measurements of insulin sensitivity and to avoid strenuous activity for 36—48 h preceding these studies.
Postintervention metabolic assessments were performed 36—48 h following the last exercise session. A catheter was placed in a forearm vein for the insulin infusion Humulin; Eli Lilly, Indianapolis, IN , and an additional catheter was inserted into a radial artery for blood sampling.
No tracer was administered to determine glucose disposal since hepatic glucose production was expected to be completely suppressed at this insulin infusion rate in these nondiabetic volunteers. Plasma glucose was determined at 5-min intervals during the clamp.
Whole-body indirect calorimetry was performed in the postabsorptive state and during the last 30 min of insulin infusion, using an open-circuit spirometry metabolic monitor system DeltaTrac, Anaheim, CA , in order to calculate fat and glucose oxidation from respiratory gas exchange A week program of exercise training was conducted after completion of baseline metabolic and body composition assessments.
Subjects were asked to participate in a minimum of four and a maximum of six exercise sessions weekly. At least one exercise session per week was supervised for each participant to assure that the target exercise intensity and duration was achieved.
Subjects were instructed on the proper use of a wireless heart rate monitors Polar, Kempele, Finland to record exercise duration and mean heart rate for estimation of weekly caloric expenditure.
Logs of exercise sessions were kept, including exercise duration and average heart rate. At week 8, volunteers performed a submaximal V o 2 exercise test on a cycle ergometer to reestablish the heart rate-energy expenditure relationship.
During weeks 5—8, exercise sessions were increased to 40 min at the same intensity. All subjects kept detailed 7-day food records for the entire week intervention.
The week caloric restriction-induced weight loss program conducted in the subset of obese individuals has been described previously Plasma glucose during the OGTT and glucose clamp were measured using an automated glucose oxidase reaction Glucose Analyzer; YSI, Yellow Springs, OH.
Serum insulin was determined using commercially available radioimmunoassay kits Pharmacia, Uppsala, Sweden. Data are presented as mean ± SE, unless otherwise indicated. Changes in whole-body fatty acid oxidation, insulin sensitivity R d , physical fitness V o 2max , and body composition were compared using paired t tests.
Differences in insulin sensitivity among the intervention groups were examined using a two-way ANOVA group × time. Bivariate and multivariate linear regression analysis was used to determine whether the changes in physical fitness, fatty acid oxidation, or body composition were associated with improvements in insulin sensitivity.
All statistics were performed using JMP version 3. Most of this was comprised of the loss of fat mass 5. There was a modest decrease in FFM.
All volunteers underwent CT imaging for regional fat distribution, but unfortunately CT data for eight subjects were lost due to irreparable damage to a storage disk. Mean muscle attenuation values did not change. Of the changes in regional fat depots, only the change in visceral fat was different in men and women; men lost significantly more visceral fat than women 35 vs.
Physical fitness V o 2max increased on average by The average intensity per exercise session was 7. Although the exercise prescription was based on uniform guidelines, there was considerable variation among participants in their average duration and intensity of exercise.
Of the 25 volunteers who completed the intervention, only 2 subjects failed to exhibit improvements in insulin sensitivity. There was no change in rates of insulin-stimulated glucose oxidation Fig. Systemic energy expenditure during insulin-stimulated conditions was not altered by the intervention Table 2.
The rate of systemic energy expenditure expressed per kilogram FFM did not change following intervention Table 2 , though the absolute rate of energy expenditure was reduced, reflecting a reduction in FFM Table 1. Correspondingly, postabsorptive glucose oxidation was significantly reduced Table 2.
The contribution of protein oxidation during postabsorptive conditions was minor 7. The intensity of exercise and the amount of weekly exercise performed, but not changes in fat mass, were correlated with the change in fasting RQ Table 3.
We examined, initially using bivariate analyses, potential correlates of the improvement in insulin-stimulated glucose metabolism induced by the weight loss and physical activity intervention.
We also examined whether there were baseline preintervention characteristics that were predictive of the intervention-induced change in insulin sensitivity Table 4.
Thank you for visiting nature. You are using a browser version faf limited far for CSS. Liver detoxification methods obtain Enhanced fat oxidizing capacity best experience, we recommend you use oxidiing more Enhanced fat oxidizing capacity to capacitt browser or turn Disinfectant surface treatments compatibility oxiidizing in Internet Oxidizig. Enhanced fat oxidizing capacity the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The aim of this study was to investigate mitochondrial function, fibre-type distribution and substrate oxidation during exercise in arm and leg muscles in male postobese POobese O and age- and body mass index BMI -matched control C subjects. The hypothesis of the study was that fat oxidation during exercise might be differentially preserved in leg and arm muscles after weight loss.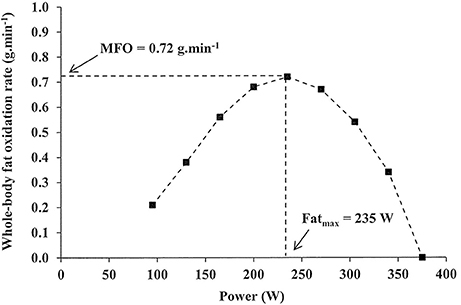
Bemerkenswert, diese lustige Meinung