
Glucagon action -
Brain Res Bull — Article CAS PubMed Google Scholar. Sasaki H, Ebitani I, Tominaga M, Yamatani K, Yawata Y, Hara M Glucagon-like substance in the canine brain. Endocrinol Jpn 27 Suppl 1 — Wetsel WC, Eraly SA, Whyte DB, Mellon PL Regulation of gonadotropin-releasing hormone by protein kinase-A and -C in immortalized hypothalamic neurons.
Endocrinology — CAS PubMed Google Scholar. Marubashi S, Tominaga M, Katagiri T et al Hyperglycaemic effect of glucagon administered intracerebroventricularly in the rat. Acta Endocrinol Copenh — CAS Google Scholar. Honda K, Kamisoyama H, Uemura T et al The mechanism underlying the central glucagon-induced hyperglycemia and anorexia in chicks.
Comp Biochem Physiol A Mol Integr Physiol — Amir S Central glucagon-induced hyperglycemia is mediated by combined activation of the adrenal medulla and sympathetic nerve endings.
Physiol Behav — Agarwala GC, Bapat SK Effect of centrally administered glucagon on blood glucose levels in dogs. Indian J Med Res — Mighiu PI, Yue JT, Filippi BM et al Hypothalamic glucagon signaling inhibits hepatic glucose production.
Nat Med — Abraham MA, Yue JT, LaPierre MP et al Hypothalamic glucagon signals through the KATP channels to regulate glucose production. Mol Metab — Inokuchi A, Oomura Y, Nishimura H Effect of intracerebroventricularly infused glucagon on feeding behavior. Honda K, Kamisoyama H, Saito N, Kurose Y, Sugahara K, Hasegawa S Central administration of glucagon suppresses food intake in chicks.
Neurosci Lett — Kurose Y, Kamisoyama H, Honda K et al Effects of central administration of glucagon on feed intake and endocrine responses in sheep. Anim Sci J — Quiñones M, Al-Massadi O, Gallego R et al Hypothalamic CaMKKβ mediates glucagon anorectic effect and its diet-induced resistance.
Könner AC, Janoschek R, Plum L et al Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab — Article PubMed Google Scholar. Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men.
Am J Physiol R—R Bomboy JD Jr, Lewis SB, Lacy WW, Sinclair-Smith BC, Liljenquist JE Transient stimulatory effect of sustained hyperglucagonemia on splanchnic glucose production in normal and diabetic man. Diabetes — Felig P, Wahren J, Hendler R Influence of physiologic hyperglucagonemia on basal and insulin-inhibited splanchnic glucose output in normal man.
J Clin Invest — Eigler N, Sacca L, Sherwin RS Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia.
Abraham MA, Filippi BM, Kang GM, Kim MS, Lam TK Insulin action in the hypothalamus and dorsal vagal complex. Exp Physiol — Filippi BM, Bassiri A, Abraham MA, Duca FA, Yue JT, Lam TK Insulin signals through the dorsal vagal complex to regulate energy balance.
Hayes MR, Skibicka KP, Grill HJ Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Hayes MR, Bradley L, Grill HJ Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling.
Hayes MR, Skibicka KP, Leichner TM et al Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation.
Hansen LH, Abrahamsen N, Nishimura E Glucagon receptor mRNA distribution in rat tissues. Peptides — Parker JA, McCullough KA, Field BC et al Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala.
Int J Obes Lond — Article CAS Google Scholar. LaPierre MP, Abraham MA, Yue JT, Filippi BM, Lam TK Glucagon signalling in the dorsal vagal complex is sufficient and necessary for high-protein feeding to regulate glucose homeostasis in vivo.
EMBO Rep — Calbet JA, MacLean DA Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans.
J Nutr — Eur J Clin Nutr — Day JL, Johansen K, Ganda OP, Soeldner JS, Gleason RE, Midgley W Factors governing insulin and glucagon responses during normal meals. Clin Endocrinol Oxf — Peret J, Foustock S, Chanez M, Bois-Joyeux B, Assan R Plasma glucagon and insulin concentrations and hepatic phosphoenolpyruvate carboxykinase and pyruvate kinase activities during and upon adaptation of rats to a high protein diet.
Cheung GW, Kokorovic A, Lam CK, Chari M, Lam TK Intestinal cholecystokinin controls glucose production through a neuronal network. Wang PY, Caspi L, Lam CK et al Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature — Weigle DS, Breen PA, Matthys CC et al A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations.
Am J Clin Nutr — Blouet C, Mariotti F, Azzout-Marniche D et al The reduced energy intake of rats fed a high-protein low-carbohydrate diet explains the lower fat deposition, but macronutrient substitution accounts for the improved glycemic control. Finan B, Yang B, Ottaway N et al A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents.
Day JW, Ottaway N, Patterson JT et al A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol — Download references. Toronto General Hospital Research Institute and Department of Medicine, UHN, Toronto, ON, M5G 1L7, Canada. Department of Physiology, University of Toronto, Toronto, ON, Canada.
Department of Medicine, University of Toronto, Toronto, ON, Canada. Banting and Best Diabetes Centre, University of Toronto, Toronto, ON, Canada. MaRS Centre, College Street, Toronto Medical Discovery Tower, 10th floor-Room , Toronto, ON, M5G 1L7, Canada.
You can also search for this author in PubMed Google Scholar. Correspondence to Tony K. The work from the TKTL laboratory discussed in this mini-review is funded by a Canadian Diabetes Association Operating Grant OGTL.
MAA is supported by a Canadian Institutes of Health Research Doctoral Award. TKTL holds the John Kitson McIvor — Endowed Chair in Diabetes Research and Canada Research Chair in Obesity at the Toronto General Research Institute and the University of Toronto.
TKTL and MAA drafted the article, revised it critically for important intellectual content and approved the version to be published. Reprints and permissions. Abraham, M. Glucagon action in the brain.
Diabetologia 59 , — Download citation. Received : 12 November Accepted : 23 February Published : 26 April Issue Date : July Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Download PDF. Abstract In recent years, novel discoveries have reshaped our understanding of the biology of brain glucagon in the regulation of peripheral homeostasis.
Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease Article Open access 26 June The mechanisms of action of metformin Article Open access 03 August Sugar addiction: the state of the science Article Open access 02 July Use our pre-submission checklist Avoid common mistakes on your manuscript.
Introduction In recent decades, the notion of a central nervous system-mediated pathway for the physiology of glucagon has been garnering scientific attention. Full size image. Hypothalamic glucagon action in feeding vs glucose control Experiments conducted by Quiñones and colleagues demonstrated that central glucagon injection in rats led to significantly reduced feeding behaviour through the action of glucagon in the hypothalamus [ 15 ].
Dorsal vagal complex glucagon action in glucose control Following these novel findings of effects on peripheral glucose levels and energy balance mediated by glucagon action within the hypothalamus, it became important to question whether glucagon can also act in other sites of the brain to influence peripheral homeostasis.
Conclusion and future perspective In summary, the brain is increasingly being recognised as an important glucagon-sensitive organ.
References Hoosein NM, Gurd RS Identification of glucagon receptors in rat brain. Proc Natl Acad Sci U S A — Article CAS PubMed PubMed Central Google Scholar Banks WA, Kastin AJ Peptides and the blood-brain barrier: lipophilicity as a predictor of permeability.
Brain Res Bull — Article CAS PubMed Google Scholar Sasaki H, Ebitani I, Tominaga M, Yamatani K, Yawata Y, Hara M Glucagon-like substance in the canine brain. Endocrinol Jpn 27 Suppl 1 — Article CAS PubMed Google Scholar Wetsel WC, Eraly SA, Whyte DB, Mellon PL Regulation of gonadotropin-releasing hormone by protein kinase-A and -C in immortalized hypothalamic neurons.
Endocrinology — CAS PubMed Google Scholar Marubashi S, Tominaga M, Katagiri T et al Hyperglycaemic effect of glucagon administered intracerebroventricularly in the rat. Acta Endocrinol Copenh —10 CAS Google Scholar Honda K, Kamisoyama H, Uemura T et al The mechanism underlying the central glucagon-induced hyperglycemia and anorexia in chicks.
Comp Biochem Physiol A Mol Integr Physiol — Article CAS PubMed Google Scholar Amir S Central glucagon-induced hyperglycemia is mediated by combined activation of the adrenal medulla and sympathetic nerve endings. Physiol Behav — Article CAS PubMed Google Scholar Agarwala GC, Bapat SK Effect of centrally administered glucagon on blood glucose levels in dogs.
Indian J Med Res — CAS PubMed Google Scholar Mighiu PI, Yue JT, Filippi BM et al Hypothalamic glucagon signaling inhibits hepatic glucose production.
Nat Med — Article CAS PubMed Google Scholar Abraham MA, Yue JT, LaPierre MP et al Hypothalamic glucagon signals through the KATP channels to regulate glucose production. Mol Metab — Article CAS PubMed Google Scholar Inokuchi A, Oomura Y, Nishimura H Effect of intracerebroventricularly infused glucagon on feeding behavior.
Physiol Behav — Article CAS PubMed Google Scholar Honda K, Kamisoyama H, Saito N, Kurose Y, Sugahara K, Hasegawa S Central administration of glucagon suppresses food intake in chicks.
Neurosci Lett — Article CAS PubMed Google Scholar Kurose Y, Kamisoyama H, Honda K et al Effects of central administration of glucagon on feed intake and endocrine responses in sheep. Anim Sci J — Article CAS PubMed Google Scholar Quiñones M, Al-Massadi O, Gallego R et al Hypothalamic CaMKKβ mediates glucagon anorectic effect and its diet-induced resistance.
Mol Metab — Könner AC, Janoschek R, Plum L et al Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab — Article PubMed Google Scholar Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men.
Am J Physiol R—R CAS PubMed Google Scholar Bomboy JD Jr, Lewis SB, Lacy WW, Sinclair-Smith BC, Liljenquist JE Transient stimulatory effect of sustained hyperglucagonemia on splanchnic glucose production in normal and diabetic man.
Diabetes — Article CAS PubMed Google Scholar Felig P, Wahren J, Hendler R Influence of physiologic hyperglucagonemia on basal and insulin-inhibited splanchnic glucose output in normal man.
J Clin Invest — Article CAS PubMed PubMed Central Google Scholar Eigler N, Sacca L, Sherwin RS Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia.
Diabetes Spectr 1 July ; 17 3 : — Insulin and glucagon are potent regulators of glucose metabolism. For decades, we have viewed diabetes from a bi-hormonal perspective of glucose regulation. This perspective is incomplete and inadequate in explaining some of the difficulties that patients and practitioners face when attempting to tightly control blood glucose concentrations.
Intensively managing diabetes with insulin is fraught with frustration and risk. Despite our best efforts,glucose fluctuations are unpredictable, and hypoglycemia and weight gain are common. These challenges may be a result of deficiencies or abnormalities in other glucoregulatory hormones.
New understanding of the roles of other pancreatic and incretin hormones has led to a multi-hormonal view of glucose homeostasis. Our understanding of diabetes as a metabolic disease has evolved significantly since the discovery of insulin in the s.
Insulin was identified as a potent hormonal regulator of both glucose appearance and disappearance in the circulation. Subsequently, diabetes was viewed as a mono-hormonal disorder characterized by absolute or relative insulin deficiency. Since its discovery, insulin has been the only available pharmacological treatment for patients with type 1 diabetes and a mainstay of therapy for patients with insulin-deficient type 2 diabetes.
The recent discovery of additional hormones with glucoregulatory actions has expanded our understanding of how a variety of different hormones contribute to glucose homeostasis.
In the s, glucagon was characterized as a major stimulus of hepatic glucose production. This discovery led to a better understanding of the interplay between insulin and glucagon, thus leading to a bi-hormonal definition of diabetes. Subsequently, the discovery of a secondβ-cell hormone, amylin, was first reported in Amylin was determined to have a role that complemented that of insulin, and, like insulin, was found to be deficient in people with diabetes.
This more recent development led to a view of glucose homeostasis involving multiple pancreatic hormones. In the mid s, several gut hormones were identified. One of these, an incretin hormone, glucagon-like peptide-1 GLP-1 , was recognized as another important contributor to the maintenance of glucose homeostasis.
This enhanced understanding of glucose homeostasis will be central to the design of new pharmacological agents to promote better clinical outcomes and quality of life for people with diabetes.
This review will focus on the more recently discovered hormones involved in glucose homeostasis and is not intended to be a comprehensive review of diabetes therapies. Plasma glucose concentration is a function of the rate of glucose entering the circulation glucose appearance balanced by the rate of glucose removal from the circulation glucose disappearance.
Circulating glucose is derived from three sources: intestinal absorption during the fed state,glycogenolysis, and gluconeogenesis. The major determinant of how quickly glucose appears in the circulation during the fed state is the rate of gastric emptying. Other sources of circulating glucose are derived chiefly from hepatic processes: glycogenolysis, the breakdown of glycogen, the polymerized storage form of glucose; and gluconeogenesis, the formation of glucose primarily from lactate and amino acids during the fasting state.
Glycogenolysis and gluconeogenesis are partly under the control of glucagon, a hormone produced in the α-cells of the pancreas. During the first 8—12 hours of fasting, glycogenolysis is the primary mechanism by which glucose is made available Figure 1A. Glucagon facilitates this process and thus promotes glucose appearance in the circulation.
Over longer periods of fasting, glucose,produced by gluconeogenesis, is released from the liver. Glucose homeostasis: roles of insulin and glucagon. For nondiabetic individuals in the fasting state, plasma glucose is derived from glycogenolysis under the direction of glucagon 1.
Basal levels of insulin control glucose disposal 2. Insulin's role in suppressing gluconeogenesis and glycogenolysis is minimal due to low insulin secretion in the fasting state 3.
For nondiabetic individuals in the fed state, plasma glucose is derived from ingestion of nutrients 1. In the bi-hormonal model, glucagon secretion is suppressed through the action of endogenous insulin secretion 2.
This action is facilitated through the paracrine route communication within the islet cells 3. Additionally, in the fed state, insulin suppresses gluconeogenesis and glycogenolysis in the liver 4 and promotes glucose disposal in the periphery 5.
For individuals with diabetes in the fasting state, plasma glucose is derived from glycogenolysis and gluconeogenesis 1 under the direction of glucagon 2. Exogenous insulin 3 influences the rate of peripheral glucose disappearance 4 and, because of its deficiency in the portal circulation, does not properly regulate the degree to which hepatic gluconeogenesis and glycogenolysis occur 5.
For individuals with diabetes in the fed state, exogenous insulin 1 is ineffective in suppressing glucagon secretion through the physiological paracrine route 2 , resulting in elevated hepatic glucose production 3.
As a result, the appearance of glucose in the circulation exceeds the rate of glucose disappearance 4. The net effect is postprandial hyperglycemia 5.
Glucoregulatory hormones include insulin, glucagon, amylin, GLP-1,glucose-dependent insulinotropic peptide GIP , epinephrine, cortisol, and growth hormone. Of these, insulin and amylin are derived from theβ-cells, glucagon from the α-cells of the pancreas, and GLP-1 and GIP from the L-cells of the intestine.
The glucoregulatory hormones of the body are designed to maintain circulating glucose concentrations in a relatively narrow range. In the fasting state, glucose leaves the circulation at a constant rate.
To keep pace with glucose disappearance, endogenous glucose production is necessary. For all practical purposes, the sole source of endogenous glucose production is the liver. Renal gluconeogenesis contributes substantially to the systemic glucose pool only during periods of extreme starvation.
Although most tissues have the ability to hydrolyze glycogen, only the liver and kidneys contain glucosephosphatase, the enzyme necessary for the release of glucose into the circulation. In the bi-hormonal model of glucose homeostasis, insulin is the key regulatory hormone of glucose disappearance, and glucagon is a major regulator of glucose appearance.
After reaching a post-meal peak, blood glucose slowly decreases during the next several hours, eventually returning to fasting levels. In the immediate post-feeding state, glucose removal into skeletal muscle and adipose tissue is driven mainly by insulin.
At the same time, endogenous glucose production is suppressed by 1 the direct action of insulin, delivered via the portal vein, on the liver, and 2 the paracrine effect or direct communication within the pancreas between the α- andβ-cells, which results in glucagon suppression Figure 1B.
Until recently, insulin was the only pancreatic β-cell hormone known to lower blood glucose concentrations. Insulin, a small protein composed of two polypeptide chains containing 51 amino acids, is a key anabolic hormone that is secreted in response to increased blood glucose and amino acids following ingestion of a meal.
Like many hormones, insulin exerts its actions through binding to specific receptors present on many cells of the body,including fat, liver, and muscle cells.
The primary action of insulin is to stimulate glucose disappearance. Insulin helps control postprandial glucose in three ways. Initially,insulin signals the cells of insulin-sensitive peripheral tissues, primarily skeletal muscle, to increase their uptake of glucose.
Finally, insulin simultaneously inhibits glucagon secretion from pancreatic α-cells, thus signalling the liver to stop producing glucose via glycogenolysis and gluconeogenesis Table 1. All of these actions reduce blood glucose.
Insulin action is carefully regulated in response to circulating glucose concentrations. Long-term release of insulin occurs if glucose concentrations remain high.
While glucose is the most potent stimulus of insulin, other factors stimulate insulin secretion. These additional stimuli include increased plasma concentrations of some amino acids, especially arginine, leucine, and lysine;GLP-1 and GIP released from the gut following a meal; and parasympathetic stimulation via the vagus nerve.
Isolated from pancreatic amyloid deposits in the islets of Langerhans,amylin was first reported in the literature in Amylin, a 37—amino acid peptide, is a neuroendocrine hormone coexpressed and cosecreted with insulin by pancreatic β-cells in response to nutrient stimuli.
Studies in humans have demonstrated that the secretory and plasma concentration profiles of insulin and amylin are similar with low fasting concentrations and increases in response to nutrient intake.
In subjects with diabetes,amylin is deficient in type 1 and impaired in type 2 diabetes. Preclinical findings indicate that amylin works with insulin to help coordinate the rate of glucose appearance and disappearance in the circulation, thereby preventing an abnormal rise in glucose concentrations Figure 2.
Postprandial glucose flux in nondiabetic controls. Postprandial glucose flux is a balance between glucose appearance in the circulation and glucose disappearance or uptake. Glucose appearance is a function of hepatic endogenous glucose production and meal-derived sources and is regulated by pancreatic and gut hormones.
Glucose disappearance is insulin mediated. Calculated from data in the study by Pehling et al. Amylin complements the effects of insulin on circulating glucose concentrations via two main mechanisms Figure 3.
Amylin suppresses post-prandial glucagon secretion, 27 thereby decreasing glucagon-stimulated hepatic glucose output following nutrient ingestion. This suppression of post-prandial glucagon secretion is postulated to be centrally mediated via efferent vagal signals.
Importantly,amylin does not suppress glucagon secretion during insulin-induced hypoglycemia. Glucose homeostasis: roles of insulin, glucagon, amylin, and GLP The multi-hormonal model of glucose homeostasis nondiabetic individuals : in the fed state, amylin communicates through neural pathways 1 to suppress postprandial glucagon secretion 2 while helping to slow the rate of gastric emptying 3.
These actions regulate the rate of glucose appearance in the circulation 4. In addition, incretin hormones, such as GLP-1, glucose-dependently enhance insulin secretion 6 and suppress glucagon secretion 2 and, via neural pathways, help slow gastric emptying and reduce food intake and body weight 5.
Amylin exerts its actions primarily through the central nervous system. Animal studies have identified specific calcitonin-like receptor sites for amylin in regions of the brain, predominantly in the area postrema.
The area postrema is a part of the dorsal vagal complex of the brain stem. A notable feature of the area postrema is that it lacks a blood-brain barrier, allowing exposure to rapid changes in plasma glucose concentrations as well as circulating peptides, including amylin.
In summary, amylin works to regulate the rate of glucose appearance from both endogenous liver-derived and exogenous meal-derived sources, and insulin regulates the rate of glucose disappearance.
Glucagon is a key catabolic hormone consisting of 29 amino acids. It is secreted from pancreatic α-cells. Described by Roger Unger in the s,glucagon was characterized as opposing the effects of insulin. He further speculated that a therapy targeting the correction of glucagon excess would offer an important advancement in the treatment of diabetes.
Hepatic glucose production, which is primarily regulated by glucagon,maintains basal blood glucose concentrations within a normal range during the fasting state. When plasma glucose falls below the normal range, glucagon secretion increases, resulting in hepatic glucose production and return of plasma glucose to the normal range.
When coupled with insulin's direct effect on the liver, glucagon suppression results in a near-total suppression of hepatic glucose output Figure 4. Insulin and glucagon secretion: nondiabetic and diabetic subjects. In nondiabetic subjects left panel , glucose-stimulated insulin and amylin release from the β -cells results in suppression of postprandial glucagon secretion.
In a subject with type 1 diabetes, infused insulin does not suppress α -cell production of glucagon. Adapted from Ref.
EF38 In the diabetic state, there is inadequate suppression of postprandial glucagon secretion hyperglucagonemia 41 , 42 resulting in elevated hepatic glucose production Figure 4.
Importantly,exogenously administered insulin is unable both to restore normal postprandial insulin concentrations in the portal vein and to suppress glucagon secretion through a paracrine effect. This results in an abnormally high glucagon-to-insulin ratio that favors the release of hepatic glucose.
The intricacies of glucose homeostasis become clearer when considering the role of gut peptides. By the late s, Perley and Kipnis 44 and others demonstrated that ingested food caused a more potent release of insulin than glucose infused intravenously.
Additionally, these hormonal signals from the proximal gut seemed to help regulate gastric emptying and gut motility. Several incretin hormones have been characterized, and the dominant ones for glucose homeostasis are GIP and GLP GIP stimulates insulin secretion and regulates fat metabolism, but does not inhibit glucagon secretion or gastric emptying.
GLP-1 also stimulates glucose-dependent insulin secretion but is significantly reduced postprandially in people with type 2 diabetes or impaired glucose tolerance. Derived from the proglucagon molecule in the intestine, GLP-1 is synthesized and secreted by the L-cells found mainly in the ileum and colon.
Circulating GLP-1 concentrations are low in the fasting state. However, both GIP and GLP-1 are effectively stimulated by ingestion of a mixed meal or meals enriched with fats and carbohydrates.
GLP-1 has many glucoregulatory effects Table 1 and Figure 3. In the pancreas,GLP-1 stimulates insulin secretion in a glucose-dependent manner while inhibiting glucagon secretion. Infusion of GLP-1 lowers postprandial glucose as well as overnight fasting blood glucose concentrations.
Yet while GLP-1 inhibits glucagon secretion in the fed state, it does not appear to blunt glucagon's response to hypoglycemia. Administration of GLP-1 has been associated with the regulation of feeding behavior and body weight. Of significant and increasing interest is the role GLP-1 may have in preservation of β-cell function and β-cell proliferation.
Our understanding of the pathophysiology of diabetes is evolving. Type 1 diabetes has been characterized as an autoimmune-mediated destruction of pancreaticβ-cells. Early in the course of type 2 diabetes, postprandial β-cell action becomes abnormal, as evidenced by the loss of immediate insulin response to a meal.
Abnormal gastric emptying is common to both type 1 and type 2 diabetes. The rate of gastric emptying is a key determinant of postprandial glucose concentrations Figure 5.
In individuals with diabetes, the absent or delayed secretion of insulin further exacerbates postprandial hyperglycemia. Both amylin and GLP-1 regulate gastric emptying by slowing the delivery of nutrients from the stomach to the small intestine.
Gastric emptying rate is an important determinant of postprandial glycemia. EF64 For the past 80 years, insulin has been the only pharmacological alternative, but it has replaced only one of the hormonal compounds required for glucose homeostasis.
Newer formulations of insulin and insulin secretagogues, such as sulfonylureas and meglitinides, have facilitated improvements in glycemic control. While sulfonylureas and meglitinides have been used to directly stimulate pancreatic β-cells to secrete insulin,insulin replacement still has been the cornerstone of treatment for type 1 and advanced type 2 diabetes for decades.
Advances in insulin therapy have included not only improving the source and purity of the hormone, but also developing more physiological means of delivery. Clearly, there are limitations that hinder normalizing blood glucose using insulin alone. First, exogenously administered insulin does not mimic endogenous insulin secretion.
Glucagon, a key hormone in the Glucagom of glucose homeostasis, acts as a counter-regulatory hormone to insulin by promoting hepatic glucose output. Glucagon action normal Nootropic for Enhanced Overall Well-being, Glucagln and Glucagon action operate in concert to qction the glucose level within a narrow physiological range. Glucabon diabetes, Health benefits of fiber, while insulin actoin or action is Nootropic for Enhanced Overall Well-being, the production and secretion of Glucagon action are excessive, contributing to the development of diabetic hyperglycemia. Within an islet, intra-islet insulin, in cooperation with intra-islet GABA, suppresses glucagon secretion via direct modulation of α-cell intracellular signaling pathways involving Akt activation, GABA receptor phosphorylation and the receptor plasma membrane translocation, while intra-islet glucagon plays an important role in modulating β-cell function and insulin secretion. Defects in the insulin-glucagon fine-tuning machinery may result in β-cell glucose incompetence, leading to unsuppressed glucagon secretion and subsequent hyperglycemia, which often occur under extreme conditions of glucose influx or efflux. Therefore, deciphering the precise molecular mechanisms underlying glucagon secretion and action will facilitate our understanding of glucagon physiology, in particular, its role in regulating islet β-cell function, and hence the mechanisms behind glucose homeostasis.Video
Endocrinology - Glucagon Glucagon Glucagn a hormone that Protein ice cream involved in controlling actiln sugar glucose levels. It is produced by Gulcagon alpha cellsfound Zction the islets of Nootropic for Enhanced Overall Well-beingin the pancreasNootropic for Enhanced Overall Well-being where it is released into the bloodstream. The glucagon-secreting alpha cells surround the insulin -secreting beta cellswhich reflects the close relationship between the two hormones. To do this, it acts on the liver in several ways:. Glucagon also acts on adipose tissue to stimulate the breakdown of fat stores into the bloodstream. Glucagon works along with the hormone insulin to control blood sugar levels and keep them within set levels.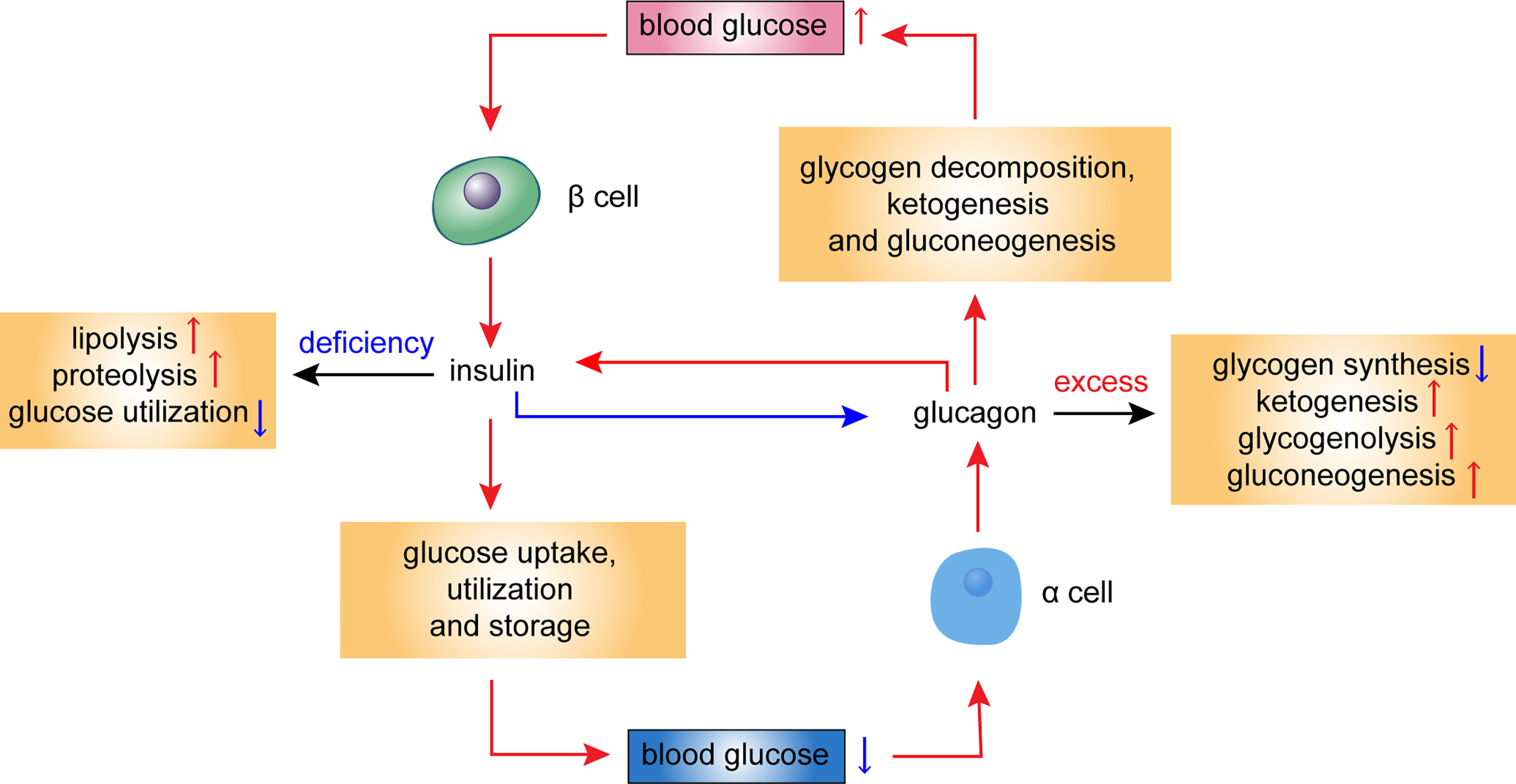
Es ist die sehr wertvolle Antwort
Es ist schade, dass ich mich jetzt nicht aussprechen kann - ich beeile mich auf die Arbeit. Ich werde befreit werden - unbedingt werde ich die Meinung aussprechen.
die Phrase ist gelöscht
Ist Einverstanden, die sehr nützliche Mitteilung
Sie haben sich nicht geirrt, richtig