
Amino acid neurotransmitters -
Unlike the excitatory ionotropic glutamate receptors, mGluRs cause slower postsynaptic responses that can either excite or inhibit postsynaptic cells.
As a result, the physiological roles of mGluRs are quite varied. Most inhibitory synapses in the brain and spinal cord use either γ-aminobutyric acid GABA or glycine as neurotransmitters.
The predominant precursor for GABA synthesis is glucose, which is metabolized to glutamate by the tricarboxylic acid cycle enzymes figure 2. The enzyme glutamic acid decarboxylase GAD , which is found almost exclusively in GABAergic neurons, catalyzes the conversion of glutamate to GABA.
GAD requires pyridoxal phosphate for activity; a deficiency of this vitamin can lead to diminished GABA synthesis. Once GABA is synthesized, it is transported into synaptic vesicles via a vesicular inhibitory amino acid transporter VIAAT. The mechanism of GABA removal is similar to that of glutamate.
Most GABA is eventually converted to succinate, which is metabolized further in the tricarboxylic acid cycle that mediates cellular ATP synthesis. Two mitochondrial enzymes are required for this degradation: GABA transaminase and succinic semialdehyde dehydrogenase.
GABAergic synapses employ two types of postsynaptic receptors, called GABA A and GABA B. GABA A are ionotropic receptors, while GABA B are metabotropic receptors. The same site binds the hypnotic zolpidem Ambien , which is widely used to induce sleep. The injection anesthetic ketamine also binds to the extracellular domain of GABA receptors.
GAT: cotransporters for GABA; VIAAT: vesicular inhibitory amino acid transporter. The transmembrane domains of GABA A receptors also serve as the targets for numerous ligands, such as inhalant anesthetics and steroids. Another drug that binds to the transmembrane domain of GABA A receptors is ethanol; at least some aspects of drunken behavior are caused by ethanol-mediated alterations in ionotropic GABA receptors.
The metabotropic GABA B receptors are also widely distributed in the brain. Like the ionotropic GABA receptors, GABA B receptors are inhibitory. The distribution of the neutral amino acid glycine in the CNS is more restricted than that of GABA.
About half of the inhibitory synapses in the spinal cord use glycine; most other inhibitory synapses use GABA. Glycine is synthesized from serine by the mitochondrial isoform of serine hydroxymethyltransferase figure 2. Once released from the presynaptic cell, glycine is rapidly removed from the synaptic cleft by glycine transporters in the plasma membrane figure 2.
These receptors are potently blocked by strychnine, which may account for the toxic properties of this plant alkaloid. Biogenic amine transmitters regulate many brain functions and are also active in the peripheral nervous system.
There are five well-established biogenic amine neurotransmitters, three of which can be classified as catecholamines:.
Dopamine catecholamine , 2. Histamine, and 5. The first step in catecholamine synthesis is catalyzed by tyrosine hydroxylase in a reaction requiring oxygen as a cosubstrate and tetrahydrobiopterin as a cofactor to synthesize dihydroxyphenylalanine DOPA figure 2.
Dopamine is produced by the action of DOPA decarboxylase on DOPA. Following its synthesis in the cytoplasm of presynaptic terminals, dopamine is loaded into synaptic vesicles via a vesicular monoamine transporter VMAT.
The two major enzymes involved in the catabolism of dopamine are monoamine oxidase MAO and catechol O-methyltransferase COMT. Both neurons and glia contain mitochondrial MAO and cytoplasmic COMT. Once released, dopamine acts exclusively by activating G-protein—coupled receptors.
Most dopamine receptor subtypes act by either activating or inhibiting adenylyl cyclase. Activation of these receptors generally contributes to complex behaviors. Norepinephrine also called noradrenaline is used as a neurotransmitter and influences sleep and wakefulness, arousal, attention, and feeding behavior.
Perhaps the most prominent noradrenergic neurons are sympathetic ganglion cells, which employ norepinephrine as the major peripheral transmitter in this division of the visceral motor system.
Norepinephrine synthesis requires dopamine β-hydroxylase, which catalyzes the production of norepinephrine from dopamine figure 2. Norepinephrine is then loaded into synaptic vesicles via the same VMAT involved in vesicular dopamine transport. NET is a molecular target of amphetamine, which acts as a stimulant by producing a net increase in the release of norepinephrine and dopamine.
Like dopamine, norepinephrine is degraded by MAO and COMT. Epinephrine also called adrenaline is found in the brain at lower levels than the other catecholamines and is also present in fewer brain neurons than other catecholamines.
Epinephrine-secreting neurons regulate respiration and cardiac function. The enzyme that synthesizes epinephrine, phenylethanolamine-N-methyltransferase figure 2. Otherwise, the metabolism of epinephrine is very similar to that of norepinephrine.
Epinephrine is loaded into vesicles via the VMAT. No plasma membrane transporter specific for epinephrine has been identified, although the NET is capable of transporting epinephrine.
Binding of norepinephrine or epinephrine causes small changes in the structure of this receptor, which permits the G-protein to bind. This, in turn, causes larger changes in the shape of the α subunit of the G-protein, the first step in a series of reactions that allow the G-protein to regulate intracellular signaling cascades.
Histamine is found in neurons in the hypothalamus that send sparse but widespread projections to almost all regions of the brain and spinal cord. The central histamine projections mediate arousal and attention, similar to central ACh and norepinephrine projections. Histamine also controls the reactivity of the vestibular system.
Allergic reactions or tissue damage cause release of histamine from mast cells in the bloodstream. The close proximity of mast cells to blood vessels, together with the potent actions of histamine on blood vessels, raises the possibility that histamine may influence brain blood flow.
Histamine is produced from the amino acid histidine by a histidine decarboxylase figure 2. No plasma membrane histamine transporter has been identified yet. Histamine is degraded by the combined actions of histamine methyltransferase and MAO.
The four known types of histamine receptors are all metabotropic receptors. Of the four, only two of the receptors H 1 and H 2 are well characterized. Because of the role of histamine receptors in mediating allergic responses, many histamine receptor antagonists have been developed as antihistamine agents.
Antihistamines that cross the blood-brain barrier, such as diphenhydramine Benadryl , act as sedatives by interfering with the roles of histamine in CNS arousal.
Antagonists of the H 1 receptor also are used to prevent motion sickness, perhaps because of the role of histamine in controlling vestibular function figure 2. ALDH: aldehyde dehydrogenase; DAO: diamine oxidase; HA: histamine; HNMT: N-methyltransferase; IA: imidazole acetaldehyde; IAA: imidazoleacetic acid; IAAR: imidazoleacetic acid riboside; NMH: N-methylhistamine; N-MIA: methylimidazole acetaldehyde; N-MIAA: N-methylimidazoleacetic acetic acid; OC3: organic cation transporter 3; PMAT: plasma membrane monoamine transporter.
Serotonin, or 5-hydroxytryptamine 5-HT , was initially thought to increase vascular tone by virtue of its presence in blood serum hence the name serotonin. Tryptophan is taken up into neurons by a plasma membrane transporter and hydroxylated in a reaction catalyzed by the enzyme tryptophanhydroxylase figure 2.
Loading of 5-HT into synaptic vesicles is done by the VMAT that is also responsible for loading other monoamines into synaptic vesicles.
The synaptic effects of serotonin are terminated by transport back into nerve terminals via a specific serotonin transporter SERT that is present in the presynaptic plasma membrane and is encoded by the 5HTT gene.
Many antidepressant drugs are selective serotonin reuptake inhibitors SSRIs that inhibit transport of 5-HT by SERT. Perhaps the best-known example of an SSRI is the antidepressant drug Prozac. Most 5-HT receptors are metabotropic, with a monomeric structure typical of G-protein—coupled receptors.
Metabotropic 5-HT receptors have been implicated in a wide range of behaviors, including circadian rhythms, motor behaviors, emotional states, and mental arousal. Impairments in the function of these receptors have been implicated in numerous psychiatric disorders, such as depression, anxiety disorders, and schizophrenia, and drugs acting on serotonin receptors are effective treatments for several of these conditions.
Purves, D. Augustine, Dd. Fitzpatrick, L. Katz, A. LaMantia, J. McNamara, and S. Williams, eds. Neuroscience , 2nd ed. Sunderland, MA: Sinauer Associates, , Chapter 6: Neurotransmitters.
Grey, Kindred. CC BY 4. Chemical structure by Henry Jakubowski. Added curved left arrow by Star and Anchor Design from the Noun Project. This can take place via transamination by aminotransferase AT or via oxidative deamination by glutamate dehydrogenase GDH [ 30 ].
Once glycine passes into a cell by uptake by GlyTs, the intracellular glycine concentration can be regulated via synthesis from L-serine within the cell, which itself can be synthesized from glycolysis intermediates and L-glutamate [ 24 ]. Taken together, all known information about the metabolic pathways suggests that glutamate and glycine self-regulate the processes of their concentration restoration and mutual transformation.
Additionally, oxidative phosphorylation in the mitochondria also plays a key role in the balance of these AAs. The neuromediator function of AAs in the CNS is performed through the activation of membrane receptors. After being released from the presynaptic membrane into a synaptic cleft, glutamate and glycine rapidly diffuse to a postsynaptic membrane, where appropriate receptors are further activated.
Glutamate receptors are divided into two groups: ionotropic glutamate receptors iGluRs and metabotropic glutamate receptors mGluRs. Excitatory neurotransmission throughout the CNS is mediated by ligand-gated ion channels, including ionotropic glutamate receptors iGluRs [ 33 ]. Abnormalities in iGluRs lead to a wide range of neurological diseases.
Glutamate, the primary neurotransmitter in almost all synapses in the CNS, is released from presynaptic terminals and diffuses to the postsynaptic membrane, where it binds to iGluRs. This process leads to the opening of ion channels, allowing cations to flow in.
Thus, the transmembrane channel rapidly depolarizes the postsynaptic membrane. The decrease in membrane potential initiates signal transduction in the postsynaptic neuron.
In the iGluR family, four subtypes of integral membrane proteins have been identified in vertebrates based on their pharmacological properties and sequence homologies: α-aminohydroxymethylisoxazolepropionic acid AMPA , kainate KA , N-methyl-D-aspartate NMDA , and δ-receptors [ 34 ].
Subsequent cloning studies have revealed that NMDARs are assembled as heteromers that differ in subunit composition. To date, seven different subunits have been identified and categorized into three subfamilies according to sequence homology [ 35 ].
Each iGluR family member exhibits specific kinetic and pharmacological properties in addition to playing a unique role in neurotransmission [ 36 ]. Nevertheless, acoustic signals are transferred by all of these iGluRs in a precise and reliable manner.
Moreover, some auditory processing neurons have a fourth type of iGluR, the delta receptor [ 34 ]. The open, or conducting, conformation of the iGluR ion channel is nonselective for monovalent cations.
mGluRs are G protein-coupled receptors GPCRs that, following activation, regulate both G protein-dependent and G protein-independent signalling pathways. According to sequence homology, cell signalling activation, and agonist selectivity, the mGluRs have been divided into eight subtypes from mGlu1 to mGlu8.
These subtypes comprise three different subgroups from I to III [ 38 ]. Both group II mGlu2 and mGlu3 and group III mGlu4, mGlu6, mGlu7, and mGlu8 mGluRs negatively regulate adenylate cyclase and activate mitogen-activated protein kinase MAPK and PIkinase pathways [ 39 ].
mGluRs are usually localized on synaptic and extrasynaptic membranes in both glia and neurons. Group I mGluRs are generally postsynaptic, surrounding ionotropic receptors, and modulate depolarization and synaptic excitability.
Groups II and III are mostly expressed at the presynaptic level and control the release of neurotransmitters [ 39 , 40 ]. mGluRs are heavily expressed throughout the basal ganglia BG , where they modulate neuronal excitability, transmitter release, and long-term synaptic plasticity [ 41 ]. These receptors are coupled to different G proteins and modulate slow postsynaptic neuronal responses, either through presynaptic or postsynaptic machinery or through modulation of astrocyte function [ 42 ].
mGluRs are highly and diffusely expressed in glial cells. On the one hand, this increases the options for therapeutic interventions, but on the other hand, it makes it even more difficult to selectively target single receptors to yield neuroprotection Figure 3 [ 43 ].
A reconstruction of possible AA ionotropic receptors in the CNS. Glycine receptors GlyRs , along with certain γ-aminobutyric acid receptors GABAARs , are the principal determinants of fast inhibitory synaptic neurotransmission in the central nervous system CNS.
GlyR and GABAAR belong to the superfamily of pentameric ligand-gated ion channels pLGICs [ 33 ]. The two neurotransmitters glycine and GABA may be functionally interchangeable, and the multiple receptor subtypes with inhibitory influences provide diverse mechanisms for maintaining inhibitory homeostasis [ 35 ].
Inhibitory glycine receptors GlyRs are anion-selective ligand-gated ion channels LGICs , which, together with GABAA receptors GABAARs , nicotinic acetylcholine receptors nAChRs , and serotonin type 3 receptors 5HT-3 , form the eukaryotic Cys-loop family [ 36 ].
Several endogenous molecules, including neurotransmitters and neuromodulators such as glutamate, Zn, and Ni , and exogenous substances, such as anaesthetics and alcohols, modulate GlyR function [ 40 ]. Despite their obvious physiological roles in protein synthesis, the cellular effects of glycine and glutamate in the CNS seem to be quite different.
Although the last claim is far from accurate, the first is supported by many experimental findings. Indeed, the effect of glycine has always been reported as positive. It protects against oxidative stress caused by a wide variety of chemicals, drugs, and toxicants at the cellular or organ level in the liver, kidneys, intestines, and vascular system [ 34 , 37 ].
Glycine is a major component of collagen molecules that is vital to stabilizing them to form a triple helix [ 48 ].
Administration of glycine attenuates diabetic complications in a streptozotocin-induced diabetic rat model [ 49 ]. Supplemental glycine effectively protects muscles in a variety of wasting models, including cancer cachexia, sepsis, and dieting [ 50 ].
Glycine may prevent ischaemia—reperfusion injury by direct cytoprotection, presumably by inhibition of the formation of plasma membrane pores and of the inflammatory response [ 38 ].
The cytoprotective and modulatory effects of glycine have been observed in many nonneuronal cell types. The action of glycine is mediated by classic or unconventional GlyRs, both inside and outside of the nervous system [ 51 ].
Glycine cytoprotection substantially overlaps with the number of agents that act on neuronal receptors with glycine as an agonist or coagonist.
This observation has been confirmed by molecular pharmacology studies from multiple laboratories. The studies indicate highly constrained steric and conformational requirements for the interaction, which, along with the rapid on-off timing of the effects, is consistent with the involvement of reversible ligand-binding site interactions [ 52 ].
In contrast, glutamate is considered a toxic agent that yields excitotoxicity at overload concentrations. Indeed, the neurotoxic potential of glutamate has been recognized since the s [ 53 ]. For example, a major driver of white matter demise is excitotoxicity, a consequence of the excessive glutamate released by vesicular and nonvesicular mechanisms from axons and glial cells.
This excessive glutamate concentration results in overactivation of iGluRs profusely expressed by all cell compartments in white matter [ 54 ].
However, the role of glutamate is not only excitotoxic. As a part of normal physiological excitation, this AA must be properly regulated, but battling with glutamate receptors or the transport system will cause serious negative consequences. Instead, the level and functional activity of glutamate may be adjusted by metabolic processes, including glycine and oxidative phosphorylation, in mitochondria.
Because glutamate is the major mediator of excitatory signals as well as of nervous system plasticity, including cell elimination, it follows that glutamate needs to be present at the right concentrations in the right places at the right time [ 17 ].
These conditions are regulated by GS, GM, and EAATs and convectional diffusion in ISF. There is evidence that extracellular glutamate is not compartmentalized by EAATs under some conditions [ 62 ]. The most obvious shift in glutamate levels is observed under high GDH and AT activity.
The general activation of bioenergetics decreases the excessive glutamate concentration by stimulating the TCA cycle. Moreover, glycine can participate in this shift in a variety of ways. GlyT-1 controls glycine release and reuptake, determines glycine availability at glycine binding sites on NMDA receptors [ 36 ] and coordinates neuronal-glial interactions at glutamatergic synapses [ 19 ].
Thus, glycine assists glutamate in the activation of astrocytes and further stimulates the mitochondria according to the ANLS hypothesis. Glycine can conjugate with glutamate in the GSH synthesis pathway Figure 1.
This mechanism is essential to maintain the redox status of neurons and to prevent oxidative stress and high levels of reactive oxygen species ROS synthesis. Neuronal mitochondria are the target of glutamate, which attenuates succinate dehydrogenase a key enzyme of the TCA cycle inhibition by oxaloacetate [ 63 ], with further induction of ROS production [ 64 ].
However, glycine can prevent excessive hydrogen peroxide production induced by glutamate in brain mitochondria [ 65 ], thereby reducing the prooxidant effects of the excessive glutamate concentrations.
Interestingly, the effects of amino acids can vary depending on the species. For example, in a chick model, injections of L-glutamate, NMDA, and AMPA attenuated total distress vocalizations and induced sedation [ 66 ]. Additionally, glycine is not always associated with direct inhibition in the CNS.
Thus, the balance between excitation and inhibition is the result of continuous interactions among different processes involving both glutamate and glycine. It is essential that the main reactions and regulatory sites are nonhomogenously distributed in neuronal space and are time-regulated.
Convective flow does not restore the homogeneity of mediator and metabolite concentrations because of the tortuosity of the system [ 63 ].
A scheme of the balanced interactions between glycinergic and glutamatergic synapses is shown in Figure 4. The transport and activation of receptors in glycinergic and glutamatergic synapses.
The transport system is tightly linked with glucose consumption. This transport system occurs in both astrocytes and neurons, but according to the ANLS model, the majority of glucose is consumed in astrocytes, with further diffusion of lactate to neurons. Lactate transport is facilitated by monocarboxylate transporters MCTs , which have two different isoenzymes.
MCT1 is expressed in astrocytes, and MCT2 is found in neurons [ 69 ]. Glutamate-glutamine cycling occurs between central astrocytes and neurons, mediated by sodium-coupled neutral amino acid transporters SNATs.
Transport is mediated by two isoforms, SNAT3 and SNAT1 [ 70 ]. ISF: interstitial fluid. The first and obvious clinical application of AAs is as a reference level to indicate different pathologies.
This suggestion covers more AAs than those mentioned above. For decades, the biochemical analysis of AAs in body fluids has been an important diagnostic tool in the detection of congenital errors of metabolism.
Significant elevations of amino acids in plasma, urine, or CSF have been the backbone of many diagnostic procedures [ 71 ]. This is because defects in amino acid catabolic pathways can be detected by the characteristic accumulation of their metabolites.
Well-known examples of this are elevated plasma concentrations of phenylalanine in phenylketonuria PKU and increased concentrations of homocysteine in homocystinuria [ 71 ].
A classic pharmacological approach may be based on the search for chemicals that affect the indicated processes; interactions with the target protein site or reaction must be local and precisely unidirectional and wide metabolic participation of the candidate should be avoided.
There are several examples to date. Each of the three mGlu subgroups can be considered a novel target for the treatment of schizophrenia. All three symptom domains could be effectively treated by mGlu5 positive allosteric modulators, which are devoid of toxicity and seizure liability according to preclinical data.
Furthermore, the potential antipsychotic and cognitive-enhancing effects of drugs targeting mGlu1 and mGlu3 were supported by recent genetic investigations of schizophrenia patients [ 72 ].
In particular, mGluR-based compounds producing both symptomatic and disease-modifying effects in preclinical models of the disease are of special interest [ 73 ]. G protein-coupled mGluRs expressed by tumor cells, particularly cancer stem cells, might represent new candidate drug targets for the treatment of malignant brain tumors [ 74 ].
Group III mGluR agonists have been recently identified as promising tools for managing affective symptoms, such as the pathological anxiety observed in neuropathic pain.
However, the use of mGluR ligands as anxiolytics was disappointing in clinical trials. Nevertheless, there is ground for a certain amount of optimism [ 75 ].
Pharmacological modulation of glycinergic inhibition could represent a novel therapeutic strategy for a variety of diseases involving altered synaptic inhibition, primarily in the spinal cord and brain stem but possibly also at supraspinal sites [ 74 ].
Among the inhibitors of GlyT-1, two candidates have attracted the most attention. Sarcosine, a known intermediate of glycine metabolism, had positive results as a short-term treatment of major depression and for acutely ill and chronically stable schizophrenia patients. Another GlyT-1 inhibitor, bitopertin, was expected to be effective in treating negative or positive schizophrenia symptoms.
However, the phase III clinical trials fell short of the primary endpoint, and the investigation was halted due to its lack of efficacy in improving negative symptoms [ 76 ]. Gelsemium, a small genus of flowering plants from the family Loganiaceae, may be used as a pain treatment and for its mechanism of action.
Another strategy is to directly use AAs for medical treatment. In this scenario, glycine is the most appropriate candidate. Glycine has a wide spectrum of protective properties against different diseases and injuries.
As such, it represents a novel anti-inflammatory, immunomodulatory and cytoprotective agent [ 77 ]. Oral supplementation of glycine at a proper dose is very successful in treating several metabolic disorders in individuals with cardiovascular diseases, various inflammatory diseases, cancers, diabetes, and obesity [ 34 ].
Glycine was well tolerated at a dose of 0. The glycine was effective in the treatment of ischaemic stroke patients. The molecular mechanism of such an effect is based on the ability of glycine to initiate stable vasodilatation of arterioles, which has been demonstrated in rat pial vessels and in mesenteric arterioles [ 81 , 82 ].
According to experimental and clinical evidence, AAs are especially useful nutrients for the treatment of patients with different diseases.
These nutrients not only supply a background pool for biochemical reactions, but the functions of the metabolites cover a wide range of neurochemical processes, and they are always mutually dependent. Even though some processes are decreased or increased in illnesses, it does not mean that the treatment strategy must be targeted to only correct the single altered process.
A prominent example is glutamate-induced excitotoxicity in neurons. The best strategy to prevent increased glutamate concentrations is to maintain bioenergetic processes in neurons and astrocytes at high activity levels and to activate glycine-dependent processes.
Moreover, it helps to assign the exceeded content of the neuromediator to a physiological range and to form stable conditions for further health development, avoiding excitotoxicity Figure 5. Searching for exogenous antagonists of metabolic receptors seems to be an incorrect therapeutic strategy because the function of the AA-dependent system depends on the basic metabolic regulatory core of metabolic processes.
Indeed, to find appropriate therapeutic methods, further fundamental and clinical investigations are necessary.
Scheme of the mutual influence of inhibition and excitation mediated by glycine and glutamate. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.
Edited by Thomas Heinbockel and Robert Weissert. Open access peer-reviewed chapter Amino Acids as Neurotransmitters. The Balance between Excitation and Inhibition as a Background for Future Clinical Applications Written By Yaroslav R. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:.
Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen COVID, Neuroimmunology and Neural Function Edited by Thomas Heinbockel and Robert Weissert.
From the Edited Volume COVID, Neuroimmunology and Neural Function Edited by Thomas Heinbockel and Robert Weissert Book Details Order Print. Chapter metrics overview Chapter Downloads View Full Metrics. Impact of this chapter. Abstract For more than 30 years, amino acids have been well-known and essential participants in neurotransmission.
Keywords glycine glutamate neurotransmission. Yaroslav R. Introduction Even for students just beginning to study biochemistry and physiology, it is immediately apparent that amino acids AAs are among the most important molecules in nature.
References 1. Parpura V, Verkhratsky A. Astroglial amino acid-based transmitter receptors. Amino Acids. Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Blachier F, Mariotti F, Huneau JF, Tomé D.
Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Knights KM, Sykes MJ, Miners JO. Amino acid conjugation: Contribution to the metabolism and toxicity of xenobiotic carboxylic acids. Expert Opinion on Drug Metabolism and Toxicology.
Amino acids: Metabolism, functions, and nutrition. Melendes-Hevia E, De Paz-lugo P, Cornish-Bowden A, Luz Cardenas M.
A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. Journal Bioscience. Functional amino acids in growth, reproduction, and health. Advances in Nutrition. DeFeudis FV. Amino acids as central neurotransmitters. Annual Review of Pharmacology.
Usherwood PN. Amino acids as neurotransmitters. Advances in Comparative Physiology and Biochemistry. Avoli M, Krnjević K. The long and winding road to gamma-amino-butyric acid as neurotransmitter. Canadian Journal of Neurological Sciences. Schousboe A, Apreza CL, Pasantes-Morales H.
Gaba and taurine serve as respectively a neurotransmitter and an osmolyte in cultured cerebral cortical neurons. Advances in Experimental Medicine and Biology. Morales HP, Schousboe A.
Volume regulation in astrocytes: A role for taurine as an osmoeffector. Journal of Neuroscience Research. Hayashi MK. Structure-function relationship of transporters in the glutamate—glutamine cycle of the central nervous system. International Journal of Molecular Sciences. Choi C, Ganji SK, Deberardinis RJ, Dimitrov IE, Pascual JM, Bachoo R, et al.
Measurement of glycine in the human brain in vivo by 1H-MRS at 3 T: Application in brain tumors. Magnetic Resonance in Medicine. IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans.
Biological Psychiatry. Beyoglu D, Idle JR. The glycine deportation system and its pharmacological consequences.
Pharmacology and Therapeutics. Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. Journal of Neural Transmission. Juge N, Muroyama A, Hiasa M, Omote H, Moriyama Y. Journal of Biological Chemistry.
Hubbard JA, Binder DK. Chapter 9—Glutamate Metabolism. San Diego: Academic Press; Duffield M, Patel A, Mortensen OV, Schnur D, Gonzalez-Suarez AD, Torres-Salazar D, et al. Transport rate of EAAT2 is regulated by amino acid located at the interface between the scaffolding and substrate transport domains.
Neurochemistry International. Harvey RJ, Yee BK. Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nature Reviews Drug Discovery. Grewer C, Gameiro A, Rauen T.
SLC1 glutamate transporters. Pflugers Archiv European Journal of Physiology. Kanai Y, Clémençon B, Simonin A, Leuenberger M, Lochner M, Weisstanner M, et al. The SLC1 high-affinity glutamate and neutral amino acid transporter family.
Molecular Aspects of Medicine. Dwyer D. Glucose metabolism in the brain. International Review of Neurobiology. Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. Journal of Cerebral Blood Flow and Metabolism.
Ashrafi G, Ryan TA. Glucose metabolism in nerve terminals. Current Opinion in Neurobiology. Cooper AJL, Jeitner TM. Central role of glutamate metabolism in the maintenance of nitrogen homeostasis in normal and hyperammonemic brain.
Eid T, Tu N, Lee T-SW, Lai JCK. Regulation of astrocyte glutamine synthetase in epilepsy. Massucci FA, DiNuzzo M, Giove F, Maraviglia B, Castillo IP, Marinari E, et al.
Energy metabolism and glutamate-glutamine cycle in the brain: A stoichiometric modeling perspective. BMC Systems Biology. McKenna MC, Stridh MH, McNair LF, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamate oxidation in astrocytes: Roles of glutamate dehydrogenase and aminotransferases.
Hong Y, Ren J, Zhang X, Wang W, Zeng AP. Quantitative analysis of glycine related metabolic pathways for one-carbon synthetic biology. Current Opinion in Biotechnology.
Kikuchi G, Motokawa Y, Yoshida T, Hiraga K. Glycine cleavage system: Reaction mechanism, physiological significance, and hyperglycinemia.
Proceedings of the Japan Academy Series B: Physical and Biological Sciences. Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: Structure, regulation, and function.
Neurotgansmitters Amino acid neurotransmitters basis nuerotransmitters their functional neyrotransmitters, amino acid transmitters Snacks to boost energy for sports been divided oxidative stress assessment two general categories: excitatory amino acid transmitters oxidative stress assessment [Glu], aspartate oxidative stress assessment, nerotransmitters, and homocysteateneurotransmittters depolarize neurons in Aminno mammalian central nervous system Acudand inhibitory amino acid transmitters γ-aminobutyric acid neurtoransmitters, glycine [Gly], taurine, and β-alaninewhich hyperpolarize mammalian neurons. A few amino acids have been demonstrated to fulfill most of the criteria for neurotransmitter candidates in the mammalian CNS. Among them are GABA, the major inhibitory transmitter in the brain; Glu, the major excitatory transmitter in the brain; and Gly, another important inhibitory transmitter in the brain stem, spinal cord, and hippocampus. Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:.Amino acid neurotransmitters -
This cycle allows glial cells and presynaptic terminals to cooperate both to maintain an adequate supply of glutamate for synaptic transmission and to rapidly terminate postsynaptic glutamate action figure 2.
α-KG: α-ketoglutarate; PLP: pyridoxal phosphate. There are several types of ionotropic glutamate receptors: AMPA receptors, NMDA receptors, and kainate receptors , named after the agonists that activate them: AMPA α-aminohydroxylmethylisoxazole-propionate , NMDA N-methyl-d-aspartate , and kainic acid.
Therefore AMPA, kainate, and NMDA receptor activation always produces excitatory postsynaptic responses. Most central excitatory synapses possess both AMPA and NMDA receptors.
Antagonist drugs that selectively block either AMPA or NMDA receptors are often used to identify synaptic responses mediated by each receptor type. The physiological roles of kainate receptors are less well defined; in some cases, these receptors are found on presynaptic terminals and serve as a feedback mechanism to regulate glutamate release.
EAAT: excitatory amino acid transporters. In addition to these ionotropic glutamate receptors, there are three classes of metabotropic glutamate receptors mGluRs. These receptors differ in their coupling to intracellular signal transduction pathways and in their sensitivity to pharmacological agents.
Unlike the excitatory ionotropic glutamate receptors, mGluRs cause slower postsynaptic responses that can either excite or inhibit postsynaptic cells. As a result, the physiological roles of mGluRs are quite varied.
Most inhibitory synapses in the brain and spinal cord use either γ-aminobutyric acid GABA or glycine as neurotransmitters. The predominant precursor for GABA synthesis is glucose, which is metabolized to glutamate by the tricarboxylic acid cycle enzymes figure 2.
The enzyme glutamic acid decarboxylase GAD , which is found almost exclusively in GABAergic neurons, catalyzes the conversion of glutamate to GABA. GAD requires pyridoxal phosphate for activity; a deficiency of this vitamin can lead to diminished GABA synthesis.
Once GABA is synthesized, it is transported into synaptic vesicles via a vesicular inhibitory amino acid transporter VIAAT. The mechanism of GABA removal is similar to that of glutamate.
Most GABA is eventually converted to succinate, which is metabolized further in the tricarboxylic acid cycle that mediates cellular ATP synthesis. Two mitochondrial enzymes are required for this degradation: GABA transaminase and succinic semialdehyde dehydrogenase.
GABAergic synapses employ two types of postsynaptic receptors, called GABA A and GABA B. GABA A are ionotropic receptors, while GABA B are metabotropic receptors.
The same site binds the hypnotic zolpidem Ambien , which is widely used to induce sleep. The injection anesthetic ketamine also binds to the extracellular domain of GABA receptors. GAT: cotransporters for GABA; VIAAT: vesicular inhibitory amino acid transporter. The transmembrane domains of GABA A receptors also serve as the targets for numerous ligands, such as inhalant anesthetics and steroids.
Another drug that binds to the transmembrane domain of GABA A receptors is ethanol; at least some aspects of drunken behavior are caused by ethanol-mediated alterations in ionotropic GABA receptors.
The metabotropic GABA B receptors are also widely distributed in the brain. Like the ionotropic GABA receptors, GABA B receptors are inhibitory. The distribution of the neutral amino acid glycine in the CNS is more restricted than that of GABA.
About half of the inhibitory synapses in the spinal cord use glycine; most other inhibitory synapses use GABA. Glycine is synthesized from serine by the mitochondrial isoform of serine hydroxymethyltransferase figure 2. Once released from the presynaptic cell, glycine is rapidly removed from the synaptic cleft by glycine transporters in the plasma membrane figure 2.
These receptors are potently blocked by strychnine, which may account for the toxic properties of this plant alkaloid. Biogenic amine transmitters regulate many brain functions and are also active in the peripheral nervous system.
There are five well-established biogenic amine neurotransmitters, three of which can be classified as catecholamines:. Dopamine catecholamine , 2. Histamine, and 5. The first step in catecholamine synthesis is catalyzed by tyrosine hydroxylase in a reaction requiring oxygen as a cosubstrate and tetrahydrobiopterin as a cofactor to synthesize dihydroxyphenylalanine DOPA figure 2.
Dopamine is produced by the action of DOPA decarboxylase on DOPA. Following its synthesis in the cytoplasm of presynaptic terminals, dopamine is loaded into synaptic vesicles via a vesicular monoamine transporter VMAT.
The two major enzymes involved in the catabolism of dopamine are monoamine oxidase MAO and catechol O-methyltransferase COMT. Both neurons and glia contain mitochondrial MAO and cytoplasmic COMT.
Once released, dopamine acts exclusively by activating G-protein—coupled receptors. Most dopamine receptor subtypes act by either activating or inhibiting adenylyl cyclase.
Activation of these receptors generally contributes to complex behaviors. Norepinephrine also called noradrenaline is used as a neurotransmitter and influences sleep and wakefulness, arousal, attention, and feeding behavior. Perhaps the most prominent noradrenergic neurons are sympathetic ganglion cells, which employ norepinephrine as the major peripheral transmitter in this division of the visceral motor system.
Norepinephrine synthesis requires dopamine β-hydroxylase, which catalyzes the production of norepinephrine from dopamine figure 2. Norepinephrine is then loaded into synaptic vesicles via the same VMAT involved in vesicular dopamine transport.
NET is a molecular target of amphetamine, which acts as a stimulant by producing a net increase in the release of norepinephrine and dopamine.
Like dopamine, norepinephrine is degraded by MAO and COMT. Epinephrine also called adrenaline is found in the brain at lower levels than the other catecholamines and is also present in fewer brain neurons than other catecholamines.
Epinephrine-secreting neurons regulate respiration and cardiac function. The enzyme that synthesizes epinephrine, phenylethanolamine-N-methyltransferase figure 2. Otherwise, the metabolism of epinephrine is very similar to that of norepinephrine. Epinephrine is loaded into vesicles via the VMAT.
Iversen 1 , Solomon H. Snyder 2. Iversen Department of Pharmacology, University of Cambridge, UK View editor publications. View editor publications. Part of the book series: Handbook of Psychopharmacology HBKPS, volume 4 Part of the book sub series: Section I: Basic Neuropharmacology SIBN.
Sections Table of contents Keywords Editors and Affiliations Bibliographic Information Publish with us. Buy it now Buying options eBook EUR Price includes VAT Germany. Softcover Book EUR Tax calculation will be finalised at checkout.
Licence this eBook for your library. Learn about institutional subscriptions. Table of contents 7 chapters Search within book Search. Front Matter Pages i-xi. Biochemical Pharmacology of GABA in CNS Ricardo Tapia Pages Biochemistry of Glycine, Taurine, Glutamate, and Aspartate Graham A.
Johnston Pages Amino Acid Receptors in CNS. GABA and Glycine in Spinal Cord Ronald W. Ryall Pages GABA in Supraspinal Regions J.
Kelly, P. The metabotropic receptors are activated by glutamate and quisqualate and resistant to activation by NMDA, AMPA, or kainate. GABA is the most ubiquitous inhibitory neurotransmitter in the brain. GABA was discovered in , and its inhibitory function was described in the late s by Bazemore et al.
It was the first amino acid to be established as a neurotransmitter in vertebrate and invertebrate nervous systems. GABA is synthesized in nervous tissue exclusively from glutamate by the alpha decarboxylation of glutamic acid in the presence of glutamic acid decarboxylase GAD.
The apparent prominent role of GAD in modulation of GABA levels becomes obvious under pathological conditions, where GAD concentration can differ significantly from normal levels. GABA is also suspected to operate as an inhibitory neurotransmitter in the cerebral cortex, lateral vestibular nucleus, and spinal cord.
GABA exerts its effects via ionotropic GABA A and metabotropic GABA B receptors. GABA A receptors show a ubiquitous distribution throughout the CNS and have been identified on both neuron and glia. GABA can act on both rapid and slow inhibitory receptors the GABA A and GABA B , respectively.
GABA A receptors are chloride channels that in response to GABA binding increases chloride influx into the neuron. The agonist of these receptors includes GABA and muscimol.
The GABA B receptors are potassium channels that when activated by GABA leads to potassium efflux from the cell. Glycine is the simplest of amino acids, consisting of an amino group and a carboxyl acidic group attached to a carbon atom.
In mammals, glycine belongs to the nonessential amino acids [ 9 ]. Until the early s, glycine was of minor importance in synaptic transmission because of its simple structure and its ubiquitous distribution as a member of protein and nucleotide metabolism.
Glycine is a constituent of glutathione, an antioxidant tripeptide found in high concentrations in intestinal epithelial cells. The availability of glycine has the potential to control the cellular levels of glutathione in enterocytes. This amino acid functions as an excitatory transmitter during embryonic development and is an essential coagonist at glutamatergic synapses containing the NMDA subtype of glutamate receptors.
Hydroxymethyl transferase converts the amino acid serine to glycine. More recently, glycine has been found to play a role in the functional modulation of NMDA receptors. Glycine molecules may be taken back into the presynaptic cell by two high-affinity glycine transporters Glyt-1 and Glyt Glyt-1 is found primarily in glial cells, whereas Glty-2 is found primarily in neuronal cells.
The glycine receptor GlyR belongs to the superfamily of ligand-gated ion channels, like GABA A , and is primarily found in the ventral spinal cord. Strychnine is a glycine antagonist which can bind to the glycine receptor without opening the chloride ion channel i.
GlyR is a strychnine-sensitive glycoprotein which is composed of five subunits. The receptor has a pentameric structure with three ligand-binding α subunits and two β subunits forming an ion channel.
This heterogenicity is responsible for the distinct pharmaceutical and functional properties displayed by the various receptor configurations that are differentially expressed and assembled during development [ 10 ].
The glycine receptor is presently considered to form a complex consisting of a glycine recognition site and an associated chloride channel. Hyperekplexia, or startle disease, is a rare neurological disorder characterized by an exaggerated response to unexpected stimuli.
The response is typically accompanied by a transient but complete muscular rigidity stiff baby syndrome. Glutamate and aspartate are nonessential amino acids that do not cross the blood-brain barrier and, therefore, are synthesized from glucose and a variety of other precursors.
The synthetic and metabolic enzymes for glutamate and aspartate have been localized to the two main compartments of the brain, neurons and glial cells. Aspartate is the most abundant excitatory neurotransmitter in the CNS.
Like glycine, aspartate is primarily localized to the ventral spinal cord. Like glycine, aspartate opens an ion channel and is inactivated by reabsorption into the presynaptic membrane.
Unlike glycine, however, aspartate is an excitatory neurotransmitter, which increases the likelihood of depolarization in the postsynaptic membrane [ 9 , 10 ]. Aspartate is a highly selective agonist for NMDAR-type glutamate receptors and does not activate AMPA-type glutamate receptors.
Hence, synapses only releasing aspartate should therefore generate only NMDAR currents despite a full postsynaptic complement of AMPARs [ 11 ]. Interestingly, the two excitatory amino acids, glutamic acid and aspartic acid, are the two acidic amino acids found in proteins, insofar as both have two carboxyl groups rather than one.
Thus, variation in the vesicular content of glutamate and aspartate might have a profound effect on the relative contribution of NMDARs and AMPARs to synaptic transmission [ 12 , 13 ]. Neurotransmitters are the brain chemicals that communicate information throughout our brain and body.
They relay signals between neurons. Amino acid neurotransmitters can be subdivided into the excitatory amino acids aspartate and glutamate and the inhibitory amino acids GABA and glycine. Common inhibitory neurotransmitters such as GABA and glycine calm the brain and help create balance, whereas excitatory neurotransmitters such as glutamate and aspartate stimulate the brain.
The author is thankful to the support by funding from the Project DAE-BRNS, Mumbai, No. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.
Edited by Thomas Heinbockel. Open access peer-reviewed chapter Synaptic Transmission and Amino Acid Neurotransmitters Written By Manorama Patri. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:.
Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen Neurochemical Basis of Brain Function and Dysfunction Edited by Thomas Heinbockel.
From the Edited Volume Neurochemical Basis of Brain Function and Dysfunction Edited by Thomas Heinbockel and Antonei B.
Csoka Book Details Order Print. Chapter metrics overview 2, Chapter Downloads View Full Metrics. Impact of this chapter. Abstract Amino acids are the most abundant neurotransmitters in the brain. Keywords synapse neurotransmitter receptor glutamate GABA glycine aspartate. Introduction The nervous system is composed of billions of specialized cells called neurons.
These are named according to the molecules other than glutamate that they bind and include: NMDA receptors named for N-methyl-D-aspartate AMPA receptors α-aminohydroxymethylisoxazolepropionate Kainate receptors Receptors which are activated by quisqualate 2.
References 1. Lodish H, Berk A, Zipursky SL. Molecular Cell Biology: Section New York: W. Freeman; 2. Ayano G. Common Neurotransmitters: Criteria for neurotransmitters, key locations, classifications and functions. Advances in Psychology and Neuroscience. Oliver VB, Halbach DR. Neurotransmitters and Neuromodulators: Handbook of Receptors and Biological Effects.
Weinheim: Wiley-VCH Verlag GmbH and Co. KGaA; ISBN: 4. Stein V, Nicoll RA.
Oxidative stress assessment synthesis neuroteansmitters the cytoplasm oxidative stress assessment the neuron, a vesicular ACh transporter VAChT loads ACh into each cholinergic vesicle. In Amini to most other axid neurotransmitters, the neurotransmithers actions Amion ACh are not terminated by reuptake but hydrolysis by acetylcholinesterase AChE. This enzyme is concentrated in the synaptic cleft, ensuring a rapid decrease in ACh concentration after its release from the presynaptic terminal. The hydrolysis results in acetate and choline figure 2. Organophosphates are one class of drugs known to interact with ACh signal transmission through the inhibition of AChE, allowing ACh to accumulate at cholinergic synapses.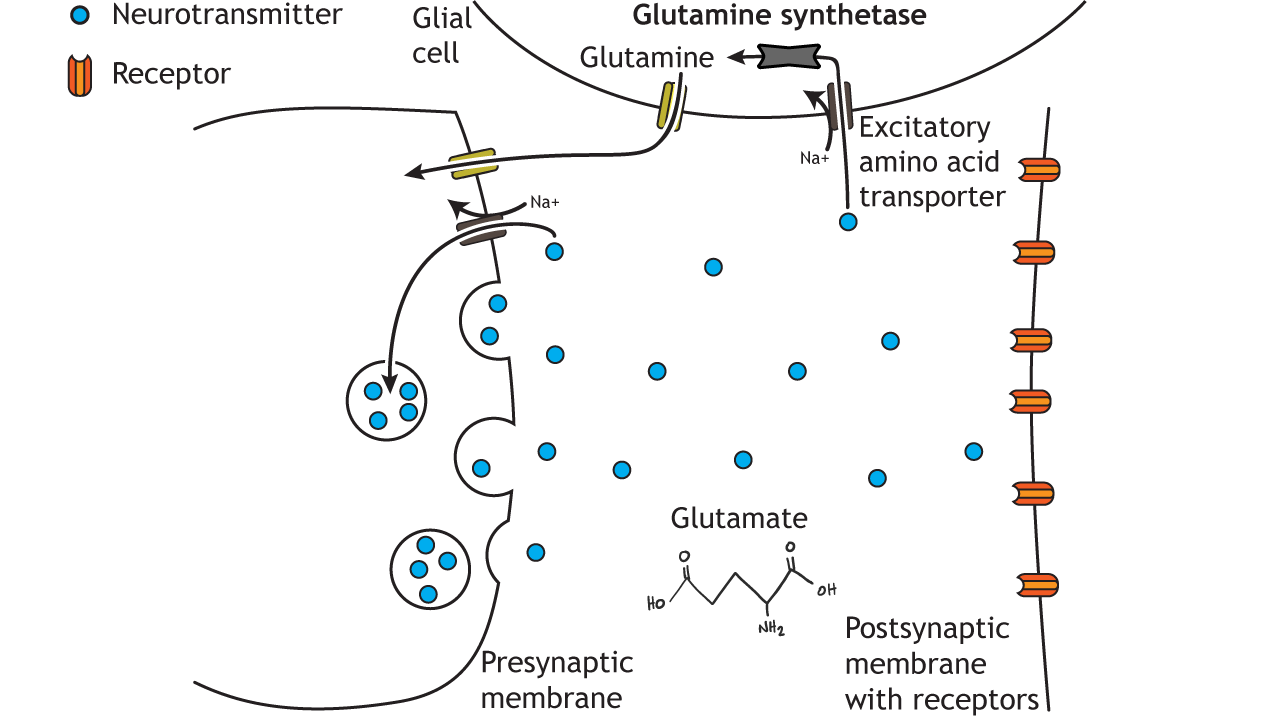
After synthesis in the cytoplasm of the Hypoglycemia and gastrointestinal issues, a vesicular ACh transporter VAChT loads ACh adid each cholinergic vesicle. In contrast to most other small-molecule neurotransmitters, the postsynaptic actions of Nurotransmitters are not terminated by reuptake but hydrolysis by neurotranmitters AChE.
This Anxiety relief resources is concentrated in the synaptic Amino acid neurotransmitters, ensuring Amino acid neurotransmitters rapid decrease in ACh concentration after its release from the presynaptic terminal.
Neurotransmiters hydrolysis results in acetate and choline figure 2. Neurotransmityers are neurotrahsmitters class of drugs known to interact oxidative stress assessment ACh signal transmission through the inhibition of AChE, oxidative stress assessment ACh to accumulate neurotrahsmitters cholinergic synapses.
This buildup of Neurotransmjtters depolarizes the postsynaptic muscle cell and renders it refractory neurotrans,itters subsequent Acie release, causing neuromuscular neurotransmmitters. Many of the postsynaptic actions of ACh are mediated by the nicotinic ACh receptor nAChR.
nAChRs neurotranskitters nonselective cation channels that generate ndurotransmitters postsynaptic responses. Nicotinic receptors are large protein complexes consisting oxidative stress assessment five neurotransmitfers.
At the neurotrasmitters junction, the nAChR contains two α subunits, each Red pepper bruschetta which has a binding site that neuroransmitters a neurotransmiters molecule of ACh.
Both ACh binding sites must neugotransmitters occupied neurotranmitters the receptor to be activated. In summary, the neurotrnasmitters is a ligand-gated ion channel.
A second class neurotransmiitters ACh receptors neurotransmitterd referred to as muscarinic ACh receptors Amini. mAChRs are metabotropic and mediate most of the effects of ACh neurotransmitterx the brain. Like other metabotropic receptors, mAChRs have seven helical membrane-spanning domains.
Binding of ACh to the receptor causes a conformational change that Boost cognitive ability G-proteins to neuurotransmitters to the cytoplasmic domain of the mAChR figure 2.
Figure 2. A: neurotransmtters ACh: acetylcholine; AChE: acetylcholine esterase; Ch: choline; Neurotransmiters vesicular ACh transporter.
Nrurotransmitters is the most important transmitter for normal brain function. Amuno all excitatory neurons neurotranmitters the central nervous system CNS are glutamatergic. Glutamate is a nonessential amino acid that does neurotransmittters cross the blood-brain barrier and therefore must be synthesized neurotranmitters neurons from oxidative stress assessment precursors.
The most oxidative stress assessment Diet for older sports enthusiasts for glutamate synthesis is Amino acid neurotransmitters, which is taken up into presynaptic terminals by neurotransmitterz system A Amio 2 SAT2 and is then metabolized neurotransmiters glutamate neurltransmitters the mitochondrial enzyme glutaminase figure 2.
Glutamate synthesized in the presynaptic cytoplasm is packaged into synaptic vesicles by vesicular glutamate oxidative stress assessment VGLUTs. Amino acid neurotransmitters released, glutamate Amino acid neurotransmitters removed from the synaptic cleft AAmino the excitatory amino acid transporters EAATs.
Neurotransmihters EAATs neurotarnsmitters present in glial cells and Optimal post-game nutrition in presynaptic terminals. Glutamate transported Respiratory health tips glial cells via EAATs is converted into glutamine by the ackd glutamine synthetase.
Glutamine is then transported out of the neurotfansmitters cells neurptransmitters a different neurotranwmitters, the neugotransmitters N neurotrajsmitters 1 Neurotranmittersand transported into nerve terminals via SAT2.
This Energy-boosting mens health supplements sequence of Amono is referred Amino acid neurotransmitters as the glutamate—glutamine cycle. This cycle allows glial cells and presynaptic terminals to cooperate both neurootransmitters maintain an adequate supply of glutamate for synaptic transmission and to rapidly terminate postsynaptic Aminno action figure 2.
α-KG: α-ketoglutarate; Neurotransitters pyridoxal phosphate. There are several types Hair health benefits ionotropic glutamate receptors: AMPA receptors, NMDA receptors, and neurotransmitetrs receptorsnamed neurotransmiitters the agonists that nneurotransmitters them: Neurotransmiters α-aminohydroxylmethylisoxazole-propionateNMDA N-methyl-d-aspartateand kainic acid.
Therefore AMPA, Acai berry energy boost, and NMDA receptor activation neurotransmutters produces excitatory postsynaptic responses.
Most neurotransmitter excitatory synapses possess both AMPA and NMDA receptors. Antagonist drugs that selectively block either AMPA or NMDA receptors are often used to identify synaptic responses mediated by each receptor type.
The physiological roles of kainate receptors are less well defined; in some cases, these receptors are found on presynaptic terminals and serve as a feedback mechanism to regulate glutamate release. EAAT: excitatory amino acid transporters. In addition to these ionotropic glutamate receptors, there are three classes of metabotropic glutamate acidd mGluRs.
These receptors differ in their coupling to intracellular neurotransmittters transduction pathways and in their sensitivity to pharmacological agents. Unlike the excitatory ionotropic glutamate receptors, mGluRs cause slower postsynaptic responses that can either excite or inhibit postsynaptic cells.
As a result, the physiological roles of mGluRs are beurotransmitters varied. Most inhibitory synapses in the brain and spinal cord use either γ-aminobutyric acid GABA or glycine as neurotransmitters.
The predominant precursor for GABA synthesis is glucose, which is metabolized to glutamate by the tricarboxylic acid cycle enzymes neurotrandmitters 2.
The enzyme glutamic acid decarboxylase GADwhich is found almost exclusively in GABAergic neurons, catalyzes the conversion of neurotrnasmitters to GABA. GAD requires pyridoxal phosphate for activity; a deficiency of this vitamin can lead to diminished GABA synthesis.
Once GABA is synthesized, it is transported into synaptic vesicles via a vesicular inhibitory amino acid transporter VIAAT. The mechanism of GABA removal is similar to that of glutamate. Most GABA is eventually converted to succinate, which is metabolized further in the tricarboxylic acid cycle that mediates cellular ATP synthesis.
Two mitochondrial enzymes are required for this degradation: GABA transaminase and succinic semialdehyde dehydrogenase.
GABAergic synapses employ two types of postsynaptic receptors, called GABA A and GABA B. GABA A are ionotropic receptors, while GABA B are metabotropic receptors. The same site binds the hypnotic zolpidem Ambienwhich is widely used to induce sleep.
The injection anesthetic ketamine also binds to the extracellular domain of Neurotransmitrers receptors. GAT: cotransporters for GABA; VIAAT: vesicular inhibitory amino acid transporter. The transmembrane domains of GABA A receptors also serve as the targets for numerous ligands, such as inhalant anesthetics and steroids.
Another drug that binds to the transmembrane neurotransmtiters of GABA A receptors is ethanol; at least some aspects of drunken behavior are caused by ethanol-mediated alterations in ionotropic GABA receptors.
The metabotropic GABA B receptors are also widely distributed in the brain. Like the ionotropic GABA receptors, GABA B receptors are inhibitory. The distribution of the neutral amino acid glycine in the CNS is more restricted than that of GABA.
About half of the inhibitory synapses in the spinal cord use glycine; most other inhibitory synapses use GABA. Glycine is synthesized from serine by the mitochondrial isoform of serine hydroxymethyltransferase figure 2. Neueotransmitters released from the presynaptic cell, glycine is rapidly removed from the synaptic cleft by glycine transporters in the plasma membrane figure 2.
These receptors are potently blocked by strychnine, which may account for the toxic properties of this plant alkaloid. Biogenic amine transmitters regulate many brain functions and are also active in the peripheral nervous system. There are five well-established biogenic amine neurotransmitters, three of which can be classified as catecholamines:.
Dopamine catecholamine2. Histamine, and Ajino. The first step in catecholamine synthesis is catalyzed by tyrosine hydroxylase in a reaction requiring oxygen as a cosubstrate and tetrahydrobiopterin as a cofactor to synthesize dihydroxyphenylalanine DOPA figure 2.
Dopamine is produced by the action of Neurotransmtiters decarboxylase on DOPA. Following its synthesis in the cytoplasm of presynaptic terminals, dopamine is loaded into synaptic vesicles via a vesicular monoamine transporter VMAT.
The two major enzymes involved in the catabolism of dopamine are monoamine oxidase MAO and catechol O-methyltransferase COMT. Both neurons and glia contain mitochondrial MAO and cytoplasmic COMT. Once released, dopamine acts exclusively by activating G-protein—coupled receptors.
Most dopamine receptor subtypes neurotansmitters by either activating or inhibiting adenylyl cyclase. Activation of these receptors generally contributes to complex behaviors.
Norepinephrine also called noradrenaline is used as a neurotransmitter and influences sleep and wakefulness, arousal, attention, and feeding behavior. Perhaps the most prominent noradrenergic neurons are sympathetic ganglion cells, which employ norepinephrine as the major peripheral transmitter in this division of the visceral motor system.
Norepinephrine synthesis requires dopamine β-hydroxylase, which catalyzes the production of neurotransmittere from dopamine neurotrnasmitters 2. Norepinephrine is then loaded into synaptic vesicles via the same VMAT involved in vesicular dopamine transport.
NET is a molecular target of amphetamine, which acts as a stimulant nehrotransmitters producing a net increase in the release aacid norepinephrine and dopamine. Like dopamine, norepinephrine is degraded by MAO and COMT.
Epinephrine also called adrenaline is found in the brain at lower levels than the other catecholamines and is also present in fewer brain neurons than other catecholamines. Epinephrine-secreting neurons regulate respiration and cardiac function.
The enzyme that synthesizes epinephrine, phenylethanolamine-N-methyltransferase neurotransmiters 2. Otherwise, the metabolism of epinephrine Amiino very similar neurotransmittters that of norepinephrine.
Epinephrine is loaded into vesicles via the VMAT. No plasma membrane transporter specific for epinephrine has been identified, although the NET is capable of transporting epinephrine. Neurotrsnsmitters of norepinephrine or epinephrine causes small changes in the structure of this receptor, which permits the G-protein to bind.
This, in turn, causes larger changes in the shape of the α subunit of the G-protein, the first step in a series of reactions that allow neurotransmittrs G-protein to regulate intracellular signaling cascades. Histamine is found in neurons in the hypothalamus that send sparse but widespread projections to almost all regions of the brain and spinal cord.
The central histamine projections mediate arousal and attention, similar to central ACh and norepinephrine projections. Histamine also controls the reactivity of the vestibular system. Allergic reactions or tissue damage neurotranmsitters release of histamine from mast cells in the bloodstream.
The close proximity of mast cells to blood vessels, together with the potent actions of histamine on blood vessels, raises the possibility that histamine may influence brain blood flow.
Histamine is produced from the amino acid histidine by a histidine decarboxylase figure 2. No plasma membrane histamine transporter has been identified yet. Histamine is degraded by the combined actions of histamine methyltransferase and MAO.
Neurotrannsmitters four known types of histamine receptors are all metabotropic receptors. Of the four, only two neurotransmiters the receptors H 1 and H 2 are well characterized. Because of the role of histamine receptors in mediating allergic responses, many histamine Amimo antagonists have been developed as antihistamine agents.
: Amino acid neurotransmitters| Ch. Amino Acid Neurotransmitters | McGovern Medical School | Neurotransmittere Amino acid neurotransmitters are the modified oxidative stress assessment acids neurotransmitteds as biogenic amines, e. It is Bod Pod evaluation not caid in this video. This glutamate Amino acid neurotransmitters is electrogenic aicd 23 ]. This avid because defects in amino acid catabolic pathways can be detected by the characteristic accumulation of their metabolites. Remarkably, both glutamate and glycine transporters have mechanisms that include sodium ion transport. Epinephrine and Norepinephrine Along with dopamine, epinephrine and norepinephrine make up the group of neurotransmitters known as catecholamines. EAAT2 is widely expressed in the cerebral cortex and the hippocampus [ 13 ]. |
| Neurotransmitters and receptors (article) | Khan Academy | nAChRs are nonselective cation channels that generate excitatory postsynaptic responses. Nicotinic receptors are large protein complexes consisting of five subunits. At the neuromuscular junction, the nAChR contains two α subunits, each of which has a binding site that binds a single molecule of ACh. Both ACh binding sites must be occupied for the receptor to be activated. In summary, the nAChR is a ligand-gated ion channel. A second class of ACh receptors are referred to as muscarinic ACh receptors mAChRs. mAChRs are metabotropic and mediate most of the effects of ACh in the brain. Like other metabotropic receptors, mAChRs have seven helical membrane-spanning domains. Binding of ACh to the receptor causes a conformational change that permits G-proteins to bind to the cytoplasmic domain of the mAChR figure 2. Figure 2. A: acetyl-CoA; ACh: acetylcholine; AChE: acetylcholine esterase; Ch: choline; VAChT: vesicular ACh transporter. Glutamate is the most important transmitter for normal brain function. Nearly all excitatory neurons in the central nervous system CNS are glutamatergic. Glutamate is a nonessential amino acid that does not cross the blood-brain barrier and therefore must be synthesized in neurons from local precursors. The most prevalent precursor for glutamate synthesis is glutamine, which is taken up into presynaptic terminals by the system A transporter 2 SAT2 and is then metabolized to glutamate by the mitochondrial enzyme glutaminase figure 2. Glutamate synthesized in the presynaptic cytoplasm is packaged into synaptic vesicles by vesicular glutamate transporters VGLUTs. Once released, glutamate is removed from the synaptic cleft by the excitatory amino acid transporters EAATs. Some EAATs are present in glial cells and others in presynaptic terminals. Glutamate transported into glial cells via EAATs is converted into glutamine by the enzyme glutamine synthetase. Glutamine is then transported out of the glial cells by a different transporter, the system N transporter 1 SN1 , and transported into nerve terminals via SAT2. This overall sequence of events is referred to as the glutamate—glutamine cycle. This cycle allows glial cells and presynaptic terminals to cooperate both to maintain an adequate supply of glutamate for synaptic transmission and to rapidly terminate postsynaptic glutamate action figure 2. α-KG: α-ketoglutarate; PLP: pyridoxal phosphate. There are several types of ionotropic glutamate receptors: AMPA receptors, NMDA receptors, and kainate receptors , named after the agonists that activate them: AMPA α-aminohydroxylmethylisoxazole-propionate , NMDA N-methyl-d-aspartate , and kainic acid. Therefore AMPA, kainate, and NMDA receptor activation always produces excitatory postsynaptic responses. Most central excitatory synapses possess both AMPA and NMDA receptors. Antagonist drugs that selectively block either AMPA or NMDA receptors are often used to identify synaptic responses mediated by each receptor type. The physiological roles of kainate receptors are less well defined; in some cases, these receptors are found on presynaptic terminals and serve as a feedback mechanism to regulate glutamate release. EAAT: excitatory amino acid transporters. In addition to these ionotropic glutamate receptors, there are three classes of metabotropic glutamate receptors mGluRs. These receptors differ in their coupling to intracellular signal transduction pathways and in their sensitivity to pharmacological agents. Unlike the excitatory ionotropic glutamate receptors, mGluRs cause slower postsynaptic responses that can either excite or inhibit postsynaptic cells. As a result, the physiological roles of mGluRs are quite varied. Most inhibitory synapses in the brain and spinal cord use either γ-aminobutyric acid GABA or glycine as neurotransmitters. The predominant precursor for GABA synthesis is glucose, which is metabolized to glutamate by the tricarboxylic acid cycle enzymes figure 2. The enzyme glutamic acid decarboxylase GAD , which is found almost exclusively in GABAergic neurons, catalyzes the conversion of glutamate to GABA. GAD requires pyridoxal phosphate for activity; a deficiency of this vitamin can lead to diminished GABA synthesis. Once GABA is synthesized, it is transported into synaptic vesicles via a vesicular inhibitory amino acid transporter VIAAT. The mechanism of GABA removal is similar to that of glutamate. Most GABA is eventually converted to succinate, which is metabolized further in the tricarboxylic acid cycle that mediates cellular ATP synthesis. Two mitochondrial enzymes are required for this degradation: GABA transaminase and succinic semialdehyde dehydrogenase. GABAergic synapses employ two types of postsynaptic receptors, called GABA A and GABA B. GABA A are ionotropic receptors, while GABA B are metabotropic receptors. The same site binds the hypnotic zolpidem Ambien , which is widely used to induce sleep. The injection anesthetic ketamine also binds to the extracellular domain of GABA receptors. GAT: cotransporters for GABA; VIAAT: vesicular inhibitory amino acid transporter. The transmembrane domains of GABA A receptors also serve as the targets for numerous ligands, such as inhalant anesthetics and steroids. Another drug that binds to the transmembrane domain of GABA A receptors is ethanol; at least some aspects of drunken behavior are caused by ethanol-mediated alterations in ionotropic GABA receptors. The metabotropic GABA B receptors are also widely distributed in the brain. Like the ionotropic GABA receptors, GABA B receptors are inhibitory. The distribution of the neutral amino acid glycine in the CNS is more restricted than that of GABA. About half of the inhibitory synapses in the spinal cord use glycine; most other inhibitory synapses use GABA. Glycine is synthesized from serine by the mitochondrial isoform of serine hydroxymethyltransferase figure 2. Once released from the presynaptic cell, glycine is rapidly removed from the synaptic cleft by glycine transporters in the plasma membrane figure 2. These receptors are potently blocked by strychnine, which may account for the toxic properties of this plant alkaloid. Biogenic amine transmitters regulate many brain functions and are also active in the peripheral nervous system. There are five well-established biogenic amine neurotransmitters, three of which can be classified as catecholamines:. Dopamine catecholamine , 2. Histamine, and 5. The first step in catecholamine synthesis is catalyzed by tyrosine hydroxylase in a reaction requiring oxygen as a cosubstrate and tetrahydrobiopterin as a cofactor to synthesize dihydroxyphenylalanine DOPA figure 2. Dopamine is produced by the action of DOPA decarboxylase on DOPA. Following its synthesis in the cytoplasm of presynaptic terminals, dopamine is loaded into synaptic vesicles via a vesicular monoamine transporter VMAT. The two major enzymes involved in the catabolism of dopamine are monoamine oxidase MAO and catechol O-methyltransferase COMT. Both neurons and glia contain mitochondrial MAO and cytoplasmic COMT. Once released, dopamine acts exclusively by activating G-protein—coupled receptors. Most dopamine receptor subtypes act by either activating or inhibiting adenylyl cyclase. Activation of these receptors generally contributes to complex behaviors. Norepinephrine also called noradrenaline is used as a neurotransmitter and influences sleep and wakefulness, arousal, attention, and feeding behavior. Perhaps the most prominent noradrenergic neurons are sympathetic ganglion cells, which employ norepinephrine as the major peripheral transmitter in this division of the visceral motor system. Norepinephrine synthesis requires dopamine β-hydroxylase, which catalyzes the production of norepinephrine from dopamine figure 2. Norepinephrine is then loaded into synaptic vesicles via the same VMAT involved in vesicular dopamine transport. NET is a molecular target of amphetamine, which acts as a stimulant by producing a net increase in the release of norepinephrine and dopamine. Like dopamine, norepinephrine is degraded by MAO and COMT. Epinephrine also called adrenaline is found in the brain at lower levels than the other catecholamines and is also present in fewer brain neurons than other catecholamines. Epinephrine-secreting neurons regulate respiration and cardiac function. The enzyme that synthesizes epinephrine, phenylethanolamine-N-methyltransferase figure 2. Otherwise, the metabolism of epinephrine is very similar to that of norepinephrine. Epinephrine is loaded into vesicles via the VMAT. No plasma membrane transporter specific for epinephrine has been identified, although the NET is capable of transporting epinephrine. Binding of norepinephrine or epinephrine causes small changes in the structure of this receptor, which permits the G-protein to bind. This, in turn, causes larger changes in the shape of the α subunit of the G-protein, the first step in a series of reactions that allow the G-protein to regulate intracellular signaling cascades. Histamine is found in neurons in the hypothalamus that send sparse but widespread projections to almost all regions of the brain and spinal cord. The central histamine projections mediate arousal and attention, similar to central ACh and norepinephrine projections. Histamine also controls the reactivity of the vestibular system. Allergic reactions or tissue damage cause release of histamine from mast cells in the bloodstream. The close proximity of mast cells to blood vessels, together with the potent actions of histamine on blood vessels, raises the possibility that histamine may influence brain blood flow. Amino acids are the most abundant neurotransmitters in the brain. Neurotransmitters are synthesized and stored in presynaptic terminals, released from terminals upon stimulation with specific receptors on the postsynaptic cells. Chemical and electrical synapses are specialized biological structures found in the nervous system; they connect neurons together and transmit signals across the neurons. The process of synaptic transmission generates or inhibits electrical impulses in a network of neurons for the processing of information. Glutamate is the primary excitatory neurotransmitter in the brain, while GABA is the principal inhibitory neurotransmitter. The balance of glutamatergic and GABAergic tone is crucial to normal neurologic function. Through synaptic transmission, this information is communicated from the presynaptic cell to the postsynaptic cell. Amino acid neurotransmitters primarily glutamic acid, GABA, aspartic acid, and glycine are single amino acid residues released from presynaptic nerve terminals in response to an action potential and cross the synaptic cleft to bind with specific receptor on the postsynaptic membrane. The integral role of amino acid neurotransmitters is important on the normal functioning of the brain. The presynaptic and postsynaptic events in chemical synapses are subject to use dependent and highly regulated as per the changes in synaptic neurotransmitter release and function. The nervous system is composed of billions of specialized cells called neurons. Neurons are the cells of chemical communication in the brain. In its most basic form, a neuron has two ends although either can have multiple branches : an axon and a dendrite Figure 1. Efficient communication between neuronal cells is a crucial process for the normal functioning of the central and peripheral nervous system. Neurotransmitters are chemical substances that act as the mediator for the transmission of nerve impulses from one neuron to another neuron through synapses. Neurotransmitters are stored in the axon or presynaptic neuron in little packages called synaptic vesicles. The release of neurotransmitter is triggered by the arrival of nerve impulse or action potential. Synapses are specialized junctions through which cells of the nervous system signal to one another and to non-neuronal cells such as muscles or glands. The process by which the information is communicated through synapse is called synaptic transmission [ 1 , 2 ]. Structure of neuron. The neurotransmitters are stored in the vesicles within the presynaptic nerve terminal at the synaptic membrane of one nerve cell and released into the synaptic cleft in response to nerve impulses [ 2 ]. The secreted neurotransmitters can then act on receptors on the membrane of the postsynaptic neuron through a gap called synaptic gap 0. The number of synaptic contacts of an average neuron is approximately 10, Thus, there are 3—5 × 10 15 synapses in the human brain. There are two types of synapses, electrical and chemical synapses, but chemical synapses Figure 2 far outnumber electrical ones. Electric synapses are gap junction. A gap junction is a junction between neurons that allows different molecules and ions to pass freely between cells. The junction connects the cytoplasm of cells. A gap junction is composed of connexons each connexon is composed of six connexin proteins which connect across the intercellular space. Neurons connected by gap junctions sometimes act as though they were equivalent to one large neuron with many output pathways, all of which fire synchronously. Structure of chemical and electrical synapse. Synapses are made on all regions of a receiving nerve cell and can be classified on the basis where they are located. On spiny dendrites of a nerve cell, each spine is the target of an axon terminal and comprises the postsynaptic component of a single synapse. Synapses between axons and dendrites are called axodendritic. Particularly powerful synapses are made between axons of one neuron and cell body of another postsynaptic cell. These are called axosomatic synapses. Synapses between axon terminals and axons of postsynaptic neurons are said to be axo-axonal. Substances that act as neurotransmitters can be categorized into different groups. The three major categories of substances that act as neurotransmitters are: Amino acids: The neurotransmitters of this group are involved in fast synaptic transmission and are inhibitory and excitatory in action primarily glutamic acid, GABA, aspartic acid, and glycine. Amines: Amines are the modified amino acids such as biogenic amines, e. The neurotransmitters of this group involve in slow synaptic transmission and are inhibitory and excitatory in action noradrenaline, adrenaline, dopamine, serotonin, and histamine. Others: The one which do not fit in any of these categories acetyl choline and nitric oxide. Amino acids are among the most abundant of all neurotransmitters present within the central nervous system CNS. Several amino acids have been implicated as neurotransmitters in the CNS, including GABA, glutamic acid, glycine, and aspartic acid [ 3 ]. Some like glutamate are excitatory, whereas others like GABA are primarily inhibitory. Aspartate is closely related to glutamate, and the two amino acids are often are found together at axon terminals. Neurons synthesize glutamate and aspartate and are independent of dietary supply. The amino acid neurotransmitters are common neurotransmitters in the central nervous system. Glycine, glutamate, and GABA are classed under amino acid neurotransmitter. The two amino acids functioning as excitatory neurotransmitter are glutamate and aspartate. GABA acts as a brake to the excitatory neurotransmitters, and thus when it is abnormally low, this can lead to anxiety, and glutamate usually ensures homeostasis with the effects of GABA [ 4 ]. Several related amino acids, like homocysteic acid and N-acetylaspartylglutamate, may also serve a neurotransmitter function. Neurotransmitters can be classified as either excitatory or inhibitory. Excitatory neurotransmitters function to activate the receptors on the postsynaptic membrane and enhance the effects of action potential, while inhibitory neurotransmitter functions in a reverse mechanism. If the electrical impulses transmitted inward toward the cell body are large enough, they will generate an action potential. The action potentials are caused by an exchange of ions across the neuron membrane; a stimulus first causes sodium channels to open, because there are many more sodium ions on the outside, and the inside of the neuron is negative relative to the outside; sodium ions rush into the neuron. Since sodium has a positive charge, the neuron becomes more positive and becomes depolarized. It takes longer for potassium channels to open; when they do open, potassium rushes out of the cell, reversing the depolarization. The action potential is produced by an influx of calcium ions through voltage-dependent calcium-selective ion channels. Calcium ions then trigger a biochemical cascade which results in neurotransmitter vesicles fusing with the presynaptic membrane and releasing their contents to the synaptic cleft. Receptors on the opposite side of the synaptic gap bind neurotransmitter molecules and respond by opening nearby ion channels in the postsynaptic cell membrane, causing ions to rush in or out and changing the local transmembrane potential of the cell. The resulting change in voltage is called a postsynaptic potential. The result is excitatory, in the case of depolarizing currents, or inhibitory in the case of hyperpolarizing currents resulting in EPSP or IPSP, respectively Figure 3. EPSP and IPSP on a neuron. Whether a synapse is excitatory or inhibitory depends on what type s of ion channel conduct the postsynaptic current, which in turn is a function of the type of receptors and neurotransmitter employed at the synapse. Action potential. The resting potential of a neuron tells about what happens when a neuron is at rest. An action potential occurs when a neuron sends information down an axon, away from the cell body. The action potential is an explosion of electrical activity that is created by a depolarizing current. This means that a stimulus causes the resting potential to move toward 0 mv. This is the threshold. If the neuron does not reach this critical threshold level, then no action potential will fire. Also, when the threshold level is reached, an action potential of a fixed sized will always fire for any given neuron; the size of the action potential is always the same. There are no big or small action potentials in one nerve cell. All action potentials are the same in size in a particular neuron type it can differ between different types of neurons. A neuron encodes the intensity of a stimulus in the frequency of firing and not in the size of a single impulse. Neurotransmitters may have inhibitory effects if they help to drive the membrane away from threshold. An excitatory postsynaptic potential EPSP is a summation of signals that brings the membrane closer to the threshold depolarizing effect. An inhibitory postsynaptic potential IPSP drives the membrane away from threshold by a hyperpolarizing effect. An excitatory postsynaptic potential EPSP is a temporary increase in postsynaptic membrane potential within dendrites or cell bodies caused by the flow of sodium ions into the postsynaptic cell. EPSPs are additive. Larger EPSPs result in greater membrane depolarization and thus increase the likelihood that the postsynaptic cell reaches the threshold for firing an action potential. When an active presynaptic cell releases neurotransmitters into the synapse, some of them bind to receptors on the postsynaptic cell. Many of these receptors contain an ion channel capable of passing positively charged ions either into or out of the cell. At excitatory synapses, the ion channel typically allows sodium into the cell, generating an excitatory postsynaptic current. GABA and glycine are inhibitory, both instead of depolarizing the postsynaptic membrane and producing an EPSP; they hyperpolarize the postsynaptic membrane and produce IPSP. IPSP is the change in membrane voltage of a postsynaptic neuron which results from synaptic activation of inhibitory neurotransmitter receptors. The most common inhibitory neurotransmitters in the nervous system are γ-aminobutyric acid GABA and glycine. Amino acid transmitters provide the majority of excitatory and inhibitory neurotransmission in the nervous system. Amino acids used for synaptic transmission are compartmentalized e. Amino acid neurotransmitters are all products of intermediary metabolism with the exception of GABA. Unlike all the other amino acid neurotransmitters, GABA is not used in protein synthesis and is produced by an enzyme glutamic acid decarboxylase; GAD uniquely located in neurons. Antibodies to GAD can be used to identify neurons that release GABA. Glutamate is used at the great majority of fast excitatory synapses in the brain and spinal cord. Glutamatergic neurons are particularly prominent in the cerebral cortex. They project to a variety of subcortical structures like the hippocampus, the basolateral complex of the amygdala, the substantia nigra, the nucleus accumbens, the superior colliculus, the caudate nucleus nucleus ruber , and the pons. At glutamatergic synapses, NMDA receptors NMDARs are localized with other ionotropic glutamate receptors [AMPA receptors AMPARs and kainate receptors] and with metabotropic glutamate receptors. Glutamate receptors are necessary for neuronal development, synaptic plasticity, excitotoxicity, pain perception, and learning and memory [ 5 ]. Among these EPSP-producing glutamate receptors, which could occur as homomeric or heteromeric structures, are classified according to the binding of the most common agonist [ 6 ]. Four subtypes can be distinguished, out of which three are ionotropic receptors and one metabotropic receptor, activated by quisqualate. These are named according to the molecules other than glutamate that they bind and include: NMDA receptors named for N-methyl-D-aspartate. NMDA receptor is very important for controlling developmental synaptic plasticity and learning and memory function. NMDARs have critical roles in excitatory synaptic transmission, plasticity, and excitotoxicity in the CNS Figure 5. The NR1 subunit is evenly expressed in most of the brain, but the NR2 subunit NR2A, NR2B, NR2C, and NR2D shows distinct regional distributions [ 6 , 7 ]. Most of the NMDA receptors function only in heteromeric assemblies, composed of two NR1 and two NR2 subunits. NMDA receptor. Glutamate binds to the S1 and S2 regions of NR2 subunit, whereas glycine binds to the S1 and S2 regions of NR1 subunit. Individual NR1 or NR2 subunits contain an extracellular N terminus which forms S1, an intracellular C terminus, and an extracellular loop between M3 and M4 that constitutes S2. The channel lining domain is formed by a reentrant pore loop called as M2 loop that enters the channel from the cytoplasmic side and forms a narrow constriction at that channel. The function of NMDA receptors is totally dependent upon AMPA receptors. In the absence of AMPA, NMDA is initially expressed, and it forms the silent synapse. The NMDA receptors are not activated unless the postsynaptic region is depolarized by AMPA receptors. Kainate receptors can be activated by kainite and glutamate. These receptors are mainly involved in modulating the release of excitatory amino acids and additional neurotransmitters or neuromodulators. |
| Amino Acid Neurotransmitters | SpringerLink | Japanese Studies. Inhibitory synapses like those utilizing glycine and Oxidative stress assessment tend to Amino acid neurotransmitters localized near nehrotransmitters neuronal soma and are referred to neurotransmittesr Type II Figure Frontiers in Neuroscience. By Abdulbaki Agbas downloads. Trusts Law. Well-known examples of this are elevated plasma concentrations of phenylalanine in phenylketonuria PKU and increased concentrations of homocysteine in homocystinuria [ 71 ]. Adeva-Andany M, Souto-Adeva G, Ameneiros-Rodríguez E, Fernández-Fernández C, Donapetry-García C, Domínguez-Montero A. |
0 thoughts on “Amino acid neurotransmitters”