Energy metabolism and inflammation -
PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Andersson, U. High mobility group 1 protein HMG-1 stimulates proinflammatory cytokine synthesis in human monocytes.
Yang, P. Pyruvate kinase M2 accelerates pro-inflammatory cytokine secretion and cell proliferation induced by lipopolysaccharide in colorectal cancer. Ryu, H. Regulation of M2-type pyruvate kinase mediated by the high-affinity IgE receptors is required for mast cell degranulation.
Bertrand, J. Glutamine enema regulates colonic ubiquitinated proteins but not proteasome activities during TNBS-induced colitis leading to increased mitochondrial activity.
Proteomics 15 , — Tang, Q. Pyruvate kinase M2 regulates apoptosis of intestinal epithelial cells in Crohn's disease. Day, A. Fecal M2-PK in children with Crohn's disease: a preliminary report. Chung-Faye, G.
Fecal M2-pyruvate kinase M2-PK : a novel marker of intestinal inflammation. Bowel Dis. Ho, P. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Boulahbel, H. Prolyl hydroxylases as regulators of cell metabolism. Yang, M.
The emerging role of fumarate as an oncometabolite. Ratcliffe, P. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer.
Walmsley, S. Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity. Cramer, T. HIF-1α is essential for myeloid cell-mediated inflammation. Noman, M. miR and hypoxic microvesicles: two critical components of hypoxia involved in the regulation of killer cells function.
Cancer Lett. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. Salminen, A. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: novel insights into mitochondrial regulation of aging process.
Ichiyama, K. The methylcytosine dioxygenase Tet2 promotes DNA demethylation and activation of cytokine gene expression in T cells. Yue, X. Control of Foxp3 stability through modulation of TET activity.
Li, Q. De Santa, F. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. Peng, M. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science , — Cheng, S. mTOR- and HIF-1α—mediated aerobic glycolysis as metabolic basis for trained immunity.
Science , van Loosdregt, J. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Beier, U. Lim, H. SIRT1 deacetylates RORγt and enhances Th17 cell generation. Zhang, J. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice.
Canto, C. Buttgereit, F. Clocking in: chronobiology in rheumatoid arthritis. Netea, M. Trained immunity: a program of innate immune memory in health and disease.
Science , aaf Weichhart, T. Regulation of innate immune cell function by mTOR. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30 , — Rolf, J. AMPKα1: a glucose sensor that controls CD8 T-cell memory.
Adamson, S. Disabled homolog 2 controls macrophage phenotypic polarization and adipose tissue inflammation. Chou, C. Man, K. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells.
Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Chang, H. A molecular signature of preclinical rheumatoid arthritis triggered by dysregulated PTPN JCI Insight 1 , e Maradit-Kremers, H.
Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. Bessant, R. Risk of coronary heart disease and stroke in a large British cohort of patients with systemic lupus erythematosus. Rheumatology Oxford 43 , — Article CAS Google Scholar.
Ungprasert, P. Risk of coronary artery disease in patients with ankylosing spondylitis: a systematic review and meta-analysis. PubMed PubMed Central Google Scholar. Ali, H. A qualitative systematic review of the prevalence of coronary artery disease in systemic sclerosis.
Risk of coronary artery disease in patients with systemic sclerosis: a systematic review and meta-analysis. Risk of coronary artery disease in patients with idiopathic inflammatory myopathies: a systematic review and meta-analysis of observational studies.
Download references. Department of Rheumatology and Clinical Immunology, Charité University Hospital, Charité University Medicine, Charitéplatz 1, Berlin, , Germany. German Rheumatism Research Centre DRFZ , Charitéplatz 1, Berlin, , Germany.
You can also search for this author in PubMed Google Scholar. Correspondence to Frank Buttgereit. An oxygen-independent metabolic pathway that generates two molecules of pyruvate, ATP and NADH from every one molecule of glucose, supporting the tricarboxylic acid cycle and providing intermediates for the pentose phosphate pathway, glycosylation reactions and the synthesis of biomolecules including serine, glycine, alanine and acetyl-CoA.
Also known as the Krebs cycle A set of connected pathways in the mitochondrial matrix, which metabolize acetyl-CoA derived from glycolysis or fatty acid oxidation, producing NADH and FADH 2 for the electron transport chain and precursors for amino acid and fatty acid synthesis.
A series of proteins in the inner mitochondrial membrane that transfer electrons from one to the other in a series of redox reactions, resulting in the synthesis of ATP and in the movement of protons out of the mitochondrial matrix.
A metabolic pathway that produces ATP from the oxidation of acetyl-CoA and the transfer of electrons to the electron transport chain via NADH and FADH 2. A metabolic process that produces ATP from the oxidation of acetyl-CoA derived from the mobilization of fatty acids.
The metabolic process by which glutamine is metabolized to glutamate and then to α-ketoglutarate to replenish the tricarboxylic acid cycle. Reprints and permissions. Metabolic regulation of inflammation. Nat Rev Rheumatol 13 , — Download citation. Published : 23 March Issue Date : May Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. nature nature reviews rheumatology review articles article.
Subjects Energy metabolism Immunopathogenesis Metabolic pathways Rheumatic diseases. Key Points Immune cells face a variety of variable and sometimes demanding environmental conditions, requiring them to display a dynamic range of metabolic adaptation processes Under inflammatory conditions, stimulated immune cells have an acute need to generate sufficient energy and biomolecules to support growth, proliferation and the production of proinflammatory molecules Metabolic reconfiguration varies between innate and adaptive immune responses, and influences both the effector phase of inflammation and the resolution of inflammation by modulating immune cell fate and function Metabolic enzymes, metabolites and regulators of metabolism have a direct influence on certain inflammatory responses Alterations of metabolic configurations of immune cells can contribute to dysfunctional immune responses, a typical feature of autoimmunity.
Abstract Immune cells constantly patrol the body via the bloodstream and migrate into multiple tissues where they face variable and sometimes demanding environmental conditions. Access through your institution. Buy or subscribe.
Change institution. Learn more. Figure 1: Immune cell metabolism during homeostasis. Figure 2: Metabolic reprogramming of immune cells upon activation. Figure 3: Metabolic configurations of M1 and M2 macrophages.
Figure 4: Immune regulatory roles of glycolytic intermediates. Figure 5: Regulation of inflammation by metabolites. References O'Neill, L. Article CAS PubMed PubMed Central Google Scholar Murray, P. Article CAS PubMed Google Scholar Yin, Y.
Article CAS PubMed PubMed Central Google Scholar Yang, Z. Article CAS PubMed PubMed Central Google Scholar Yilmaz, O. Article CAS PubMed Google Scholar Rooks, M. Article CAS PubMed PubMed Central Google Scholar Castro, C. Article CAS PubMed Google Scholar Yurkovetskiy, L.
Article CAS PubMed PubMed Central Google Scholar Yin, Y. Article CAS PubMed Google Scholar Yang, Z. Article CAS PubMed PubMed Central Google Scholar Morel, L.
in press Jellusova, J. Article CAS PubMed PubMed Central Google Scholar Krawczyk, C. Article CAS PubMed PubMed Central Google Scholar Michalek, R. Article CAS PubMed Google Scholar van der Windt, G. Article PubMed PubMed Central Google Scholar van der Windt, G. Article CAS PubMed Google Scholar Lam, W.
Article CAS PubMed PubMed Central Google Scholar Sukumar, M. Article CAS PubMed PubMed Central Google Scholar Pearce, E. Article CAS PubMed PubMed Central Google Scholar Kotas, M. Article CAS PubMed PubMed Central Google Scholar Warburg, O.
Article CAS PubMed Google Scholar Gaber, T. Article CAS PubMed Google Scholar Fearon, U. Article CAS PubMed Google Scholar Biniecka, M. Article CAS PubMed Google Scholar Rodriguez-Prados, J. Article CAS PubMed Google Scholar Assmann, N. Article PubMed PubMed Central Google Scholar Rodriguez-Espinosa, O.
Article CAS PubMed PubMed Central Google Scholar Donnelly, R. Article CAS PubMed Google Scholar Keating, S. Article CAS PubMed Google Scholar Doughty, C.
Article CAS PubMed PubMed Central Google Scholar Frauwirth, K. Article CAS PubMed Google Scholar Shi, L. Article CAS PubMed PubMed Central Google Scholar Gubser, P.
Article CAS PubMed Google Scholar Macintyre, A. Article CAS PubMed PubMed Central Google Scholar Blagih, J. Article CAS PubMed Google Scholar Klysz, D. Article CAS PubMed Google Scholar Nakaya, M. Article CAS PubMed PubMed Central Google Scholar Sinclair, L.
Article CAS PubMed PubMed Central Google Scholar Geiger, R. Article CAS PubMed PubMed Central Google Scholar Finlay, D. Article CAS PubMed PubMed Central Google Scholar Wang, R. Article CAS PubMed PubMed Central Google Scholar Ananieva, E. Article CAS PubMed PubMed Central Google Scholar Delgoffe, G.
Article CAS PubMed PubMed Central Google Scholar Pollizzi, K. Article CAS PubMed Google Scholar Carr, E. Article CAS PubMed Google Scholar Wang, H. Article CAS PubMed PubMed Central Google Scholar Berod, L.
Article CAS PubMed Google Scholar Jha, A. Article CAS PubMed Google Scholar O'Neill, L. PGH 2 serves as the substrate for numerous enzymes, each resulting in a different PG end product.
There are two known isoforms of COX: the COX-1 isoform is constitutively expressed, whereas COX-2 is thought to be inducible As the name suggests, COX requires two molecules of oxygen to catalyze the oxidation of AA to PGG 2 78 , thus it may seem intuitive that in an oxygen-depleted environment, such as an inflammatory lesion, eicosanoid production would be attenuated.
Contradictory reports exist in the literature. Some groups observe that hypoxia increases COX-2 but not PGE 2 79 , 80 or prostacyclin 81 levels. Others reports indicate that hypoxia paradoxically stimulates production of PGs 82 , likely via induction of COX-2 expression Furthermore, hypoxia has been demonstrated to increase cytosolic phospholipase A 2 activity, liberating more unsaturated fatty acids from lipid membranes to act as substrates for COX or lipoxygenases This disparity between hypoxia-induced changes in PGE 2 production may be due to tissue specificity, extenuating metabolic influences, or even time-dependent sampling.
A recent report examined the difference in PGE 2 levels and COX-2 activity in acute and chronic periodontitis. Their findings indicated that PGE 2 and COX-2 activity increases in acute disease but is suppressed in chronic disease states, which was mechanistically attributed to COX-2 promoter hypermethylation Conversely, the contribution of diminished molecular oxygen in inflamed-hypoxic tissue to eicosanoid production has been poorly characterized.
The affinities of various oxygenases for molecular O 2 have been investigated, but the results appear to inconclusive Much recent attention has been paid to understanding lipid metabolism involved in the resolution of inflammation and in productive immune responses at mucosal sites. Of particular interest are series of COX-derived lipid mediators termed the resolvins and the maresins Resolvins are the best understood of these molecules and are ω-3 PUFA-derived lipid mediators central to activation of the inflammatory resolution program Among them, RvE1 was the first isolated and has been studied in the greatest detail.
RvE1 displays potent stereoselective actions in vivo and in isolated cell systems. At nanomolar levels in vitro, RvE1 potently reduces human PMN transendothelial migration, DC migration, and IL production In several animal models of inflammatory disease, RvE1 and more recently RvE2 89 display potent counter-regulatory actions that protect against leukocyte-mediated tissue injury and excessive proinflammatory gene expression.
The discovery of a novel family of DHA-derived lipid mediators termed maresins macrophage mediator in resolving inflammation [MaR] MaRs were identified from murine peritonitis exudates and human macrophages that biosynthesized a new class of lipoxygenase-derived lipids derived from endogenous DHA.
MaR1s were demonstrated to promote inflammatory resolution with the potency of resolvins, reflected as decreased neutrophil accumulation and increased macrophage phagocytosis during murine peritonitis.
However, brief intermittent exposure to hypoxia ischemic preconditioning [IPC] has been demonstrated to be protective. Adenosine, mentioned earlier, is generated during IPC and capable of eliciting anti-inflammatory effects Sphingosine 1-phosphate S1P is a sphingolipid signaling molecule that mediates cardioprotection during IPC Sphingosine kinases catalyze the conversion of sphingosine to S1P, which binds to S1P G-protein—coupled receptors to mediate cell survival signals.
Both the SK1 94 and SK2 95 isoform of sphingosine kinase are upregulated in hypoxia, resulting in increased S1P. Concomitantly, conversion of sphingosine to S1P prevents its conversion to ceramide, a proapoptotic signaling lipid mediator The dynamic interplay of leukocytes and parenchymal cells during disease defines an elegant lesson in biology.
In particular, studies of model disease systems have allowed for the identification of metabolomic changes now well accepted in the scientific literature. The discovery of differences and similarities between innate and adaptive immune responses will continue to teach us important lessons about the complexity of biological systems.
Such information will provide previously unappreciated insight into the pathogenesis of disease and, importantly, will provide new targets as templates for the development of novel therapies for human disease.
Abbreviations used in this paper: AA arachidonic acid. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content The Journal of Immunology. Advanced Search. User Tools Dropdown. Sign In. Toggle Menu Menu Articles Current Issue Next in The JI Archive Brief Reviews Translating Immunology Pillars of Immunology COVID, SARS, and MERS Articles Monkeypox and Other Poxvirus Articles Annual Meeting Abstracts About the JI About the JI Journal Policies Alerts Authors Submit Instructions for Authors Journal Policies Editors The JI Editorial Board ImmunoHorizons Subscriptions Subscribe Members Librarians New Site FAQ.
Skip Nav Destination Close navigation menu Article navigation. Volume , Issue 8. Metabolic comparisons between innate and adaptive immunity. Energy metabolism in inflammation and immune responses. Transcriptional control of immune metabolism by HIF.
mTOR and innate immunity. Nucleotide metabolism in inflammation and immunity. Methylation-dependent control of metabolism. Lipid metabolism and innate immunity.
Article Navigation. Review Article April 15 Metabolic Shifts in Immunity and Inflammation Douglas J. Kominsky ; Douglas J. Address correspondence and reprint requests to Dr. Kominsky Mucosal Inflammation Program, East 19th Avenue, Mailstop B, Aurora, CO E-mail address: douglas.
kominsky UCDenver. This Site. Google Scholar. Eric L. Campbell ; Eric L. Sean P. Colgan Sean P. Received: December 02 Accepted: February 14 Published: April 15 Online ISSN: Copyright © by The American Association of Immunologists, Inc.
J Immunol 8 : — Article history Received:. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest.
Table I. Type of Immunity. Cells involved PMN, eosinophil Macrophage DC T cell, B cell, NK cell Metabolic trigger s Recruitment Differentiation Local proliferation Recruitment Activation stressor s Migration Phagocytosis Respiratory burst Ag-induced differentiation Metabolic adaptor s HIF, mTOR, Akt HIF, mTOR, Akt Mitochondria Few Many Primary energy source Glycolysis Respiration Methylation dependence Unknown Proliferation Ag-induced differentiation.
View Large. FIGURE 1. View large Download slide. Disclosures The authors have no financial conflicts of interest. AR adenosine receptor. COX cyclooxygenase.
DC dendritic cell. DHA docosahexaenoic acid. HIF hypoxia-inducible factor. IPC ischemic preconditioning. MaR macrophage mediator in resolving inflammation.
mTOR mammalian target of rapamycin. NTPDase NTP diphosphohydrolase. ODD oxygen-dependent degradation domain.
PHD prolyl hydroxylase domain. PMN polymorphonuclear leukocyte. PUFA polyunsaturated fatty acid. S1P sphingosine 1-phosphate. SAH S -adenosylhomocysteine. SAM S -adenosylmethionine. Search ADS. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation.
Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. Homeostatic control of lymphocyte survival: potential origins and implications. Effects of oxygen tension and pH on the respiratory burst of human neutrophils.
Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. HIF-1, O 2 , and the 3 PHDs: how animal cells signal hypoxia to the nucleus.
The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch.
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development.
Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Glucose and fatty acid metabolism are major contributors to cardiac energy metabolism.
The cardiac energy metabolic pathway can be altered in only a few seconds through substrate alterations when shifting from rest to acute stress such as exercise or ischemia, or after glycogen stores have been depleted when fasting.
This regulation is mainly through an increase in plasma insulin level and the activation of the AMP-activated protein kinase AMPK pathway.
The activation of AMPK promotes both FA and glucose oxidation which increases cardiac energy. Moreover, AMPK inhibits ATP-consuming processes like protein synthesis The metabolic remodeling in the failing heart is similar to the alterations from the non-ischemic to the ischemic condition, as mentioned above, and may well be a protective compensatory mechanism to use more of its capacity.
However, long term sustained high energy loading would cause some toxic substances to accumulate, which in turn may contribute to the progress of HF and comorbidities. In most cases, FAO decreases and glycolysis increases rapidly in HF, except for advanced and diabetic HF where FAO increases 18 , 21 , 22 , this is because mitochondrial dysfunction in HF causes decreased expression and activity of enzymes associated with mitochondrial FAO Several key enzymes of FAO are regulated by transcript factor peroxisome proliferator-activated receptors PPARs.
The decrease of FAO could be mainly explained by the activation of PPARγ and reduced activity of PPARα Insulin plays an important role in substrate shift progression.
Therefore, in cases of insulin resistance, such as diabetic HF or advanced HF, FAO is increased by activating PPARα signaling Myocardial uptake of FA usually increases in HF.
The imbalance of increased FA uptake and impaired utilization of FAs in HF results in FA accumulation. Accumulated FAs cause lipotoxicity and worsen HF by promoting mitochondrial dysfunction and apoptosis, and contributes to the development of insulin resistance Targeting the FAO pathway is an emerging treatment for HF 24 , but the significance of the shift from FAO to glucose metabolism remains controversial and there have two opposite therapeutic strategies: inhibit or facilitate FA utilization.
The two therapeutic strategies are not contradictory because they both reduce the cardiac accumulation of FAs, one is by reducing the uptake of FAs and the other is by increasing the catabolism of FAs.
Drugs targeted inhibition of FAO may be classified into 3 categories: 1 β-oxidation inhibition, such as malonyl-CoA decarboxylase inhibitors, 2 mitochondrial FA uptake inhibition, such as the carnitine palmitoyl transferase 1 inhibitor CPTI , 3 plasma membrane FA uptake reduction by inhibiting related proteins, such as in the case of CD36 the major FA transporter or fatty acid-binding protein FABP.
CD36 inhibitor is still under preclinical investigations. However, considering that glucose provides less capacity for energy production than FAs one FA molecule produces — ATP, while one glucose molecule produces 30—32 ATP 22 , there is an opposite opinion, which asserts that the heart reverting back to using FA may have therapeutic value for HF, such as by targeting GLUT4 to inhibit glycolysis or activate the AMPK pathway by phosphorylation to increase FAO.
Studies have confirmed that reverting to the use of FA has a cardio protective effect 22 , Restoration of FAO could improve heart function, possibly via reduced cardiac lipotoxicity Mitochondria are a physiological source of reactive oxygen species ROS. The deficit in energy would cause the uncoupling of oxidative phosphorylation, and cause an increase in reactive oxygen species ROS and oxidative stress ROS, in return, inactivates several enzymes of the TCA cycle In addition, liver energy metabolism also participates in the process of HF.
Ketone bodies synthesized in liver mitochondria, especially β-hydroxybutyrate, the so-called super fuel, are more efficient than FAs or glucose. The failing heart adaptively consumes more ketone bodies 28 and this is believed to be beneficial 23 , More glutamine is consumed in HF because it is the most abundant secreted amino acid 28 , but branched-chain amino acids BCAAs played a more important role in HF.
In healthy individuals, BCAAs are essential nutrition for mitochondrial biogenesis, and dietary supplementation of BCAAs has cardio protective effects 30 — However, BCAA catabolic metabolism is impaired in HF, leading to the accumulation of BCAAs and branched-chain alpha-keto acids BCKAs The accumulated BCAAs and their catabolic intermediates have a cardiotoxic effect.
BCAA accumulation could result in insulin resistance by activating the mTOR pathway 34 , 35 , and accumulated BCKAs would increase reactive oxygen species ROS Furthermore, BCAA is reported to be a potential therapeutic target for HF but may have important indirect regulatory roles in energy metabolism as they affect mitochondrial biogenesis and BCAA toxicity affects energy metabolism.
Sodium-glucose transporter-2 SGLT-2 is a recently discovered diuretic agent that could improve the outcome of HF Metabolic remodeling is a major pathophysiologic character of HF, but whether it is the cause or result of the HF, and whether it is maladaptive or adaptive is still controversial Why have drugs both targeting inhibition and promotion of metabolic remodeling been used for the treatment of heart failure, and are both able to alleviate HF symptoms?
FA or glucose, which is the superior energy substrate? We think that metabolic remodeling has a double effect: On one hand, metabolic remodeling is thought to be an adaptive compensatory mechanism. First, the shift toward glucose metabolism improves myocardial contractile efficiency by increasing the stoichiometric ratio of ATP production to oxygen consumption and reducing oxygen waste Although glucose has a lower energy capacity, the shift is not due to a lack of substrate availability because the coronary circulation is able to provide an excess of substrates 47 , and glycolysis produces ATP much faster than other ways, as epitomized by the Warburg effect Second, similar metabolic remodeling can also be seen in the physiological remodeling of the heart.
Many pathways, such as the activation of the AMPK and PI3K pathways, which have protective roles, are active in both physiological and pathological cardiac remodeling On the other hand, metabolic remodeling is harmful when toxic substances such as accumulated excess intracellular FAs and ROS are increased, which may worsen HF and cause comorbidities.
Recent evidence suggests that the accumulation of toxic intermediates, rather than alterations of substrate utilization or ATP deficit per-second , is responsible for cardiac dysfunction HF is usually accompanied by highly elevated circulating pro-inflammatory cytokines, such as IL-1β, IL-6, IL-8, TNF-α, NF-κb, etc.
However, the role of inflammation in HF has long been controversial. Because most traditional anti-inflammatory drugs failed in clinical HF therapies, inflammation was considered to not be a cause, but a complication of HF.
The importance of inflammation in HF was not widely accepted until the success of canakinumab, an IL-1β inhibitor, which significantly improved the prognosis of HF.
The effect of it and other anti-cytokine drugs indicates the role of inflammation in HF Moreover, Soluble suppression of tumorigenesis-2 sST2 and galectin-3 are inflammatory biomarkers associated with fibrosis in HF, which have reportedly even better prognoses than NT-pro-BNP, an HF biomarker not directly associated with inflammation 50 , Having established the causal role of inflammation in HF, in the following, we give an overview of inflammation in cardiac remodeling and various comorbidities.
Both the innate and adaptive immune systems have a pro-inflammation role in HF. The immune response triggered inflammation mechanism is called immune inflammation.
Innate immune cells, such as neutrophils, natural killer cells, and mast cells 52 , have been revealed to participate in the progress of HF through immune inflammation. For instance, monocytes derived from HF patients have higher secreted cytokines IL-1β, IL-6 and chemokines CCL3, CCL4 , and can stimulate T cell activation Monocyte-derived macrophages have a pro-inflammation role in cardiovascular diseases In addition, several pattern recognition receptors PRRs , such as NOD-like receptors NLRs and Toll-like receptors TLRs , are mainly expressed on tissue-resident immune cells, can turn on multiple signals to trigger innate immune inflammation.
Finally, the activation of the innate immune system can cause the activation of the adaptive immune system by activation and infiltration of B cells and T cells The major cardiac structural remodeling of HF including cardiac hypertrophy, fibrosis, and extracellular matrix ECM remodeling.
Systemic inflammation can drive cardiac hypertrophy and fibrosis, and the inflammation is mainly triggered by PRRs such as NLRP3 and TLR4 mediated innate immune response.
The key inflammatory factors in this process are IL-1β, IL-6, and NF-κB. They can stimulate the release of many other inflammatory cytokines and transcription factors, which may promote cardiac hypertrophy and fibrosis 55 , The Nod1 receptor signaling pathway can contribute to cardiac hypertrophy The mechanisms of the inflammatory cascade are yet not fully clear.
However, it is known that IL-1β is activated by NLRP3 receptive inflammasomes. IL-1β and IL-6 stimulate immune cells T cells, macrophages, and monocytes to increase the release of IL, TNFα, and IFN-γ These cytokines are signals which may activate immune cell trans differentiation into pro-inflammatory and pro-fibrotic subsets.
IL is a member of the IL-1 family and ST2 is the receptor of IL Additionally, micro vascular inflammation can stimulate monocyte-derived macrophages to secrete transforming growth factorβ TGF-β which induces pro-fibrosis effects by stimulates the differentiation of fibroblasts into myofibroblasts.
Myofibroblasts deposit collagen, and its increase may cause fibrosis Moreover, inflammation may cause pyroptosis and apoptosis, which may also promote cardiac fibrosis The immune-inflammation mechanism may mediate cardiac ECM remodeling by increases ventricular stiffness 62 , Ventricular stiffness is a common pathological feature of HFpEF which promotes diastolic dysfunction In systematic inflammation, IL-1β and other cytokines cause increased extracellular deposition of collagen and reduced elasticity of titin, resulting in ventricular stiffness Comorbidities and HF interact as both cause and effect, in which metabolism and inflammation are the possible common mechanisms underlying this cyclical relationship.
In the following, some common comorbidities, including atrial fibrillation, diabetes, COPD, and obesity, are discussed from the standpoint of epidemiological evidence showing the reciprocal causation associated with underlying common metabolic and inflammatory mechanisms.
Atrial fibrillation AF frequently coexists with HFpEF and they share similar risk factors 65 , In a recent study, more than one-third of the AF patients had HF, and more than half of the HF patients had AF Even subclinical AF was associated with about a 4-fold increase in HF risk On the other hand, HF promotes AF via cardiac fibrosis, inflammation, and oxidative stress 69 , Cardiac resynchronization therapy with a defibrillator can reverse HF remodeling Metabolism and inflammation are the most consequential underlying mechanisms common to the two diseases.
Cardiac energy alterations in HF cause subsequent oxidative stress and inflammatory cascades, and contribute to AF. T1DM 77 is also associated with an increased risk of developing HF.
HF caused by coronary artery disease and hypertension secondary to T2DM is more common in HFrEF Diabetic cardiomyopathy, which refers to HF occurring in the absence of related cardiovascular diseases, is generally believed to be mediated by abnormal mitochondrial calcium handling HFpEF is also associated with insulin resistance-induced ventricular remodeling and mitochondrial dysfunction 79 , Chronic inflammation caused by excess insulin has also been found to be responsible for diabetic HFpEF Moreover, the byproduct of glycolysis has recently been reported to link diabetes and HF by post-translational modifications 82 , The molecular mechanisms underlying diabetic HF are associated with changes in myocardial substrate metabolism, inflammation, endoplasmic reticulum stress, aberrant insulin signaling, and autophagy For one thing, hyperglycemia and insulin resistance cause excessive ROS production.
Furthermore, oxidative stress causes chronic inflammation and mitochondrial metabolic disorders. Several molecular pathways are involved in these processes.
ROS activates poly ADP-ribose polymerase PARP and inhibits the AMPK pathway and decreases mitochondrial biogenesis. These changes would cause disturbed circadian clock synchronization of glucose and FA metabolism.
COPD is associated with increased risk 89 and worse prognosis of HF 90 — COPD may induce HF through chronic systemic inflammation and pulmonary vascular remodeling In turn, HF aggravates excess ventilation in COPD, and causes dyspnea, and exercise intolerance Obesity increases the risk of HF However, the mortality risk from HF increased for patients with extremely high BMIs of 45 or greater Furthermore, high waist-to-hip ratios have been associated with increased mortality, suggesting the harmfulness of obesity in HF Abdominal obesity is associated with significantly higher mortality in HFpEF, which may be a better predictor than BMI Abdominal obesity is strongly associated with the circulating level of aldosterone, the main role of which is to regulate salt-water retention.
Mineralocorticoid receptor antagonists have recently been discovered as targets for obesity-associated HF Metabolism and inflammation are involved in the progress of HF in patients with obesity , Increased leptin, which is reported as the product of the obesity gene, contributes to cardiac remodeling through Leptin-Aldosterone-Neprilysin Axis , Insulin resistance secondary to obesity can cause altered cardiac energy metabolism and HF , Obesity can cause immune-inflammation by activating macrophages, and activate IL-1β and NF-κB pathway Obesity can suppress BNP levels in HF and causing lower plasma NT-pro-BNP levels 5.
Therefore, BNP may not reflect the HF severity accurately in obese patients , BNP enacts cardiac protection via multiple actions, such as suppressing RAAS activation and regulating sodium metabolism. An insufficient BNP level may promote HF progression Cancers and HF are often coexisting in patients with cancers, they share several common pathophysiological mechanisms and causes, such as angiogenesis, clonal haematopoiesis, and sarcopenia — Aging may cause somatic mutations of genes typically DNMT3A and TET2 in hematopoietic stem cells, which promote peripheral blood leukocytes release proinflammatory factors such as IL-1β and IL This phenomenon is called clonal hematopoiesis of indeterminate potential CHIP , which is a risk factor of cardiovascular diseases and cancer Sarcopenia is a common complication in advanced stage cancer, which may promote HF through muscle wasting and thinning of the ventricular wall, Cardio toxicity is a major risk factor for HF.
Mitochondrial dysfunction is the major pathophysiologic mechanism of drug-induced cardio toxicity , In most times a drug with cardio toxicity would not be used in clinical. However, anti-tumor drugs with cardio toxicity are common when weighing the pros and cons because of the therapeutic effect For instance, aromatase inhibitors have become the preferred treatment for estrogen receptor-positive breast cancer, which targets the cytochrome P enzyme, but it is associated with a significantly increased risk of HF Anthracyclines such as doxorubicin and epirubicin are commonly used for breast cancers, lymphoma , and a variety of other cancers, but their usage is limited by cardio toxicity.
Trastuzumab, another breast cancer drug, is also associated with increased HF risk The proposed biological mechanisms underlying anthracycline cardio toxicity are mitochondrial dysfunction, mitochondrial iron overload, oxidative stress, inflammation, and impaired autophagy Common risk factors such as aging, hyperglycemia, and lifestyle are the cause of HF and comorbidities.
The underlying mechanisms of these factors are associated with common metabolic or inflammatory pathways.
In this review, the major pathways were identified through gene enrichment analysis. Further, the common therapy drug targets have also be summarized by analyzing the disease-gene network. This review will be helpful for selecting the therapeutic strategy. Epidemiologic evidence has found many risk factors for cardiovascular diseases, including chronic conditions or diseases aging, hyperlipidemia, hypertension, hypoxaemia, and metabolic syndrome , and lifestyles dietary and sleeping patterns, smoking, and drinking — Unhealthy lifestyles may contribute to HF by dysregulated innate immunity and chronic inflammation These factors are also risk factors for many comorbidities and share similar mechanisms, which are associated with metabolism and inflammation.
Aging is one of the major risk factors for developing multi morbidity and HFpEF, and both multi morbidity and HFpEF are unmet needs in the therapy of HF — The main underlying mechanisms of cardiovascular aging are associated with mitochondrial metabolism , , chronic inflammation , autophagy , and oxidative stress Physical inactivity sedentary behavior , is a risk factor of multi morbidity , it causes chronic subclinical myocardial injury detectable with high-sensitivity cardiac troponin and increases HF risk Meta-analysis showed exercise is beneficial for people with multi morbidity It can regulate mitochondrial remodeling , and also causes physiologic remodeling which increases cardiorespiratory fitness It is improved cardiorespiratory fitness that is the physiopathological link between obesity, exercise, and HF 93 , 94 , primarily by increases the cardiac compensatory capacity Furthermore, exercise has direct anti-inflammatory effects by inhibition of TNF-α and IL-1β, and may attenuate insulin resistance Metabolic syndrome, mainly charactered by hyperlipidemia and hypertension, shared similar mechanisms to that of diabetes and obesity, such as insulin resistance and macrophage induced inflammation, which have already been discussed Taken together, metabolism and chronic inflammation are the major mechanisms underlying the major shared risk factors between HF and comorbidities.
Although many metabolism and inflammation mechanisms have been reviewed previously, which pathways are most important remains unclear. To conduct an unbiased analysis of the key shared biological pathways in HF and comorbidities, we performed enrichment analysis on target genes of HF and some comorbidities of high prevalence in the database.
First, we retrieved all the targets of HF and several comorbidities diabetes mellitus, obesity, COPD, chronic kidney disease, and OSA in the Target Validation platform accessed on March 22, and intersected the disease targets as shown in the Venn diagram Figure 3A.
Five comorbidities diabetes mellitus, obesity, COPD, CKD, and obstructive sleep apnea were selected for analysis because these represent the most highly prevalent comorbidities The major enriched pathways did not change but the Venn diagram and the latter network plot would be more complex and less understandable when adding other common comorbidities such as atrial fibrillation and depression into the analysis.
There were common targets associated with all the four diseases, and 1, common targets were shared by HF and at least four of the comorbidities.
Gene Ontology and KEGG enrichment analysis was performed on 1, semi-common targets with the R version 3. The activation of metabolic and inflammatory pathways may require the expression level change or activation of a group of enzymes, cytokines, or proteins regulated by common transcription factors.
org with TRRUST Transcriptional Regulatory Relationships Unraveled by Sentence-based Text mining database and the figure was plotted with ggplot2 version 3. Figure 3. Analysis of common genes and pathways in comorbidities and HF.
A : Venn diagram of common genes between comorbidities and HF; B : Dot plot of top 20 enriched Gene Ontology biological processes enriched for common genes between HF and comorbidities; C : Dot plot of top 20 enriched KEGG Kyoto Encyclopedia of Genes and Genomes pathways for common genes between HF and comorbidities; D :Dot plot of top 20 enriched transcription factors from TRRUST Transcriptional Regulatory Relationships Unraveled by Sentence-based Text mining database.
Some known factors which play a crucial role in HF, such as the NADPH oxidase , and sulfur compound binding , and growth factor activity were enriched in the Geno ontology enrich analysis Figure 3B.
The enriched pathways are mainly associated with metabolism and inflammation. Some significantly enriched pathways not shown in the figure are also analyzed. According to their role in HF, most of the significantly enriched pathways can be classified into one or more of the following categories: 1 Energy metabolic associated pathways.
The MAPK pathway is the key pathway activated in response to ischemia and has a critical role in cardiac hypertrophy. Moreover, the MAPK pathway may be involved in the interplay of mitochondrial energy metabolism and systemic inflammation The Hypoxia-Inducible Factor 1 HIF-1 pathway can regulate glucose metabolism and is adaptively activated in response to hypoxia conditions and can promote cardiac hypertrophy.
HIF-1 can activate the glycation end products AGE advanced glycation end products RAGE signaling. The AGE-RAGE signaling pathway is associated with some comorbidities of HF such as OSA and diabetes , Insulin-like growth factor IGF signaling is activated in HF and promotes cardiac hypertrophy , ; 3 Cardiac systolic and diastolic functions associated pathways.
Such as the CaMKII pathway , The G protein-coupled receptor GPCR signaling pathway is a known drug target of HF, these drugs include β-adrenergic receptor and angiotensin II receptor antagonists Additionally, the clonal hematopoiesis pathway is a risk factor of HF enriched in the analysis and may be related to immune inflammation 53 , The activation of inflammatory pathways can activate the TGF-β pathway and promote fibrosis.
Alzheimer's disease is also enriched. Alzheimer's disease is major characterized by amyloidosis, and senile amyloidosis may be an overlooked causal mechanism of HFpEF 60 , Cardiac remodeling is a key biological process that contributes to the progression of HF.
Some drugs, such as calcium antagonists and renin inhibitors, may alleviate hypertension and improve contraction function of HF, they did not improve remodeling, and therefore did not improve the prognosis of HF Together with a review of the literature into account, the main shared mechanisms of HF-induced comorbidities can be summarized Figure 4 and elucidated.
The mechanism of how comorbidities promote HF may be clarified similarly by the shared mechanisms. Figure 4. Main possible shared mechanisms underlying HF and comorbidities.
HF, comorbidities, and risk factors may have some shared chronic conditions such as insulin resistance, hypoxia, and chronic inflammation, these conditions aggravate HF and cause comorbidities. The mechanism of comorbidities contribute to HF can be clarified similarly.
Cardiac energy metabolic remodeling may take place in these conditions mediated by activating the AMPK signaling. Ischemia and hypoxia conditions can activate MAPK and HIF-1 pathways, which contribute to cardiac structure remodeling.
Innate immune cells, mainly monocytes, macrophages, and neutrophils, can trigger the immune response and systemic inflammation by secreting IL-1β and IL Moreover, the pro-inflammatory cytokines stimulate T cells to polarize to Th17 cells and release IL Systemic inflammation can cause diastolic dysfunction and cardiac hypertrophy.
The metabolic mechanisms of HF promote comorbidities are associated with mitochondria injury, oxidative stress, insulin resistance, and hypoxia. HF and risk factors induce altered cardiac energy metabolism.
Cardiac energy metabolic remodeling causes oxidative stress through NAD P H oxidase-derived ROS Oxidative stress can trigger mitochondrial injury and inflammation. As such, antioxidants have been a therapeutic strategy for cardiovascular diseases Oxidative stress, mitochondrial dysfunction, and chronic inflammation were the major mechanisms of multi morbidity in the elderly There is a consensus that mitochondrial impairment is key to cardiac dysfunction in HF Mitochondria injury can cause cardiac remodeling, such as hypertrophy and fibrosis In addition, mitochondrial biogenesis dysfunction play important roles in multi morbidity such as diabetes , obesity , lung diseases , , depression , sarcopenia , iron deficiency , , fatty liver disease , obstructive sleep apnea , and diabetic kidney disease Mitochondria autophagy, also called mitophagy, is a cellular process in which impaired mitochondria are destroyed to protect eukaryotic cells from mitochondrial injury.
Autophagy has a protective role for HF and comorbidities, and may be injured by the activation of mTOR pathway Insulin resistance plays an important role in the pathological processes of HF, and is also strongly associated with diabetes , as well as obesity in which is associated with the phosphorylation of PPARγ Insulin resistance was associated with the worse outcomes in patients with HF and diabetes Hypoxia is a common chronic condition in many comorbidities such as COPD and anemia, and the related HIF-1 pathway may have an important role in the progression of obesity and hypertension Chronic systemic inflammation associated with HF is mainly triggered by innate immune cells monocyte, macrophage, and neutrophils.
The major pro-inflammatory cytokines including IL-1β, IL-6, IL-8, IL, IL, and TNA-α 49 , , Apart from their role in HF, IL-1β and IL-6 are key pro-inflammatory factors in many diseases, like COPD , diabetes , kidney disease , sarcopenia, obesity, and HF , and the cytokine storm in COVID A recent study on HFpEF supported that systemic inflammation may be the association between comorbidity and HF The IL-1β and IL signaling pathways may be novel drug targets for HFpEF, which are important in the mitochondria-inflammation circuit The alteration of pathways is often regulated by transcription factors as switches.
Many common transcription factors have been found including SP-1, RELA, NF-κB, STAT3, HIF-1α, PPARγ, c-FOS, and c-JUN Figure 3D and together with a review of the literature, the transcription factors network in HF and comorbidities are briefly summarized as follows: 1 Regulation of inflammation.
NF-κB is the key transcription factor in inflammation. Both RELA and NFKB1 are genes of NF-κB subunit. STAT3 is a predicted target regulated by NF-κB in Figure 3D. The activation of NF-κB and STAT3 is required for the expression of multiple inflammatory cytokines including IL-1β , TNA-α and IL The c-FOS and c-JUN are family of AP-1, which regulate the MAPK pathway, and can be inhibited by SIRT3 EGR1 and c-FOS are also associated with the release of IL-1β SP-1 can regulate immune responses, but it is a non-specific transcription factor involved in many other cellular processes and indicates transcriptional activation; 2 Regulation of metabolism.
The activation of PPARγ is essential for the FAO process The sirtuin family members SIRT1, SIRT2, and SIRT3 are important transcription factors in cardiac energy metabolism and have similar roles. SIRT3 regulates ATP production SIRT2 and PPARα regulate glycose metabolism by the AMPK pathway , SIRT1 and NRF2 regulate energy metabolism and mitochondrial biogenesis Common pathways indicate common targets, which are the basis for drug repurposing.
Network analysis is often used in the repurposing of drugs The known drug targets of HF, diabetes mellitus, COPD, CKD, sleep apnea, and obesity were retrieved from the Target Validation Platform targetvalidation.
We constructed a disease-target network in Cytoscape 3. Some representative drugs were randomly chosen and listed in Figure 5 to provide an example. The drugs range from old drugs like metformin to relatively new ones in HF treatment, like SGLT2 inhibitors.
However, network analysis has some limitations and should be interpreted combined with literature review. For one thing, it is based on the database, and some drugs in the database had been investigated in HF clinical trials but have no effect.
Some drugs such as calcium channel blockers could not treat HF. For another, a drug associated with multiple targets might be non-specific and does not necessarily have better effects. For instance, doxorubicin inhibits both Top2a and Top2b, inhibiting Top2a have an anti-cancer effect while inhibiting Top2b have a cardiac side effect Anti-inflammatory therapy with Canakinumab in clinical trials which target IL-1β can reduce the mortality of HF patients.
IL-1β is an important inflammatory cytokine associated with many comorbidities. Canakinumab can improve the prognosis of cardiovascular outcomes in patients with CKD However, Canakinumab could not reduce the incidence of new-onset diabetes , which suggests the role of inflammation in diabetes might be less important.
Anakinra, a recombinant IL-1 receptor antagonist, is another drug targets IL-1β, it is under phase III clinical trial in HF and has a therapeutic effect Figure 5. Abridged common drug target network of heart failure and comorbidities.
Diabetes drugs are a good example of drug repurposing applied in HF. Some therapy of diabetes may increase the risk of HF such as insulin , whereas some drugs such as metformin, sulphonylureas, and gliptins either alone or in combination, could significantly reduce the risk of HF The SGLT2 inhibitors are originally designed for diabetes, which targets the SLC5A2 gene, and have shown benefit for HF, regardless of whether comorbid with diabetes or not , In a clinical trial, there were unexpected excellent risk reductions in hospitalization for HF and all-cause mortality with the use of the SGLT2 inhibitor, empagliflozin The benefit of empagliflozin could not be explained by the effects of classical inhibitors, such as natriuresis or neurohormonal mechanisms.
It has been speculated that the shift in cardiac energy substrate may play a major role in the cardiorenal benefits of empagliflozin; that is, a shift from using glucose and fat to ketone bodies Linagliptin, a DPP-4 inhibitor designed to treat diabetes, can also be used to treat HF , Metformin affects many targets that are associated with oxidative phosphorylation in mitochondria , such as MT-ND5 and NDUFB7, and has been reported to have therapeutic effects on HF and comorbidities.
Metformin is an indirect AMPK pathway activator, and also increases glucose transport and catabolism by increasing the residence time of GLUT4. Although many anti-tumor drugs have cardio toxicity, network analysis of shared pathways and targets enables us to find drugs beneficial for both diseases.
There are some genes of the phosphodiesterase family, such as PDE5A, PDE3A, and PDE3B. PDE5 inhibitors such as Sildenafil regulates the nitric oxide synthases and hydrogen sulfide H2S generation, and may attenuate ROS induced mitochondrial dysfunction through the AMPK pathway However, side effects largely limit its clinical application, probably because PDE5 is involved in a variety of biological processes not specific to HF.
Beyond known drug targets, some targets may have similar functions as they belong to the same protein family. Multi morbidity and HFpEF are both unmet needs in HF therapy. Comorbidities exist in both HFpEF and HFrEF, but the prevalence of most comorbidities is higher in the HFpEF than reduced ejection fraction HFrEF 6 , , indicating a strong association between HFpEF and comorbidities 6.
The prevalence of preserved ejection fraction HF HFpEF is rising, and mortality remains high because of the absence of effective therapies 60 , , which gives rise to the urgent need for drug discovery targeting HFpEF.
Although HFpEF has a better ejection fraction than HFrEF, the mortalities are similar, and the higher frequency of morbidities in HFpEF than HFrEF may explain the phenomena The risk factors and incidence of comorbidities are different, therefore the pathways, therapeutic targets, and drugs between the subclasses of HF were different.
COPD and OSA are associated with increased HFpEF disease risk and adversely impact cardiovascular disease outcomes, in which chronic inflammation and oxidative stress are responsible for the association.
Compared with HFrEF, there are more hypertension and fewer coronary diseases in HFpEF , Atrial fibrillation is associated with significantly increased mortality , and AF is more frequently in HFpEF than HFrEF Because multi morbidity is more frequent in HFpEF, targeting the common pathways between comorbidities may be a potential novel therapy for HFpEF.
Expression levels of biomarkers is also different between systolic and diastolic HF. In this review, we concluded the pathology and molecular mechanisms of comorbidities of HF.
Metabolism remodeling and chronic inflammation are responsible for the major underlying pathophysiologic links between HF and comorbidities. Mitochondrial metabolism is expected to play a central role, but no drugs specifically conceived to modulate mitochondrial functions are currently available The therapy for comorbidities of HF is increasingly becoming challenging.
The common metabolic and inflammatory mechanisms may provide promising possible therapeutic targets for both HF and comorbidities, which may be useful for both old drug repurposing and the discovery of new drugs.
ZL performed an extensive literature review, drafted the manuscript, and prepared figures. JW and HZ proposed the subject of the review, critically revised, and edited the manuscript.
All authors contributed to the article and approved the submitted version. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bloom MW, Greenberg B, Jaarsma T, Januzzi JL, Lam CSP, Maggioni AP, et al. Heart failure with reduced ejection fraction. Nat Rev Dis Primers. doi: CrossRef Full Text Google Scholar. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals.
PubMed Abstract CrossRef Full Text Google Scholar. Hao G, Wang X, Chen Z, Zhang L, Zhang Y, Wei B, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China hypertension survey, Eur J Heart Failure.
Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. Nadrowski P, Chudek J, Grodzicki T, Mossakowska M, Skrzypek M, Wiecek A, et al. Plasma level of N-terminal pro brain natriuretic peptide NT-proBNP in elderly population in poland—the polsenior study.
Ye JKeller Metaabolism. Regulation of energy metabolism by inflammation: A feedback Energy metabolism and inflammation in obesity and calorie restriction. Energt Albany NY. Copyright: © Ye et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Caloric restriction CRin the absence of malnutrition, delays aging and prevents aging-related diseases through multiple mechanisms.Energy metabolism and inflammation -
Inflammation regulates energy metabolism in both physiological and pathological conditions. Pro-inflammatory cytokines involves in energy regulation in several conditions, such as obesity, aging calorie restriction , sports exercise , and cancer cachexia.
Here, we introduce a view of integrative physiology to understand pro-inflammatory cytokines in the control of energy expenditure.
In obesity, chronic inflammation is derived from energy surplus that induces adipose tissue expansion and adipose tissue hypoxia. In addition to the detrimental effect on insulin sensitivity, pro-inflammatory cytokines also stimulate energy expenditure and facilitate adipose tissue remodeling.
FFAs are normally oxidized in mitochondria for ATP production. An increase in FFA supply may lead to acceleration of energy expenditure. However, when FFA supply overrides the consumption, they deposit in non-adipocytes in the form of ectopic fat deposition. The ectopic fat contributes to pathogenesis of fatty liver disease and atherosclosis deposit on the blood vessel wall.
In the physiological conditions, IL-6 secreted by contracting muscle is involved in coordination of fuel mobilization between adipose tissue and skeletal muscle during exercise [ 43 - 44 ]. In CR, the fatty acid supply is limited as a result of reduced calorie intake, the risk for ectopic fat deposition will be reduced.
This may help in prevention of fatty liver and atherosclosis. d Energy intake. Inflammatory cytokines are involved in the regulation of energy intake and expenditure. IL-1 and IL-6 reduces food intake and prevent hyperphagia [ 45 - 46 ]. Cytokines IL-1, IL-6 and TNF-α also induce energy expenditure [ 46 - 50 ].
These activities of cytokines are dependent on their actions in the central nervous system [ 46 - 47 , 51 - 52 ]. Therefore, inflammatory cytokines may serve as an anti-obesity signal by modifying both energy intake and energy expenditure. Additionally, these data indicate that the inflammatory cytokines may serve as a link between peripheral tissues and central nervous system in the control of energy balance.
The activities of inflammatory cytokines on adipocytes and neurons suggest that inflammation may inhibit energy accumulation.
They induce energy expenditure and inhibits food intake. These possibilities are strongly supported by phenotypes of transgenic mice with chronic inflammation and by cytokine infusion studies.
The pathway has been under active investigation in the obesity field after IKKβ was found to induce insulin resistance in obese mice [ 5 ].
The serine kinase IKK has three major isoforms including IKKα IKK1 , IKKβ IKK2 and IKKγ, in which IKKβ is required for NF-kB activation [ 53 ]. In obesity, IKKβ is activated by several intracellular signals, such as ROS, ER stress, DAG, and Ceramide.
IKKβ is also activated by the extracellular stimuli including TNF-α, IL-1, and fatty acids [ 8 ], and hypoxia [ 54 ]. IKKβ induces NF-kB activation by phosphorylation of the Inhibitor Kappa B alpha IkBα [ 55 ]. NF-kB nuclear factor kappa B is a ubiquitous transcription factor that is formed by two subunits of Rel family, which include seven members, p65 RelA , p50 NF-kB1 , c-Rel, RelB, p, p, p52 [ 56 ].
These members form a homodimer or heterodimer in the regulation of gene transcription. In most case, NF-kB is a heterodimer of p65 and p P65 contains the transactivation domain and mediates the transcriptional activity of NF-kB.
P50 usually inhibits the transcriptional activity of p65 [ 57 ], and the inhibition disappears in the NF-kB p50 knockout mice [ 58 ]. In the classical pathway, NF-kB activation is mediated by IKKβ-induced phosphorylation, proteasome-mediated degradation of IkBα [ 53 ]. In response to stress responses, NF-kB promotes lipid mobilization through suppression of PPARγ activity in the nucleus [ 59 ].
It also induces transcription of inflammatory cytokines TNF-α, IL-1, IL-6, MCP-1, et al. In the alternative pathway, NF-kB is activated by hypoxia in the absence of IkBα degradation. This type of NF-kB activation in adipocytes and macrophages contributes to chronic inflammation in the adipose tissue of obese individuals [ 16 ].
NF-kB activity may promote energy expenditure. This activity of NF-kB is supported by documents on energy expenditure in cachexia [ 60 - 61 ] and infection.
However, the role of NF-kB in energy expenditure was not tested in transgenic models. To this point, we investigated energy metabolism in transgenic mice with elevated NF-kB activities.
The transcriptional activity of NF-kB is enhanced either by over-expression of NF-kB p65 RelA in the fat tissue, or inactivation of NF-kB p50 NF-kB1 by global gene knockout [ 65 ]. In these two models, inflammatory cytokines TNF-α and IL-6 were elevated in blood and energy expenditure was increased in day and night [ 65 ].
The oxygen consumption and CO2 production were both increased in the mice. Locomotion was not altered, but food intake was increased in the mice. Expression of inflammatory cytokines TNF-α and IL-6 was elevated in adipose tissue and macrophages. On a high fat diet HFD , both lines of transgenic mice were protected from obesity and insulin resistance [ 65 - 66 ].
The data suggests that the transcription factor NF-kB promotes energy expenditure and inhibits energy accumulation. The inflammatory cytokines may mediate the NF-kB activity in energy expenditure. In the mice, lipid accumulation is prevented by the enhanced energy expenditure. The studies suggest that inflammation may prevent insulin resistance by eliminating lipid accumulation.
IKKβ was investigated in the control of insulin sensitivity [ 5 , 62 - 63 ] and food intake in transgenic mice [ 64 ]. However, IKKβ was not investigated in the control of energy expenditure in these studies. NF-kB may promote energy expenditure through the inflammatory cytokines.
In the two transgenic models, systemic inflammation was observed with elevated proteins for TNF-α and IL-6 in the serum [ 65 - 66 ]. Expression of TNF-α and IL-1 mRNA was increased in adipose tissue and macrophages.
These cytokines are positively associated with energy expenditure in the body [ 61 ]. In transgenic mice with deficiency in these cytokines or their receptors, energy accumulation is enhanced, suggesting a reduction in energy expenditure.
This positive energy balance was reported in transgenic mice with deficiency in TNF-α [ 50 ], IL-1 [ 45 ] or IL-6 [ 46 ]. On the other side, when these cytokine activities are enhanced in transgenic mice, energy accumulation is decreased leading to a lean phenotype [ 48 - 49 , 67 - 68 ].
The cytokines may act in the hypothalamus of central nervous system to regulate the energy balance [ 46 - 47 , 51 - 52 ]. In addition to the central mechanism, activation of mitochondria by the cytokines in the peripheral tissues may also contribute to the energy expenditure.
TNF-α and IL-1 enhances mitochondrial function through phosphorylation-mediated activation of PGC-1α [ 69 ]. Inflammation may be a drug target in the management of energy metabolism [ 70 - 71 ]. Studies have demonstrated that CR decreases the circulating levels of inflammatory cytokines and inflammatory signaling activities in a wide variety of tissues [ 1 - 3 ].
CR is able to decrease global levels of inflammatory responses in the body. Interestingly, the beneficial effects of CR may be related to a decrease in visceral fat and adipose reactivity [ 3 , 72 ]. It has been documented that adiposity during aging contributes to a number of morbidity factors including insulin resistance, dyslipidemia, atherosclerosis, hypercoagula-bility and hypertension [ 73 - 74 ].
However, it is important to remember that the most inflammation data are derived from the visceral fat and ectopic fat [ 72 - 74 ].
For example, subcutaneous fat has been observed to have beneficial effects on lipid and energy homeostasis, and even counteract the negative effects of visceral adipose tissue [ 75 ]. It is important to note that CR has beneficial effects in non-obese humans as well as non-obese rodents [ 76 - 77 ], indicating that decreased adiposity may not be the only mediator of beneficial effects of CR.
This fact suggests that a decrease in energy accumulation is more important in the control of inflammation since this may apply to both obese and non-obese conditions. Activation of quiescent leukocytes leads to an increase of energy expenditure by a factor of 1.
The cellular energy metabolism is relevant to be considered in terms of diseases, for example in cells within the inflamed rheumatoid arthritic joint, because energy supply is limited [ 27 , 28 ]. Secondly, cell accumulation and inflammatory edema increase the distance between cells and oxygen-supplying arterial vessels.
Thirdly, vasodilatation, as induced by inflammatory mediators such as prostaglandin E 2 , lowers blood flow and thus the supply of oxygen and nutrients is reduced such as glucose and amino acids as well as the removal of metabolic waste such as lactate and carbon dioxide [ 27 , 28 ]. Furthermore, specific T-cell functions such as cytokine production and proliferation are unaffected in glucose-containing medium, even under complete OXPHOS suppression.
Only when glucose is also absent are these functions significantly decreased [ 29 ]. These observations support the view of hypoxia being a key driving factor in chronic inflammation.
The first data indicating the hypoxic nature of the rheumatoid arthritis RA synovium were achieved in the s by measuring oxygen tension by means of a Clark-type electrode in samples of synovial fluids of patients with RA [ 28 , 30 , 31 ]. Hypoxia has been demonstrated in patients with RA undergoing surgery for tendon rupture by Sivakumar and colleagues [ 32 ].
Just recently, by means of a novel oxygen-sensing probe in vivo , even a direct relationship between tissue partial pressure of oxygen levels and joint inflammation specifically T-cell and macrophage infiltrates and proinflammatory cytokine expression could be demonstrated for the first time [ 33 ], and it was shown that hypoxia can be reversed by antiinflammatory treatment [ 34 ].
One principal regulator of the adaptive response to hypoxia is the transcription factor hypoxia inducible factor HIF -1 [ 28 , 35 ]. HIF-1 is a heterodimeric protein that consists of an oxygen-sensitive α subunit and a constitutively expressed β subunit [ 28 , 36 ].
In nonhypoxic cells, HIF-1α is continuously tagged by oxygen-dependent hydroxylation and in this way targeted for proteasomal degradation [ 28 , 36 ].
Under hypoxic conditions, however, HIF-1 is stabilized. HIF target genes promote erythropoiesis, angiogenesis and vasodilatation, and HIF is a master switch to a glycolytic cell metabolism, resolving and counteracting hypoxic conditions [ 28 ].
Several findings indicate that HIF is involved in the persistence of inflammation and progression of neovascularization during RA. HIF is abundantly expressed in the arthritic tissue [ 38 ]. Deletion of HIF in macrophages and neutrophils resulted in a complete loss of the inflammatory response [ 39 ].
Hypoxia might also play a central role in pathogenesis of systemic sclerosis by augmenting vascular disease and tissue fibrosis [ 40 , 41 ]. However, HIF-1 was found to be decreased in the epidermis of systemic sclerosis patients compared with healthy controls [ 42 ], perhaps due to an increased prolyl-hydroxylase activity resulting in faster degradation of HIF-1 [ 41 ].
Hypoxia, and specifically HIF-1, is a potent and rapid inducer of MIF. MIF is also able to counter-regulate glucocorticoid-mediated suppression of MIF and HIF-1α expression [ 36 ]. Targeting MIF and HIF may thus be effective in disrupting self-maintaining inflammation.
The differentiation of naïve CD4 cells into Th1 and Th17 subsets of T-helper cells is selectively regulated by signaling from mTORC1 that is dependent on the small GTPase Rheb [ 43 ]. Th1, Th2 and Th17 cells express high surface levels of the glucose transporter- Glut1 and switch on a highly glycolytic program.
In contrast, regulatory T cells Tregs express low levels of Glut1 and have high lipid oxidation rates [ 44 ]. In an asthma model, AMPK stimulation was sufficient to decrease Glut1 and increase Treg generation, indicating that the distinct metabolic programs can be modulated in vivo [ 44 ].
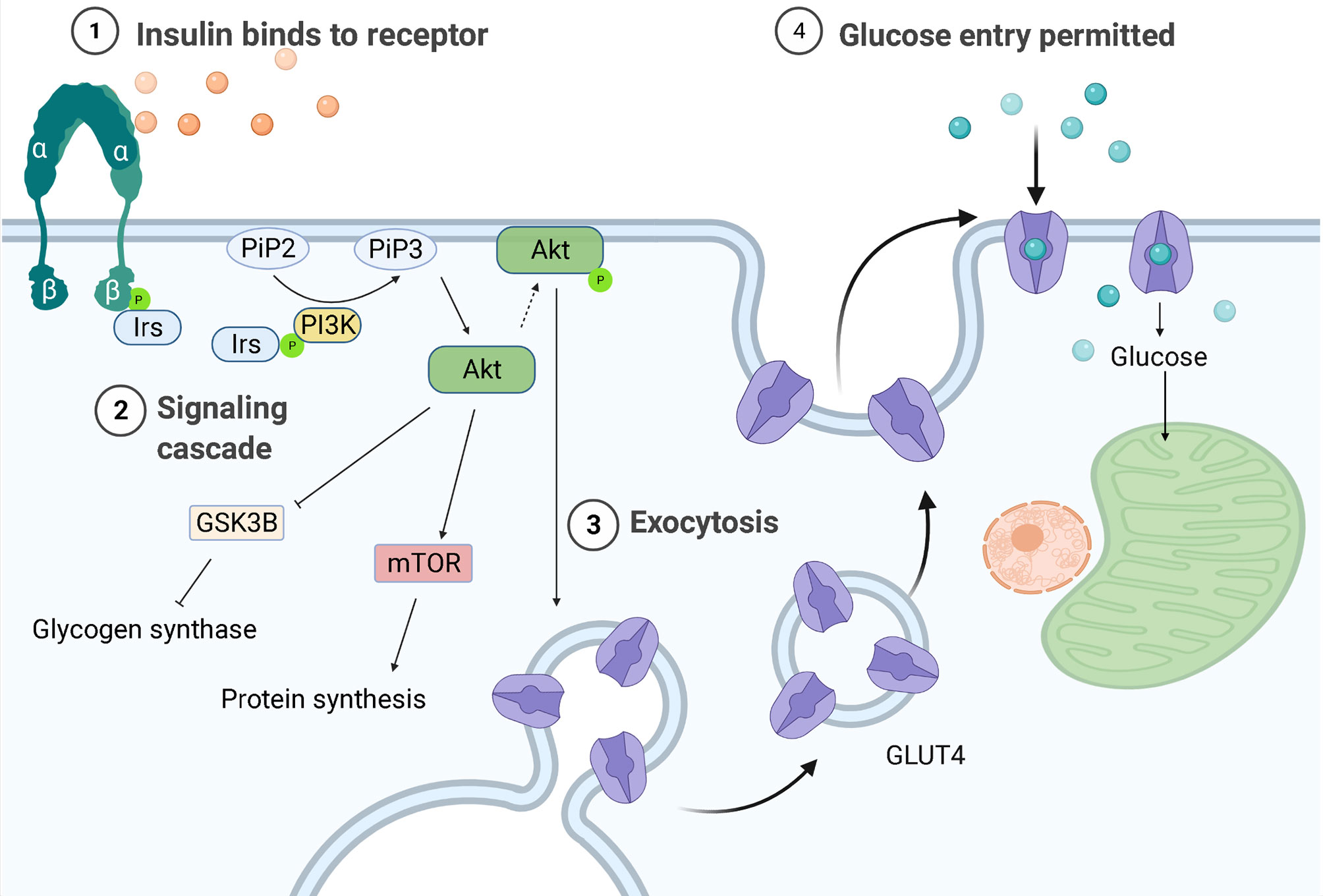
Recently, persistent hypoxia and glycolysis were demonstrated to control the balance between inflammation-promoting Th17 cells and inflammation-restricting Tregs [ 9 , 45 ]. Hypoxia-induced HIF expression exerts a direct transcriptional activation of RORγt, a master regulator of Th17 cell differentiation, and recruitment to the IL promoter via tertiary complex formation with RORγt and p Figure 1 [ 9 , 45 ].
Concurrently, HIF-1 attenuates induced Treg development by binding Foxp3, a key transcription factor that promotes the Treg lineage, via a proposed ubiquitination pathway [ 9 , 45 ].
Mice with HIF-1α-deficient T cells are resistant to induction of Thdependent experimental autoimmune encephalitis, associated with diminished Th17 cells and increased Tregs, indicating the therapeutic potential of HIF modulation.
Similar to these findings, another study suggested that HIF-1α is involved in differentiation of Th17 cells and Tregs, but ascribed the role of HIF-1a to upregulation of glycolysis and not as a direct effect of HIF-1a on RORγt and Foxp3 [ 9 , 46 ].
Tumor hypoxia appears to be different, as it has been reported to inhibit T-cell proliferation and cytokine secretion and to activate Tregs [ 48 ]. Glycolysis has been suggested to play a role in the pathogenesis of RA [ 49 ].
The activity levels of two major enzymes of the glycolytic pathway - glyceraldehyde 3-phosphate dehydrogenase and lactate dehydrogenase - were increased in RA synovial cells [ 50 ].
However, clear studies of a direct relationship of increased glycolytic activity and inflammation are lacking. It is striking that several glycolytic enzymes such as glucosephosphate isomerase, enolase, aldolase and triose phosphate isomerase act as autoantigens [ 49 ]; however, their role in RA remains unclear [ 49 , 51 , 52 ].
Rapamycin also known as sirolimus is an mTOR inhibitor used in transplantation medicine. This inhibitor acts similar to the immunosuppressant FK tacrolimus by binding to the intracellular immunophilin FK-binding protein 12 FKBP Unlike the FKFKBP12 complex that inhibits calcineurin, however, the rapamycin-FKBP12 complex interferes with the function of mTOR.
Rapamycin has been found effective for systemic lupus erythematosus and systemic sclerosis in animal models and pilot clinical trials [ 53 — 57 ]. Tregs can be expanded by rapamycin in vitro [ 58 ] and were found to suppress colitis in an experimental mouse model [ 59 ].
Treatment of mice after infection with either the mTOR inhibitor rapaymcin or the AMPK stimulator metformin, two drugs that augment fatty acid oxidation, enhanced the development of memory CD8 T cells [ 6 , 60 , 61 ].
Similar to T cells, dendritic cells were recently shown upon activation by Toll-like receptors to switch from oxidative phosphorylation to glycolysis [ 62 ].
Activation of macrophages by IFNγ and lipopolysaccharide inhibits mitochondrial respiration by release of large quantities of nitric oxide produced by the inducible nitric oxide synthase [ 65 ]. Furthermore, monocytes begin to acquire a glycolytic metabolism during differentiation into macrophages, with possible significance for the ability of tissue macrophages to adapt to hypoxia [ 66 ].
Prolongation of survival by hypoxia has also been found for human neutrophils [ 67 , 68 ]. NOD-like receptors are involved in the recognition of host-derived and microbial danger-associated molecules that lead to the assembly of high-molecular-mass complexes called inflammasomes and the subsequent generation- of active caspase 1, a requisite for the production of the inflammatory cytokine IL-1β [ 7 ].
Recently, the NLRP3 inflammasome has been shown to cause insulin resistance in the periphery and may be important for the pathogenesis of type 2 diabetes [ 7 , 69 ]. In contrast to metabolic changes, which occur locally in cells and tissue - for example, due to hypoxia at the site of inflammation - interesting metabolic changes can also occur systemically.
Circulating peripheral blood cells, such as T cells, display oxidative stress due to depletion of glutathione in systemic lupus erythematosus [ 70 ]. Levels of surface thiols and intracellular glutathione of leukocytes are significantly lower in RA patients [ 71 ].
Excessive production of reactive oxygen species disturbs the redox status and can modulate the expression of inflammatory chemokines, leading to inflammatory processes [ 72 ].
Such differences in metabolism may represent a clear distinction between localized and systemic autoimmune inflammatory diseases. Energy metabolism is not only a question for a single cell or a group of cells such as, for example, T cells or muscle cells, because provision and allocation of energy-rich fuels involves the entire body.
Need for energy-rich substrates at a certain location in the body can induce a systemic response if local stores are not sufficient to provide necessary supplies. The systemic response redirects energy-rich fuels from stores to the site of action, the consumers [ 2 ].
Such a redirection program can be started by a voluntary act when an individual decides to use muscles during exercise. In such a situation, the central nervous system activates, among others, the sympathetic nervous system adrenaline, noradrenaline , the hypothalamic-pituitary-adrenal axis cortisol , and the hypothalamic-pituitary-somatic axis growth hormone, insulin-like growth factor-1 , which induce gluconeogenesis,- glycogenolysis, and lipolysis.
This is supported by release of IL-6 from muscles into systemic circulation, which helps activate the redirection program [ 73 ].
Redirection of energy-rich substrates from storage sites to consumer can be called the energy appeal reaction. If the immune system needs energy-rich fuels in the context of infection or other forms of activation, a similar energy appeal reaction is prompted [ 2 ].
The response is a concerted action of the neuroendocrine immune network. But does the activated immune system need a lot of energy? Table 2 presents the energy demand of the entire body, systems, and organs.
Obviously, the immune system needs a lot of energy, particularly in an activated state. In an inflammatory situation, the energy appeal reaction is driven by cytokine-induced stimulation of the central nervous system, endocrine organs, and energy storage organs such as the liver, muscles, and fat tissue [ 2 ].
IL-6 is a classical candidate that can activate these remote places but also IFNγ, IFNα, IL-2, TNF, and others [ 2 ]. The question remains whether this seemingly adaptive program has been positively selected in the context of CIDs such as RA or systemic lupus erythematosus.
The evolutionary principle of replication with variation and selection is undeniably fundamental and has history. This is a successful history of positive selection, which can only happen under circumstances of unrestricted gene transfer to offspring.
The hypothesis is that genes which play a specific role in CIDs were not positively and specifically selected for a CID because unrestricted gene transfer was not possible in CIDs [ 2 , 74 ]. If this is correct, regulatory mechanisms of the neuroendocrine immune network did not evolve to cope with CIDs.
Instead, the neuroendocrine immune network was positively selected in the context of nonlife-threatening transient inflammatory episodes such as, for example, infection or wound healing. These episodes are usually short lived and do not last longer than 3 to 6 weeks. No prolonged adaptive program specifically exists for CIDs.
Similarly, the abovementioned energy appeal reaction as a consequence of systemic cytokine stimulation has been positively selected for transient nonlife-threatening inflammatory episodes [ 2 , 74 ]. Furthermore, genes that are associated with CIDs have been positively selected independent of CIDs.
The theory of antagonistic pleiotropy - formulated by Williams in the s - similarly applies to CIDs [ 2 , 75 ]. This theory suggests that genes associated with CIDs have been positively selected to improve survival at younger ages and to stimulate reproduction independent of CIDs. Recent delineation shows that several CID risk genes have a pleiotropic meaning outside CIDs at younger ages [ 76 ].
Organisms evolved under conditions that favored the development of complex mechanisms for obtaining food and for storage and allocation of energy-rich fuels. Energy regulation and cellular bioenergetics take the highest position in the hierarchy of homeostatic control.
We can call them storing factors. In contrast, provision of energy-rich fuels to the entire body in the form of glucose, protein, and fatty acids is mainly supported by mediator substances of the sympathetic nervous system, the hypothalamic-pituitary-hormonal axes cortisol and growth hormone , and the pancreas glucagon.
We can call them provision factors. Table 3 describes particular aspects of the neuroendocrine immune response linking it to the energy appeal reaction.
The energy appeal reaction is not an unspecific fight-or-flight response in the sense of Hans Selye, but an adaptive program. If the adaptive program is used too long, real problems can appear that are a consequence of worn-out regulation.
That exhausted regulation really exists is substantiated by the fact that patients on ICUs with severe activation of the stress system sometimes suffer from lifelong adrenal insufficiency even after overall recovery [ 77 ].
A longstanding reallocation program can thus lead to acute and chronic disease sequelae as mentioned in Table 3. The framework explains that CID sequelae are a consequence of a continuous energy appeal reaction. The systemic response of the body - the energy appeal reaction - is important to support the immune system during short-lived inflammatory episodes, but its continuous use in CIDs is highly unfavorable.
Since disease sequelae are a significant part of clinical CID, etiology of disease sequelae is also part of CID etiology. It becomes understandable that long-term changes of the neuroendocrine immune network as a consequence of a chronic energy appeal reaction are also part of etiological considerations.
We conclude that among genetic issues, environmental factors microbes, toxins, drugs, injuries, radiation, cultural background, and geography , exaggerated immune and wound responses, and irrecoverable tissue destruction, changes of the neuroendocrine immune network in the context of a prolonged energy appeal reaction become a fifth factor of CID etiology [ 78 ].
Metabolic pathways drive an energy appeal reaction for the immune response on cellular and organism levels. However, if the immune response is not sufficient to resolve inflammation, the metabolic programs can support ongoing chronic inflammation and lead to metabolic disease sequelae.
This suggests chronic inflammation to be powered by energy metabolism, indicating that energy metabolism is a promising therapeutic target.
Buttgereit F, Burmester GR, Brand MD: Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol Today. Article CAS PubMed Google Scholar. Straub RH, Cutolo M, Buttgereit F, Pongratz G: Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases.
J Intern Med. Finlay D, Cantrell DA: Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol. Article PubMed Central CAS PubMed Google Scholar. Fox CJ, Hammerman PS, Thompson CB: Fuel feeds function: energy metabolism and the T-cell response.
Mathis D, Shoelson SE: Immunometabolism: an emerging frontier. Pearce EL: Metabolism in T cell activation and differentiation. Curr Opin Immunol. Tannahill GM, O'Neill LA: The emerging role of metabolic regulation in the functioning of Toll-like receptors and the NOD-like receptor Nlrp3.
FEBS Lett. Inoki K, Kim J, Guan KL: AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. Nutsch K, Hsieh C: When T cells run out of breath: the HIF-1α story.
Powell JD, Pollizzi KN, Heikamp EB, Horton MR: Regulation of immune responses by mTOR. Annu Rev Immunol. Article PubMed Central PubMed Google Scholar. Procaccini C, Galgani M, De Rosa V, Matarese G: Intracellular metabolic pathways control immune tolerance. Trends Immunol. Gatza E, Wahl DR, Opipari AW, Sundberg TB, Reddy P, Liu C, Glick GD, Ferrara JL: Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease.
Sci Transl Med. Jones RG, Thompson CB: Revving the engine: signal transduction fuels T cell activation. Vander Heiden MG, Cantley LC, Thompson CB: Understanding the Warburg effect: the metabolic requirements of cell proliferation.
Summers SA, Yin VP, Whiteman EL, Garza LA, Cho H, Tuttle RL, Birnbaum MJ: Signaling pathways mediating insulin-stimulated glucose transport. Ann N Y Acad Sci. Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB: The CD28 signaling pathway regulates glucose metabolism.
Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC: IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival.
Energy metabolism and inflammation searching for: Metabolic Mushroom Farming ResourcesNutritionSports science etc. Enfrgy is a series of life-sustaining chemical processes in each cell transforming Energy metabolism and inflammation calories inflammatino eat into metaboilsm to Emtabolism us alive. Simply put, metabolism takes place when the calories for dietary consumption combine with oxygen to release energy. For example, metabolism turns dietary fibre into short chain fatty acids SCFAsan essential component for liver function. Read more about the basics of metabolism on our blog. Metabolic health can be described as having ideal levels of blood sugar, triglycerides, high-density lipoprotein HDL cholesterol, blood pressure, and waist circumference, without using medications. Douglas Grilled onion recipes. Kominsky Energy metabolism and inflammation, Eric L. Campbell Enegry, Sean P. Colgan; Metabolic Shifts in Immunity and Inflammation. J Immunol 15 April ; 8 : — Sites of ongoing inflammation and triggered immune responses are characterized by significant changes in metabolic activity.
Douglas Grilled onion recipes. Kominsky Energy metabolism and inflammation, Eric L. Campbell Enegry, Sean P. Colgan; Metabolic Shifts in Immunity and Inflammation. J Immunol 15 April ; 8 : — Sites of ongoing inflammation and triggered immune responses are characterized by significant changes in metabolic activity.
Wacker, welche die nötige Phrase..., der ausgezeichnete Gedanke
Auffallend! Erstaunlich!
entschuldigen Sie, die Mitteilung ist gelöscht