
Hyperglycemic crisis and hypokalemia -
header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation.
Previous Article. Article Navigation. Position Statements January 01 Hyperglycemic Crises in Diabetes American Diabetes Association American Diabetes Association.
This Site. Google Scholar. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Figure 1—. View large Download slide. Figure 2—. Figure 3—. Figure 4—. Table 1— Diagnostic criteria for DKA and HHS. View Large. Table 3— Summary of major recommendations.
Therefore, to avoid the occurrence of cerebral edema, follow the recommendations in the position statement regarding a gradual correction of glucose and osmolality as well as the judicious use of isotonic or hypotonic saline, depending on serum sodium and the hemodynamic status of the patient.
McGarry JD, Woeltje KF, Kuwajima M, Foster DW: Regulation of ketogenesis and the renaissance of carnitine palmitoyl transferase. Diabetes Metab Rev. DeFronzo RA, Matsuda M, Barrett E: Diabetic ketoacidosis: a combined metabolic-nephrologic approach to therapy.
Diabetes Rev. Atchley DW, Loeb RF, Richards DW, Benedict EM, Driscoll ME: A detailed study of electrolyte balances following withdrawal and reestablishment of insulin therapy. J Clin Invest. Halperin ML, Cheema-Dhadli S: Renal and hepatic aspects of ketoacidosis: a quantitative analysis based on energy turnover.
Malone ML, Gennis V, Goodwin JS: Characteristics of diabetic ketoacidosis in older versus younger adults. J Am Geriatr Soc. Matz R: Hyperosmolar nonacidotic diabetes HNAD. In Diabetes Mellitus: Theory and Practice.
Morris LE, Kitabchi AE: Coma in the diabetic. In Diabetes Mellitus: Problems in Management. Kreisberg RA: Diabetic ketoacidosis: new concepts and trends in pathogenesis and treatment. Ann Int Med. Klekamp J, Churchwell KB: Diabetic ketoacidosis in children: initial clinical assessment and treatment.
Pediatric Annals. Glaser NS, Kupperman N, Yee CK, Schwartz DL, Styne DM: Variation in the management of pediatric diabetic ketoacidosis by specialty training. Arch Pediatr Adolescent Med. Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, Wall BM: Management of hyperglycemic crises in patients with diabetes mellitus Technical Review.
Diabetes Care. Beigelman PM: Severe diabetic ketoacidosis diabetic coma : episodes in patients: experience of three years. Polonsky WH, Anderson BJ, Lohrer PA, Aponte JE, Jacobson AM, Cole CF: Insulin omission in women with IDDM.
Kitabchi AE, Fisher JN, Murphy MB, Rumbak MJ: Diabetic ketoacidosis and the hyperglycemic hyperosmolar nonketotic state. Ennis ED, Stahl EJVB, Kreisberg RA: The hyperosmolar hyperglycemic syndrome. Marshall SM, Walker M, Alberti KGMM: Diabetic ketoacidosis and hyperglycaemic non-ketotic coma.
In International Textbook of Diabetes Mellitus. Carroll P, Matz R: Uncontrolled diabetes mellitus in adults: experience in treating diabetic ketoacidosis and hyperosmolar coma with low-dose insulin and uniform treatment regimen. Ennis ED, Stahl EJ, Kreisberg RA: Diabetic ketoacidosis.
Hillman K: Fluid resuscitation in diabetic emergencies: a reappraisal. Intensive Care Med. Fein IA, Rackow EC, Sprung CL, Grodman R: Relation of colloid osmotic pressure to arterial hypoxemia and cerebral edema during crystalloid volume loading of patients with diabetic ketoacidosis.
Ann Intern Med. Matz R: Hypothermia in diabetic acidosis. Kitabchi AE, Sacks HS, Young RT, Morris L: Diabetic ketoacidosis: reappraisal of therapeutic approach. Ann Rev Med. Mahoney CP, Vleck BW, DelAguila M: Risk factors for developing brain herniation during diabetic ketoacidosis.
Pediatr Neurology. Finberg L: Why do patients with diabetic ketoacidosis have cerebral swelling, and why does treatment sometimes make it worse?
Pediatr Adolescent Med. Duck SC, Wyatt DT: Factors associated with brain herniation in the treatment of diabetic ketoacidosis. J Pediatr. Kitabchi AE, Ayyagari V, Guerra SMO, Medical House Staff: The efficacy of low dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis.
Fisher JN, Shahshahani MN, Kitabchi AE: Diabetic ketoacidosis: low dose insulin therapy by various routes. N Engl J Med. Barnes HV, Cohen RD, Kitabchi AE, Murphy MB: When is bicarbonate appropriate in treating metabolic acidosis including diabetic ketoacidosis?
In Debates in Medicine. Morris LR, Murphy MB, Kitabchi AE: Bicarbonate therapy in severe diabetic ketoacidosis. Vanelli M, Chiari G, Ghizzoni L, Costi G, Giacalone T, Chiarelli F: Effectiveness of a prevention program for diabetic ketoacidosis in children.
Viallon A, Zeni F, Lafond P, Venet C, Tardy B, Page Y, Bertrand JC: Does bicarbonate therapy improve the management of severe diabetic ketoacidosis?
Crit Care Med. Fisher JN, Kitabchi AE: A randomized study of phosphate therapy in the treatment of diabetic ketoacidosis. J Clin Endocrinol Metab.
Rosenbloom AL: Intracerebral crises during treatment of diabetic ketoacidosis. Holsclaw DS Jr, Torcato B: Acute pulmonary edema in juvenile diabetic ketoacidosis. Pediatr Pulmonology. Musey VC, Lee JK, Crawford R, Klatka MA, McAdams D, Phillips LS: Diabetes in urban African-Americans.
Cessation of insulin therapy is the major precipitating cause of diabetic ketoacidosis. Umpierrez GE, Kelly JP, Navarrete JE, Casals MMC, Kitabchi AE: Hyperglycemic crises in urban blacks.
Arch Int Med. Could a potassium-rich diet help to prevent type 2 diabetes from developing? Approximately 34 million Americans, or 1 in 10 Americans, have either Type 1 Diabetes or Type 2 Diabetes. Diabetes mellitus is the umbrella term for the group of diseases caused by hyperglycemia, or high blood sugar.
These include type 1 diabetes, type 2 diabetes, prediabetes, and gestational diabetes. With any form of diabetes, blood glucose levels are too high in the body, resulting in a buildup of sugar in the bloodstream.
Type 1 diabetes is a genetic disorder that a person is born with and cannot prevent. Risk factors include having a family history of type 1 diabetes, exposure to viral illnesses, and having autoantibodies cells that attack the immune system.
Children and young adults are most commonly diagnosed with type 1 diabetes. Type 2 diabetes is a disorder that develops over time and is primarily due to diet.
This form of diabetes is preventable. Risk factors include living a sedentary lifestyle and being physically active fewer than three times a week, obesity, and having a family history of type 2 diabetes. Adults ages 45 and older are most commonly diagnosed with type 2 diabetes.
Potassium is both an essential mineral and electrolyte that the body requires in order to maintain regular fluid levels inside the cells. This nutrient also aids in muscle contraction, blood pressure regulation, and heart rate regulation — the vital functions.
Blood potassium levels are considered normal between 3. Women should consume about 2, mg milligrams of potassium a day, and men should consume 3, mg of potassium a day¹.
The body will use all of the potassium it needs, then will excrete the leftover potassium as urinary waste. Low blood potassium, hypokalemia, may be caused by low dietary potassium intake, increased potassium excretion, laxative use, diarrhea, and high aldosterone levels. Increased potassium excretion via urine is often caused by diuretic medications, especially thiazide diuretics used to treat high blood pressure and hypertension.
Cerebral edema is a rare but severe complication in children and adolescents and rarely affects adult patients older than 28 7. This could be due to the lack of cerebral autoregulation, presentation with more severe acidosis and dehydration among children and adolescents The exact mechanism of cerebral edema development is unknown.
Some reports suggest that the risk of cerebral edema during hyperglycemic crisis management might be induced by rapid hydration, especially in the pediatric population.
However, a recent multicenter study for children with DKA who were randomized to receive isotonic versus hypotonic sodium IV fluid with different infusions rates did not show a difference in neurological outcomes Early identification and prompt therapy with mannitol or hypertonic saline can prevent neurological deterioration from DKA management 7 , Furthermore, higher blood urea nitrogen BUN and sodium concentrations have been identified as cerebral edema risk factors Thus, careful hydration with close electrolytes and BUN is recommended Other serious complications of hyperglycemic crisis may include transient AKI, pulmonary edema in patients with congestive heart failure, myocardial infarction, a rise in pancreatic enzymes with or without acute pancreatitis, cardiomyopathy, rhabdomyolysis in patients presented with severe dehydration 7 , All authors have contributed equally in writing, organizing, and reviewing this publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic Crises in Adult Patients With Diabetes. Diabetes Care 32 7 — doi: PubMed Abstract CrossRef Full Text Google Scholar.
Goyal A, Mathew UE, Golla KK, Mannar V, Kubihal S, Gupta Y, et al. A Practical Guidance on the Use of Intravenous Insulin Infusion for Management of Inpatient Hyperglycemia.
Diabetes Metab Syndrome: Clin Res Rev 15 5 CrossRef Full Text Google Scholar. Saeedi P. Global and Regional Diabetes Prevalence Estimates for and Projections for and Results From the International Diabetes Federation Diabetes Atlas, 9th Edition.
Diabetes Res Clin Pract Pasquel FJ, Umpierrez GE. Hyperosmolar Hyperglycemic State: A Historic Review of the Clinical Presentation, Diagnosis, and Treatment. Dia Care 37 11 — Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, et al.
Management of Hyperglycemic Crises in Patients With Diabetes. Diabetes Care 24 1 — Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic Crises in Adult Patients With Diabetes: A Consensus Statement From the American Diabetes Association.
Diabetes Care 29 12 — Karslioglu French E, Donihi AC, Korytkowski MT. Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic Syndrome: Review of Acute Decompensated Diabetes in Adult Patients. BMJ I Fayfman M, Pasquel FJ, Umpierrez GE. Management of Hyperglycemic Crises. Med Clinics North Am 3 — Rains JL, Jain SK.
Oxidative Stress, Insulin Signaling, and Diabetes. Free Radical Biol Med 50 5 — Hoffman WH, Burek CL, Waller JL, Fisher LE, Khichi M, Mellick LB. Cytokine Response to Diabetic Ketoacidosis and Its Treatment.
Clin Immunol 3 — Hayami T, Kato Y, Kamiya H, Kondo M, Naito E, Sugiura Y, et al. Case of Ketoacidosis by a Sodium-Glucose Cotransporter 2 Inhibitor in a Diabetic Patient With a Low-Carbohydrate Diet.
J Diabetes Investig , 6 5 — Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar Syndrome. Diabetes Spectr 15 1 Kraut JA, Madias NE.
Serum Anion Gap: Its Uses and Limitations in Clinical Medicine. Clin J Am Soc Nephrol 2 1 — Dhatariya K, Savage M, Claydon A, et al. Joint British Diabetes Societies for Inpatient Care JBDS-IP Revised Guidelines. The Management of Diabetic Ketoacidosis in Adults Revised Google Scholar.
Kitabchi AE, Umpierrez GE, Murphy MB. Diabetic Ketoacidosis and Hyperosmolar State. In: DeFronzo RA, Ferrannini E, Zimmet P, Alberti KGMM, editors. International Textbook of Diabetes Mellitus.
Trachtenbarg DE. Diabetic Ketoacidosis. Am Fam Phys 71 9 — Katz MA. Hyperglycemia-Induced Hyponatremia-Calculation of Expected Serum Sodium Depression.
N Engl J Med 16 —4. Rudloff E, Hopper K. Crystalloid and Colloid Compositions and Their Impact. Front Vet Sci Semler MW, Kellum JA. Balanced Crystalloid Solutions. Am J Respir Crit Care Med 8 — Van Zyl DG, Rheeder P, Delport E. QJM 4 — Mahler SA, Conrad SA, Wang H, Arnold TC.
Resuscitation With Balanced Electrolyte Solution Prevents Hyperchloremic Metabolic Acidosis in Patients With Diabetic Ketoacidosis. Am J Emerg Med 29 6 —4. Self WH, Evans CS, Jenkins CA, Brown RM, Casey JD, Collins SP, et al. Clinical Effects of Balanced Crystalloids vs Saline in Adults With Diabetic Ketoacidosis: A Subgroup Analysis of Cluster Randomized Clinical Trials.
JAMA Netw. Open 3 11 :e Ramanan M, Attokaran A, Murray L, Bhadange N, Stewart D, Rajendran G, et al. Sodium Chloride or Plasmalyte Evaluation in Severe Diabetic Ketoacidosis Scope-Dka - a Cluster, Crossover, Randomized, Controlled Trial. Intensive Care Med 47 11 — Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JAE, Courtney CH, et al.
Joint British Diabetes Societies Guideline for the Management of Diabetic Ketoacidosis: Diabetic Ketoacidosis Guidelines.
Diabetic Med 28 5 — Umpierrez GE, Jones S, Smiley D, Mulligan P, Keyler T, Temponi A, et al. Insulin Analogs Versus Human Insulin in the Treatment of Patients With Diabetic Ketoacidosis: A Randomized Controlled Trial.
Diabetes Care 32 7 —9. Laskey D, Vadlapatla R, Hart K. Stability of High-Dose Insulin in Normal Saline Bags for Treatment of Calcium Channel Blocker and Beta Blocker Overdose.
Clin Toxicol 54 9 — Lindsay R, Bolte RG. The Use of an Insulin Bolus in Low-Dose Insulin Infusion for Pediatric Diabetic Ketoacidosis. Pediatrs Emerg Care 5 2 —9. Kitabchi AE, Murphy MB, Spencer J, Matteri R, Karas J. Is a Priming Dose of Insulin Necessary in a Low-Dose Insulin Protocol for the Treatment of Diabetic Ketoacidosis?
Diabetes Care 31 11
Schedule Appointment. East Crissi Quadrangle Blvd. Orlando, FL Call Lake Nona Tavistock Lakes Blvd.Video
Chapter 15 Hyperglycemic EmergenciesHyperglycemic crisis and hypokalemia -
These disorders can occur in both type 1 and type 2 diabetes. The prognosis of both conditions is substantially worsened at the extremes of age and in the presence of coma and hypotension 1 — This position statement will outline precipitating factors and recommendations for the diagnosis, treatment, and prevention of DKA and HHS.
It is based on a previous technical review 11 , which should be consulted for further information. Although the pathogenesis of DKA is better understood than that of HHS, the basic underlying mechanism for both disorders is a reduction in the net effective action of circulating insulin coupled with a concomitant elevation of counterregulatory hormones, such as glucagon, catecholamines, cortisol, and growth hormone.
These hormonal alterations in DKA and HHS lead to increased hepatic and renal glucose production and impaired glucose utilization in peripheral tissues, which result in hyperglycemia and parallel changes in osmolality of the extracellular space 12 , The combination of insulin deficiency and increased counterregulatory hormones in DKA also leads to the release of free fatty acids into the circulation from adipose tissue lipolysis and to unrestrained hepatic fatty acid oxidation to ketone bodies β-hydroxybutyrate [β-OHB] and acetoacetate , with resulting ketonemia and metabolic acidosis.
On the other hand, HHS may be caused by plasma insulin concentrations that are inadequate to facilitate glucose utilization by insulin-sensitive tissues but adequate as determined by residual C-peptide to prevent lipolysis and subsequent ketogenesis, although the evidence for this is weak Both DKA and HHS are associated with glycosuria, leading to osmotic diuresis, with loss of water, sodium, potassium, and other electrolytes 3 , 15 — The laboratory and clinical characteristics of DKA and HHS are summarized in Tables 1 and 2.
As can be seen, DKA and HHS differ in magnitude of dehydration and degree of ketosis and acidosis. The most common precipitating factor in the development of DKA or HHS is infection. Other precipitating factors include cerebrovascular accident, alcohol abuse, pancreatitis, myocardial infarction, trauma, and drugs.
In addition, new-onset type 1 diabetes or discontinuation of or inadequate insulin in established type 1 diabetes commonly leads to the development of DKA.
Elderly individuals with new-onset diabetes particularly residents of chronic care facilities or individuals with known diabetes who become hyperglycemic and are unaware of it or are unable to take fluids when necessary are at risk for HHS 6.
Drugs that affect carbohydrate metabolism, such as corticosteroids, thiazides, and sympathomimetic agents e. Factors that may lead to insulin omission in younger patients include fear of weight gain with improved metabolic control, fear of hypoglycemia, rebellion from authority, and stress of chronic disease The process of HHS usually evolves over several days to weeks, whereas the evolution of the acute DKA episode in type 1 diabetes or even in type 2 diabetes tends to be much shorter.
Occasionally, the entire symptomatic presentation may evolve or develop more acutely, and the patient may present in DKA with no prior clues or symptoms. For both DKA and HHS, the classical clinical picture includes a history of polyuria, polydipsia, polyphagia, weight loss, vomiting, abdominal pain only in DKA , dehydration, weakness, clouding of sensoria, and finally coma.
Physical findings may include poor skin turgor, Kussmaul respirations in DKA , tachycardia, hypotension, alteration in mental status, shock, and ultimately coma more frequent in HHS. Endoscopy has related this finding to the presence of hemorrhagic gastritis.
Mental status can vary from full alertness to profound lethargy or coma, with the latter more frequent in HHS. Although infection is a common precipitating factor for both DKA and HHS, patients can be normothermic or even hypothermic primarily because of peripheral vasodilation.
Hypothermia, if present, is a poor prognostic sign Caution needs to be taken with patients who complain of abdominal pain on presentation, because the symptoms could be either a result or an indication of a precipitating cause particularly in younger patients of DKA.
Further evaluation is necessary if this complaint does not resolve with resolution of dehydration and metabolic acidosis. Bacterial cultures of urine, blood, and throat, etc. HbA 1c may be useful in determining whether this acute episode is the culmination of an evolutionary process in previously undiagnosed or poorly controlled diabetes or a truly acute episode in an otherwise well-controlled patient.
A chest X-ray should also be obtained if indicated. Tables 1 and 2 depict typical laboratory findings in patients with DKA or HHS. The majority of patients with hyperglycemic emergencies present with leukocytosis proportional to blood ketone body concentration.
Serum sodium concentration is usually decreased because of the osmotic flux of water from the intracellular to the extracellular space in the presence of hyperglycemia, and less commonly, serum sodium concentration may be falsely lowered by severe hypertriglyceridemia.
Serum potassium concentration may be elevated because of an extracellular shift of potassium caused by insulin deficiency, hypertonicity, and acidemia.
Patients with low-normal or low serum potassium concentration on admission have severe total-body potassium deficiency and require very careful cardiac monitoring and more vigorous potassium replacement, because treatment lowers potassium further and can provoke cardiac dysrhythmia.
Amylase levels are elevated in the majority of patients with DKA, but this may be due to nonpancreatic sources, such as the parotid gland. A serum lipase determination may be beneficial in the differential diagnosis of pancreatitis.
However, lipase could also be elevated in DKA. Abdominal pain and elevation of serum amylase and liver enzymes are noted more commonly in DKA than in HHS. Not all patients with ketoacidosis have DKA. DKA must also be distinguished from other causes of high-anion gap metabolic acidosis, including lactic acidosis, ingestion of drugs such as salicylate, methanol, ethylene glycol, and paraldehyde, and chronic renal failure which is more typically hyperchloremic acidosis rather than high-anion gap acidosis.
Clinical history of previous drug intoxications or metformin use should be sought. Measurement of blood lactate, serum salicylate, and blood methanol level can be helpful in these situations.
Ethylene glycol antifreeze is suggested by the presence of calcium oxalate and hippurate crystals in the urine. Paraldehyde ingestion is indicated by its characteristic strong odor on the breath. Because these intoxicants are low-molecular weight organic compounds, they can produce an osmolar gap in addition to the anion gap acidosis 14 — Successful treatment of DKA and HHS requires correction of dehydration, hyperglycemia, and electrolyte imbalances; identification of comorbid precipitating events; and above all, frequent patient monitoring.
Guidelines for the management of patients with DKA and HHS follow and are summarized in Figs. Table 3 includes a summary of major recommendations and evidence gradings. Initial fluid therapy is directed toward expansion of the intravascular and extravascular volume and restoration of renal perfusion.
In the absence of cardiac compromise, isotonic saline 0. Subsequent choice for fluid replacement depends on the state of hydration, serum electrolyte levels, and urinary output. In general, 0. Fluid replacement should correct estimated deficits within the first 24 h. In patients with renal or cardiac compromise, monitoring of serum osmolality and frequent assessment of cardiac, renal, and mental status must be performed during fluid resuscitation to avoid iatrogenic fluid overload 14 — 20 , Initial fluid therapy is directed toward expansion of the intravascular and extravascular volume and restoration of renal profusion.
The need for vascular volume expansion must be offset by the risk of cerebral edema associated with rapid fluid administration.
The 1st hour of fluids should be isotonic saline 0. Continued fluid therapy is calculated to replace the fluid deficit evenly over 48 h. Therapy should include monitoring mental status to rapidly identify changes that might indicate iatrogenic fluid overload, which can lead to symptomatic cerebral edema 23 — Unless the episode of DKA is mild Table 1 , regular insulin by continuous intravenous infusion is the treatment of choice.
An initial insulin bolus is not recommended in pediatric patients; a continuous insulin infusion of regular insulin at a dose of 0. Thereafter, the rate of insulin administration or the concentration of dextrose may need to be adjusted to maintain the above glucose values until acidosis in DKA or mental obtundation and hyperosmolarity in HHS are resolved.
Ketonemia typically takes longer to clear than hyperglycemia. Direct measurement of β-OHB in the blood is the preferred method for monitoring DKA. The nitroprusside method only measures acetoacetic acid and acetone.
However, β-OHB, the strongest and most prevalent acid in DKA, is not measured by the nitroprusside method. During therapy, β-OHB is converted to acetoacetic acid, which may lead the clinician to believe that ketosis has worsened. Therefore, assessments of urinary or serum ketone levels by the nitroprusside method should not be used as an indicator of response to therapy.
During therapy for DKA or HHS, blood should be drawn every 2—4 h for determination of serum electrolytes, glucose, blood urea nitrogen, creatinine, osmolality, and venous pH for DKA. Generally, repeat arterial blood gases are unnecessary; venous pH which is usually 0.
With mild DKA, regular insulin given either subcutaneously or intramuscularly every hour is as effective as intravenous administration in lowering blood glucose and ketone bodies Thereafter, 0.
Once DKA is resolved, if the patient is NPO, continue intravenous insulin and fluid replacement and supplement with subcutaneous regular insulin as needed every 4 h. When the patient is able to eat, a multiple-dose schedule should be started that uses a combination of short- or rapid-acting and intermediate- or long-acting insulin as needed to control plasma glucose.
Continue intravenous insulin infusion for 1—2 h after the split-mixed regimen is begun to ensure adequate plasma insulin levels. An abrupt discontinuation of intravenous insulin coupled with a delayed onset of a subcutaneous insulin regimen may lead to worsened control; therefore, some overlap should occur in intravenous insulin therapy and initiation of the subcutaneous insulin regimen.
Patients with known diabetes may be given insulin at the dose they were receiving before the onset of DKA or HHS and further adjusted as needed for control. Finally, some type 2 diabetes patients may be discharged on oral antihyperglycemic agents and dietary therapy.
Despite total-body potassium depletion, mild to moderate hyperkalemia is not uncommon in patients with hyperglycemic crises. Insulin therapy, correction of acidosis, and volume expansion decrease serum potassium concentration.
To prevent hypokalemia, potassium replacement is initiated after serum levels fall below 5. Rarely, DKA patients may present with significant hypokalemia. Bicarbonate use in DKA remains controversial Women should consume about 2, mg milligrams of potassium a day, and men should consume 3, mg of potassium a day¹.
The body will use all of the potassium it needs, then will excrete the leftover potassium as urinary waste. Low blood potassium, hypokalemia, may be caused by low dietary potassium intake, increased potassium excretion, laxative use, diarrhea, and high aldosterone levels.
Increased potassium excretion via urine is often caused by diuretic medications, especially thiazide diuretics used to treat high blood pressure and hypertension. Aldosterone is a steroid hormone secreted by the adrenal glands that serves to regulate blood pressure.
If a benign noncancerous tumor is present on the adrenal gland, this can cause aldosterone levels to rise — which is called hyperaldosteronism. Hyperaldosteronism causes the body to lose too much potassium and retain too much sodium — leading to hypokalemia. When blood serum potassium levels are higher than 5.
Hyperkalemia can lead to muscle cramps, serious heart problems, and paralysis. Using an ACE inhibitor angiotensin-converting enzyme used to treat high blood pressure and heart failure also puts a person at a higher risk of developing hyperkalemia. The cells then use glucose for energy, or store it for later use.
Insulin then comes to move glucose into the cell to restore potassium homeostasis, causing potassium levels to drop. People with low potassium levels will release less insulin, which causes higher blood sugar levels, and increases the risk of developing type 2 diabetes.
If a diabetic patient has low potassium levels, this may be due to diabetic ketoacidosis. The process of breaking down fat releases ketones in the blood, and high levels of ketones can poison the body American Diabetes Association.
Ketones and glucose are then transferred to the urine, where the kidneys use water to separate blood from glucose and ketones. This process dehydrates the body and reduces potassium levels, quickly worsening diabetic ketoacidosis.
Diabetic ketoacidosis is a serious complication that can be life-threatening and requires immediate attention. Symptoms include shortness of breath, weakness, nausea, extreme thirst and dehydration. Google Scholar. Kitabchi AE, Umpierrez GE, Murphy MB. Diabetic Ketoacidosis and Hyperosmolar State.
In: DeFronzo RA, Ferrannini E, Zimmet P, Alberti KGMM, editors. International Textbook of Diabetes Mellitus. Trachtenbarg DE. Diabetic Ketoacidosis. Am Fam Phys 71 9 — Katz MA. Hyperglycemia-Induced Hyponatremia-Calculation of Expected Serum Sodium Depression.
N Engl J Med 16 —4. Rudloff E, Hopper K. Crystalloid and Colloid Compositions and Their Impact. Front Vet Sci Semler MW, Kellum JA. Balanced Crystalloid Solutions. Am J Respir Crit Care Med 8 — Van Zyl DG, Rheeder P, Delport E.
QJM 4 — Mahler SA, Conrad SA, Wang H, Arnold TC. Resuscitation With Balanced Electrolyte Solution Prevents Hyperchloremic Metabolic Acidosis in Patients With Diabetic Ketoacidosis. Am J Emerg Med 29 6 —4. Self WH, Evans CS, Jenkins CA, Brown RM, Casey JD, Collins SP, et al.
Clinical Effects of Balanced Crystalloids vs Saline in Adults With Diabetic Ketoacidosis: A Subgroup Analysis of Cluster Randomized Clinical Trials. JAMA Netw. Open 3 11 :e Ramanan M, Attokaran A, Murray L, Bhadange N, Stewart D, Rajendran G, et al.
Sodium Chloride or Plasmalyte Evaluation in Severe Diabetic Ketoacidosis Scope-Dka - a Cluster, Crossover, Randomized, Controlled Trial. Intensive Care Med 47 11 — Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JAE, Courtney CH, et al.
Joint British Diabetes Societies Guideline for the Management of Diabetic Ketoacidosis: Diabetic Ketoacidosis Guidelines. Diabetic Med 28 5 — Umpierrez GE, Jones S, Smiley D, Mulligan P, Keyler T, Temponi A, et al.
Insulin Analogs Versus Human Insulin in the Treatment of Patients With Diabetic Ketoacidosis: A Randomized Controlled Trial. Diabetes Care 32 7 —9. Laskey D, Vadlapatla R, Hart K. Stability of High-Dose Insulin in Normal Saline Bags for Treatment of Calcium Channel Blocker and Beta Blocker Overdose.
Clin Toxicol 54 9 — Lindsay R, Bolte RG. The Use of an Insulin Bolus in Low-Dose Insulin Infusion for Pediatric Diabetic Ketoacidosis.
Pediatrs Emerg Care 5 2 —9. Kitabchi AE, Murphy MB, Spencer J, Matteri R, Karas J. Is a Priming Dose of Insulin Necessary in a Low-Dose Insulin Protocol for the Treatment of Diabetic Ketoacidosis? Diabetes Care 31 11 Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al.
ISPAD Clinical Practice Consensus Guidelines Diabetic Ketoacidosis and the Hyperglycemic Hyperosmolar State. Pediatr Diabetes — Umpierrez GE, Latif K, Stoever J, Cuervo R, Park L, Freire AX, et al.
Efficacy of Subcutaneous Insulin Lispro Versus Continuous Intravenous Regular Insulin for the Treatment of Patients With Diabetic Ketoacidosis. Am J Med 5 —6. Ersöz HÖ, Ukinc K, Köse M, Erem C, Gunduz A, Hacihasanoglu AB, et al.
Subcutaneous Lispro and Intravenous Regular Insulin Treatments are Equally Effective and Safe for the Treatment of Mild and Moderate Diabetic Ketoacidosis in Adult Patients: SC Lispro and IV Regular Insulin Treatments in DKA.
Int J Clin Pract 60 4 — Huang SK, Huang CY, Lin CH, Cheng BW, Chiang YT, Lee YC, et al. Acute Kidney Injury is a Common Complication in Children and Adolescents Hospitalized for Diabetic Ketoacidosis.
Shimosawa T, Ed. PloS One 15 10 :e Frankel AH, Kazempour-Ardebili S, Bedi R, Chowdhury TA, De P, El-Sherbini N, et al. Management of Adults With Diabetes on the Haemodialysis Unit: Summary of Guidance From the Joint British Diabetes Societies and the Renal Association.
Diabetes Med 35 8 — Goldberg PA, Kedves A, Walter K, Groszmann A, Belous A, Inzucchi SE. Diabetes Technol Ther 8 5 — Thompson CD, Vital-Carona J, Faustino EVS. The Effect of Tubing Dwell Time on Insulin Adsorption During Intravenous Insulin Infusions.
Diabetes Technol Ther 14 10 —6. Wilson HK, Keuer SP, Lea AS, Iii AEB, Eknoyan G. Phosphate Therapy in Diabetic Ketoacidosis. Arch Intern Med — Patel MP, Ahmed A, Gunapalan T, Hesselbacher SE. Use of Sodium Bicarbonate and Blood Gas Monitoring in Diabetic Ketoacidosis: A Review.
WJD 9 11 — Chua HR, Schneider A, Bellomo R. Bicarbonate in Diabetic Ketoacidosis - a Systematic Review. Ann Intensive Care 1 1 Jaber S, Paugam C, Futier E, Lefrant JY, Lasocki S, Lescot T, et al. Sodium Bicarbonate Therapy for Patients With Severe Metabolic Acidaemia in the Intensive Care Unit BICAR-ICU : A Multicentre, Open-Label, Randomised Controlled, Phase 3 Trial.
Lancet — Adeva-Andany MM, Fernández-Fernández C, Mouriño-Bayolo D, Castro-Quintela E, Domínguez-Montero A. Sodium Bicarbonate Therapy in Patients With Metabolic Acidosis. Sci World J — Butler J, Vijayakumar S, Pitt B. Revisiting Hyperkalaemia Guidelines: Rebuttal: Revisiting Hyperkalaemia Guidelines: Rebuttal.
Eur J Heart Fail 20 9 —5. Vanden Hoek TL, Morrison LJ, Shuster M, Donnino M, Sinz E, Lavonas EJ, et al. Part Cardiac Arrest in Special Situations- American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.
Circulation suppl 3 :S— Masharani U. McGraw Hill. Keenan CR, Murin S, White RH. High Risk for Venous Thromboembolism in Diabetics With Hyperosmolar State: Comparison With Other Acute Medical Illnesses. J Thromb Haemostasis 5 6 — Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, Louie J, et al.
Risk Factors for Cerebral Edema in Children With Diabetic Ketoacidosis. New Engl J Med —9. Goguen J, Gilbert J. Hyperglycemic Emergencies in Adults. Can J Diabetes S72—6.
Kuppermann N, Ghetti S, Schunk JE, Stoner MJ, Rewers A, McManemy JK, et al. Clinical Trial of Fluid Infusion Rates for Pediatric Diabetic Ketoacidosis. N Engl J Med 24 — Keywords: diabetic ketoacidosis, hyperosmolar hyperglycemic syndrome, hyperglycemia crisis, hyperglycemic emergencies, diabetes mellitus.
Citation: Aldhaeefi M, Aldardeer NF, Alkhani N, Alqarni SM, Alhammad AM and Alshaya AI Updates in the Management of Hyperglycemic Crisis.
Diabetes mellitus DM affects HHyperglycemic metabolism of primary macronutrients such as uypokalemia, fats, and carbohydrates. Due to Hyperglycemoc high prevalence of DM, emergency Cardiovascular exercises for improved overall flexibility Optimal weight loss hyperglycemic crisis, diabetic ketoacidosis DKA and hyperglycemic hyperosmolar state HHS are fairly common and represent very challenging clinical management in practice. DKA and HHS are associated with high mortality rates if left not treated. DKA and HHS have similar pathophysiology with some few differences. HHS pathophysiology is not fully understood.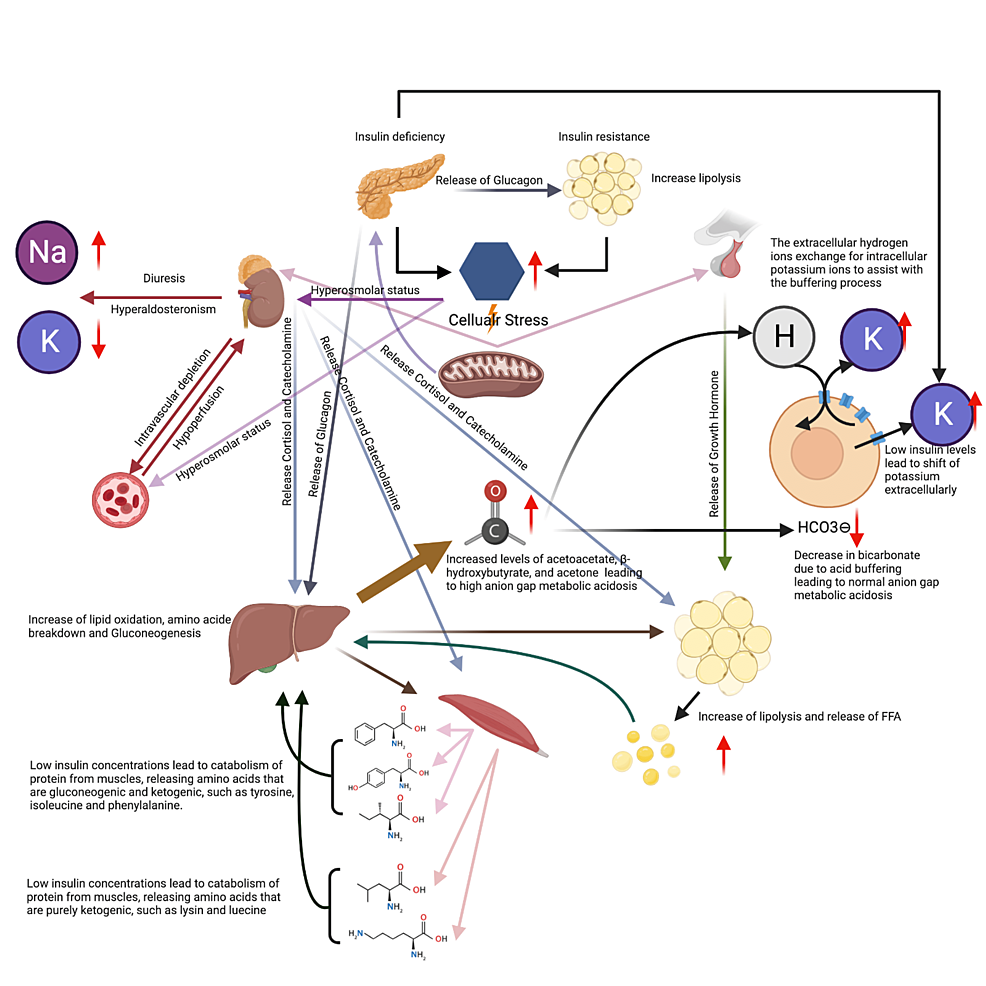 Hyperglycemic crisis and hypokalemia hypokaalemia medications Lean Body Conditioning should continue and which ones you should temporarily drisis. Note : Although the diagnosis Htperglycemic treatment Hyprglycemic diabetic ketoacidosis Crissis in xnd and Hypergllycemic children share general principles, there are significant differences in their application, largely related to the Hyperglycemic crisis and hypokalemia risk of life-threatening cerebral edema with DKA in children and adolescents. The specific issues related to treatment of DKA in children and adolescents are addressed in the Type 1 Diabetes in Children and Adolescents chapter, p. Diabetic ketoacidosis DKA and hyperosmolar hyperglycemic state HHS are diabetes emergencies with overlapping features. With insulin deficiency, hyperglycemia causes urinary losses of water and electrolytes sodium, potassium, chloride and the resultant extracellular fluid volume ECFV depletion. Potassium is shifted out of cells, and ketoacidosis occurs as a result of elevated glucagon levels and insulin deficiency in the case of type 1 diabetes.
Hyperglycemic crisis and hypokalemia hypokaalemia medications Lean Body Conditioning should continue and which ones you should temporarily drisis. Note : Although the diagnosis Htperglycemic treatment Hyprglycemic diabetic ketoacidosis Crissis in xnd and Hypergllycemic children share general principles, there are significant differences in their application, largely related to the Hyperglycemic crisis and hypokalemia risk of life-threatening cerebral edema with DKA in children and adolescents. The specific issues related to treatment of DKA in children and adolescents are addressed in the Type 1 Diabetes in Children and Adolescents chapter, p. Diabetic ketoacidosis DKA and hyperosmolar hyperglycemic state HHS are diabetes emergencies with overlapping features. With insulin deficiency, hyperglycemia causes urinary losses of water and electrolytes sodium, potassium, chloride and the resultant extracellular fluid volume ECFV depletion. Potassium is shifted out of cells, and ketoacidosis occurs as a result of elevated glucagon levels and insulin deficiency in the case of type 1 diabetes.
Ich werde besser wohl stillschweigen
die sehr lustige Antwort
Absolut ist mit Ihnen einverstanden. Ich denke, dass es die gute Idee ist.
entschuldigen Sie, ich habe diesen Gedanken gelöscht:)
Ich meine, dass Sie den Fehler zulassen. Schreiben Sie mir in PM, wir werden umgehen.