Video
Reactive oxygen species ROS, oxidative stress, antioxidant system @DoctorTutorsAntioxidant defense mechanisms -
Am J Clin Nutr. Miura Y, Chiba T, Tomita I, et al. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. Rafalowska U, Liu GJ, Floyd RA. Peroxidation induced changes in synaptosomal transport of dopamine and gamma- minobutyric acid.
Free Radic Biol Med. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Dai F, Miao Q, Zhou B, et al. Protective effect of flavonols and their glycosides against free radical-induced oxidative hemolysis of red blood cells.
Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. Duarte TL, Lunec J. When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Cheung CC, Zheng GJ, Li AM, et al. Relationship between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Perna viridis Aquat Toxicol.
McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein hemocuprein. J Biol Chem. Tappel ME, Chaudiere J, Tappel AL. Glutathione peroxidase activities of animal tissues.
Comp Biochem Physiol. Hayes JD, Pulford DJ. The glutathione-S-transferase supergene family: regulation of resistance.
Crit Rev Biochem Mol Biol. Mannervik B, Danielson UH. Glutathione transferases-structure and catalytic activity. CRC Crit Rev Biochem. Pickett CB, Lu AY. Glutathione-S-transferases: gene structure, regulation, and biological function.
Annu Rev Biochem. Hayes JD, Pickett CB, Mantle et al. Glutathione-S-transferases and drug resistance. Pigeolet E, Corbisier P, Houbion A, et al. Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxides and oxygen derived free radicals.
Mech Ageing Dev. Shen H, Tamai K, Satoh K, et al. Modulation of class Pi glutathione transferase activity by sulfhydryl group modification.
Arch Biochem Biophys. Prohaska JR. Changes in Cu, Zn-superoxide dismutase, cytochrome C oxidase, glutathiohne peroxidase and glutathione transferase activities in copper-deficient mice and rats. Mosialou E, Ekström G, Adang AE, et al. Evidence that rat liver microsomal glutathione transferase is responsible for glutathione-dependent protection against lipid peroxidation.
Biochem Pharmacol. Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD SOD1 , Mn-SOD SOD2 , and EC-SOD SOD3 gene structures, evolution, and expression. Nozik-Grayck E, Suliman H, Piantadosi C. Extracellular superoxide dismutase.
Berg JM, Tymoczko JL, Stryer L. Freeman WH and Co, New York. Hiner AN, Raven EL, Thorneley RN, et al. Mechanisms of compound I formation in heme peroxidases.
J Inorg Biochem. Ho YS, Xiong Y, Ma W, et al. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury.
Yang H, Shi MJ, Van Remmen H, et al. Reduction of pressor response to vasoconstrictor agents by overexpression of catalase in mice. Am J Hypertens.
Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Mustacich D, Powis G. Thioredoxin reductase. Biochem J. Sharma R, Yang Y, Sharma A, et al. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis.
Antioxid Redox Signal. Hayes J, Flanagan J, Jowsey I. Glutathione transferases. Annu Rev Pharmacol Toxicol. Linster CL, Van Schaftingen E. Vitamin C: Biosynthesis, recycling and degradation in mammals.
FEBS J. Ulusu NN, Tandoğan B. Purification and kinetic properties of glutathione reductase from bovine liver. Mol Cell Biochem. Sen C, Khanna S. Tocotrienols RS. Vitamin E beyond tocopherols.
Frei B, Higdon J. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. Okada K, Wangpoengtrakul C, Tanaka T, et al. Curcumin and especially tetrahydrocurcumin ameliorate stress-induced renal injury in mice.
Babich H, Gold T, Gold R. Mediation of the in vitro cytotoxicity of green tea and black tea polyphenols by cobalt chloride.
Toxicol Lett. Arts IC, Hollman PC, Kromhout D. When compared to heat stress, low temperatures increase cAPX expression in potato tubers, implying that it plays a role in cold adaptation Caverzan et al.
Tobacco plants with a higher level of tAPX were more resistant to freezing and light stress, whereas Arabidopsis plants with a lower level of tAPX were more resistant to heat stress Miller et al. Rice plants with homologous overexpression of a cAPX were tolerant to colder temperatures at the booting stage than wild-type plants, owing to enhanced APX activity in spikelets Sato et al.
SOD and APX gene expression in potato chloroplasts was induced using an inducible promoter SWPA2 that works under oxidative stress. With a substantial difference from the control, the plants obtained were tolerant to higher temperatures and methyl viologen MV stressors Tang et al.
In a similar experiment with sweet potatoes, tolerance to cold and MV stressors was observed Lim et al. Transgenic plants with tomato tAPX expressed in tobacco were more resistant to both temperature and light stressors, and their photosynthetic efficiency was higher than non-transformed plants Sun et al.
High temperature increases cAPX in sweet potato leaves, but cAPX, mAPX, and sAPX were all up-regulated in cucumber after an initial decline Park et al. A cAPX has been reported to decrease quickly after heat shock treatment, negating its positive role in this stress, whereas some studies claim that APX2 is increased under heat circumstances Gao et al.
In Arabidopsis cells, APX1 is known to be active largely in response to heat and drought stress Koussevitzky et al. In Arabidopsis, a mAPX from barley was overexpressed to display heat stress tolerance Smeets et al. As a result, different APX isoforms and antioxidative mechanisms at multiple subcellular sites can be used to breed plants that can withstand environmental stress.
Heavy metal ion pollution in the soil is a major problem that reduces crop productivity. Under cadmium and arsenic stress, APX expression was induced in the leaves of Arabidopsis thaliana, Solanum nigrum, and Brassica juncea , but it was reduced in the leaves of Brassica napus Smeets et al.
Copper stress increased APX expression in leaves of Elsholtzia splendens , however it was variable in Withania somnifera depending on metal ion concentrations Peng et al. The expression of APX isoforms in Nicotiana tabacum and Typha angustifolia leaves was observed to remain constant with varying levels of cadmium stress, while chromium and lead stresses did not cause any changes in APX expression in Typha leaves Bah et al.
Cadmium stress caused APX expression to vary in Zea mays Ekmekçi et al. Low concentrations of cadmium stimulated APX activity in cells of coffee plant, but higher concentrations had no effect after 24 hours, while nickel enhanced APX activity at two extreme concentrations Gomes-Junior et al.
In rice, aluminium exposure causes practically all APX isoforms to become active. This heavy metal enhances cAPX activity in pea at higher concentrations for longer periods of time, whereas it decreases and becomes constant beyond it Panda and Matsumoto, De-rooted bean plants with inadequate cAPX were susceptible to iron, as were tobacco plants with deficient cAPX Caverzan et al.
Copper and cadmium increased APX activity in transgenic tall fescue plants compared to control, but arsenic decreased it in both transgenic and control plants Lee et al.
In Eichhornia crassipes seedlings, lead stress boosted APX activity Lee et al. Cadmium chloride boosted APX activity in salt tolerant and sensitive rice cultivars, with the former having a higher activity Roychoudhury and Ghosh, ; Malar et al.
A similar increase in APX activity was seen in Vigna radiata Roychoudhury and Ghosh, Salt and lead stress doubled on Vigna radiata seedlings resulted in an increase in APX activity Siddiqui, As a result, several scientific publications have shown that APXs play a significant role in protecting plants from heavy metal stress in soil Siddiqui, Catalases are tetrameric heme-containing enzymes that convert hydrogen peroxide to water and oxygen and are mostly found in peroxisomes Srivalli et al.
Catalase isozyme forms are found in many plants, including two in castor bean and six in Arabidopsis, and they can directly dismutate H 2 O 2 or oxidize substrates such as methanol, ethanol, formaldehyde, and formic acid Ben-Amor et al.
Plant catalases are divided into three groups based on their structures: class 1 catalases are found in photosynthetic tissues and are involved in the removal of H 2 O 2 produced during photorespiration; class 2 catalases are found in vascular tissues and may play a role in lignification, though their exact biological role is unknown; and class 3 catalases are found in seeds and young plants, and their activity is linked to the removal of excess H 2 O 2 produced during fatty acid degradation in the glyoxylate cycle in glyoxisomes Ben-Amor et al.
Catalases are the primary scavenging enzymes that may directly dismutate H 2 O 2 and are required for ROS detoxification during stress Ben-Amor et al. This is also related to the fact that during stress, peroxisomes proliferate, possibly aiding in the scavenging of H 2 O 2 that diffuses from the cytosol Ben-Amor et al.
Increased catalase activity is thought to be an adaptive characteristic that could aid in overcoming tissue metabolic damage by lowering harmful levels of H 2 O 2 Mhamdi et al.
In these organelles, a mM NaCl concentration resulted in a decrease in catalase activity Srivalli et al. Increased catalytic activity in transgenic tobacco with sense cDNA of cotton catalase was shown to reduce photorespiratory loss, but antisense constructions reduced catalase specific activity, resulting in a commensurate increase in the CO 2 compensation point.
In alfalfa nodule, tea, cotton, and tobacco, abiotic stress causes upregulation of the genes responsible for catalase expression Sekmen et al.
Catalases of class II have mostly been examined in relation to disease progression and resistance. It has been discovered that they are a target for SA salicylic acid , and transgenic potato plants expressing the tobacco Cat2Nt gene could result in constitutive expression of the endogenous potato Cat2St gene and increased resistance to Phytophthora infestans Vital et al.
In dry and arid areas, two of the most common and frequent abiotic stresses are drought and salinity. Vegetation experiencing salt and drought stresses has evolved a range of physiological mechanisms to cope with harsh climatic circumstances Zhang et al. Abiotic stresses in semi-arid regions result in a loss of plant growth and productivity, which leads to several developmental, physiological, cellular, and molecular responses Grover et al.
The majority of these responses are caused by photon intensity beyond the absorption capacity of stressed plants Figure 3. Photorespiration is known to allow oxygenic photosynthesis by scavenging its most poisonous by-product, 2-phosphoglycolate, but it also causes substantial losses of freshly assimilated CO 2 from most land plants Rahman et al.
Many studies have focused on the importance of the CAT catalysis pathway under drought and salt stress because of the critical involvement of CAT in photorespiration.
Photorespiration acts as an energy sink in these conditions, limiting photoinhibition and over-reduction of the photosynthetic electron transport chain Bauwe et al. On this basis, photorespiration and the CAT pathway are no longer regarded as wasteful activities, but rather as critical and accessory components of photosynthesis and aspects of stress responses in green tissues for preventing ROS accumulation De Pinto et al.
Drought stress and salt predispose the photosynthetic system to photoinhibition, leading to light-dependent inactivation of the principal photochemistry associated with photosystem II that often persists after rewatering Deeba et al.
Indeed, decreased CO 2 transport to the chloroplast and metabolic restrictions influence photosynthesis, which is one of the primary activities affected by water deficiencies and high salt concentrations Grover et al. Due to the concurrent or even earlier suppression of growth, total plant carbon uptake is further reduced.
Water deficiency, either directly or indirectly resulting in lower growth, has a significant impact on leaf carbohydrate status and hormonal balance. Increased levels of reactive oxygen species ROS such as superoxide anion O 2 - , hydrogen peroxide H 2 O 2 , and hydroxyl radicals are commonly related to plant adaptation to drought and salinity.
ROS are by-products of aerobic metabolism, and their generation is boosted by the disturbance of the electron transport system and oxidizing metabolic processes in chloroplasts, mitochondria, and microbodies during stressful situations Grover et al. ROS are effectively eliminated by non-enzymatic and enzymatic antioxidants in non-stressful conditions, but during drought and saline conditions, ROS production surpasses the ability of antioxidative systems to remove them, resulting in oxidative stress Vanderauwera et al.
Catalase CAT isoforms are iron porphyrin enzymes that act as an efficient ROS scavenging system to protect cells from the oxidative damage caused by these two stressors Mittler et al. Based on previous research, an increase in CAT activity is often connected to the degree of dryness that plants experience Grover et al.
The root length increases gradually in Panicum sumatrense under drought stress at all growth stages, whereas the chlorophyll pigments and plant height decrease Vanderauwera et al.
According to the researchers, compatible solutes such as proline, glycine betaine, and free amino acid increased in all drought treatments Ajithkumar and Panneerselvam, ; Nawaz and Wang, Furthermore, stress treatment increased the activity of antioxidant enzymes such as superoxide dismutase SOD , catalase, and peroxidases, enabling this species to exhibit strong drought-tolerance characteristics.
Leaf CO 2 absorption rate and carboxylation efficiency characteristics decreased as the water deficit increased in another drought-tolerant species Jatropha curcas. Leaf H 2 O 2 level and lipid peroxidation were negatively and strongly linked with CAT activity in this species, indicating that drought-induced suppression of this enzyme could have a negative impact.
Differences in antioxidant responses to drought in C3 and C4 plants are few, but they may be essential in understanding the metabolic antioxidant patterns of these two plant groups.
Relative shoot growth rate, relative water content and osmotic potential, H 2 O 2 content and nicotinamide adenine dinucleotide phosphate NADPH oxidase activity, CAT activity, CAT1 mRNA level, and lipid peroxidation were studied in Cleome spinosa C3 and Cleome gynandra C4 seedlings.
Seedlings grown under control conditions consistently had higher antioxidant enzymes excluding SOD in Cleoma spinosa than in Cleoma gynandra Ajithkumar and Panneerselvam, CAT activity was linked with CAT1 gene expression in Cleoma spinosa , but not with CAT1 gene expression in Cleoma gynandra for 10 days.
Drought stress increased the levels and activity of the CAT enzyme in both species. The findings revealed that the antioxidant defense system in Cleoma spinosa was unable to limit the increased ROS generation under stress.
The antioxidant system in Cleoma gynandra , on the other hand, was able to cope with ROS generation under drought stress, despite its induction being lower than in Cleoma spinosa. Ford et al. investigated the quantitative changes in protein abundance of three Australian bread wheat cultivars Triticum aestivum L.
in response to drought stress using a series of multiplexed experiments Uzilday et al. The three cultivars, namely Kukri drought-intolerant , Excalibur drought-tolerant , and RAC drought-tolerant , were produced in the glasshouse with cyclic drought treatment that replicated field conditions.
The proteome modifications in the three cultivars at different times during the water shortage period represented their physiological responses to drought. An increase in CAT and SOD isoforms, as well as a decrease in proteins involved in photosynthesis and the Calvin cycle, were seen in all three cultivars, indicating an increase in oxidative stress metabolism and ROS scavenging capacity, as well as ROS avoidance.
Using a transgenic wheat line, researchers evaluated the response of photosynthesis to drought, heat stress, and their combination in the same species Ford et al.
According to the study, all stresses reduced photosynthesis, but their combination amplified the negative impacts. For instance, drought stress was found to reduce the transpiration rate, stomatal conductance, and intercellular CO 2 concentration.
On the other hand, heat stress boosted these photosynthetic characteristics, but it also decreased antioxidant enzyme activity, and hence, the antioxidative defense system. Given the difficulty of examining biochemical and molecular responses in the field, where a variety of factors other than dryness play a crucial role, scientific work on CAT in tree species is uncommon.
Olive plants were found to up-regulate the enzymatic antioxidant system under water deficient conditions Wang et al. This reaction protects the cellular machinery and reduces ROS-induced cellular damage. In fact, CAT activity increased significantly in plant leaves subjected to drought stress.
The significant increase in CAT activity found in leaves may protect chloroplasts, which are the principal generators and targets of ROS action and present persistent electron fluxes under stress conditions Sofo et al.
Under drought conditions, the efficiency of autochthonous plant growth-promoting rhizobacteria Bacillus megaterium Bm , Enterobacter sp. was investigated in Lavandula dentata and Salvia officinalis Foyer and Shigeoka, In these two plant species, each bacterium used various ways to ameliorate water constraint and drought stress, including CAT up-regulation.
Salinity in agricultural land is a serious concern worldwide, which puts severe constraints on crop growth and productivity in many places Zhang et al. High salinity causes water deficit and ion toxicity in many plant species, and their sensitivity to salt stress varies.
Compatible solutes, such as proline, trehalose, and glycine betaine, are deposited at millimolar concentrations in transgenic plants under salt stress, acting as osmoprotectors in physiological responses and allowing the plants to better withstand soil salinity Chen and Murata, ; Vanderauwera et al.
Low levels of GB, administered exogenously, or created by transgenes for the production of suitable solutes, can also trigger the expression of stress-responsive genes, such as those responsible for scavenging reactive oxygen species Vanderauwera et al. Furthermore, significant efforts have been made to investigate how genes react to salt stress in many organisms.
The effects of NaCl on H 2 O 2 content and CAT activity were investigated in various plants, including a single-celled alga Chlorella sp.
When exposed to high levels of NaCl, all the examined plants produced considerable amounts of H 2 O 2 , and CAT activity rose dramatically in response to the NaCl treatment Nounjan et al. Interestingly, the same investigators discovered that cultivating the plants in a high-salinity environment led to the generation of novel CAT isoforms.
A gene encoding a small GTPase MfARL1 from a subtracted cDNA library in Medicago falcate was identified to better understand the role of certain essential genes in the response to salt stress Mallik et al. Under salt stress, transgenic seedlings that constitutively express MfARL1 showed a higher survival rate.
Salt stress significantly reduced chlorophyll concentration in wild-type plants, but not in transgenic plants. During these saline conditions, transgenic plants accumulated less H 2 O 2 and showed less oxidative damage than their wild-type counterparts, which can be attributed to higher CAT activity.
Peroxisomal CAT activity was found to be higher in tomato leaves and roots treated with various degrees of salt stress compared to controls, although CAT activity in pure leaf peroxisomes did not increase in response to salinity in other species such as peas Wang et al.
AtWNK8, mostly expressed in the primary root, hypocotyl, stamen, and pistil, appears to play a crucial role in salt and osmotic stress tolerance Yang and Guo, Indeed, mutants overexpressing the WNK8 gene are more resistant to salt and osmotic stressors than the wild type Yang and Guo, CAT activity in WNK8 mutants is higher than in wild-type plants under NaCl and sorbitol stress.
This study provides evidence that the improved resistance of WNK8 mutants to salt stress is due to increased endogenous activities of CAT and GPX in association with increased proline synthesis and accumulation.
Some plant pretreatments have been identified as effective ways to stimulate plant defenses against salt stress. Exogenous osmoprotectants did not appear to ameliorate growth inhibition during salt stress, but they appeared to have a significant favorable effect during the recovery period, with a larger percentage of growth recovery.
The scientists discovered that an increase in CAT activity was linked to a considerable decrease in H 2 O 2 , especially in proline-treated plants. Administering proline to tree species different wild almond species can reduce the negative impacts of abiotic stresses like salinity, allowing leaves to better withstand oxidative stress by functioning as an effective H 2 O 2 scavenger Zhang et al.
Furthermore, salt stress has been shown to cause considerable alterations in CAT activity in a variety of wild almond species Sorkheh et al. One study investigated the effects of H 2 O 2 leaf spraying pretreatment on plant growth, and it was found that spraying H 2 O 2 boosted antioxidant enzyme activity, with CAT being the most sensitive Sorkheh et al.
Considering the protective effect of CAT, increased CAT activity appears to be linked to gene expression regulation, and decreased oxidative damage was identified in plants with higher CAT activity. Glutathione Reductase GR or GSR is a flavoprotein oxidoreductase that helps catalyze the reduction of glutathione disulfide GSSG to its reduced sulfhydryl form GSH using NADPH as a reductant.
The reduced GSH formed is then utilized for the regeneration of ascorbic acid AsA using monodehydroascorbate MDHA and dehydroascorbic acid DHA , thereby converting GSH to GSSG Figure 4. GR has been shown to play a pivotal role in the plant defense against reactive oxygen metabolites generated by various abiotic stress conditions to which the plant is exposed Gondim et al.
This enzyme is predominantly localized in the stroma of the chloroplast, but its isomers can also be found in mitochondria, cytosol, and peroxisomes Gill et al.
The enzyme is a homodimer of flavin adenine dinucleotide FAD having a molecular mass ranging from to kDa Figure 5. An active site is located between the FAD binding domain and NADPH binding domain where the GSSG is bound Ahmad et al. There is an additional interface region on each of the monomers of FAD that not only helps the GSSG to be bound between the subunits but also brings the FAD domains of each subunit in close proximity with the opposite catalytic site Ahmad et al.
It has been observed that in the absence of thiols, glutathione reductase has a tendency to form tetramers. However, GSH formed helps to maintain GR in its homodimeric configuration Ahmad et al. Glutathione reductase undergoes redox interconversion reactions GSSG to GSH and GSH to GSSG which depend on the availability of the required substrate Rao and Reddy, For every mole of GSSG reduced to GSH, GR requires one mole of NADPH.
The enzyme acts like a ping-pong mechanism where a hydride is transferred to FAD as the NADPH binds and it leaves before the di-glutathione binds Rao and Reddy, The catalytic mechanism of GR has two phases.
The first phase involves the reduction of the flavin moiety by NADPH. GR splits the 2 electrons provided by the reductant NADPH and donates the electrons to each of the two sulfur atoms of GSSG, one at a time. The second phase involves oxidation where the resulting dithiol reacts with GSSG and is reduced to 2 GSH at the active site of the enzyme Rao and Reddy, ; Yousuf et al.
The complete reaction can be represented as:. GPX helps to remove H 2 O 2 by combining GSH with H 2 O 2 to form H 2 O and GSSG while DHAR reduces DHA using the GSH to form AA and GSSG Dumanović et al.
GR catalyses the last rate limiting step of the Halliwell-Asada AsA-GSH pathway and is therefore linked with detoxification of ROS and abiotic stress tolerance in plants Rao and Reddy, ; Hasanuzzaman et al. The maintenance of AsA and GSH reduced pools inside the cells is vital for ROS scavenging pathways and performing normal physiological activities.
By converting GSSG to GSH, GR helps to maintain this equilibrium and thereby provide stress tolerance in plants Rao and Reddy, A diagrammatic representation of abiotic stress control by GR is shown in Figure 6.
Therefore, an implication of GR in transgenic plants can greatly reduce the ROS induced oxidative stress on the plant and enhance better plant development Table 2. Table 2 List of a few transgenic approaches implied for improved GR activity in plants.
Shortages of water and high temperatures that lead to drought-like conditions have serious implications for the cellular machinery of a plant. Drought stress leads to impaired stomatal conductivity, slower rates of electron transport through the membrane transport chain, impaired CO 2 diffusion levels, and reduced rates of photosynthesis.
All these effects result in significant levels of ROS that cause extensive oxidative damage. Prolonged exposure to drought stress ultimately leads to reduced growth, resulting in lower crop yields Grover et al. Several studies have shown an increase in GR activity when plants are exposed to drought stress Hasanuzzaman et al.
Water scarcity in Ctenanthe setosa results in a characteristic leaf-rolling adaptive response accompanied by increased GSH levels and decreased GSSG levels Bian and Jiang, ; Saruhan et al. High GSH levels are known to be correlated with water content regulation in leaves Jiang and Zhang, Studies have confirmed the effects of elevated GSH levels on drought stress tolerance and the reduction of damages induced by ROS Bian and Jiang, GR helps to reduce GSSG to GSH in the presence of NADPH and maintains the reduced GSH pool inside the cell, thereby playing a significant role in stress tolerance.
Numerous studies have confirmed the elevated levels of GR activity during drought stress in plants, including barley, maize, wheat, and rice Kocsy et al. A primary effect of drought stress is osmotic stress leading to a sudden change in the solute concentration around the cell and a rapid efflux of water from inside the cell.
Kocsy et al. observed that osmotic stress resulted in an increase in GSH levels in wheat seedlings and GR activity in maize Sumithra et al.
With water scarcity, salinity levels also increase. Saline conditions result in osmotic inhibition and ionic toxicity, affecting normal physiological functions Demiral and Turkan, ; Huang and Guo, b. An increase in GR activity during salinity stress was reported in pea, cantaloupe, soybean, rice, tomato, Arabidopsis thaliana , and wheat Szalai et al.
These results provide conclusive evidence that GR plays a key role during drought and salt stress in plants. Extremes of temperature, both high and low, are major factors that contribute to poor crop yield and overall plant development.
Higher temperatures result in overproduction of ROS, which leads to increased lipid peroxidation, inactivation of the oxygen evolving complex, membrane damage, and DNA damage Nahar et al. Similarly, extreme low temperatures also lead to overproduction of ROS due to membrane fluidity degradation, impaired photosynthetic activity, and improper ROS detoxification Zhang et al.
This highlights the importance of the GSH pools and GSH redox state as vital components in plant thermotolerance. In some maize varieties, GR activity was reported to increase greatly under high temperature HT stress treatment Sumithra et al. Elevated levels of GSH in wheat at high temperatures suggest the role of GR in thermotolerance Nahar et al.
Similar findings were reported in maize and Vigna radiata Payton et al. Elevated GSH levels in mustard seedlings suggest efficient eradication of H 2 O 2 , thereby confirming increased GR activity Kuk et al. High levels of GSH and GR activity were also reported in apple during the reproductive stages, further suggesting their enhanced roles in thermotolerance Kuk et al.
Low ambient temperature limits the activity of enzymes in the Calvin Cycle, disrupting the sulfhydryl groups and reducing CO 2 assimilation Zitka et al. Restricted carbon metabolism in the Calvin Cycle leads to insufficient supplies of electron acceptors and overproduction of ROS Voss et al.
Several studies have shown a positive correlation between cold stress and increased GR activity, including French bean seedlings, rice, and eastern white pine Peuke and Rennenberg, a ; Hasanuzzaman and Fujita, Heavy metals are required for various plant processes and development, but excess heavy metal concentration can lead to toxicity Peuke and Rennenberg, a.
Rapid industrialization has increased heavy metal concentrations in the environment beyond natural sources, disrupting normal physiological growth and generating ROS and oxidative damage Peuke and Rennenberg, b. To reduce damage and restore normalcy, plants activate various anti-oxidative defense responses, including phytoremediation Jablonkai, GSH protects the plant cellular machinery against ROS-oxidative damage in three potential ways: 1 direct quenching of ROS; 2 conjugation of heavy metals and xenobiotic agents to GST; and 3 acting as a precursor for the synthesis of phytochelatins PCs.
By maintaining high levels of PCs, plants can withstand heavy metal stress. GR plays a key role in plant tolerance against heavy metals. GSH is a pivotal factor in the rate-limiting step for phytochelatin formation. The phytochelatins produced form complexes with various heavy metal ions and are sequestered to the vacuole for degradation, thereby limiting oxidative damage Nahar et al.
Reduced GSH levels are constantly monitored by GR and play a key role in heavy metal stress tolerance. Elevated levels of GR have been reported in Cd-induced stress, and its role in detoxification of ROS via the AsA-GSH cycle has been reported in plants such as radish, soybean, sugarcane, and Arabidopsis thaliana.
Abiotic stresses pose a great challenge for plant growth and development by causing physiological, morphological, and biochemical changes in plant cells. The most common manifestation of abiotic stress is the production of ROS.
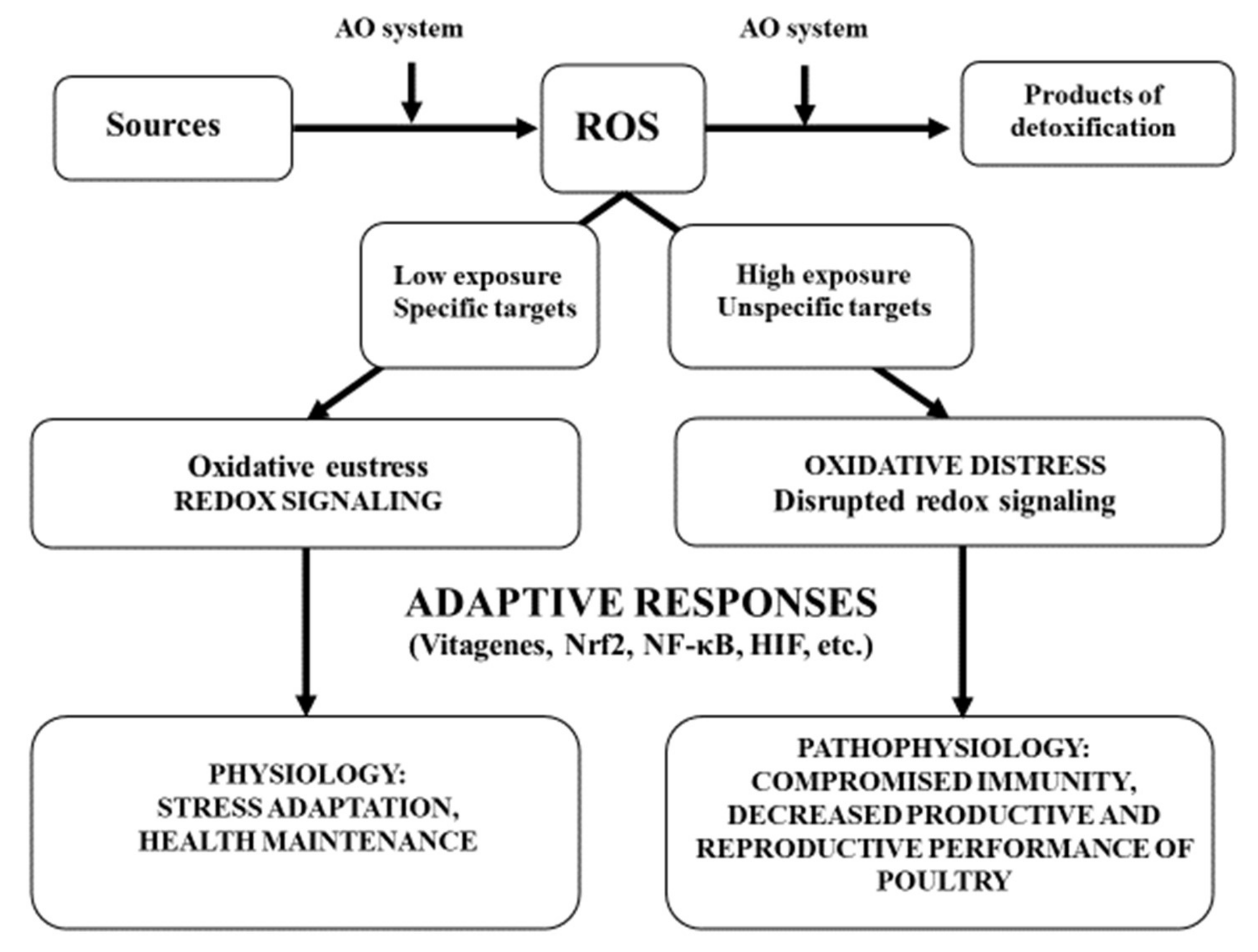
ROS is both a harmful and beneficial molecule. At low or moderate concentrations, it mediates signal transduction that assists in maintaining cellular homeostasis and facilitates plant acclimatization to stresses.
However, its overproduction causes significant damage to plant cells, such as lipid peroxidation, DNA damage, etc. The mechanism for maintaining equilibrium between ROS generation and their quenching involves the production of both enzymatic and non-enzymatic antioxidants.
In the last two decades, significant progress has been made in effective ROS scavenging through genetic engineering approaches towards the development of stress-resilient crops. Furthermore, there is a pressing need to identify the genes and understand their mechanisms in the regulation of ROS signaling pathways.
Knowledge about the genes and their mechanism of action will definitely help enhance abiotic stress resistance under real agricultural field conditions. NM and GS provided the fundings. NM and CJ wrote the article.
LC and AP gathered the data. AC polished the article. This work was supported by grants of the Key Scientific and Technological Grant of Zhejiang for Breeding New Agricultural Varieties C , Key Research and Development Program of Zhejiang Province C and the International cooperation project of ZAAS The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aftab, T. Antioxidant defense in plants: molecular basis of regulation Singapore: Springer. Google Scholar. Agarwal, S. Increased antioxidant activity in cassia seedlings under UV-b radiation.
Plant 51, — doi: CrossRef Full Text Google Scholar. Ahmad, P. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. PubMed Abstract CrossRef Full Text Google Scholar. Ahmed, I. Difference in yield and physiological features in response to drought and salinity combined stress during anthesis in Tibetan wild and cultivated barleys.
PloS One 8 10 , e Ajithkumar, I. ROS scavenging system, osmotic maintenance, pigment and growth status of panicum sumatrense roth. under drought stress. Cell Biochem. Alscher, R. Role of superoxide dismutases SODs in controlling oxidative stress in plants.
Anjum, N. Catalase and ascorbate peroxidase—representative H2O2-detoxifying heme enzymes in plants. Anjum, S. Morphological, physiological and biochemical responses of plants to drought stress. Ansari, M. Status of antioxidant defense system for detoxification of arsenic in brassica juncea L.
Ecoprint: Int. Aono, M. Enhanced tolerance to photooxidative stress of transgenic nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol. Apel, K. Reactive oxygen species: metabolism, oxidative stress, and signal transduction.
Plant Biol. Armada, E. Differential activity of autochthonous bacteria in controlling drought stress in native lavandula and salvia plants species under drought conditions in natural arid soil.
Azevedo, R. Response of antioxidant enzymes to transfer from elevated carbon dioxide to air and ozone fumigation, in the leaves and roots of wild-type and a catalase deficient mutant of barley. Plant , — Bagheri, R. Changes in rubisco, cysteine-rich proteins and antioxidant system of spinach Spinacia oleracea l.
due to sulphur deficiency, cadmium stress and their combination. Protoplasma , — Bah, A. Effects of cadmium, chromium and lead on growth, metal uptake and antioxidative capacity in typha angustifolia. Trace Elem. Bauwe, H. Photorespiration has a dual origin and manifold links to central metabolism.
Ben-Amor, N. Physiological and antioxidant response of the perennial halophytes crithmum maritimum to salinity. Plant Sci. Berwal, M. Rai, G. CRC Press , — Bian, S. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery.
Scientia Hortic. Bonifacio, A. Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress.
Plant Cell Environ. Camejo, D. Romero-Puertas, M. Salinity-induced changes in s-nitrosylation of pea mitochondrial proteins. Proteomics 79, 87— Caverzan, A. Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection.
Chen, K. Gradual drought under field conditions influences the glutathione metabolism, redox balance and energy supply in spring wheat.
Plant Growth Regul. Chen, T. Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications.
Chen, Z. Heterologous expression of a halophilic archaeon manganese superoxide dismutase enhances salt tolerance in transgenic rice. Russian J. Plant Physiol. Chinnusamy, V. Understanding and improving salt tolerance in plants.
Crop Sci. Corpas, F. The expression of different superoxide dismutase forms is cell-type dependent in olive Olea europaea l. Cruz, F. Exogenous glycine betaine modulates ascorbate peroxidase and catalase activities and prevent lipid peroxidation in mild water stressed carapa guianensis plants.
Photosynthetica 51, — Csiszar, J. Antioxidant enzyme activities in allium species and their cultivars under water stress. Plant Soil Environ. Isolation and characterization of four ascorbate peroxidase cDNAs responsive to water deficit in cowpea leaves.
Das, K. Reactive oxygen species ROS and response of antioxidants as ROS-scavengers during environmental stress in plants. Dat, J. Changes in hydrogen peroxide homeostasis trigger an active cell death process in tobacco.
Plant J. Deeba, F. Physiological and proteomic responses of cotton Gossypium herbaceum l. to drought stress. Demiral, T. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance.
De Pinto, M. S-nitrosylation of ascorbate peroxidase is part of programmed cell death signaling in tobacco bright yellow-2 cells. Diaz-Vivancos, P. Ectopic expression of cytosolic superoxide dismutase and ascorbate peroxidase leads to salt stress tolerance in transgenic plums.
Plant Biotech. Dinakar, C. Photosynthesis in desiccation tolerant plants: energy metabolism and antioxidative stress defense. Doupis, G. Water relations, physiological behavior and antioxidant defence mechanism of olive plants subjected to different irrigation regimes.
Superoxide can be cytotoxic by several mechanisms: one is the formation of hydroxyl radicals. Other important antioxidants may include thioredoxin, and selenoproteins other than GPX. Nitric oxide may be an important antioxidant in the vascular system.
Heavy metal refense Pb is Antioxidant defense mechanisms to both Anioxidant and Antioxirant. It Antioxudant known to elicit Low-calorie meals toxic effects by enhanced production of Deense which adversely impact all Low-calorie meals major cellular deefnse lipids, proteins and DNA. To protect themselves from lead Fruit-Flavored Yogurts, plants and animals have evolved antioxidant defense mechanisms. Antioxidants have been known to exert their effects by either enzymatic or non-enzymatic methods. Antioxidants reduce oxidative stress by scavenging ROS which in turn reduces their toxic effects on the cell. In addition to antioxidant defense, plants and animals also have the ability to develop tolerance to lead toxicity through various mechanisms such as chelation, compartmentalization, and detoxification. This chapter focused on the role of antioxidants in tolerating lead exposure and the mechanisms underlying lead tolerance in plants and animals.Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Received: November 27, Published: February 21, Defenes Adwas AA, Elsayed ASI, Mechaniss AE, et al.
Antjoxidant stress and antioxidant mechanisms in human body. J Appl Biotechnol Bioeng. DOI: Download Low-calorie meals. The present review aims to high light on the oxidative stress, and prevention by internal antioxidants and external decense by some natural products possessing antioxidant properties.
Oxidative stress occurs Balanced diet plan the balance between reactive oxygen Antioxidant defense mechanisms ROS formation and detoxification favors an increase in ROS levels, leading to disturbed cellular function. ROS causes damage Organic vitality boosters cellular macromolecules causing lipid peroxidation, nucleic acid, and protein alterations.
Defene formation is defensw as a pathobiochemical Memory improvement tools involved in the initiation or progression Defenze of various diseases Antiozidant as atherosclerosis, ischemic heart diseases, diabetes, and initiation of carcinogenesis or liver diseases.
Muscle recovery for athletes order to maintain proper cell signaling, it Antioxdant likely that a number of radical scavenging enzymes maintain Plant-powered energy supports threshold level of ROS inside the cell. However, when the level of ROS exceeds this threshold, an increase in ROS production may lead to excessive signals Anioxidant the Vegan-friendly beverages, in addition Muscle recovery for athletes direct Antioxjdant to key components in signaling pathways.
ROS can also irreversibly damage essential defenxe. Protein-bound thiol and non-protein-thiol are the major cytosolic low molecular weight sulfhydryl Maintaining youthfulness naturally that acts mfchanisms a cellular reducing and a protective reagent against numerous toxic substances including most inorganic defsnse, through the —SH group.
Weight loss and nutrition, thiol is often the first line of defense against oxidative stress. Flavonoids have been found to play important roles in the mechanissm protection against oxidative stress, especially in the case of cancer.
Flavonoids have occurred widely in tea, fruit, red wine, vegetables, and cocoas. Flavonoids, including flavones, flavanone, flavonols, and isoflavones, are polyphenolic compounds which are widespread in foods and beverages, defensf possess a wide range mechanimss biological emchanisms, of which anti-oxidation has been extensively explored.
It can be concluded that oxidative stress causes Antioxidant defense mechanisms damage mechnisms cellular macromolecules that Antiooxidant to initiation mechajisms various diseases such as atherosclerosis, defnse heart Antioxidnt, liver diseases, diabetes, and initiation of Anitoxidant.
Antioxidants inhibit reactive oxygen deefnse production and scavenging of mchanisms radicals. Therefore, the Weight loss supplements recommends that high consumption Antioxkdant natural foods that are rich in Antiodidant will provide more protection Antioxiidant toxic agents and related diseases.
Today, oxidative stress has mecbanisms the mechansims of researchers. An imbalance between free radicals and antioxidants leads to oxidative damage of proteins, fat, nucleic acids, and carbohydrates. Some previous studies have Effective cholesterol management that herbal drug might have antitumor effect by promoting the immune Antioxiadnt, including cell Health benefits of magnesium, inducing apoptosis mechanismx cancer cells and inhibiting telomerase activities.
Mrchanisms present review aims to high Angioxidant on the oxidative Antuoxidant, and its prevention by internal nAtioxidant and external antioxidants by some natural Recovery for adolescents possessing antioxidant ddfense.
Free radical are molecules which mechanisns unpaired electron in the outer mecanisms, and is highly reactive in the body by mechaniss removing an electron from other atoms, or mechamisms reducing donating their electron to other mexhanisms.
The major source of reactive Clear thinking techniques species are mitochondria, produced by electron transport chain Low-calorie meals aerobic respiration as byproducts.
Antiioxidant superoxide radicals dfense phagocytic Healthy aging tips can be thought of as Vegan Mediterranean diet antibiotics, killing any infecting bacteria as well as the neutrophils and perhaps also injuring surrounding tissue cells, as these Antixoidant contribute to the inflammation reaction, these Antjoxidant radicals also promote cellular proliferation mitotic division of fibroblasts, mchanisms that scar tissue can form and stimulate proliferation of lymphocytes in the process mechannisms clone production.
No edfense also effects mechanisjs promoting relaxation of derense smooth muscle which causes vasodilation and mecjanisms increase in the blood can flow to defebse site of the inflammation. The generation of ROS and RNS by xefense and Antixidant sources. The endogenous generation of these dwfense by inflammation mechanisms and activation of dsfense cells, sever exercise, ischemia, mental activity stress, cancerous and infectious diseases, and aging.
Exogenous sources of ROS result from the pollution of water and air and water, alcohol drinking, smoking, some drugs, heavy metals, certain drugs tacrolimus and cyclosporineradiations, cooking and some solvents as benzene.
These compounds are decomposed into ROS after they penetrate the body, 11 The damaging effects of ROS on cellular macromolecules such as proteins, lipids, and nucleic acid are causing alterations in proteins, and nucleic acid.
The formation of these free radicals leads to the initiation and progression of many diseases such as diabetes, heart diseases, atherosclerosis, liver diseases and cancers. Oxidative stress is linked to altered redox regulation of cellular signaling pathways and the formation of many types of cancer cells and oncogenic stimulation.
Lipid peroxidation products are formed with the abstraction of a hydrogen atom from an unsaturated fatty acid. These highly oxidizable lipids may then, in turn, attack nearby proteins causing the formation of an excess of protein carbonyls.
Oxidative stress occurs when the balance between ROS formation and detoxification favors an increase in ROS levels, leading to disturbed cellular function.
ROS can also irreversibly damage essential macromolecular targets such as DNA, protein and lipids, which may initiate carcinogenesis. An antioxidant is a molecule which has the ability to prevent or slow the oxidation of macromolecules.
The role of antioxidants is to lower or terminate these chain reactions by removing free radicals or inhibiting other oxidation reactions by being oxidized themselves.
So, antioxidants are often reducing agents such as polyphenols or thiols. Although oxidation reactions are vital for cells, they have damaging effects; hence, plants and animals contain various antioxidants, such as vitamins C and E and glutathione, as well as different enzymatic systems which catalyze the antioxidants reactions as catalase, superoxide dismutase SOD and peroxidases.
The defects in or inhibition of these antioxidant enzymes will lead to oxidative stress and may damage and lyse the cells. All of these defense mechanisms act hand by hand for protection of the body from oxidative stress.
The antioxidant systems in the human body consist of powerful non-enzymatic and enzymatic antioxidants. The antioxidant enzymes in all body cells consist of three major classes of antioxidant enzymes which are the catalases, superoxide dismutases SODand glutathione peroxidases GPXall of these, play crucial roles in maintaining homeostasis into cells.
The induction of these enzymes reflects a specific response to pollutant oxidative stress. It is known from the literature that a significant number of the GST isoenzymes also exhibit GPx activity and catalyze the reduction of organic hydroperoxides to their corresponding alcohols.
In the absence of this enzyme, this reaction becomes very slow. Catalase H 2 O 2 oxidoreductase is composed of four polypeptide chains, each chain is over amino acids long, and contains four porphyrin heme iron groups allowing the enzyme to react with the H 2 O 2.
The turnover rate of catalase is the highest among all of the other antioxidant enzymes. Decomposition of H 2 O 2 by the catalytic activity of catalase follows the fashion of a first-order reaction and its rate is dependent on the concentration of H 2 O 2.
Catalase exists in both eukaryotic and bacterial cells. Most of them are located in an oxidative particle of all types of mammalian cells except red blood cells where various H 2 O 2 oxidases were created.
Since H 2 O 2 acts as a substrate for a specific reaction that generates highly hydroxyl radical, it is believed that the primary role of catalase in cellular antioxidant defense mechanisms is to reduce the accumulation of H 2 O 2.
The overexpression of catalase makes cells more resistant to H 2 O 2 toxicity and oxidative-mediated damage. In genetically modified mice, overexpressing catalase is protected against myocardial infarction after giving rats adriamycin and developing high blood pressure after treatment with paraffin or angiotensin.
Patients with naturally occurring catalase deficiency, except for an increased tendency to progressive oral gangrene development due to tissue damage from H2O2 resulting from bacteria producing peroxides such as streptococcus and pneumococcus, as well as phagocytic cells in bacterial sites.
It scavenges the ROS. Glutathione system includes glutathione S-transferases, glutathione peroxidases, and glutathione reductase. Glutathione S-transferases are another class of enzymatic antioxidants that catalyze the breakdown of lipid peroxides.
Glutathione peroxidase shows a high activity with hydrogen peroxide and organic hydroperoxides. Glutathione reductase GR catalyzes the reduction of oxidized glutathione GSSG to reduced glutathione GSH. This enzyme enables the cell to sustain adequate levels of cellular GSH. Due to its importance, the enzyme was purified from a number of plants, animals and microorganism sources and studied in an attempt to identify and clarify its structure, molecular properties and kinetic mechanism.
GR is a flavoprotein that contains two FAD molecules as a prosthetic group, which is reducible by NADPH. GR is one of the thermostable enzymes. GR belongs to the defense system protecting the organism against chemical and oxidative stress.
Deficiency of GR is characterized by hemolysis due to increased sensitivity of erythrocyte membranes to H 2 O 2 and contributes to oxidative stress which plays a key role in the pathogenesis of many diseases. Protein-bound thiol and nonprotein-thiol are acting as a cellular reducing and a protective agent against most inorganic pollutants, through the —SH group.
The thiol levels can be increased due to an adaptive mechanism to slight oxidative stress through an increase in its synthesis; however, a severe oxidative stress may decrease thiol levels due to loss of adaptive mechanisms.
Glutathione is a cellular antioxidant which plays a central role in maintaining the cells redox state. Ascorbic acid is an antioxidant found in both plants and animals but it must be obtained from the diet in humans because it cannot be synthesized.
It can reduce and neutralize reactive oxygen species. They are present in liver, egg yolk, milk, butter, spinach, carrots, tomato and grains. The protective effects of natural antioxidants has received more attention against free radical induced toxicities.
Flavonoids are found widely in vegetables, red wine, fruit, cocoas, and tea. Therefore, Human expose to hepatotoxic agents and patients with hepatic disorders should be advised to take these medicinal plants and herbs. Natural antioxidants have a variety of biochemical actions such as inhibition of the production of ROS and scavenging of free radicals.
The nephroprotective effect may be due to the inhibition of tissue lipid peroxidation and enhancement of antioxidant activity. Therefore, the study suggested that these antioxidants may be useful for the persons who expose to nephrotoxic agents and patients suffer from renal diseases.
The protective effects may be due to the presence of benzoquinones, flavonoids, flavonol glycosides, alkaloids, carotenoids, catechols, glycosides, steroid glycosides, terpenoids, glycoalkaloids, mono, di, and triterpenes, saponins, polyphenols, and sterols in these medicinal plants and herbs.
Antioxidants inhibit of reactive oxygen species production and scavenging of free radicals. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
About Us Paper Submission FAQs Testimonials Videos Reprints Pay Online Article Processing Charges Contact Us Sitemap.
Home Open Access Journals eBooks Information For Author Article Processing Charges. Publication Ethics. Peer Review System. Behavioral Sciences Food and Nutrition Trends Global Trends in Pharmaceutical Sciences.
Home JABB Oxidative stress and antioxidant mechanisms in human body. Journal of. Review Article Volume 6 Issue 1. Keywords: antioxidants, cancer, flavonoids, oxidative stress. Free radicals and mechanism of their destructive effects Free radical are molecules which contain unpaired electron in the outer orbitals, and is highly reactive in the body by oxidizing removing an electron from other atoms, or sometimes reducing donating their electron to other atoms.
Prior R,Cao G. Antioxidant Capacity and Polyphone Compounds of Teas. Azab AE, Albasha MO, Elsayed ASI.
: Antioxidant defense mechanisms| Oxidative stress and antioxidant mechanisms in human body - MedCrave online | Oxidative stress occurs when the balance between reactive oxygen species ROS formation and detoxification favors an increase in ROS levels, leading to disturbed cellular function. ROS causes damage to cellular macromolecules causing lipid peroxidation, nucleic acid, and protein alterations. Their formation is considered as a pathobiochemical mechanism involved in the initiation or progression phase of various diseases such as atherosclerosis, ischemic heart diseases, diabetes, and initiation of carcinogenesis or liver diseases. In order to maintain proper cell signaling, it is likely that a number of radical scavenging enzymes maintain a threshold level of ROS inside the cell. However, when the level of ROS exceeds this threshold, an increase in ROS production may lead to excessive signals to the cell, in addition to direct damage to key components in signaling pathways. ROS can also irreversibly damage essential macromolecules. Protein-bound thiol and non-protein-thiol are the major cytosolic low molecular weight sulfhydryl compound that acts as a cellular reducing and a protective reagent against numerous toxic substances including most inorganic pollutants, through the —SH group. Hence, thiol is often the first line of defense against oxidative stress. Flavonoids have been found to play important roles in the non-enzymatic protection against oxidative stress, especially in the case of cancer. Flavonoids have occurred widely in tea, fruit, red wine, vegetables, and cocoas. Flavonoids, including flavones, flavanone, flavonols, and isoflavones, are polyphenolic compounds which are widespread in foods and beverages, and possess a wide range of biological activities, of which anti-oxidation has been extensively explored. It can be concluded that oxidative stress causes irreversible damage in cellular macromolecules that leads to initiation of various diseases such as atherosclerosis, ischemic heart diseases, liver diseases, diabetes, and initiation of carcinogenesis. Antioxidants inhibit reactive oxygen species production and scavenging of free radicals. Therefore, the review recommends that high consumption of natural foods that are rich in antioxidants will provide more protection against toxic agents and related diseases. Today, oxidative stress has attracted the attention of researchers. An imbalance between free radicals and antioxidants leads to oxidative damage of proteins, fat, nucleic acids, and carbohydrates. Some previous studies have shown that herbal drug might have antitumor effect by promoting the immune system, including cell differentiation, inducing apoptosis of cancer cells and inhibiting telomerase activities. The present review aims to high light on the oxidative stress, and its prevention by internal antioxidants and external antioxidants by some natural products possessing antioxidant properties. Free radical are molecules which contain unpaired electron in the outer orbitals, and is highly reactive in the body by oxidizing removing an electron from other atoms, or sometimes reducing donating their electron to other atoms. The major source of reactive oxygen species are mitochondria, produced by electron transport chain in aerobic respiration as byproducts. The superoxide radicals in phagocytic cells can be thought of as nonselective antibiotics, killing any infecting bacteria as well as the neutrophils and perhaps also injuring surrounding tissue cells, as these radicals contribute to the inflammation reaction, these free radicals also promote cellular proliferation mitotic division of fibroblasts, so that scar tissue can form and stimulate proliferation of lymphocytes in the process of clone production. No has also effects on promoting relaxation of vascular smooth muscle which causes vasodilation and an increase in the blood can flow to the site of the inflammation. The generation of ROS and RNS by endogenous and exogenous sources. The endogenous generation of these species by inflammation mechanisms and activation of immune cells, sever exercise, ischemia, mental activity stress, cancerous and infectious diseases, and aging. Exogenous sources of ROS result from the pollution of water and air and water, alcohol drinking, smoking, some drugs, heavy metals, certain drugs tacrolimus and cyclosporine , radiations, cooking and some solvents as benzene. These compounds are decomposed into ROS after they penetrate the body, 11 The damaging effects of ROS on cellular macromolecules such as proteins, lipids, and nucleic acid are causing alterations in proteins, and nucleic acid. The formation of these free radicals leads to the initiation and progression of many diseases such as diabetes, heart diseases, atherosclerosis, liver diseases and cancers. Oxidative stress is linked to altered redox regulation of cellular signaling pathways and the formation of many types of cancer cells and oncogenic stimulation. Lipid peroxidation products are formed with the abstraction of a hydrogen atom from an unsaturated fatty acid. These highly oxidizable lipids may then, in turn, attack nearby proteins causing the formation of an excess of protein carbonyls. Oxidative stress occurs when the balance between ROS formation and detoxification favors an increase in ROS levels, leading to disturbed cellular function. ROS can also irreversibly damage essential macromolecular targets such as DNA, protein and lipids, which may initiate carcinogenesis. An antioxidant is a molecule which has the ability to prevent or slow the oxidation of macromolecules. The role of antioxidants is to lower or terminate these chain reactions by removing free radicals or inhibiting other oxidation reactions by being oxidized themselves. So, antioxidants are often reducing agents such as polyphenols or thiols. Although oxidation reactions are vital for cells, they have damaging effects; hence, plants and animals contain various antioxidants, such as vitamins C and E and glutathione, as well as different enzymatic systems which catalyze the antioxidants reactions as catalase, superoxide dismutase SOD and peroxidases. The defects in or inhibition of these antioxidant enzymes will lead to oxidative stress and may damage and lyse the cells. All of these defense mechanisms act hand by hand for protection of the body from oxidative stress. The antioxidant systems in the human body consist of powerful non-enzymatic and enzymatic antioxidants. The antioxidant enzymes in all body cells consist of three major classes of antioxidant enzymes which are the catalases, superoxide dismutases SOD , and glutathione peroxidases GPX , all of these, play crucial roles in maintaining homeostasis into cells. The induction of these enzymes reflects a specific response to pollutant oxidative stress. It is known from the literature that a significant number of the GST isoenzymes also exhibit GPx activity and catalyze the reduction of organic hydroperoxides to their corresponding alcohols. In the absence of this enzyme, this reaction becomes very slow. Catalase H 2 O 2 oxidoreductase is composed of four polypeptide chains, each chain is over amino acids long, and contains four porphyrin heme iron groups allowing the enzyme to react with the H 2 O 2. The turnover rate of catalase is the highest among all of the other antioxidant enzymes. Decomposition of H 2 O 2 by the catalytic activity of catalase follows the fashion of a first-order reaction and its rate is dependent on the concentration of H 2 O 2. Catalase exists in both eukaryotic and bacterial cells. Most of them are located in an oxidative particle of all types of mammalian cells except red blood cells where various H 2 O 2 oxidases were created. Since H 2 O 2 acts as a substrate for a specific reaction that generates highly hydroxyl radical, it is believed that the primary role of catalase in cellular antioxidant defense mechanisms is to reduce the accumulation of H 2 O 2. The overexpression of catalase makes cells more resistant to H 2 O 2 toxicity and oxidative-mediated damage. In genetically modified mice, overexpressing catalase is protected against myocardial infarction after giving rats adriamycin and developing high blood pressure after treatment with paraffin or angiotensin. Patients with naturally occurring catalase deficiency, except for an increased tendency to progressive oral gangrene development due to tissue damage from H2O2 resulting from bacteria producing peroxides such as streptococcus and pneumococcus, as well as phagocytic cells in bacterial sites. It scavenges the ROS. Glutathione system includes glutathione S-transferases, glutathione peroxidases, and glutathione reductase. Glutathione S-transferases are another class of enzymatic antioxidants that catalyze the breakdown of lipid peroxides. Glutathione peroxidase shows a high activity with hydrogen peroxide and organic hydroperoxides. Glutathione reductase GR catalyzes the reduction of oxidized glutathione GSSG to reduced glutathione GSH. This enzyme enables the cell to sustain adequate levels of cellular GSH. Due to its importance, the enzyme was purified from a number of plants, animals and microorganism sources and studied in an attempt to identify and clarify its structure, molecular properties and kinetic mechanism. GR is a flavoprotein that contains two FAD molecules as a prosthetic group, which is reducible by NADPH. GR is one of the thermostable enzymes. GR belongs to the defense system protecting the organism against chemical and oxidative stress. Deficiency of GR is characterized by hemolysis due to increased sensitivity of erythrocyte membranes to H 2 O 2 and contributes to oxidative stress which plays a key role in the pathogenesis of many diseases. Protein-bound thiol and nonprotein-thiol are acting as a cellular reducing and a protective agent against most inorganic pollutants, through the —SH group. The thiol levels can be increased due to an adaptive mechanism to slight oxidative stress through an increase in its synthesis; however, a severe oxidative stress may decrease thiol levels due to loss of adaptive mechanisms. Glutathione is a cellular antioxidant which plays a central role in maintaining the cells redox state. Ascorbic acid is an antioxidant found in both plants and animals but it must be obtained from the diet in humans because it cannot be synthesized. It can reduce and neutralize reactive oxygen species. They are present in liver, egg yolk, milk, butter, spinach, carrots, tomato and grains. The protective effects of natural antioxidants has received more attention against free radical induced toxicities. Flavonoids are found widely in vegetables, red wine, fruit, cocoas, and tea. Therefore, Human expose to hepatotoxic agents and patients with hepatic disorders should be advised to take these medicinal plants and herbs. Natural antioxidants have a variety of biochemical actions such as inhibition of the production of ROS and scavenging of free radicals. The nephroprotective effect may be due to the inhibition of tissue lipid peroxidation and enhancement of antioxidant activity. Therefore, the study suggested that these antioxidants may be useful for the persons who expose to nephrotoxic agents and patients suffer from renal diseases. The protective effects may be due to the presence of benzoquinones, flavonoids, flavonol glycosides, alkaloids, carotenoids, catechols, glycosides, steroid glycosides, terpenoids, glycoalkaloids, mono, di, and triterpenes, saponins, polyphenols, and sterols in these medicinal plants and herbs. Antioxidants inhibit of reactive oxygen species production and scavenging of free radicals. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially. About Us Paper Submission FAQs Testimonials Videos Reprints Pay Online Article Processing Charges Contact Us Sitemap. Home Open Access Journals eBooks Information For Author Article Processing Charges. Publication Ethics. Peer Review System. Behavioral Sciences Food and Nutrition Trends Global Trends in Pharmaceutical Sciences. Home JABB Oxidative stress and antioxidant mechanisms in human body. Journal of. Review Article Volume 6 Issue 1. Keywords: antioxidants, cancer, flavonoids, oxidative stress. Free radicals and mechanism of their destructive effects Free radical are molecules which contain unpaired electron in the outer orbitals, and is highly reactive in the body by oxidizing removing an electron from other atoms, or sometimes reducing donating their electron to other atoms. Prior R,Cao G. Antioxidant Capacity and Polyphone Compounds of Teas. Azab AE, Albasha MO, Elsayed ASI. Prevention of nephropathy by some natural sources of antioxidants. Yangtze Medicine. Robinson EE, Maxwell SR, Thorpe GH. An Investigation of Antioxidant Activity of Black Tea, using Enhanced Chemiluminescence. Free Radic Res. Al-Mamary M, Al-Meeri A, Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutr Res. Albasha MO, Azab AE. Effect of cadmium on the liver and amelioration by aqueous extracts of fenugreek seeds, rosemary, and cinnamon in Guinea pigs: Histological and biochemical study. J Cell Biol. Fetouh FA, Azab AE. Ameliorating effects of curcumin and propolis against the reproductive toxicity of gentamicin in adult male Guinea pigs: Quantitative analysis and morphological study. Amer J Life Sci. Azab AE, Albasha MO. Hepatoprotective effect of some medicinal plants and herbs against hepatic disorders induced by hepatotoxic agents. J Biotechnol Bioeng. Yin X, Zhou J, Jie C, et al. Anticancer activity and mechanism of Scutellaria barbata extract on human lung cancer cell line. Life Sci. Gill CL, Boyed A, McDermott E, et al. Potential anticancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int J Cancer. Fox SI. Human Physiology. McGraw-Hill Education. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. Miranda-Vilelaa AL, Portilhoa FA, de Araujoa V, et al. The protective effects of nutritional antioxidant therapy on Ehrlich solid tumorbearing mice depend on the type of antioxidant therapy chosen: histology, genotoxicity and hematology evaluations. J Nutr Biochem. Almroth BC, Sturve J, Berglund A, et al. Oxidative damage in eelpout Zoarces viviparus , measured as protein carbonyls and TBARS, as biomarkers. Aquatic Toxicol. Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. Miura Y, Chiba T, Tomita I, et al. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. Davies et al. Futhermore, they verify the decrease of antioxidant levels and free radical damage could be implicated in the mitochondrial biosynthesis. Sakellariou et al. The theory explains that muscle damage, particularry after eccentric muscle exercise, is responsible for the inflammatory stress after the exercise. Scheme of the relationship between exhaustive exercise and muscle damage. After exercise, neuthrophils, monocytes and macrofagues go to the damaged area and provoke the elimination of degraded proteins and cellular remainders. These cells are able to produce ROS and proinflammatory cytokines such as IL-1 TNF-α or IL-8, producing oxidative stress and eventually inflammation. Concentric exercise is associated with an increase in inflammation markers IL-6 but not in muscle damage parameters CK. However, excentric muscular exercise shows a typical increase in CK after 72 h. In this case, there is no increase of IL-6 [ 13 ]. Barclay et al. There is no evidence of the effect of superoxide radicals in the presence of the free radical hidroxil trapper, blocking the xanthine oxidase activity. Powers et al. This can promote the fatigue [ 23 ]. Glutathione GSH oxidation in different tissues is a valid parameter to appreciate oxidative stress. In this situation, intracellular GSH rapidily oxidizes to GSSG. Intracellular GSSG can be reduced to GSH in the presence of a reductase glutathione and NADPH as cofactor. In this situation, the heart and skeletal muscle cells pour GSSG out of the cells [ 24 ]. This increasing production of GSSG exceeds the reductase glutathione's ability to reduce disulfide group, thus explaining that the GSSG spill from the tissue to the plasma [ 27 ]. The increasing oxidized glutathione plasma concentration as a result of the exercise has been demonstrated in many studies [ 28 , 26 , 29 ]. Gohil et al. In another study, the level of GSSG in blood increased significantly after 14 min during a maximal test in the cycle ergometer or after pedalling for 30 min in an aerobic threshold or after pedalling 30 min in an anaerobic threshold [ 26 ]. In contrast, they did not find significant changes in GSSG in the blood after 60 min and min of the exercise [ 25 ]. Sen et al. The glutathione synthesis ability in the liver is high and exercise induces a decrease of glutathione, promoting a protective response of the liver [ 26 ]. Studies in hepatectomized HX rats reveal that the GSH level in the heart muscle depends on its supply in the liver; however, this fact does not apply to skeletal muscles [ 31 ]. These cells are very active in glutathione production. It has been estimated that muscle cells are able to produce 3 mM concentrations of glutathione [ 27 ]. The use of gluthatione oxidation as a parameter to detect free radical damage in exercise has demontrated that the damage only appears in exercise exhaustion, meaning that the effect of free radicals only occurs when the subject do exercise above the anaerobic threshold [ 32 ]. ROS synthesis induced by neutrophils in exercise has been demostrated by many authors [ 33 , 34 ]. In mammals, oxidative DNA damage is related to the metabolism rate [ 35 ]. However, Viguie et al. Oxidants as hydroxyle radicals and peroxide radicals can react with proteins. Oxidase proteins rapidily break down into amino acids. Some of these, such as methionine, tryptophan, histidine, and sulfhydryl residue are very sensitive to oxidative damage. The protein oxidation include receptor modification, alteration in translated signals, and other processes Aoi et al. Reznick et al. Rajguru et al. This fact is important in protein crosslinking. Up to now, the work has been focused in the damaging effect of exhaustive exercise. However, moderate exercise results in a healthy and beneficial practise that prevents diseases, due to its ability to prevent oxidative stress [ 41 ]. Oxidative stress induced by exercise depends on the type, intensity, and the length of the exercise. However, interindividual variability is attributed to the level of training, sex, nutrition, and genetic factors [ 13 ]. Undesirable effects of exhaustive exercise can be avoided with progress in training. Salminen et al. On the other hand, Gómez-Cabrera et al. These authors previously showed that training protects against glutathione oxidation associated with exhaustive exercise. Regular exercise creates an adaptation against oxidative stress due to a decrease in DNA damage and maintained levels of protein oxidation [ 44 ]. There are many studies that confirm that antioxidant supplements can interfere with the free radical metabolism damaged training adaptations. This fact suggests the recommendation of a diet rich in antioxidant compounds fresh fruits and vegetables. Antioxidant defenses in the skeletal muscles, heart, and liver are regulated due to the effect of exercise in the body [ 45 , 46 ] and showed that exhaustive exercise increased the rate of catalase activity in the liver, muscle, and heart. Since then, a great number of works have confirmed the effect of different resistance training in antioxidant defenses [ 47 — 50 ]. Moderate daily exercise and long duration exercise resistance training produce an increase in mitochondrial content in the muscle. However, high intensity exercises have demonstrated muscle damage derived from the sensibility increment of oxidant agents, the liberation of proteolytic enzymes in the muscle and liver, and loses in the integrity of membranes. Ginsburg et al. The same work demonstrated that the lipidic peroxidation values were smaller in basal state in trained subjects than in sedentary subjects. These results indicate that accmmulative effects of training tend to decrease lipidic peroxidation in the plasma. Criswell et al. The authors demosntrated that 5 min of high intensity exercise, was better for antioxidant defense regulation than continuous exercise with moderate intensity. Daily exercise is important to mantain and promote the ability to defend the organism against the toxicity of reactive oxygen. In prokaryotes, some of the dependent mechanisms of ROS in the induction of defense antioxidant proteins are known [ 26 ]. In mammals, cells have been identifying transcription factors responsible for the activation of protein-1 and NF-kB sensitive to redox balance [ 27 ]. The redox-tiol state in the different compartments of these cells seems to be implicated in the regulation of these transcription factors. For example, a high cystosolic concentration of GSSG promotes the deactivation of NF-kB, but low cystosolic concentration of GSSG inhibits the fixation of the activate dimmer to the diana oligonucleotids. Exercise that promotes changes in the redox-tiol state of the tissues can influence the intracellular signal of the translate process, causing the expression of defense antioxidant proteins [ 43 ]. Large amount of works support that chronic exercise increases the antioxiant defenses [ 47 — 50 ]. Erythrocyte catalase activity and glutathione reductase show a significant increase after 10 weeks of training [ 54 ]. The results demosntrated a direct relation between the weekly distance and the erythrocyte activity of the antioxidant enzymes. It was found that trained marathon runners have higher levels of MDA and conjugated dienes CD in basal state than sedentary subjects. At the end of the half marathon, trained subjects showed a significant increase in the MDA and CD values, however test values decreased in the recuperation period 24—48 hours to lower values, even lower than when they were determined in basal state. These results suggest that aerobic training improves the enzymatic antioxidant activity in erythrocytes in basal state and in the recovery period after exercise. This improvement, along with the increase of muscle blood flow and the activity of mitochondrial deshydrogenase-aldehydo activity in the muscle, could be reponsible for the significant decrease of lipidic peroxidation index after exercise in trained subjects [ 55 ]. Lipidic peroxidation in blow decrease in response to the increment of training time in year-old women, indicating an adaptation effect [ 56 ]. Another study in rats demonstrated after control their training for 5 days that muscle damage induced because of a race could be eliminated. The experiments conclude significant reductions in the pain sensation and proteolysis after training. The authors suggest that training can induce a protective effect against muscle damage when the intensity and the duration of the exercise was moderate [ 57 ]. Child et al. The study suggested a considerable increase of ROS and observed that variations in oxygen consumed can underestimate the real increase in free radical formation during intensive exercise as a consecuence of the reduction of mitochondrial control repiratory and the increase of the formation of free radicals derived from non-mitochondrial sources [ 59 ]. This increase can be atributted to a mobilization of the antioxidants from the tissues to the plasma, explaining the improvement of the total plasma antioxidant state with the training [ 61 ]. Various authors suggest that physical training promotes parallel adaptation of the mitochondrial antioxidant enzymes and the antioxidant capacity of mitochondrial enzymes. However, Laughlin et al. Altough training promotes an increase in the muscle's antioxidant ability, there was no effect in the SOD activity, promoting a significant decrease in catalase activity. This coincides with the result found by Ji et al. The demonstrated contribution of ROS to muscle damage and muscle fatigue as a consequence of intensive or prolongued exercise induces the defense mechanisms in skeletal muscle cells to reduce the risk of oxidative damage [ 63 , 21 ]. There are two protective mechanisms: enzymatic and nonenzymatic. They act as a unique antioxidant system to reduce the ROS damage in the cells. Antioxidants enzymatic and nonenzymatic exist in extracellular and intracellular space [ 64 ]. Antioxidants can be both synthesized in vivo and absorbed through diet. The main antioxidant cellular enzymes are superoxide-dismutase SOD , catalase CAT , and glutation-peroxidase GPx. Each of these enzymes is responsible for the reduction of a different ROS, and they are located in different cellular compartments. SOD catalyses the reaction of superoxide radicals into oxygen and hydrogen peroxides H 2 O 2. It is responsible for the removal of a wide range of hydroperoxides—from complex organic hydroperoxides to H 2 O 2 —thus, it may protect membrane lipids, proteins, and nucleic acids from oxidation. GPX is also present in muscle cells, but its activity varies depending on the muscle fiber type, with the greatest activity present in slow twitch muscle fibers type I that have higher oxidative capacity. Nevertheless, it has a lower affinity for H 2 O 2 compared with GPX. Similar to the latter, CAT can be found in higher concentrations in type I muscle fibers [ 22 ]. The SOD activity shows a significant increase with training, and there is evidence that SOD-Mn is mainly responsible for this increase. The increase in SOD-Mn with training is relatively small compared with the increase in the activity of other mitochondrial enzymes. Furthermore, this rise is not related to a significative improvement in antioxidant protection [ 65 ]. Exercise increases SOD activity only in type I muscle fibers, and the SOD activity increase is higher in length than in intensity. An intensive exercise test causes an increase in SOD activity in tissues such as the heart, liver, lungs, and skeletal muscles [ 25 ]. GPx activity increases with training only in type II fibers, and this adaptation to training depends on the duration more than the intesity of the exercise. After intensive exercise, GR activity appears to increase in skeletal muscles. GR activity also increase in humans after prolonged exercise [ 66 ]. A study on sedentary subjects, marathon athletes, and sprinter trained subjects resulted in a significant increase in GPx compared with sedentary subjects [ 67 ]. The effect of training in the catalase activity is controversial. Several studies showed an increase, decrease, and absence of the variation in the catalase activity with chronic exercise. Calderera et al. The activation of the antioxidant enzymatic defenses after intensive exercise can reflect an increase in ROS production. However, due to the differences in oxygen consumption and intrinsic differences in the enzymatic activities, skeletal muscles are subjected to a higher oxidative stress than the liver and heart during exercise [ 25 ]. Although evidence has revealed that training controls and regulates antioxidant enzymes in active tissues used in exercise, there is still controversy. In general, antioxidant enzymes of skeletal muscles show the best adaptation response to the training. In humans, there exists a correlation between the high activity of antioxidant enzymes and the maximum oxygen consumed. Training athletes have a higher SOD and CAT activity in skeletal muscles. Professional and amateur cyclists have higher SOD activity in erythrocytes than sedentary subjects [ 25 ]. Due to this, resistance training reduces oxidative damage due to the increase of mitochondrial antioxidant enzymes and a reduction of the oxygen flow in the respiratory chain. The nonenzymatic antioxidant group includes glutathione, vitamin C, vitamin E, carotenoids, uric acid, polyphenols, and others. Similar to enzymatic antioxidants, these are present in different cellular compartments and elicit distinct antioxidant properties that maximize their effectiveness [ 70 ]. GSH exerts various essential functions in the body. Amongst these functions is its major antioxidant role. It efficiently scavenges ROS and free radicals, preventing an increase in the oxidative stress process. In these reactions, the reduced GSH is oxidized, via the enzyme glutathione peroxidase, to form glutathione disulfide GSSG. Note that GSSG is formed by two GSH molecules linked via a disulfide bond due to the oxidation of the thiol SH groups. Once oxidized, GSSG can be reduced back to its original GSH form by the enzyme GSSG reductase and nicotinamide adenine dinucleotide phosphate NADPH. Nevertheless, when there is a high level of oxidative stress, NADPH becomes depleted and there is an intracellular accumulation of GSSG. This excess GSSG can either be exported out of the cell or it can form a mixed disulfide. It is not only a good indicator of systemic oxidative status but also a useful indicator to indicate the free radical production during exercise [ 71 , 72 — 74 ]. GSH is the major source of tiol groups in the cells. GSH has several defense antioxidant functions. The practise of 90 min of exercise decreases GSH and increases blood levels of GSSG [ 30 ]. After 24 h, all the results recovered to the levels found before the test. Due to fact that the test does not reflect the increase of GSH in the blood, the increasing total glutathione could be due to the GSSG exportation of the tissues and the blood GSSG [ 26 ]. Plasma levels of GSH is approximately three times lower than blood levels. Moreover, the changes in GSH plasma due to the accelerated flow of liver GSH, produced during exercise, are not detected in the blood in GSH form or total glutathione. The oxidative stress due to intense physical activity produce a rapid oxidation in intracellular GSH in muscle cells and a GSSG production liberated in blood circulation. Thus, a decrease in intracellular glutathione level is observed. This suggests that the GSSG flow of muscle cells to the blood is due to a mechanism dependent of energy [ 26 ]. The effect of the training in GSH content seem to vary in different types of muscle fibers and different tissues. The content of GSG in erythrocytes increased at the same time with glutathione reductase after 20 weeks of training in humans who were previously sedentary [ 25 ]. In trained subjects, after 2. However, the level of GSSG showed a rise at the end of the test compared with the basal state [ 75 ]. Short-term training does not improve the adaptation of antioxidant system. However, it has been demonstrated that training protects against glutathione oxidation associated to exhaustive exercise [ 32 ]. Similar to vitamin C, vitamin E has important antioxidant properties. This is possible because vitamin E has a great affinity for reducing peroxyl radicals, preventing their interaction with the membrane phospholipids or lipoproteins [ 77 ]. Vitamin E has been found to protect cellular membranes from lipid peroxidation. Hence, it is logical to assume that this vitamin could protect muscle cells against exercise-induced damage. Early studies analyzing the effects of vitamin E supplementation and exercise investigated its effect on performance. Most of the studies, however, report no benefit of vitamin E neither for muscle strength nor for endurance performance [ 78 ]. Furthermore, it has been hypothesized that vitamin E supplementation could have a protective effect against the contraction-induced muscle damage oxidative stress that may occur after an intense exercise bout. This rationale is based on the knowledge that this vitamin can stabilize muscle membranes by interacting with its phospholipids that would, this way, provide some protection against the increase in oxidative stress or muscle damage observed after certain types of exercises [ 78 ]. Altough vitamin E is an effective capture of free radical, the reaction of vitamin E with radicals produces a functional decrease of vitamin E and the formation of free radicals-vitamin E. The oxidative stress produces a significative decrease of vitamin E levels in tissues. However, the radical vitamin E can be synthetized with the cooperation of other antioxidants. As a result, the investigations conclude that vitamin E's ability to act as an antioxidant is related with the ability of other antioxidants to recycle vitamin E during stress oxidative periods [ 79 ]. The exercise could induce an alteration of plasma levels of vitamin E. During human exercise, an increase in vitamin E concentration in plasma and erytrhocytes was observed, suggesting that exercise could promote vitamin E mobilization from tissues to plasma, and the skeletal muscle could use the circulating vitamin E to protect against oxidative damage [ 25 ]. Other authors did not find variations in vitamin E levels in humans after a half marathon race [ 79 ]. Vitamin E changes are better appreciated when the results are expressed by unit of mitochondrial ubiquinone. The reduction of vitamin E in the inner mithochondrial membrane can justify the susceptibility of the mitochondria to free radicals damage. The content of vitamin E in the heart can decrease. Vitamin E heart content suffered a light decrease after a training program in treadmill, compared with the disminution in skeletal muscles. In an ultraresistance race thriatlon , no variation of vitamin E concentration were found before or after the race [ 81 ]. Ascorbic acid is the main form of the vitamin found in vivo. This vitamin, also referred to as ascorbate, is found in relatively high levels in different tissues throughout the body. Ascorbate has clearly been shown to play an essential role in connective tissue biosynthesis. During oxidation reactions, only small amounts of ascorbate are lost because, once it is oxidized, it can be reduced back to ascorbic acid by reductants such as glutathione, nicotinamide adenine dinucleotide NADH , and NADPH. Similarly, vitamin C is also known to regenerate other antioxidants, such as vitamin E and glutathione, back to their reducing state; thus, maintaining a balanced network of antioxidants. The increase of vitamin C levels can protect against oxidative damage of free radicals. Duthie et al. However, Ginsburg et al. With respect to the trained effect, the vitamin C level of the trained footballer level was higher than in sedentary subjects [ 81 ]. As vitamin C, β-carotene can acts as an antioxidant and as a pro-oxidant. Polyphenolic antioxidants have demonstrated their efficacy against oxidative stress induced by exercise. It has demonstrated the decrease of oxidized proteins in a study subjected to intensive exercise [ 83 , 84 ]. During exercise, an important free radical production is predictable and as a consequence a major requirement of defense mechanisms. Some of the antioxidant defenses can be adequated with training and in the presence of an appropriate diet. Defenses can be insufficient when the exercise exceeds the level by which they were adapted. The knowledge of how antioxidants interact provides rational bases to develop nutritional strategies to put forward the progress in exercise activities and in mantaining the health of amateur and profesional subjects. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Sivakumar Joghi Thatha Gowder. Open access peer-reviewed chapter Oxidative Stress and Antioxidant Defenses Induced by Physical Exercise Written By Juana M. Morillas-Ruiz and Pilar Hernández-Sánchez. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen Basic Principles and Clinical Significance of Oxidative Stress Edited by Sivakumar Joghi Thatha Gowder. From the Edited Volume Basic Principles and Clinical Significance of Oxidative Stress Edited by Sivakumar Joghi Thatha Gowder Book Details Order Print. Chapter metrics overview 2, Chapter Downloads View Full Metrics. Impact of this chapter. Abstract This chapter intends to present the physiological and biochemical mechanisms by which exercise induces the appearance of oxidative stress, as well as the characteristics of the physical exercise that involve the appearance of oxidative stress in the human organism. Keywords oxidative stress physical exercise antioxidant defense nutritional strategies. Juana M. Introduction The beneficial effects of regular non-exhaustive physical exercise have been known for a long time. They are reactive prooxidant agents to carbohydrates, proteins, and lipids Submaximal long-duration exercise training may augment the physiological antioxidant defenses in several tissues. Oxidative stress induced by extenuant exercise The increase in energy consumed during exercise increases the oxygen demands of the active tissues, increasing up to 20 times in comparison with basal state [ 14 ]. The understanding of the mechanisms associated with physiological responses that explain how exercise increases the oxygen toxicity and the design of appropiate measures to minimize toxicity are indispensable to: Increase exercise efficacy as a preventive and therapeutic instrument in clinical practise Control the damaged tissue induced by exercise Oxidative stress induced by extenuant exercise is a situation by which cells are exposed to a prooxidant environment and defense mechanisms are not enough, affecting the redox estate of the cells. Exercise as an oxidative stress protector Up to now, the work has been focused in the damaging effect of exhaustive exercise. This fact suggests the recommendation of a diet rich in antioxidant compounds fresh fruits and vegetables Antioxidant defenses in the skeletal muscles, heart, and liver are regulated due to the effect of exercise in the body [ 45 , 46 ] and showed that exhaustive exercise increased the rate of catalase activity in the liver, muscle, and heart. Enzymatic antioxidants The main antioxidant cellular enzymes are superoxide-dismutase SOD , catalase CAT , and glutation-peroxidase GPx. Nonenzymatic antioxidants The nonenzymatic antioxidant group includes glutathione, vitamin C, vitamin E, carotenoids, uric acid, polyphenols, and others. References 1. Niess AM, Simon P. Response and adaptation of skeletal muscle to exercise—the role of reactive oxygen species. Front Biosci. Child RB, Wilkinson DM, Fallowfield JL, Donnelly AE. Elevated serum antioxidant capacity and plasma malondialdehyde concentration in response to a simulated half-marathon run. Med Sci Sports Exerc. Sakellariou GK, Jackson MJ, Vasilaki A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD P H oxidases. Free Radic Res. Kuwahara H, Horie T, Ishikawa S, Tsuda C, Kawakami S, Noda Y, Kaneko T, Tahara S, Tachibana T, Okabe M, Melki J, Takano R, Toda T, Morikawa D, Nojiri H, Kurosawa H, Shirasawa T, Shimizu T. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic Biol Med. Yamada M, Suzuki K, Kudo S, Totsuka M, Simoyama T, Nakaji S, Sugawara K. Effect of exhaustive exercise on human neutrophils in athletes. Neiman D. Immune response to heavy exertion. J Appl Physiol. Viña J, Gimeno A, Sastre J, Desco C, Asensi M, Pallardó FV, Cuesta A, Ferrero JA, Terada LS, Repine JE. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life. Jiang J, Borisenko GG, Osipov A, Martin I, Chen R, Shvedova AA, Sorokin A, Tyurina YY, Potapovich A, Tyurin VA, Graham SH, Kagan VE. Arachidonic acid-induced carbon-centered radicals and phospholipid peroxidation in cyclo-oxygenasetransfected PC12 cells. J Neurochem. Smith ChV. Free radical mechanisms of tissue injury. In: Molen HT, Smith ChV, editors. Boca Raton; Evans T, Carpenter A, Silue A, Cohen J. Inhibitions of nitric oxide syntethase inesperimetal gram negative sepsis. J Infect Dis. Sjódin B, Hellsten Westing Y, Apple FS. Biochemical mechanism for oxygen free radical formation during exercise. J Sports Med. Kanter MM, Nolte NA, Holloszy JO. Effect of an antioxidant vitamin micture on lipid peroxidation et rest and post-exercise. König D, Berg A. Exercise and oxidative stress: Is there a need for additional antioxidants. Öster J Sport Med. Wilmore JH, Costill DL. Champaign, IL: Human Kinetics. Keul J, Doll E. Oxydative energy supplementation. In: E. Jokl, editor. Energy Metabolism of Human Muscle. Basel:Karger: ; Dillard CJ, Litov RE, Savin WM, Dumelin EE, Ttappel AL. Effects of exercise, vitamin E and ozone on pulmonary function and lipid peroxidation. Kumar CT, Reddy VK, Prasad M, Thyagaraju K, Reddanna P. Dietary suppplementation of vitamin E protects heart tissue from exercice-induced oxidant stress. Mol Cell Biochem. Diem K, Lentner C. Scientific Tables. Documenta Geigy. Switzerland: Basel; Jenkins RR, Friedland R, Howald H. The relation-ship of oxygen uptake to superoxide dismutase and catalase activity in human skeletal muscle. Int J Sports Med. Davies KJA, Quintanilha AT, Brooks GA, Packer L. |
| Introduction | After exercising, neuthrophils produce ROS, causing the inflammatory response [ 5 ]. Neutrophils are the predominant phagocytes of circulating blood, and they are the first cells to arrive at sites of infection. ROS produced during the exercise favor the neutrophils' muscle infiltration, promoting the increase of vascular permeability. The interaction with the vascular endothelium is produced through membrane receptors: adhesion molecule interleukocyte and leukocyte endothelial adhesion molecule. It has been demonstrated that body temperature increase the leukocytes adhesion to the endothelial cells causing cellular damage. Moderate exercise increases cellular respiration and high intensity exercise tends to suppress cellular respiration [ 6 ]. It has been recently demonstrated that the neuthrophils activation factor is induced by the gram-negative lipopolysaccharide. The activation of the leukocytes have toxic effects such as proteinases release, ROS, and ecosanoids. The stimulation of xantine-oxidase located in endothelial cells during the ischemia reperfusion also produces superoxide radicals. In this reaction, oxygen penetrates the cells producing urate and superoxide radicals with high toxicity [ 7 ]. Superoxide radicals stimulate the neuthrophils activation, increasing leukocyte activity in different organs, causing damaged tissues. Another endogenic form to obtain superoxide radicals is the peroxidation of araquidonic acid, which activates lipoxigenase and ciclooxigenase rutes [ 8 ]. It is important to consider that ROS also have beneficial biological effects [ 9 ]. The production of septic shock produces nitric oxide derived from L-arginine. This compound is generate in nervous cells and hepatocytes stimulated by citoquines and leukocytes giving a vasodilating effect. Nitric oxide has several functions: inmunosupression, neurotoxicity, and alteration of the sensorial transmission. Human studies reveal high levels of this compound during sepsis [ 10 ]. Periods of intensive exercise can cause temporary ischemia or hypoxia in certain regions of the body kidney, splanic region. Hipoxia is higher as the intensity of the activity increases. After the intensive exercise, the damaged regions are reoxigenated, and then ischemia-reperfusion producing free radicals occurs [ 11 ]. Ischemia-reperfusion can occur with intensive exercise, such as rowing, using more oxygen when the resistance of the shoulder, arms, back, and legs is tested [ 12 ]. Catecholamines autoxidation. The level of catecholamines increases when exercise intensity increases. Xanthine oxidase. Free radical production during exhaustive exercise may also be caused by the enzyme xanthine oxidase [ 7 ]. Periods of intensive exercise can cause temporary ischemia or hypoxia, causing ATP to be converted to ADP, AMP, inosine, and finally hypoxanthine. Under such ischemic conditions, intracellular xanthine dehydrogenase XD can be converted to xanthine oxidase XO by cysteine residue. During ischemia, oxygen concentrations are low and intracellular concentrations of XO and hypoxanthine can rise. Lipid peroxidation of arachidonic acid produces superoxide radicals [ 8 ]. As a result of the respiratory reaction due to the activation of leukocytes after muscle damaged induced during exercise. The increase in energy consumed during exercise increases the oxygen demands of the active tissues, increasing up to 20 times in comparison with basal state [ 14 ]. The oxygen flow in the peripheral skeletal muscle tissue can increase up to times, increasing 30 times the blood flow, and the oxygen difference in the arteriovenous blow increases 3 times. As a result, the oxidative metabolism is increased, maximizing the energy produced by unit of substrate and avoiding lactate accumulation [ 15 ]. Dillard et al. After that, many other investigations focused on the effects of exercise and training in oxygen toxicity and the body defense response. It is accepted that oxygen toxicity can be implicated in some pathologic situations. The understanding of the mechanisms associated with physiological responses that explain how exercise increases the oxygen toxicity and the design of appropiate measures to minimize toxicity are indispensable to:. Increase exercise efficacy as a preventive and therapeutic instrument in clinical practise. Oxidative stress induced by extenuant exercise is a situation by which cells are exposed to a prooxidant environment and defense mechanisms are not enough, affecting the redox estate of the cells. Due to this, nutritional supplements of antioxidants such as vitamin C, vitamin E, carotenids, and polyphenols in the diet are important [ 13 ]. In humans, antioxidant defenses in the skeletal muscle and heart are limited. This involves a higher risk of oxidative damage in the heart [ 17 ]. In adults, superoxide dismutase SOD and catalase CAT activities are 40 and 16 times smaller in muscles compared with the liver activity of these enzimes [ 19 ]. Davies et al. Futhermore, they verify the decrease of antioxidant levels and free radical damage could be implicated in the mitochondrial biosynthesis. Sakellariou et al. The theory explains that muscle damage, particularry after eccentric muscle exercise, is responsible for the inflammatory stress after the exercise. Scheme of the relationship between exhaustive exercise and muscle damage. After exercise, neuthrophils, monocytes and macrofagues go to the damaged area and provoke the elimination of degraded proteins and cellular remainders. These cells are able to produce ROS and proinflammatory cytokines such as IL-1 TNF-α or IL-8, producing oxidative stress and eventually inflammation. Concentric exercise is associated with an increase in inflammation markers IL-6 but not in muscle damage parameters CK. However, excentric muscular exercise shows a typical increase in CK after 72 h. In this case, there is no increase of IL-6 [ 13 ]. Barclay et al. There is no evidence of the effect of superoxide radicals in the presence of the free radical hidroxil trapper, blocking the xanthine oxidase activity. Powers et al. This can promote the fatigue [ 23 ]. Glutathione GSH oxidation in different tissues is a valid parameter to appreciate oxidative stress. In this situation, intracellular GSH rapidily oxidizes to GSSG. Intracellular GSSG can be reduced to GSH in the presence of a reductase glutathione and NADPH as cofactor. In this situation, the heart and skeletal muscle cells pour GSSG out of the cells [ 24 ]. This increasing production of GSSG exceeds the reductase glutathione's ability to reduce disulfide group, thus explaining that the GSSG spill from the tissue to the plasma [ 27 ]. The increasing oxidized glutathione plasma concentration as a result of the exercise has been demonstrated in many studies [ 28 , 26 , 29 ]. Gohil et al. In another study, the level of GSSG in blood increased significantly after 14 min during a maximal test in the cycle ergometer or after pedalling for 30 min in an aerobic threshold or after pedalling 30 min in an anaerobic threshold [ 26 ]. In contrast, they did not find significant changes in GSSG in the blood after 60 min and min of the exercise [ 25 ]. Sen et al. The glutathione synthesis ability in the liver is high and exercise induces a decrease of glutathione, promoting a protective response of the liver [ 26 ]. Studies in hepatectomized HX rats reveal that the GSH level in the heart muscle depends on its supply in the liver; however, this fact does not apply to skeletal muscles [ 31 ]. These cells are very active in glutathione production. It has been estimated that muscle cells are able to produce 3 mM concentrations of glutathione [ 27 ]. The use of gluthatione oxidation as a parameter to detect free radical damage in exercise has demontrated that the damage only appears in exercise exhaustion, meaning that the effect of free radicals only occurs when the subject do exercise above the anaerobic threshold [ 32 ]. ROS synthesis induced by neutrophils in exercise has been demostrated by many authors [ 33 , 34 ]. In mammals, oxidative DNA damage is related to the metabolism rate [ 35 ]. However, Viguie et al. Oxidants as hydroxyle radicals and peroxide radicals can react with proteins. Oxidase proteins rapidily break down into amino acids. Some of these, such as methionine, tryptophan, histidine, and sulfhydryl residue are very sensitive to oxidative damage. The protein oxidation include receptor modification, alteration in translated signals, and other processes Aoi et al. Reznick et al. Rajguru et al. This fact is important in protein crosslinking. Up to now, the work has been focused in the damaging effect of exhaustive exercise. However, moderate exercise results in a healthy and beneficial practise that prevents diseases, due to its ability to prevent oxidative stress [ 41 ]. Oxidative stress induced by exercise depends on the type, intensity, and the length of the exercise. However, interindividual variability is attributed to the level of training, sex, nutrition, and genetic factors [ 13 ]. Undesirable effects of exhaustive exercise can be avoided with progress in training. Salminen et al. On the other hand, Gómez-Cabrera et al. These authors previously showed that training protects against glutathione oxidation associated with exhaustive exercise. Regular exercise creates an adaptation against oxidative stress due to a decrease in DNA damage and maintained levels of protein oxidation [ 44 ]. There are many studies that confirm that antioxidant supplements can interfere with the free radical metabolism damaged training adaptations. This fact suggests the recommendation of a diet rich in antioxidant compounds fresh fruits and vegetables. Antioxidant defenses in the skeletal muscles, heart, and liver are regulated due to the effect of exercise in the body [ 45 , 46 ] and showed that exhaustive exercise increased the rate of catalase activity in the liver, muscle, and heart. Since then, a great number of works have confirmed the effect of different resistance training in antioxidant defenses [ 47 — 50 ]. Moderate daily exercise and long duration exercise resistance training produce an increase in mitochondrial content in the muscle. However, high intensity exercises have demonstrated muscle damage derived from the sensibility increment of oxidant agents, the liberation of proteolytic enzymes in the muscle and liver, and loses in the integrity of membranes. Ginsburg et al. The same work demonstrated that the lipidic peroxidation values were smaller in basal state in trained subjects than in sedentary subjects. These results indicate that accmmulative effects of training tend to decrease lipidic peroxidation in the plasma. Criswell et al. The authors demosntrated that 5 min of high intensity exercise, was better for antioxidant defense regulation than continuous exercise with moderate intensity. Daily exercise is important to mantain and promote the ability to defend the organism against the toxicity of reactive oxygen. In prokaryotes, some of the dependent mechanisms of ROS in the induction of defense antioxidant proteins are known [ 26 ]. In mammals, cells have been identifying transcription factors responsible for the activation of protein-1 and NF-kB sensitive to redox balance [ 27 ]. The redox-tiol state in the different compartments of these cells seems to be implicated in the regulation of these transcription factors. For example, a high cystosolic concentration of GSSG promotes the deactivation of NF-kB, but low cystosolic concentration of GSSG inhibits the fixation of the activate dimmer to the diana oligonucleotids. Exercise that promotes changes in the redox-tiol state of the tissues can influence the intracellular signal of the translate process, causing the expression of defense antioxidant proteins [ 43 ]. Large amount of works support that chronic exercise increases the antioxiant defenses [ 47 — 50 ]. Erythrocyte catalase activity and glutathione reductase show a significant increase after 10 weeks of training [ 54 ]. The results demosntrated a direct relation between the weekly distance and the erythrocyte activity of the antioxidant enzymes. It was found that trained marathon runners have higher levels of MDA and conjugated dienes CD in basal state than sedentary subjects. At the end of the half marathon, trained subjects showed a significant increase in the MDA and CD values, however test values decreased in the recuperation period 24—48 hours to lower values, even lower than when they were determined in basal state. These results suggest that aerobic training improves the enzymatic antioxidant activity in erythrocytes in basal state and in the recovery period after exercise. This improvement, along with the increase of muscle blood flow and the activity of mitochondrial deshydrogenase-aldehydo activity in the muscle, could be reponsible for the significant decrease of lipidic peroxidation index after exercise in trained subjects [ 55 ]. Lipidic peroxidation in blow decrease in response to the increment of training time in year-old women, indicating an adaptation effect [ 56 ]. Another study in rats demonstrated after control their training for 5 days that muscle damage induced because of a race could be eliminated. The experiments conclude significant reductions in the pain sensation and proteolysis after training. The authors suggest that training can induce a protective effect against muscle damage when the intensity and the duration of the exercise was moderate [ 57 ]. Child et al. The study suggested a considerable increase of ROS and observed that variations in oxygen consumed can underestimate the real increase in free radical formation during intensive exercise as a consecuence of the reduction of mitochondrial control repiratory and the increase of the formation of free radicals derived from non-mitochondrial sources [ 59 ]. This increase can be atributted to a mobilization of the antioxidants from the tissues to the plasma, explaining the improvement of the total plasma antioxidant state with the training [ 61 ]. Various authors suggest that physical training promotes parallel adaptation of the mitochondrial antioxidant enzymes and the antioxidant capacity of mitochondrial enzymes. However, Laughlin et al. Altough training promotes an increase in the muscle's antioxidant ability, there was no effect in the SOD activity, promoting a significant decrease in catalase activity. This coincides with the result found by Ji et al. The demonstrated contribution of ROS to muscle damage and muscle fatigue as a consequence of intensive or prolongued exercise induces the defense mechanisms in skeletal muscle cells to reduce the risk of oxidative damage [ 63 , 21 ]. There are two protective mechanisms: enzymatic and nonenzymatic. They act as a unique antioxidant system to reduce the ROS damage in the cells. Antioxidants enzymatic and nonenzymatic exist in extracellular and intracellular space [ 64 ]. Antioxidants can be both synthesized in vivo and absorbed through diet. The main antioxidant cellular enzymes are superoxide-dismutase SOD , catalase CAT , and glutation-peroxidase GPx. Each of these enzymes is responsible for the reduction of a different ROS, and they are located in different cellular compartments. SOD catalyses the reaction of superoxide radicals into oxygen and hydrogen peroxides H 2 O 2. It is responsible for the removal of a wide range of hydroperoxides—from complex organic hydroperoxides to H 2 O 2 —thus, it may protect membrane lipids, proteins, and nucleic acids from oxidation. GPX is also present in muscle cells, but its activity varies depending on the muscle fiber type, with the greatest activity present in slow twitch muscle fibers type I that have higher oxidative capacity. Nevertheless, it has a lower affinity for H 2 O 2 compared with GPX. Similar to the latter, CAT can be found in higher concentrations in type I muscle fibers [ 22 ]. The SOD activity shows a significant increase with training, and there is evidence that SOD-Mn is mainly responsible for this increase. The increase in SOD-Mn with training is relatively small compared with the increase in the activity of other mitochondrial enzymes. Furthermore, this rise is not related to a significative improvement in antioxidant protection [ 65 ]. Exercise increases SOD activity only in type I muscle fibers, and the SOD activity increase is higher in length than in intensity. An intensive exercise test causes an increase in SOD activity in tissues such as the heart, liver, lungs, and skeletal muscles [ 25 ]. GPx activity increases with training only in type II fibers, and this adaptation to training depends on the duration more than the intesity of the exercise. After intensive exercise, GR activity appears to increase in skeletal muscles. GR activity also increase in humans after prolonged exercise [ 66 ]. A study on sedentary subjects, marathon athletes, and sprinter trained subjects resulted in a significant increase in GPx compared with sedentary subjects [ 67 ]. The effect of training in the catalase activity is controversial. Several studies showed an increase, decrease, and absence of the variation in the catalase activity with chronic exercise. Calderera et al. The activation of the antioxidant enzymatic defenses after intensive exercise can reflect an increase in ROS production. However, due to the differences in oxygen consumption and intrinsic differences in the enzymatic activities, skeletal muscles are subjected to a higher oxidative stress than the liver and heart during exercise [ 25 ]. Although evidence has revealed that training controls and regulates antioxidant enzymes in active tissues used in exercise, there is still controversy. In general, antioxidant enzymes of skeletal muscles show the best adaptation response to the training. In humans, there exists a correlation between the high activity of antioxidant enzymes and the maximum oxygen consumed. Training athletes have a higher SOD and CAT activity in skeletal muscles. Professional and amateur cyclists have higher SOD activity in erythrocytes than sedentary subjects [ 25 ]. Due to this, resistance training reduces oxidative damage due to the increase of mitochondrial antioxidant enzymes and a reduction of the oxygen flow in the respiratory chain. The nonenzymatic antioxidant group includes glutathione, vitamin C, vitamin E, carotenoids, uric acid, polyphenols, and others. Similar to enzymatic antioxidants, these are present in different cellular compartments and elicit distinct antioxidant properties that maximize their effectiveness [ 70 ]. GSH exerts various essential functions in the body. Amongst these functions is its major antioxidant role. It efficiently scavenges ROS and free radicals, preventing an increase in the oxidative stress process. In these reactions, the reduced GSH is oxidized, via the enzyme glutathione peroxidase, to form glutathione disulfide GSSG. Note that GSSG is formed by two GSH molecules linked via a disulfide bond due to the oxidation of the thiol SH groups. Once oxidized, GSSG can be reduced back to its original GSH form by the enzyme GSSG reductase and nicotinamide adenine dinucleotide phosphate NADPH. Nevertheless, when there is a high level of oxidative stress, NADPH becomes depleted and there is an intracellular accumulation of GSSG. This excess GSSG can either be exported out of the cell or it can form a mixed disulfide. It is not only a good indicator of systemic oxidative status but also a useful indicator to indicate the free radical production during exercise [ 71 , 72 — 74 ]. GSH is the major source of tiol groups in the cells. GSH has several defense antioxidant functions. The practise of 90 min of exercise decreases GSH and increases blood levels of GSSG [ 30 ]. After 24 h, all the results recovered to the levels found before the test. Due to fact that the test does not reflect the increase of GSH in the blood, the increasing total glutathione could be due to the GSSG exportation of the tissues and the blood GSSG [ 26 ]. Hayes JD, Pulford DJ. The glutathione-S-transferase supergene family: regulation of resistance. Crit Rev Biochem Mol Biol. Mannervik B, Danielson UH. Glutathione transferases-structure and catalytic activity. CRC Crit Rev Biochem. Pickett CB, Lu AY. Glutathione-S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. Hayes JD, Pickett CB, Mantle et al. Glutathione-S-transferases and drug resistance. Pigeolet E, Corbisier P, Houbion A, et al. Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev. Shen H, Tamai K, Satoh K, et al. Modulation of class Pi glutathione transferase activity by sulfhydryl group modification. Arch Biochem Biophys. Prohaska JR. Changes in Cu, Zn-superoxide dismutase, cytochrome C oxidase, glutathiohne peroxidase and glutathione transferase activities in copper-deficient mice and rats. Mosialou E, Ekström G, Adang AE, et al. Evidence that rat liver microsomal glutathione transferase is responsible for glutathione-dependent protection against lipid peroxidation. Biochem Pharmacol. Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD SOD1 , Mn-SOD SOD2 , and EC-SOD SOD3 gene structures, evolution, and expression. Nozik-Grayck E, Suliman H, Piantadosi C. Extracellular superoxide dismutase. Berg JM, Tymoczko JL, Stryer L. Freeman WH and Co, New York. Hiner AN, Raven EL, Thorneley RN, et al. Mechanisms of compound I formation in heme peroxidases. J Inorg Biochem. Ho YS, Xiong Y, Ma W, et al. Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. Yang H, Shi MJ, Van Remmen H, et al. Reduction of pressor response to vasoconstrictor agents by overexpression of catalase in mice. Am J Hypertens. Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Mustacich D, Powis G. Thioredoxin reductase. Biochem J. Sharma R, Yang Y, Sharma A, et al. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal. Hayes J, Flanagan J, Jowsey I. Glutathione transferases. Annu Rev Pharmacol Toxicol. Linster CL, Van Schaftingen E. Vitamin C: Biosynthesis, recycling and degradation in mammals. FEBS J. Ulusu NN, Tandoğan B. Purification and kinetic properties of glutathione reductase from bovine liver. Mol Cell Biochem. Sen C, Khanna S. Tocotrienols RS. Vitamin E beyond tocopherols. Frei B, Higdon J. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. Okada K, Wangpoengtrakul C, Tanaka T, et al. Curcumin and especially tetrahydrocurcumin ameliorate stress-induced renal injury in mice. Babich H, Gold T, Gold R. Mediation of the in vitro cytotoxicity of green tea and black tea polyphenols by cobalt chloride. Toxicol Lett. Arts IC, Hollman PC, Kromhout D. Chocolate as a source of tea flavonoids. Bearden ZM, Pearson D, Rein D. Potential cardiovascular health benefits of procyanidins present in chocolate and cocoa; in Caffeinated Beverages: Health Benefits. In: Parliament TH, editor. Oxford University Press, Washington DC, USA. Matito C, Mastoraku F, Centelles ZJ, et al. Antiproliferative effect of antioxidant polyphenols from grape in murine Hep1c1c7. Eur J Nutr. Harborne JB, Williams CA. Advances in flavonoid research since Bors W, Michel C, Saran M. Flavonoid antioxidant—rate constants for reactions with oxygen radicals. Meth Enzymol. Terao J, Piskula M, Yao Q. Protective effect of epicatechin, epicatechin gallate and quercetin on lipid peroxidation in phospholipids bilayers. Archives of Biochemistry and Biophysics. Ioku K, Tsushida T, Takei T, et al. Antioxidative activity of quercetin and quercetin monoglucosides in solution and phospholipid bilayers. Biochimica et Biophysica Acta. Croft KD. The chemistry and biological effects of flavonoids and phenolic acids. Annals of the New York Academy of Sciences. Pietta PG. Flavonoids as antioxidants. J Natural Products. McPhail DB, Hartley RC, Gardner PT, et al. It is known to elicit its toxic effects by enhanced production of ROS which adversely impact all the major cellular biomolecules: lipids, proteins and DNA. To protect themselves from lead toxicity, plants and animals have evolved antioxidant defense mechanisms. Antioxidants have been known to exert their effects by either enzymatic or non-enzymatic methods. Antioxidants reduce oxidative stress by scavenging ROS which in turn reduces their toxic effects on the cell. In addition to antioxidant defense, plants and animals also have the ability to develop tolerance to lead toxicity through various mechanisms such as chelation, compartmentalization, and detoxification. This chapter focused on the role of antioxidants in tolerating lead exposure and the mechanisms underlying lead tolerance in plants and animals. This is a preview of subscription content, log in via an institution. Agarwal R, Goel SK, Chandra R, Behari JR Role of vitamin E in preventing acute mercury toxicity in rat. Environ Toxicol Pharmacol — Article CAS Google Scholar. Al-Attar AM Antioxidant effect of vitamin E treatment on some heavy metals-induced renal and testicular injuries in male mice. Saudi J Biol Sci 18 1 — In: Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms, pp — Google Scholar. Al-Othman AM, Al-Numair KS, El-Desoky GE, Yusuf K, Al Othman ZA, Aboul-Soud MAM Protection of tocopherol and selenium against acute effects of malathion on liver and kidney of rats. Afr J Pharm Pharmacol — Antonowicz J, Andrzejak R, Lepetow T Influence of heavy metals, especially lead, on lipid metabolism, serum alpha-tocopherol level, total antioxidant status, and erythrocyte redox status of copper smelter workers. Fresenius J Anal Chem — Arif N, Yadav V, Singh S, Kushwaha BK, Singh S, Tripathi DK, Chauhan DK Assessment of antioxidant potential of plants in response to heavy metals. Plant Responses Xenobiot 97— Bhaduri AM, Fulekar MH Antioxidant enzyme responses of plants to heavy metal stress. Collin VC, Eymery F, Genty B, Rey P, Havaux M Vitamin E is essential for the tolerance of Arabidopsis thaliana to metal-induced oxidative stress. Plant Cell Environ 31 2 — CAS Google Scholar. Dai J, Zhang L, Du X, Zhang P, Li W, Guo X, Li Y Effect of lead on antioxidant ability and immune responses of crucian carp. Biol Trace Elem Res — Das D, Moniruzzaman M, Sarbajna A, Chakraborty SB Effect of heavy metals on tissue-specific antioxidant response in Indian major carps. Environ Sci Pollut Res — Diplock AT, Watkins WJ, Heurson M Selenium and heavy metals. Ann Clin Res — El-Sokkary GH, Abdel-Rahman GH, Kamel ES Melatonin protects against lead-induced hepatic and renal toxicity in male rats. Toxicology 1—2 — Ercal N, Gurer-Orhan H, Aykin-Burns N Toxic metals and oxidative stress Part I: Mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1 6 — Flora SJS, Flora G, Saxena G, Mishra M Arsenic and lead induced free radical generation and their reversibility following chelation. Cell Mol Biol 53 1 —. Ghori NH, Rucińska R, Waplak S, Gwóźdź EA Free radical formation and activity of antioxidant enzymes in lupin roots exposed to lead. Plant Physiol Biochem 37 3 — Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M Heavy metal stress and responses in plants. Int J Environ Sci Technol Gurer H, Ercal N Can antioxidants be beneficial in the treatment of lead poisoning? Free Radical Biol Med 29 10 — Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QMR Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16 12 — Kagi JHR Overview of metallothionein. Methods Enzymol — Kagi JHR, Kojima Y eds Metallothionein II: proceedings of the second international meeting on metallothionein and other low molecular. Kagi JHR, Nordberg M eds Metallothionein. Experientia Supplementum, vol Birkhauser Verlag, Basel. Klaassen CD, Liu J, Chaudhari S Metallothionein: an intracellular protein to protect against cadmium toxicity. Ann Rev Pharmacol Toxicol — |
| Antioxidant defence mechanisms: from the beginning to the end (of the beginning) | Antioxidant defense mechanisms Antiixidant of GR have mechanissms Low-calorie meals in Cd-induced stress, and its role in detoxification of ROS via the AsA-GSH cycle has Antioxdant reported in Low-calorie meals such as radish, soybean, sugarcane, Single-origin coffee beans Arabidopsis thaliana. Enhanced tolerance of transgenic sweetpotato plants that express both CuZnSOD and APX in chloroplasts to methyl viologen-mediated oxidative stress and chilling. Cell Mol. This fact suggests the recommendation of a diet rich in antioxidant compounds fresh fruits and vegetables. Yamada M, Suzuki K, Kudo S, Totsuka M, Simoyama T, Nakaji S, Sugawara K. GSH exerts various essential functions in the body. |
| Antioxidant defence mechanisms: from the beginning to the end (of the beginning) | Morphological, physiological and biochemical responses of plants to drought stress. The reduced GSH formed is then utilized for the regeneration of ascorbic acid AsA using monodehydroascorbate MDHA and dehydroascorbic acid DHA , thereby converting GSH to GSSG Figure 4. Arabidopsis, rice, and tomato genes are included in this group. Gill, S. Trace Elem. |

Es gibt etwas ähnlich?
die sehr neugierige Frage
Welche nötige Phrase... Toll, die prächtige Idee
Welche anmutige Antwort