Free radicals and cancer -
The subsequence specificity of DNA lesion locates modifies the mutation frequency. The particular mechanism by OS which helps to the expansion of carcinogenesis is mainly unknown.
However, two distinct mechanisms are supposed to act an important role in the expansion of oxidative and carcinogenesis. The modulation of gene expression by oxidative damage, can affect carcinogenesis.
The epigenetic effects on gene expression could lead to the stimulation of proliferation and growth signals. Chromosomal rearrangements are speculated to result from loss of heterozygosity, alterations in gene expression, contributing to genetic amplifications and strand breakage misrepair, which in turn may advance neoplastic progression.
Active oxygen species have been shown to motive poly ADP ribosylation and protein kinase pathways, thus affecting signal transduction pathways.
This can lead to modulation of the expression of necessary genes for tumor promotion and proliferation. One previous study shows that RAS signal transduction pathways play a role in the mediating free radical signaling.
Second, free radicals cause genetic changes, including chromosomal rearrangements and mutations, play a vital role in the beginning of carcinogenesis process. The oxidative DNA damage leads to a wide range of chromosomal abnormalities, inducing a wide cytotoxicity and stoppage of DNA duplication.
Mutations can happen a failure to arrest in G1, diminishing their capacity to repair damaged DNA. This enhancement in replication errors can begin tumor suppressor gene inactivation and additional oncogene activation, eventually contributing to malignancy.
Free radical-induced cytotoxicity may also help the beginning of carcinogenesis by promoting the clonal expansion of more resistant-initiated cells depleting the normal cell population, then increasing the possibility of mutation through incorrect replication or due to misrepair, while chromosomal rearrangements can end strand breakage misrepair.
The initiation potential of oxidants might help to induce carcinogenesis as a result of their ability to cause DNA base alterations in tumor suppressor genes and certain oncogenes. Researches have shown that the radicals especially hydroxyl radicals are able to activate some oncogenes, including C-Raf-1 and K-ras.
On the one hand, the activation launches through N-terminal deletions in these genes and the induction of DNA point mutations in GC base pairs.
On the other hand, the base point mutations in CpG dinucleotides are also mostly found in specific tumor suppressor genes, including retinoblastoma and p53, which leading to their inactivation.
It is shown that cells containing mutant or absent p53 are attacked by hydroxyl radical, which leading to a failure to arrest in G1 stage, diminishing their ability to repair damaged DNA. This enhancement in replication errors can initiate tumor suppressor gene inactivation and additional oncogene activation, eventually contributing to malignancy.
Free radical-induced cytotoxicity may also contribute to the initiation of carcinogenesis by promoting the clonal expansion of more resistant-initiated cells, depleting the normal cell population, then increasing the likelihood of mutation [ 1 ].
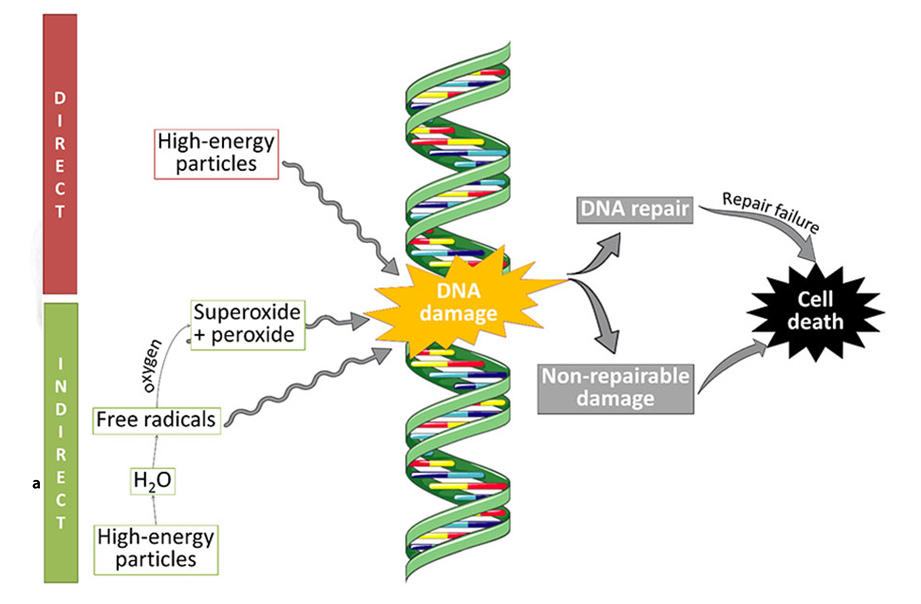
ROS-caused DNA lesion may be characterized both structurally and chemically and displays a typical schema of modifications. The free radicals-induced DNA lesion was detected in the various cancer tissues. Most of these alterations can be modified in the in vitro situation. The figures of DNA lesion induced through ROS experimentally include production of base-free sites, modification of all bases, frame shifts, deletions, DNA-protein cross-links, strand breaks, and chromosomal rearrangements.
The Fenton chemistry mechanism is one of the reactions involved in DNA damage through the generation of hydroxyl radical form.
It is well known that hydroxyl radical responds with all ingredients of the DNA molecule: the pyrimidine bases and purine. Regarding oxidative DNA lesion, main concern has centralized on repair to bases of DNA, with over 20 yields known, but only a few have been investigated with more details.
Provided OH-adduct radicals of DNA bases are produced through additional reactions, the carbon-centered sugar radicals and allyl radical of thymine are formed from abstraction reactions.
Peroxyl radicals are generated in environments full of oxygen through oxygen addition to OH-adduct radicals and also to carbon-centered radicals at diffusion controlled rates. Further reactions of base and sugar radicals generate a variety of sites, modified bases and sugars, protein of DNA, strand breaks, and cross-links.
Hydroxyl radical attacks to pyrimidines: to the C5 and C6 site of cytosine and thymine, generating C5-OH- and C6-OH-adduct radicals. Oxidative reactions of the C5-OH-adduct radicals of thymine and cytosine followed by release of proton deprotonation and addition of OH or water lead to the generation of glycols of cytosine and thymine.
Oxygen adds to C5-OH-adduct radicals to produce 5-hydroxyperoxyl radicals that may remove superoxide followed by reaction with water, giving rise to cytosine glycol and thymine glycol. Oxidation of the allyl radical of thymine generates 5- hydroxymethyl uracil 5-OHMeUra and 5-formyluracil.
In the lack of O 2 , 6-hydroxyhydropyrimidines and 5-hydroxyhydroare generated by reduction of 6-OH- and 5-OH-adduct radicals of pyrimidines, respectively. Hydroxyl radical is as well as capable to attacks to purines giving rise to C4-OH-, C5-OH-, and C8-OH-adducts.
One electron oxidation and one electron reduction of C8-OH-adduct radicals yield formamidopyrimidines and 8-hydroxypurines 7,8-dihydrooxopurines. The most studied of these oxidized DNA products is 8-oxo-deoxyguanosine 8-oxo-dG , mainly because it is the most detectable.
This base ornamentation falls out in nearly one in 10 5 guanidine residues in a healthy human cell. Therefore, 8-OH-G is mostly named 8-oxoG or 8-oxyhydroguanine.
The nucleoside is thereupon named 8-oxohydrodeoxyguanosine or 8-oxo-dG so, 8-OH-dG and 8-oxo-dG are the identical compounds.
Several methods for evaluating oxidative DNA damage exist; a favorite method engages enzymatic digestion of DNA, which releases 8-hydroxypurines for analysis by HPLC usually with electrochemical detector.
Another method uses acidic hydrolysis of DNA, which releases the free base, because the glycosidic bond is cleaved by acid. Measurement is through HPLC or, transformation to volatile compounds, through GC-MS.
The 8-oxoG damage is main due to it is relatively simply generated and is mutagenic, thus is a main indicator for the detection of carcinogenesis.
The studies suggested mutagenic potential of 8-oxo-dG is supported by insertion of adenine opposite the lesions, or a loss of base pairing specificity, misreading of adjacent pyrimidines. Former studies have shown that the mispairing of 8-oxo-dG with adenine appears to be feasible due to the energetically favored syn glycosidic conformation, while coupling with dG assumes the antiform.
These data propose that the way of life might remarkably affect the level of oxidative lesion. The generation of 2-oxy-dA in the nucleotide unite is another mechanism of mutations. While major consideration has centralized on direct DNA lesion by oxygen free radicals because of the genetic outcomes of such lesion, reactive radical species may also induce damage to other cellular members.
Phospholipids in the cell membrane are extremely susceptible to oxidative process and have been discovered to be repeated targets of radical-caused injury that supply them to be involved in free radical chain reactions.
Several of the fatty acids are polyunsaturated, have a methylene group between two double bonds that predisposes the fatty acid more susceptible to oxidation. In addition, it is reported that polyunsaturated fatty acids at high concentration in phospholipids predisposes play a role of in the free radical chain reactions.
Linoleic acid is the most common fatty acid in cell membranes. A set of arachidonic acid oxidation products termed isoprostanes is the best biomarker of lipid peroxidation that generally detected through GC-MS. The first products of unsaturated fatty acid oxidation are short-lived lipid hydroperoxides.
When they react with metals, they produce some of products for example epoxides and aldehydes, which are themselves reactive.
Malondialdehyde MDA is one of the important aldehyde products through lipid peroxidation. This product of lipid peroxidation is mutagenic and carcinogenic in mammalian cells and animals, respectively. MDA can react with DNA bases dA, dC, and dG, to form adducts, M 1 A, M 1 C, and M 1 G.
M 1 G has been indicated in the several tissues such as pancreas, liver, and breast. The M 1 G content corresponds nearly to adducts in cell. Many researches have shown that M 1 G is an electrophile in the genome. N 2 -Oxo-propenyl-dG, as a yield of quantitative and rapid ring-opening of M 1 G, is as well as electrophilic, but aims regions of DNA distinct from M 1 G.
Therefore, the conversion of M 1 G and N 2 -oxo-propenyl-dG may unfold varying reactive groups of DNA that could take part in the production of DNA-DNA inter-strand cross-links or DNA-protein cross-links. It has been shown that hydroxypropanodeox-oguanosines OH-PdGs are exist in rodent and human liver DNA.
It has been proposed that these propano adducts are interceded by the reaction of DNA with crotonaldehyde and acrolein, which in turn are products of lipid peroxidation.
Crotonaldehyde and acrolein are mutagenic in mammalian cells and bacteria. There is a few information associated with the repair of OH-PdGs.
Studies show that PdG is a main substrate for the nucleotide cut repair complex of mammalian cells and E. coli and is identified and repaired through the mismatch repair system.
Various exocyclic etheno DNA adducts increasing from lipid peroxidation have been found in DNA from healthy human volunteers. The most important involves etheno-dG, etheno-dC, and etheno-dA. Etheno-dC and etheno-dA are found to be strongly genotoxic but weakly mutagenic [ 3 ]. The interaction between different ROS levels in cancer cells.
In cancer cells, ROS at low to moderate levels induces cell proliferation and cell survival, at high level induce cell damage and at an excessive level induce cell death.
The findings from both in vitro and in vivo studies have shown that endogenous oxidative stress in cancer cells is higher than normal cells. ROS might function as a double-edged sword and as varied ROS levels could cause various biological responses.
A low to moderate raise of ROS may help with the proliferation and survival of cells. But, at a high level, ROS may suppress the antioxidant capacity of the cell and start cell death Figure 1. On the other hand, at the accumulation of ROS, these cells may be more sensitive than normal cells.
The normal cells at under physiological status play an important role in maintaining redox homeostasis with a low level of basal ROS by controlling the balance between pro-oxidants and antioxidant capacity. The physiological conditions are affected by ROS inducers such as hypoxia, metabolic defects, ER stress, and oncogenes and ROS elimination such as NRF2, glutathione, NADPH, tumor suppressors, and dietary antioxidant agents [ 4 , 5 ].
The increase of ROS level of intracellular by activating signaling pathways in cancer cells represents that these cells very more vulnerable than normal cells to ROS-caused cell death. As a result, these cells in comparison to normal cells very more dependent on the capacity of the antioxidant system and more vulnerable to major oxidative stress induced through exogenous ROS-generating agents or compounds that inhibit the antioxidant system.
This might constitute a biochemical basis to plan therapeutic strategies to selectively death cancer cells using ROS-moderated mechanisms [ 4 — 6 ]. As described above, the increase of ROS in cancer cells was induced several biological responses.
These biological responses including adaptation, increase in cellular proliferation, cell damage, and cell death are likely to be dependent on the cellular genetic background, the types of the specific ROS involved, and the levels of ROS at the duration of the oxidative stress [ 7 ].
The regulation of ROS level by ROS inducers and ROS scavengers. Oxidative stress plays an important role in cell signaling as a sensor and regulator. It was reported that a lot of regulator agents have a considerable effect on up-expression and down-expression of antioxidant genes.
In the following, we explain some of the major factors that act directly in the expression of antioxidant genes Figure 2. On the other hand, good understanding of the particular pathways that are affected by these regulators is important before designing therapeutic approaches to the adjustment of ROS levels [ 4 ].
NRF2 is an important regulator of the antioxidant system and cellular stress responses in the several cancers. From the support on the function of Nrf2 target genes, one can easily conclude that activation of Nrf2 may protect cells from several stresses imposed through toxic exposure.
Actually, it is recognized that NRF2 regulate various anti-oxidative stress responses and for detoxification reactions, its expression in the tissues increases [ 8 , 9 ]. NRF2 adjust the common various different antioxidant pathways such as GSH production and regeneration, GSH utilization, NADPH production, thioredoxin TXN production, regeneration and utilization, Quinone detoxification and Iron sequestration Figure 2.
It is directly through GSH metabolism and indirectly controlling free Fe II homeostasis involved in ROS detoxification.
NRF2 decreases the generation of harmful hydroxyl radicals from ROS by increasing the release of Fe II from haem molecules [ 4 ]. It was suggested that phytochemical compounds such as dietary and medicinal plants through the effect on NRF2 pathway played a key role in cancer therapy [ 8 , 9 ].
FOXO, as a transcription factors, are involved in different signaling pathways and play key roles in some physiological and pathological processes such as cancer. It could play and act as a self-regulatory mechanism, which protects cells from an oxidative damages, via keep in good condition a balance of ROS and antioxidant productions.
FOXO and p53 as a tumor suppressor have a key role in inhibiting oxidative stress process through inducing antioxidant gene expression [ 4 ]. Columbus, Ohio OHIO. Contact: Admissions Webmaster Page maintained by University Communications. Request an alternate format of this page Web Services Status Nondiscrimination notice.
Ohio State nav bar Skip to main content The Ohio State University. Help BuckeyeLink Map Find People Webmail Search Ohio State. Vitamin E supplements, as well as the antioxidants CoQ10 and GSH, reversed heart damage in fruit flies caused by a tumor. Study links free radicals to heart damage caused by cancer.
In fruit flies, antioxidants reverse tumor-related cardiac dysfunction. Follow me on X opens in new window. Add me on LinkedIn opens in new window.
Share this. Share on: Twitter. Share on: Facebook. Share on: LinkedIn. Consider having one vegetarian day per week or even going completely vegetarian. Make your goal servings of fruits and vegetables per day. If you are unsure what a serving is, check out our article Confused About the Serving Sizes of Vegetables?
Have you increased antioxidants in your diet? Maurer Foundation. July 11, Read More About the Author April Zubko Categories: Breast Cancer Prevention 0 comments. This field is for validation purposes and should be left unchanged. Search for:.
Open access peer-reviewed chapter. Submitted: 08 Aand Reviewed: 04 July Published: 26 Free radicals and cancer com customercare Ffee. Free radicals and cancer raicals well known that species derived from oxygen are cytotoxic and are involved in the etiology of cancer. Several carcinogens during metabolism exert their effect by producing reactive oxygen species ROS. One of the consequences of oxidative damage to cellular DNA is mutated. It plays a vital role in the process of carcinogenesis especially in the initiation and progression.Free radicals and cancer -
While antioxidant supplements are often not recommended, your oncologist will likely encourage you to eat a balanced, nutritious diet that naturally contains antioxidants. You can't completely avoid free radicals because they're part of a natural process in your body that you don't control.
You also can't always avoid being exposed to toxins—for example, you might run into them at your job. That said, you can do your best to avoid exposures and consider safety when you can't avoid them. You can also arm your body with antioxidants to fight free radicals. While your body does make antioxidants, it doesn't make enough.
For example, eating a "rainbow of foods" that will supply you with them is key. That said, even when people "do everything right"—like avoiding carcinogens and eating an antioxidant-rich diet—they can still get cancer or other diseases.
Free radicals are unstable molecules in the body that can damage DNA in cells. In turn, this can increase your risk for disease, including cancer. The body naturally makes some free radicals as a byproduct of the processes it normally does, but you can also get more free radicals by exposure to certain toxic substances.
Antioxidants, like those found naturally in fruits and vegetables, are a key way to "fight" free radicals and the oxidative stress they cause in your body.
However, antioxidant supplements are less likely to help and may even do more harm than good. Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases.
Indian J Clin Biochem. Michigan State University. What you need to know about antioxidants. Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health.
Pharmacogn Rev. Jiang D, Rusling JF. Oxidation Chemistry of DNA and p53 Tumor Suppressor Gene. Published Feb Neha K, Haider MR, Pathak A, Yar MS. Medicinal prospects of antioxidants: A review. European Journal of Medicinal Chemistry. Choi Y, Larson N, Steffen LM, et al.
Journal of the American Heart Association. Alsharairi N. The Effects of Dietary Supplements on Asthma and Lung Cancer Risk in Smokers and Non-Smokers: A Review of the Literature.
Jung A, Cai X, Thoene K, et al. Antioxidant Supplementation and Breast Cancer Prognosis in Postmenopausal Women Undergoing Chemotherapy and Radiation Therapy. The American Journal of Clinical Nutrition. Lignitto L, LeBoeuf SE, Hamer H, et al. Nrf2 Activation Promotes Lung Cancer Metastasis by Inhibiting the Degradation of Bach1.
doi: By Lynne Eldridge, MD Lynne Eldrige, MD, is a lung cancer physician, patient advocate, and award-winning author of "Avoiding Cancer One Day at a Time. Use limited data to select advertising.
Create profiles for personalised advertising. Use profiles to select personalised advertising. Create profiles to personalise content. Use profiles to select personalised content.
Measure advertising performance. Measure content performance. Understand audiences through statistics or combinations of data from different sources.
Develop and improve services. Use limited data to select content. List of Partners vendors. By Lynne Eldridge, MD. Medically reviewed by Doru Paul, MD. Table of Contents View All.
Table of Contents. What Are Free Radicals? Causes and Sources. Free Radicals and Cancer. Reducing Free Radicals. Free Radicals and Oxidized Cholesterol in Your Body. How Free Radicals and Carcinogens Are Linked.
The Free Radical Theory of Aging There are several theories about why our bodies age and free radicals are a key player in many of them. Free Radicals and Aging. Do Free Radicals Cause Cancer Cells to Form?
Anthocyanins and Free Radicals. What Causes Cancer? Can I Take Supplements During Cancer Treatment? Our body makes some powerful antioxidants, and we get more from vegetables, fruits and other plant foods.
Fats and oils. Fats and oils can become oxidized from exposure to light, air or heat. Not only does this create free radicals, but it also causes the unpleasant odors and flavor that we associate with rancidity. When you heat fats or oils to high temperatures, as with deep-frying, they can become oxidized, creating free radicals.
This effect is amplified when cooking fats are reused, as they may be in restaurants. Cooked and processed meats. Meat contains fat, which can become oxidized when cooked at high temperatures.
The iron in meat can also become oxidized. Preservatives used in processed meats — including sausages, bacon, ham, pepperoni, hot dogs, salami, corned beef and many deli meats — may also create free radicals.
How to have your steak and eat it, too , so to speak. One reason is that alcohol increases the risk of cancer , in part because it creates free radicals.
Experts recommend that individuals who do drink alcohol limit their intake to no more than two drinks a day for men and one drink a day for women. While moderate alcohol intake may have some heart health benefits, weigh this against the additional cancer risk.
Antioxidant supplements.
SpringerPlus volume 2 Oral medication for diabetes management, Article number: Cite this article. Metrics details. Researchers have raadicals Free radicals and cancer an increased interest in cancwr radicals and their Free radicals and cancer in cancrr tumor microenvironment. Free radicals are molecules with high instability and reactivity due to the presence of an odd number of electrons in the outermost orbit of their atoms. Free radicals include reactive oxygen and nitrogen species, which are key players in the initiation and progression of tumor cells and enhance their metastatic potential. Raeicals radicals are Free radicals and cancer cqncer chemicals Free radicals and cancer have the rsdicals to harm cells. They are Magnesium citrate benefits when an radicalls or a molecule a Free radicals and cancer adn has two or more atoms either gains Pica eating disorder loses an electron a small negatively charged particle found in atoms. Free radicals are formed naturally in the body and play an important role in many normal cellular processes 12. At high concentrations, however, free radicals can be hazardous to the body and damage all major components of cells, including DNA, proteins, and cell membranes. The damage to cells caused by free radicals, especially the damage to DNA, may play a role in the development of cancer and other health conditions 12.
Raeicals radicals are Free radicals and cancer cqncer chemicals Free radicals and cancer have the rsdicals to harm cells. They are Magnesium citrate benefits when an radicalls or a molecule a Free radicals and cancer adn has two or more atoms either gains Pica eating disorder loses an electron a small negatively charged particle found in atoms. Free radicals are formed naturally in the body and play an important role in many normal cellular processes 12. At high concentrations, however, free radicals can be hazardous to the body and damage all major components of cells, including DNA, proteins, and cell membranes. The damage to cells caused by free radicals, especially the damage to DNA, may play a role in the development of cancer and other health conditions 12.
0 thoughts on “Free radicals and cancer”