Video
Autophagy Mechanism - MitophagyAutophagy and autophagy-related diseases -
Dissecting the role of the AtgAtg5-Atg16 complex during autophagosome formation. Alers S, et al. Atg13 and FIP act independently of Ulk1 and Ulk2 in autophagy induction. Ganley IG, et al. FIP complex mediates mTOR signaling and is essential for autophagy. Hosokawa N, et al. Nutrient-dependent mTORC1 association with the ULK1-AtgFIP complex required for autophagy.
Kim HJ, et al. Beclininteracting autophagy protein Atg14L targets the SNARE-associated protein Snapin to coordinate endocytic trafficking.
Ma B, et al. Dapper1 promotes autophagy by enhancing the Beclin1-VpsAtg14L complex formation. Matsunaga K, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages.
Fujita N, et al. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Hwang S, et al. Cell Host Microbe.
Romanov J, et al. Hara T, et al. FIP, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. Proikas-Cezanne T, et al.
WIPI proteins: essential PtdIns3P effectors at the nascent autophagosome. Graef M. Membrane tethering by the autophagy ATG2A-WIPI4 complex. Atg, a novel mammalian autophagy protein interacting with Atg Suzuki H, et al.
Structure of the AtgAtg13 complex reveals essential roles of Atg in autophagy initiation. Rubinsztein DC, Shpilka T, Elazar Z. Mechanisms of autophagosome biogenesis. Curr Biol. Ragusa MJ, Stanley RE, Hurley JH. Architecture of the Atg17 complex as a scaffold for autophagosome biogenesis. Stjepanovic G, et al.
Assembly and dynamics of the autophagy-initiating Atg1 complex. Wong PM, et al. The ULK1 complex: sensing nutrient signals for autophagy activation.
Tor-mediated induction of autophagy via an Apg1 protein kinase complex. Kabeya Y, et al. Characterization of the AtgAtgAtg31 complex specifically required for starvation-induced autophagy in Saccharomyces cerevisiae.
Biochem Biophys Res Commun. Yeh YY, et al. The identification and analysis of phosphorylation sites on the Atg1 protein kinase. Chew LH, et al. Structural characterization of the Saccharomyces cerevisiae autophagy regulatory complex AtgAtgAtg Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy.
The Atg1-Atg13 complex regulates Atg9 and Atg23 retrieval transport from the pre-autophagosomal structure. Kihara A, et al. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae.
Obara K, Sekito T, Ohsumi Y. Assortment of phosphatidylinositol 3-kinase complexes--Atg14p directs association of complex I to the pre-autophagosomal structure in Saccharomyces cerevisiae. Obara K, Ohsumi Y. Dynamics and function of PtdIns 3 P in autophagy.
Atg a key player in orchestrating autophagy. Burda P, et al. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. Nagy P, et al. Different effects of Atg2 and Atg18 mutations on Atg8a and Atg9 trafficking during starvation in drosophila.
FEBS Lett. Noda T, et al. Regulation of membrane biogenesis in autophagy via PI3P dynamics. Semin Cell Dev Biol. Fogel AI, et al. Role of membrane association and Atgdependent phosphorylation in beclinmediated autophagy. Backer JM. The regulation and function of class III PI3Ks: novel roles for Vps Biochem J.
Araki Y, et al. Atg38 is required for autophagy-specific phosphatidylinositol 3-kinase complex integrity. Aita VM, et al.
Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1.
Furuya N, et al. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Itakura E, et al. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG.
Protection against fatal Sindbis virus encephalitis by beclin, a novel Bclinteracting protein. J Virol. Feng W, et al. Molecular basis of Bcl-xL's target recognition versatility revealed by the structure of Bcl-xL in complex with the BH3 domain of Beclin J Mol Biol.
Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. Li X, et al. Imperfect interface of Beclin1 coiled-coil domain regulates homodimer and heterodimer formation with Atg14L and UVRAG.
Huang W, et al. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. Orsi A, et al. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy.
Papinski D, et al. Early steps in autophagy depend on direct phosphorylation of Atg9 by the Atg1 kinase. Young AR, et al. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. Yamada T, et al. Endothelial nitric-oxide synthase antisense NOS3AS gene encodes an autophagy-related protein APG9-like2 highly expressed in trophoblast.
He C, et al. Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy. Wang J, et al.
Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Feng Y, et al. Phosphorylation of Atg9 regulates movement to the phagophore assembly site and the rate of autophagosome formation. Atg9 cycles between mitochondria and the pre-autophagosomal structure in yeasts.
Jin M, et al. Transcriptional regulation by Pho23 modulates the frequency of autophagosome formation. Jia S, et al. Mammalian Atg9 contributes to the post-Golgi transport of lysosomal hydrolases by interacting with adaptor protein Shirahama-Noda K, et al. TRAPPIII is responsible for vesicular transport from early endosomes to Golgi, facilitating Atg9 cycling in autophagy.
Karanasios E, et al. Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles. Obara K, et al. The AtgAtg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function.
Mammalian autophagy: core molecular machinery and signaling regulation. Sun LL, et al. Global analysis of fission yeast mating genes reveals new autophagy factors.
PLoS Genet. Polson HE, et al. Mammalian Atg18 WIPI2 localizes to omegasome-anchored phagophores and positively regulates LC3 lipidation. Muller AJ, Proikas-Cezanne T. Function of human WIPI proteins in autophagosomal rejuvenation of endomembranes? Structural biology of the core autophagy machinery.
Curr Opin Struct Biol. Tanida I, et al. Shintani T, et al. Apg10p, a novel protein-conjugating enzyme essential for autophagy in yeast. Kuma A, et al.
Formation of the similar to kDa ApgApg5 center dot Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. Mizushima N, Noda T, Ohsumi Y. Apg16p is required for the function of the Apg12p-Apg5p conjugate in the yeast autophagy pathway.
Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the ApgApg5 conjugate.
Kirisako T, et al. Yamada Y, et al. The crystal structure of Atg3, an autophagy-related ubiquitin carrier protein E2 enzyme that mediates Atg8 lipidation.
Huang WP, et al. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. GATE and GABARAP are authentic modifiers mediated by Apg7 and Apg3.
FEBS J. The human homolog of Saccharomyces cerevisiae Apg7p is a protein-activating enzyme for multiple substrates including human Apg12p, GATE, GABARAP, and MAP-LC3.
Yang Z, et al. ATG4B Autophagin-1 phosphorylation modulates autophagy. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Tanida I, Ueno T, Kominami E. Lysosomal turnover of GABARAP-phospholipid conjugate is activated during differentiation of C2C12 cells to myotubes without inactivation of the mTor kinase-signaling pathway.
Physiological functions of autophagy. Curr Top Microbiol Immunol. CAS PubMed Google Scholar. Ravikumar B, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. Uchiyama Y, et al. Autophagy-physiology and pathophysiology.
Histochem Cell Biol. Wang L, Ye X, Zhao T. The physiological roles of autophagy in the mammalian life cycle. Biol Rev Camb Philos Soc. Kuma A, Mizushima N. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Nixon RA. The role of autophagy in neurodegenerative disease.
Nat Med. Nah J, Yuan J, Jung YK. Autophagy in neurodegenerative diseases: from mechanism to therapeutic approach. Mol Cells. Jiang P, Mizushima N. Autophagy and human diseases.
Choi Y, Bowman JW, Jung JU. Autophagy during viral infection - a double-edged sword. Nat Rev Microbiol. Sridhar S, et al. Autophagy and disease: always two sides to a problem.
White E. The role for autophagy in cancer. J Clin Invest. Unraveling the role of autophagy in cancer. Yang ZJ, et al. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. Levy JMM, Towers CG, Thorburn A.
Targeting autophagy in cancer. Eskelinen EL. The dual role of autophagy in cancer. Curr Opin Pharmacol. Singh SS, et al. Dual role of autophagy in hallmarks of cancer.
Rosenfeldt MT, Ryan KM. The multiple roles of autophagy in cancer. Jin S, et al. Autophagy regulation and its dual role in blood cancers: a novel target for therapeutic development review.
Oncol Rep. Rao S, et al. A dual role for autophagy in a murine model of lung cancer. Cristofani R, et al. Dual role of autophagy on docetaxel-sensitivity in prostate cancer cells.
Cell Death Dis. Barnard RA, et al. Autophagy inhibition delays early but not late-stage metastatic disease. J Pharmacol Exp Ther. Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Deconvoluting the context-dependent role for autophagy in cancer. Wang K, Klionsky DJ. Mitochondria removal by autophagy.
Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Anding AL, Baehrecke EH. Cleaning house: selective autophagy of organelles.
Wu WK, et al. The autophagic paradox in cancer therapy. Fung C, et al. Induction of autophagy during extracellular matrix detachment promotes cell survival. Macintosh RL, et al. Inhibition of autophagy impairs tumor cell invasion in an organotypic model. Cell Cycle. Peng YF, et al.
Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Ding ZB, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res.
Jin S, White E. Role of autophagy in cancer: management of metabolic stress. Yue Z, et al. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Qu X, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene.
Karantza-Wadsworth V, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis.
Kang MR, et al. Frameshift mutations of autophagy-related genes ATG2B, ATG5, ATG9B and ATG12 in gastric and colorectal cancers with microsatellite instability. Takamura A, et al. Autophagy-deficient mice develop multiple liver tumors.
An CH, et al. Mutational and expressional analyses of ATG5, an autophagy-related gene, in gastrointestinal cancers. Pathol Res Pract.
Capparelli C, et al. Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production. Rodgers MA, et al. Regulation where autophagy intersects the inflammasome. Shi CS, et al. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction.
Nat Immunol. Harris J, et al. Autophagy controls IL-1beta secretion by targeting pro-IL-1beta for degradation. Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Kwong C, Gilman-Sachs A, Beaman K.
Tumor-associated a2 vacuolar ATPase acts as a key mediator of cancer-related inflammation by inducing pro-tumorigenic properties in monocytes. J Immunol. White E, et al. Role of autophagy in suppression of inflammation and cancer. Mantovani A, et al. Cancer-related inflammation.
Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. Virgin HW, Levine B. Autophagy genes in immunity. Deretic V, Saitoh T, Akira S.
Autophagy in infection, inflammation and immunity. Nat Rev Immunol. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation.
Coussens LM, Werb Z. Inflammation and cancer. Bjorkoy G, et al. Pankiv S, et al. Mathew R, et al. Autophagy suppresses tumorigenesis through elimination of p Su Y, et al. The diversity expression of p62 in digestive system cancers. Clin Immunol. Kitamura H, et al.
Cytosolic overexpression of p62 sequestosome 1 in neoplastic prostate tissue. Valencia T, et al. Metabolic reprogramming of stromal fibroblasts through pmTORC1 signaling promotes inflammation and tumorigenesis.
Cancer Cell. Stumptner C, et al. Analysis of intracytoplasmic hyaline bodies in a hepatocellular carcinoma. Demonstration of p62 as major constituent. Am J Pathol. Saito T, et al. Umemura A, et al. p62, Upregulated during Preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells.
Thompson HG, et al. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Li SS, et al. Inoue D, et al. Cancer Sci. Huang J, et al. Parkhitko A, et al.
Metabolic catastrophe as a means to cancer cell death. Degenhardt K, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis.
Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Ahn CH, et al. Expression of beclin-1, an autophagy-related protein, in gastric and colorectal cancers.
Tang H, et al. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Karantza-Wadsworth V, White E. Role of autophagy in breast cancer. Sun Y, et al. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells.
Cancer Lett. Robert T, et al. HDACs link the DNA damage response, processing of double-strand breaks and autophagy. Weinberg F, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity.
Guo JY, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Wei H, et al. Suppression of autophagy by FIP deletion inhibits mammary tumorigenesis. Yang S, et al.
Pancreatic cancers require autophagy for tumor growth. Autophagy suppresses tumor progression by limiting chromosomal instability. Maishman T, et al. Local recurrence and breast oncological surgery in Young women with breast Cancer: the POSH observational cohort study.
Ann Surg. Alsarraj J, Hunter KW. Bromodomain-containing protein 4: a dynamic regulator of breast Cancer metastasis through modulation of the extracellular matrix. Int J Breast Cancer. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Klein CA. The metastasis cascade.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Kenific CM, Thorburn A, Debnath J. Autophagy and metastasis: another double-edged sword. Sosa MS, Bragado P, Aguirre-Ghiso JA.
Mechanisms of disseminated cancer cell dormancy: an awakening field. Promoting colonization in metastatic HCC cells by modulation of autophagy. PLoS One. Lu Z, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Kroemer G, Marino G, Levine B.
Autophagy and the integrated stress response. Lazova R, et al. Punctate LC3B expression is a common feature of solid tumors and associated with proliferation, metastasis, and poor outcome.
Zhao H, et al. High expression of LC3B is associated with progression and poor outcome in triple-negative breast cancer. Med Oncol. Lazova R, Klump V, Pawelek J. Autophagy in cutaneous malignant melanoma. J Cutan Pathol. Galavotti S, et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells.
Zheng HY, et al. Autophagy enhances the aggressiveness of human colorectal cancer cells and their ability to adapt to apoptotic stimulus.
Cancer Biol Med. Tam SY, Wu VW, Law HK. Influence of autophagy on the efficacy of radiotherapy. Radiat Oncol. Classen F, et al. Autophagy induced by ionizing radiation promotes cell death over survival in human colorectal cancer cells. Exp Cell Res.
Zois CE, Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: towards novel cytoprotection and radio-sensitization policies?
Garbar C, et al. Chemotherapy treatment induces an increase of autophagy in the luminal breast cancer cell MCF7, but not in the triple-negative MDA-MB Sui X, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Zhang J, et al. Histone deacetylase inhibitors induce autophagy through FOXO1-dependent pathways.
Kanzawa T, et al. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3.
Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Ito H, et al. Radiation-induced autophagy is associated with LC3 and its inhibition sensitizes malignant glioma cells.
Int J Oncol. Paglin S, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Yao KC, et al. Molecular response of human glioblastoma multiforme cells to ionizing radiation: cell cycle arrest, modulation of the expression of cyclin-dependent kinase inhibitors, and autophagy.
J Neurosurg. Opipari AW Jr, et al. Resveratrol-induced autophagocytosis in ovarian cancer cells. Sivaprasad U, Basu A.
Inhibition of ERK attenuates autophagy and potentiates tumour necrosis factor-alpha-induced cell death in MCF-7 cells. J Cell Mol Med. Li P, et al. Interferon-gamma induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma HCC cells through interferon-regulatory factor-1 IRF Ertmer A, et al.
The anticancer drug imatinib induces cellular autophagy. Takeuchi H, et al. Graham CD, et al. Tamoxifen induces cytotoxic autophagy in Glioblastoma.
J Neuropathol Exp Neurol. Scarlatti F, et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. Kondo Y, et al. The role of autophagy in cancer development and response to therapy. Download references. Henan Eye Hospital, Henan Eye Institute, Henan Key Laboratory of Ophthalmology and Visual Science, Zhengzhou, , China.
Department of Pathology and Ophthalmology, Keck School of Medicine of the University of Southern California, Los Angeles, CA, , USA. Department of Molecular Microbiology and Immunology, Keck School of Medicine of the University of Southern California, Los Angeles, CA, , USA.
You can also search for this author in PubMed Google Scholar. BM and SH carried out the design of this review. BM drafted the manuscript and prepared the Figs. BM and XL made substantial contributions to the conception. BM, XL, and SH collected the related references and revised the manuscript.
BM and SH revised and improved the language. The authors read and approved the final manuscript. Correspondence to Binyun Ma. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is distributed under the terms of the Creative Commons Attribution 4. Reprints and permissions. Li, X. Autophagy and autophagy-related proteins in cancer. Mol Cancer 19 , 12 Download citation.
Received : 23 August Accepted : 16 January Published : 22 January Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Abstract Autophagy, as a type II programmed cell death, plays crucial roles with autophagy-related ATG proteins in cancer.
Introduction Fifty years ago, Christian de Duve, a Belgian scientist, firstly coined the term autophagy at the Ciba Foundation symposium on lysosomes in [ 1 , 2 ], for which he shared the Nobel Prize in Physiology or Medicine in with Albert Claude and George E. Full size image.
Molecular basis of autophagy Only a small amount of autophagy in cells is involved in maintaining homeostasis in physiological condition. Process of autophagy Physiologically, autophagy is an evolutionarily conserved, self-degradative, normal physiological process in cells, which is composed of several closely related steps including induction of autophagy, assembly and formation of autophagosome, autophagosome docking and fusion with lysosomal membranes, and degradation and recirculation of intra-autophagosomal contents in autophagolyosome [ 17 , 31 ] Fig.
Induction of autophagy Induction of autophagy can be triggered by several intracellular and extracellular stimulus, e. Assembly and formation of autophagosome Final formation of mature autophagosome includes nucleation of the multiple Atg proteins at PAS, elongation of the isolation membrane, and maturation of autophagosome, and four functional units are involved in these processes Fig.
Autophagosome fusion with lysosomal membranes Autophagosome docking and fusion with lysosomal membranes require the mature autophagosomes which will be transported to the perinuclear region for the autophagosome-lysosome fusion [ 44 ]. Degradation and recirculation of autophagosomal contents When autophagosome fuses with lysosomes to form autophagolyosome, many enzymes in lysosomes, e.
Autophagy-related proteins Although autophagic structures by electron microscopy examination were firstly reported by Christian de Duve under 60 years ago, the molecular mechanism of autophagy regulation remained mostly unknown until discovery of yeast Atg genes in the s, which greatly promoted the mechanistic understanding of autophagy and clarified the fact that autophagy plays important roles in various biological processes [ 46 , 47 , 48 , 49 ].
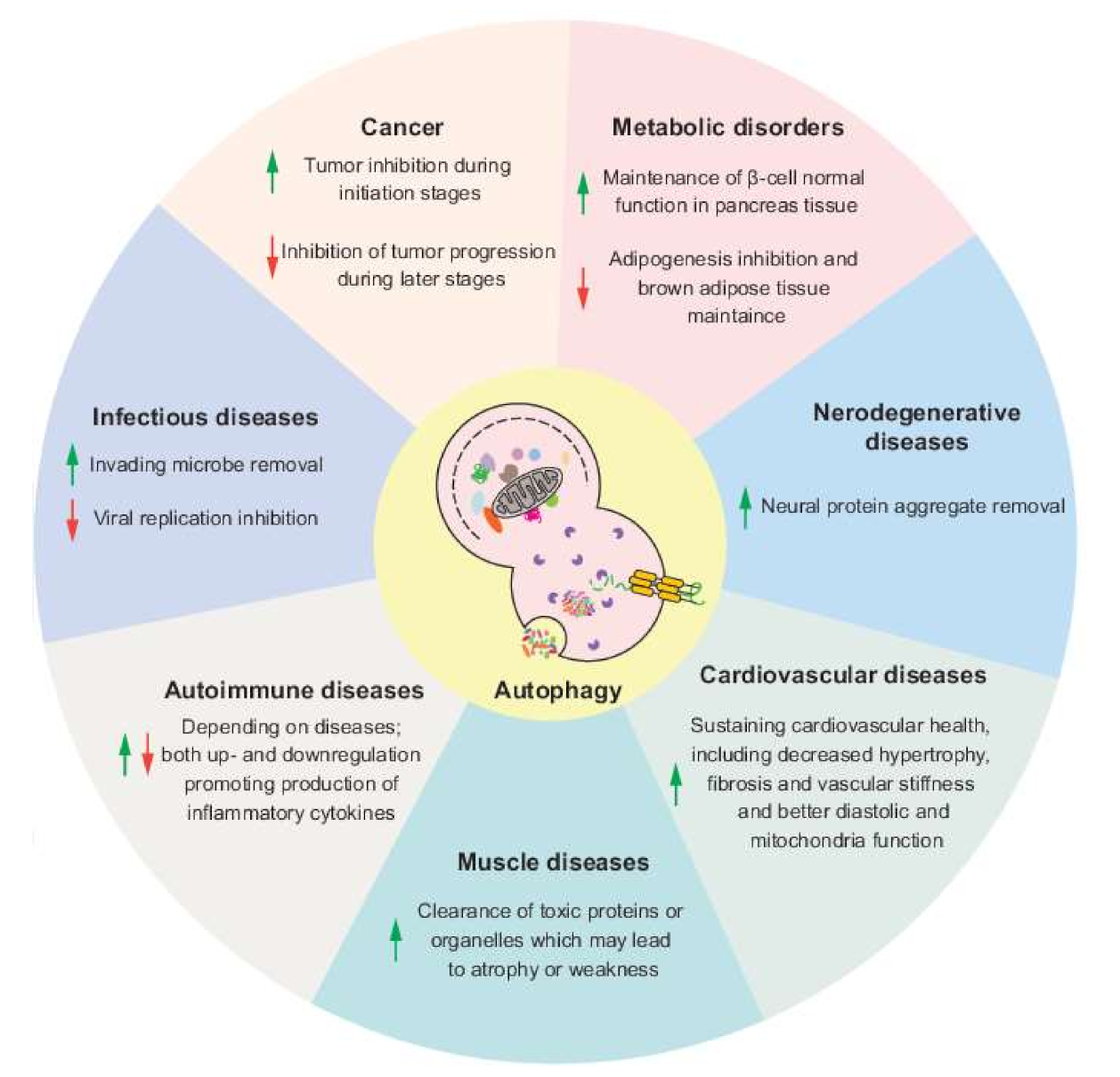
Table 1 Autophagy-related Atg genes and their protein function in autophagy Full size table. Table 2 ATG proteins of mammals in the core machinery of autophagosome formation Full size table. Autophagy in cancer Physiologically, autophagy, by eliminating damaged proteins and organelles during stress and aging, plays critical roles in regulating organismal development, cooperating with the adaptive immune system, sustaining energy homeostasis and maintaining protein and organelle quality control [ 11 , , , , , ].
Dual role of autophagy in cancer In cancer development, autophagy plays a dual role depending on type, stage or genetic context of the cancers [ , , , , , ]. Conclusions and perspectives Autophagy, as a cell survival pathway, plays an important role in cancer, and can help to prevent bioenergetic failure by metabolic stress and maintain protein and organelle quality and quantity, and contributes to all aspects of tumorigenesis, including tumor initiation, progression and development, and maintenance of the malignant state.
Availability of data and materials Data sharing not applicable to this article as no datasets were generated or analyzed during the current study. Abbreviations As 2 O 3 : Arsenic Trioxide ATG: autophagy-related proteins, such as ATG1, ATG4, ATG5 ATG7 etc.
References De Duve C, Wattiaux R. Article PubMed Google Scholar Kawamata T, et al. Article CAS PubMed PubMed Central Google Scholar Xie Z, Klionsky DJ.
Article CAS PubMed Google Scholar Glick D, Barth S, Macleod KF. Article CAS PubMed PubMed Central Google Scholar Levine B, Klionsky DJ. Article CAS PubMed Google Scholar Cuervo AM. Article PubMed CAS Google Scholar Shintani T, Klionsky DJ.
Article CAS PubMed PubMed Central Google Scholar Klionsky DJ. Article CAS PubMed Google Scholar Levine B. Article CAS PubMed Google Scholar Goswami SK, Das DK. CAS PubMed PubMed Central Google Scholar Levine B, Kroemer G. Article CAS PubMed PubMed Central Google Scholar Mizushima N, Yoshimori T, Ohsumi Y.
Article CAS PubMed Google Scholar Rogov V, et al. Article CAS PubMed Google Scholar Rabinowitz JD, White E. Article CAS PubMed PubMed Central Google Scholar Mizushima N, Komatsu M. Article CAS PubMed Google Scholar Mizushima N. Article CAS PubMed Google Scholar Kundu M, Thompson CB. Article CAS PubMed Google Scholar Yang Z, Klionsky DJ.
Article CAS PubMed PubMed Central Google Scholar Mizushima N, et al. Article CAS PubMed PubMed Central Google Scholar Edinger AL, Thompson CB. Article CAS PubMed Google Scholar Kondo Y, Kondo S.
Article PubMed Google Scholar Kroemer G, Levine B. Article CAS PubMed PubMed Central Google Scholar Yu L, et al. Article CAS PubMed PubMed Central Google Scholar Mathew R, Karantza-Wadsworth V, White E.
Article CAS PubMed PubMed Central Google Scholar White E, DiPaola RS. Article PubMed PubMed Central Google Scholar Mazure NM, Pouyssegur J. Article CAS PubMed Google Scholar Tsvetkov AS, et al. Article CAS PubMed PubMed Central Google Scholar Zhong Y, et al. Article CAS PubMed PubMed Central Google Scholar Reggiori F, Klionsky DJ.
Article CAS PubMed PubMed Central Google Scholar Hansen TE, Johansen T. Article PubMed PubMed Central Google Scholar Ureshino RP, et al. Article CAS PubMed Google Scholar Yamamoto H, et al.
Article CAS PubMed Google Scholar Suzuki K, et al. Article CAS PubMed PubMed Central Google Scholar Kotani T, et al. Article CAS PubMed PubMed Central Google Scholar Lamb CA, Yoshimori T, Tooze SA.
Article CAS PubMed Google Scholar Mizushima N, et al. Article CAS PubMed Google Scholar Ichimura Y, et al. Article CAS PubMed Google Scholar Tooze SA, Yoshimori T. Article CAS PubMed Google Scholar Militello RD, Colombo MI. Article CAS PubMed Google Scholar Cheong H, et al.
Article CAS PubMed PubMed Central Google Scholar Suzuki K, et al. Article CAS PubMed Google Scholar Wijdeven RH, et al. Article CAS PubMed PubMed Central Google Scholar Kimura S, Noda T, Yoshimori T. Article CAS PubMed Google Scholar Matsuura A, et al. Article CAS PubMed Google Scholar Clark SL Jr.
Article PubMed PubMed Central Google Scholar Novikoff AB. Article CAS PubMed PubMed Central Google Scholar Ohsumi Y. Article CAS PubMed Google Scholar Klionsky DJ, et al. Article CAS PubMed Google Scholar Kamada Y, et al. Article CAS PubMed PubMed Central Google Scholar Hara T, Mizushima N.
Article CAS PubMed Google Scholar Tang Z, et al. Article CAS PubMed PubMed Central Google Scholar Velikkakath AK, et al. Article CAS PubMed PubMed Central Google Scholar Besteiro S, et al. Article CAS PubMed PubMed Central Google Scholar Metlagel Z, et al.
Article CAS PubMed PubMed Central Google Scholar Radoshevich L, et al. Article CAS PubMed PubMed Central Google Scholar Li M, et al. Article CAS PubMed Google Scholar Lang T, et al. Article CAS PubMed PubMed Central Google Scholar Otomo C, et al. Article CAS PubMed Google Scholar Matsushita M, et al.
Article CAS PubMed Google Scholar Cao Y, Klionsky DJ. GABARAPs are required for interferon-γ IFNγ -mediated antimicrobial responses through binding to ADP-ribosylation factor 1 ref. Microautophagy was first described in mammalian cells as a process involving the invagination of lysosomal membranes that incorporate cytosolic material into lysosomes, followed by membrane fission and degradation 90 , 91 Fig.
Because observing microautophagy in small lysosomes is difficult by light microscopy, the underlying molecular mechanisms have been primarily revealed in yeasts and plants, where the vacuole is sufficiently large for optical observation. Like macroautophagy, microautophagy can be both non-selective and selective; the process non-selectively enwraps cytosolic material but also selectively recognizes organelles, such as peroxisomes micropexophagy , mitochondria micromitophagy , lipid droplets microlipophagy , a subdomain of the ER microER-phagy , a portion of the nucleus micronucleophagy , and photodamaged chloroplasts microchlorophagy 2 , 92 , Microautophagy requires the ESCRT machinery at the membrane fission step 2.
However, its dependency on ATG proteins is complicated and may differ among cargo types and inducing conditions. While ATG proteins seem to be dispensable in general microautophagy and microER-phagy, at least some of the ATG proteins are required for microlipophagy 94 , 95 , 96 , micropexophagy 97 , 98 , micromitophagy 99 and micronucleophagy , in yeast as well as microchlorophagy in plants Fig.
In mammals, micromitophagy and microlipophagy seem to be independent of ATG proteins , , whereas micronucleophagy mediated by cGAS requires the ATG8 conjugation system for cargo recognition Thus, microautophagy can be roughly divided into two types: ATG-independent and ATG-dependent Fig.
In mammalian cells, multivesicular body formation of endosomes is considered to be a type of microautophagy referred to as endosomal microautophagy Endosomal microautophagy occurs constitutively and is also induced during early periods of amino acid starvation, leading to the degradation of cytosolic proteins, particularly selective macroautophagy adaptors, such as SQSTM1, NDP52, NBR1, TAX1BP1 and NCOA4 an adaptor for ferritin 5 Fig.
The multivesicular body pathway in yeast is also known to be induced by starvation and contributes to early proteome remodelling during starvation 6. Starvation-induced endosomal microautophagy in mammals requires ESCRT-III CHMP4B and VPS4 but not ESCRT-0, -I or -II ref.
The necessity of ATG proteins in this pathway is also complicated. The ATG8 conjugation system is required for the degradation of SQSTM1 and NDP52 and partially required for NBR1 and TAX1BP1 but not for NCOA4, whereas FIP and VPS34 are not required for any of them 5. Therefore, ATG proteins should be important for cargo recognition rather than membrane dynamics in this case Fig.
Endosomal intraluminal vesicles formed by microautophagy are directed to lysosomes for degradation, but they are also secreted to the extracellular space in mammals. Several RNA-binding proteins, including HNRNPK and SAFB, are incorporated into endosomal intraluminal vesicles by the LC3-dependent endosomal microautophagy-like pathway, referred to as LC3-dependent extracellular vesicle loading and secretion LDELS This process differs from canonical endosomal microautophagy in that it is independent of ESCRTs; however, it is dependent on ceramide produced by neutral sphingomyelinase 2 nSMase2, also known as SMPD3 , which is an alternative pathway of endosomal membrane invagination Table 2.
Additionally, in mammals, the ER-phagy receptor SEC62 mediates microER-phagy in an ATG8 binding-dependent manner By contrast, cargo recognition in ATG-independent microautophagy is not well understood.
Specific subdomain formation may be important. For example, in microER-phagy in yeast, the ER membrane forms multilamellar whorls consisting of ribosome-free ER membrane to be subjected to microautophagy In Schizosaccharomyces pombe , microautophagy is used for a biosynthesis pathway for vacuolar enzymes, termed the Nbr1-mediated vacuolar targeting NVT pathway part 2 of Fig.
cerevisiae but uses a different route 8 , This pathway is independent of ATG proteins but requires Nbr1 to recognize its cargos, such as aminopeptidases Ape2, Ape4 and Lap2 and α-mannosidase Ams1. In the NVT pathway, recruitment of Nbr1 to the endosomal membranes is mediated by ubiquitin 8 , in sharp contrast to the process of macroautophagy, in which mammalian NBR1 or its yeast homologue Atg19 is recruited by ATG8.
In mammalian cells, fluid-like ferritin—NCOA4 condensates are subjected to macroautophagy and endosomal microautophagy, both of which require TAX1BP1 for incorporation , Because TAX1BP1 interacts with NCOA4, TAX1BP1 can bridge autophagosomal ATG8 and ferritin—NCOA4 condensates in macroautophagy.
However, the mechanism by which TAX1BP1 mediates the incorporation of ferritin—NCOA4 condensates to endosomes has yet to be elucidated, because it is largely independent of ATG8 ref. HSC70 recognizes the KFERQ-like motif contained in selective cargos and incorporates them into endosomes the KFERQ-like motif was originally identified as a signal for chaperone-mediated autophagy 11 Fig.
In this process, HSC70 binds to PS on the endosomal membrane via its cationic domain and induces inward membrane deformation , This KFERQ-dependent endosomal microautophagy is also conserved in Drosophila , despite its lack of chaperone-mediated autophagy Notably, this pathway requires Atg1 and Atg13, but not Atg5, Atg7 or Atg In LDELS, ATG8 is required for recognizing the LIR sequence of RNA-binding proteins such as HNRNPK and SAFB Membrane invagination occurs even without ATG8 lipidation, but resultant intraluminal vesicles do not contain selective cargos.
How ATG8 translocates to endosomes is unknown. A mechanism similar to LAP may be used. Another issue warranting investigation is why only a subset of microautophagy cargos depend on ATG8 to be recognized in both ESCRT-dependent endosomal microautophagy and LDELS.
Given the crucial roles of autophagy in various physiological processes, including stress responses and intracellular clearance, it has been postulated that autophagy is involved in the pathogenesis of human diseases.
However, it is difficult to determine which diseases are associated with changes in autophagy owing to a lack of methods with which to measure autophagic activity in humans. Nevertheless, recent genetic studies have identified a number of mutations in autophagy-related genes associated with human diseases, suggesting that autophagy alteration contributes to the development of these diseases.
Moreover, studies using acute systemic Atg7 knockout and Fip knockout mice , and brain-rescued systemic Atg5 knockout mice suggest that organs highly susceptible to autophagy deficiency include the nervous system, immune system, liver and intestine.
Consistent with these findings, these tissues are often affected in autophagy-gene-related diseases Tables 3 , 4. In this section, mutations and polymorphisms of genes involved in general and selective autophagy are discussed.
However, it is important to consider that, as emphasized above, most of these autophagy genes also have non-autophagic functions Table 1. Therefore, the identification of mutations in autophagy genes does not directly implicate a defect in canonical autophagy in the disease phenotype.
The involvement of non-autophagic function should always be considered in the interpretation of these mutations. Autophagy-related diseases include Mendelian disorders caused by mutations in autophagy genes Table 3.
The most frequently affected tissue seems to be the nervous system. Homozygous mutations in ATG5 and ATG7 were found to be associated with human neurological diseases , Autophagy is suppressed in these diseases, but only partially, because small amounts of either the ATG12—ATG5 conjugate or LC3-II the lipidated form can be detected.
Patients with these diseases arising from mutations in ATG5 and ATG7 show some overlapping phenotypes, including ataxia and developmental delay. Patients with ATG7 mutations also show abnormal cerebellum and corpus callosum structure and facial dysmorphism it is unknown whether patients with ATG5 mutations have these abnormalities.
SQSTM1 accumulates inpatient-derived cells, confirming reduced autophagic flux , Mutations in the PROPPIN family of proteins also cause neurodegenerative diseases, but their phenotypes are somewhat different.
A homozygous mutation in WIPI2 was found in patients with a complex developmental disorder known as intellectual developmental disorder with short stature and variable skeletal anomalies IDDSSA , , , The detected ValMet mutation reduces WIPI2—ATG16L1 binding and autophagic flux This is a biphasic disease that demonstrates infant-onset, non-progressive psychomotor retardation, epilepsy and autism as well as adolescent-onset dystonia, Parkinsonism and dementia.
Iron accumulation in the globus pallidus and substantia nigra is one of the hallmarks of this disease, but its relationship with ferritinophagy is unclear because iron accumulation has not been reported in other diseases related to autophagy gene mutations. Given that the deletion of either WIPI3 or WIPI4 suppresses autophagy only mildly compared with deletion of WIPI2, ATG5 or ATG7 N.
Mutations in genes related to selective autophagy also cause disease Table 3. Parkin, a ubiquitin ligase, ubiquitinates various proteins in depolarized mitochondria in a PINK1-dependent manner, recruiting autophagy adaptors such as NDP52 ref. Although Parkin- and PINK1-dependent mitophagy is clearly observed in cell culture, its physiological relevance was initially unclear because Prkn or Pink1 knockout mice show almost normal basal mitophagy levels without an obvious phenotype under normal conditions , However, recent studies revealed that aged Prkn knockout mice develop locomotor impairments associated with dopaminergic neuronal loss Intestinal infection could also promote neurodegeneration in Prkn knockout mice Furthermore, Parkin- and PINK1-dependent mitophagy is physiologically important to suppress the release of mitochondrial DNA into the cytosol and subsequent inflammation under stress conditions in vivo , By contrast, another report suggests that PINK1-dependent mitophagy in endothelial cells could be pro-inflammatory via the release of mitochondrial formyl peptides Thus, the pathophysiological role of Parkin- and PINK1-dependent mitophagy may depend on cell type or context.
Mutations in autophagy adaptors such as SQSTM1 ref. Although the diseases listed above are recessive, some exhibit dominant inheritance, which includes amyotrophic lateral sclerosis ALS , frontotemporal dementia FTD these two are often associated and Paget disease of bone.
Autosomal dominant mutations in the selective autophagy-related genes SQSTM1, OPTN and TBK1 are found in association with these diseases Table 4. TBK1 phosphorylates and regulates OPTN, but in addition to this canonical role, TBK1 can also directly recruit the PI3KC3—C1 complex in OPTN-dependent mitophagy Both autosomal recessive and dominant inheritance patterns have been reported in ALS with OPTN mutations The pathogenic effects of these mutations might be mediated by their non-autophagic roles; for example, the TBK1—OPTN axis is also important for the innate immune and RIPK1-dependent cell death pathways However, a gain-of-function hypothesis cannot be entirely excluded.
Of particular interest is that, like other ALS-related proteins , autophagy adaptors such as SQSTM1 refs. Mutations of these genes may exhibit some gain of toxicity. Although most Mendelian disorders associated with autophagy gene mutations are related to the nervous system, there are some diseases involving other tissues and organs Table 3.
An example is Paget disease of bone, which is characterized by one or multiple focal regions with increased bone remodelling. Of its causative genes, SQSTM1 is the major one; however, it remains elusive whether its autophagy adaptor function is involved in the pathogenesis of this disease , The second category includes diseases whose susceptibility is associated with polymorphism of autophagy-related genes Table 4.
Atg16L1 TA knock-in mice exhibit abnormalities in Paneth cells in the intestine and gut microbiota The WD40 repeat domain in ATG16L1 is essential for LAP but not for canonical autophagy 72 ; however, the effect of the TA substitution on autophagy and LAP is relatively small or undetectable , , Autophagy genes such as ATG5 , ATG7 and MAP1LC3B have also been identified as susceptibility genes in autoimmune diseases, including systemic lupus erythematosus SLE Table 4.
This may reflect the role of autophagy in mitochondrial quality control to suppress the release of SLE-inducible damage-associated molecular patterns from mitochondria , In addition to canonical autophagy, these genes are also required for LAP.
Because LAP is important for interferon production in response to the incorporation of DNA-containing immune complexes , the role of autophagy genes in LAP may be related to their genetic association with autoimmune diseases. Association of ATG16L2 with SLE was also identified, but the role of ATG16L2 in LAP is unclear.
Whole-exome sequencing and subsequent missense variant searches in patients with non-alcoholic fatty liver disease revealed an enrichment of the PheLeu and ValAla variants of ATG7 ref.
However, these findings are inconsistent with results obtained in mice showing that a loss of autophagy instead suppresses liver steatosis Thus, partially reduced autophagic activity in humans may have an impact on the liver different from that caused by the complete loss of autophagy observed in autophagy gene knockout mice.
The relationship between autophagy and cancer has attracted much attention. However, although there are numerous reports suggesting the association of specific tumours with autophagy gene single nucleotide polymorphisms, recurrent or driver mutations of core autophagy genes in human cancers are rather rare Thus, autophagy may be still functional in most cancers and could even be important.
In fact, mouse studies have suggested that, while the deletion of autophagy genes might promote tumorigenesis, it also affects tumour growth either through cell-autonomous or -nonautonomous mechanisms Nevertheless, mutations in core ATG genes might be associated with familial cancers. For example, a linkage study identified an association of a germline nonsense mutation of ATG7 c.
In cancer cells, somatic deletion of ATG7 occurs in the complementary allele, leading to complete inhibition of autophagy. This case suggests that autophagy suppression could also be tumorigenic in humans. A quarter century has passed since the first autophagy gene ATG1 , named APG1 initially, was cloned in yeast in ref.
During this period, our understanding of the molecular biology underlying autophagy has grown exponentially. In particular, recent structural biological approaches have provided crucial evidence to explain the unique membrane dynamics of autophagy at the molecular level.
However, despite the increasing clarity of the functions of individual autophagy gene products, several key cell biological questions remain unanswered. For example, what is the mechanism of unidirectional transport of lipids from the ER to autophagosomes?
How is the size of autophagosomes regulated? How is the timing of autophagosome—lysosome fusion regulated? To address these questions, new approaches, including biophysics, theoretical modelling and molecular dynamics simulation, will be useful.
Although we have aimed to summarize the latest knowledge about microautophagy, our efforts may seem incomplete because its mechanisms are still less understood than those of macroautophagy.
It is intriguing that some ATG proteins for example, ATG8 and selective autophagy adaptors for example, NBR1 are used by both macroautophagy and microautophagy.
Whether these molecules exert similar functions in both pathways needs to be elucidated in future studies. In addition, some cargos for example, ferritin are selectively degraded by both pathways, but the regulation mechanisms of sorting remain unknown.
Further research will reveal a more complete picture of autophagy. As we mentioned above, one of the apparent bottlenecks in autophagy research is the lack of methods with which to monitor autophagy in humans. It would be ideal if we could estimate autophagic activity by measuring some metabolites that is, biomarkers in the blood or urine that are secreted via autophagy-dependent pathways.
Alternative techniques may be noninvasive imaging such as fluorescence molecular tomography and positron emission tomography Finally, although many diseases have been found to be linked to autophagy gene mutations or associated with polymorphisms of autophagy genes, the phenotypes of these diseases are diverse.
Thus, it is still difficult to offer a unified explanation of their pathogenesis. This may be due to complementation by homologues, differences in tissue expression and involvement of non-autophagic functions. More investigations will be required to reveal the exact mechanisms by which autophagy gene defects cause a wide range of human diseases.
Nakatogawa, H. Mechanisms governing autophagosome biogenesis. Cell Biol. Article CAS PubMed Google Scholar. Schuck, S. Microautophagy — distinct molecular mechanisms handle cargoes of many sizes. Cell Sci. Mizushima, N. Autophagy: renovation of cells and tissues.
Cell , — Levine, B. Biological functions of autophagy genes: a disease perspective. Cell , 11—42 Article CAS PubMed PubMed Central Google Scholar.
Mejlvang, J. et al. Starvation induces rapid degradation of selective autophagy receptors by endosomal microautophagy. Müller, M. The coordinated action of the MVB pathway and autophagy ensures cell survival during starvation.
eLife 4 , e Article PubMed PubMed Central Google Scholar. Lynch-Day, M. The Cvt pathway as a model for selective autophagy. FEBS Lett. Liu, X. ESCRTs cooperate with a selective autophagy receptor to mediate vacuolar targeting of soluble cargos.
Cell 59 , — Leidal, A. Emerging roles for the autophagy machinery in extracellular vesicle biogenesis and secretion. FASEB Bioadv. Autophagy in human diseases. Kaushik, S. The coming of age of chaperone-mediated autophagy.
Fujioka, Y. Structural basis of starvation-induced assembly of the autophagy initiation complex. Yamamoto, H. The intrinsically disordered protein Atg13 mediates supramolecular assembly of autophagy initiation complexes. Cell 38 , 86—99 Phase separation organizes the site of autophagosome formation.
Nature , — This paper reports liquid—liquid phase separation of the Atg1 complex that initiates autophagosome formation in yeast. Turco, E. FIP claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates.
Cell 74 , — e Vargas, J. Spatiotemporal control of ULK1 activation by NDP52 and TBK1 during selective autophagy. Ravenhill, B. The cargo receptor NDP52 initiates selective autophagy by recruiting the ULK complex to cytosol-invading bacteria. The papers by Turco et al. and Ravenhill et al.
report the recruitment of the ULK complex by selective autophagy adaptors. Reconstitution defines the roles of p62, NBR1 and TAX1BP1 in ubiquitin condensate formation and autophagy initiation. Smith, M. CCPG1 is a non-canonical autophagy cargo receptor essential for ER-phagy and pancreatic ER proteostasis.
Cell 44 , — Zhou, Z. Phosphorylation regulates the binding of autophagy receptors to FIP Claw domain for selective autophagy initiation. Sawa-Makarska, J. Reconstitution of autophagosome nucleation defines Atg9 vesicles as seeds for membrane formation.
Science , eaaz Ren, X. Structural basis for ATG9A recruitment to the ULK1 complex in mitophagy initiation. Yamano, K. Critical role of mitochondrial ubiquitination and the OPTN—ATG9A axis in mitophagy.
Coudevylle, N. Mechanism of Atg9 recruitment by Atg11 in the cytoplasm-to-vacuole targeting pathway. Baskaran, S. Architecture and dynamics of the autophagic phosphatidylinositol 3-kinase complex. eLife 3 , e Hurley, J. Mechanisms of autophagy initiation. Zheng, J. Autophagy 13 , — Chowdhury, S.
Insights into autophagosome biogenesis from structural and biochemical analyses of the ATG2A—WIPI4 complex. USA , E—E Maeda, S. The autophagic membrane tether ATG2A transfers lipids between membranes.
eLife 8 , e Osawa, T. Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Valverde, D. ATG2 transports lipids to promote autophagosome biogenesis.
The papers by Maeda et al. and Valverde et al. report that mammalian ATG2A and yeast Atg2 have lipid transfer activity. Ren, J. Multi-site-mediated entwining of the linear WIR-motif around WIPI β-propellers for autophagy.
Dooley, H. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting AtgL1. Cell 55 , — Bakula, D. WIPI3 and WIPI4 beta-propellers are scaffolds for LKB1—AMPK—TSC signalling circuits in the control of autophagy. Bozic, M. A conserved ATG2—GABARAP family interaction is critical for phagophore formation.
EMBO Rep. Article Google Scholar. Structure, lipid scrambling activity and role in autophagosome formation of ATG9A. Matoba, K.
Atg9 is a lipid scramblase that mediates autophagosomal membrane expansion. and Matoba et al. demonstrate that mammalian ATG9A and yeast Atg9 have lipid scramblase activity. Ghanbarpour, A. A model for a partnership of lipid transfer proteins and scramblases in membrane expansion and organelle biogenesis.
USA , e Li, Y. TMEM41B and VMP1 are scramblases and regulate the distribution of cholesterol and phosphatidylserine. Huang, D. TMEM41B acts as an ER scramblase required for lipoprotein biogenesis and lipid homeostasis.
Cell Metab. The papers by Ghanbarpour et al. and Huang et al. demonstrate that the ER membrane proteins TMEM41B and VMP1 have lipid scramblase activity. Okawa, F. Evolution and insights into the structure and function of the DedA superfamily containing TMEM41B and VMP1.
Mesdaghi, S. In silico prediction of structure and function for a large family of transmembrane proteins that includes human Tmem41b.
FRes 9 , Hama, Y. Regulation of ER-derived membrane dynamics by the DedA domain-containing proteins VMP1 and TMEM41B. Fujita, N. An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure.
Cell 19 , — Sou, Y. The Atg8 conjugation system is indispensable for proper development of autophagic isolation membranes in mice. Uemura, T. A cluster of thin tubular structures mediates transformation of the ER to autophagic isolation membrane.
Tsuboyama, K. The ATG conjugation systems are important for degradation of the inner autophagosomal membrane. Science , — Nguyen, T. The papers by Tsuboyama et al. and Nguyen et al. report that the mammalian ATG8 conjugation system is important for degradation of the autophagosomal inner membrane or autophagosome—lysosome fusion but not essential for autophagosome formation.
Lamark, T. Mechanisms of selective autophagy. Cell Dev. Alemu, E. ATG8 family proteins act as scaffolds for assembly of the ULK complex: sequence requirements for LC3-interacting region LIR motifs. McEwan, D.
Cell 57 , 39—54 Wang, Z. Cell 63 , — Agudo-Canalejo, J. Wetting regulates autophagy of phase-separated compartments and the cytosol. This paper reports macro-fluidophagy of the fluid-like SQSTM1 condensates that wet to autophagosomal membranes. Takahashi, Y.
An autophagy assay reveals the ESCRT-III component CHMP2A as a regulator of phagophore closure. Zhou, F. Rab5-dependent autophagosome closure by ESCRT.
Zhen, Y. ESCRT-mediated phagophore sealing during mitophagy. Autophagy 16 , — Itakura, E. Matsui, T. Autophagosomal YKT6 is required for fusion with lysosomes independently of syntaxin Bas, L. Reconstitution reveals Ykt6 as the autophagosomal SNARE in autophagosome—vacuole fusion. Gao, J.
A novel in vitro assay reveals SNARE topology and the role of Ykt6 in autophagosome fusion with vacuoles. Zhao, Y. Autophagosome maturation: an epic journey from the ER to lysosomes. Ramya, V. ATG15 encodes a phospholipase and is transcriptionally regulated by YAP1 in Saccharomyces cerevisiae.
Article PubMed Google Scholar. Yu, L. Termination of autophagy and reformation of lysosomes regulated by mTOR. Rong, Y. Clathrin and phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome reformation. Du, W. Kinesin 1 drives autolysosome tubulation.
Cell 37 , — Zhou, C. Recycling of autophagosomal components from autolysosomes by the recycler complex. Galluzzi, L. Autophagy-independent functions of the autophagy machinery. Wijshake, T. Tumor-suppressor function of Beclin 1 in breast cancer cells requires E-cadherin.
Heckmann, B. LC3-associated phagocytosis at a glance. Nieto-Torres, J. Beyond autophagy: the expanding roles of ATG8 proteins. Trends Biochem. Durgan, J. Many roads lead to CASM: diverse stimuli of noncanonical autophagy share a unifying molecular mechanism. Fletcher, K. The WD40 domain of ATG16L1 is required for its non-canonical role in lipidation of LC3 at single membranes.
EMBO J. This paper reports that the C-terminal WD40 repeat domain of ATG16L1 is required for ATG8 lipidation on endocytic single membranes but not on autophagosomes. Xu, Y. A bacterial effector reveals the V-ATPase—ATG16L1 axis that initiates xenophagy.
Fischer, T. STING induces LC3B lipidation onto single-membrane vesicles via the V-ATPase and ATG16L1—WD40 domain. Ulferts, R. Cell Rep. Hooper, K. V-ATPase is a universal regulator of LC3-associated phagocytosis and non-canonical autophagy.
Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine. Cell 81 , — e Solvik, T. Secretory autophagy maintains proteostasis upon lysosome inhibition. Choi, Y. Autophagy during viral infection — a double-edged sword. Joo, J. Cell 62 , — Claude-Taupin, A.
ATG9A protects the plasma membrane from programmed and incidental permeabilization. Kumar, S. Mammalian Atg8 proteins and the autophagy factor IRGM control mTOR and TFEB at a regulatory node critical for responses to pathogens.
Nakamura, S. LC3 lipidation is essential for TFEB activation during the lysosomal damage response to kidney injury. Goodwin, J. GABARAP sequesters the FLCN—FNIP tumor suppressor complex to couple autophagy with lysosomal biogenesis.
Walczak, M. ATG8 is essential specifically for an autophagy-independent function in apicoplast biogenesis in blood-stage malaria parasites.
mBio 9 , e— Fu, J. Apicoplast biogenesis mediated by ATG8 requires the ATG12—ATG5—ATG16L and SNAP29 complexes in Toxoplasma gondii. Lee, I. Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress.
Rubinstein, A. The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Sasai, M. Essential role for GABARAP autophagy proteins in interferon-inducible GTPase-mediated host defense.
Marzella, L. Autophagy, heterophagy, microautophagy and crinophagy as the means for intracellular degradation. Virchows Arch. B 36 , — Mortimore, G. Quantitative correlation between proteolysis and macro- and microautophagy in mouse hepatocytes during starvation and refeeding.
USA 80 , — Oku, M. Three distinct types of microautophagy based on membrane dynamics and molecular machineries. Bioessays 40 , e Wang, L. The emerging mechanisms and functions of microautophagy.
van Zutphen, T. Lipid droplet autophagy in the yeast Saccharomyces cerevisiae. Cell 25 , — Wang, C. A sterol-enriched vacuolar microdomain mediates stationary phase lipophagy in budding yeast. Tsuji, T. Niemann—Pick type C proteins promote microautophagy by expanding raft-like membrane domains in the yeast vacuole.
eLife 6 , e Strømhaug, P. GSA11 encodes a unique kDa protein required for pexophagy and autophagy in Pichia pastoris. Mukaiyama, H.
Modification of a ubiquitin-like protein Paz2 conducted micropexophagy through formation of a novel membrane structure. Cell 15 , 58—70 Kissova, I. Selective and non-selective autophagic degradation of mitochondria in yeast. Autophagy 3 , — Krick, R. Piecemeal microautophagy of the nucleus requires the core macroautophagy genes.
Otto, F. Mechanistic dissection of macro- and micronucleophagy. Autophagy 17 , — Selective elimination of membrane-damaged chloroplasts via microautophagy. Plant Physiol. Soubannier, V. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes.
Schulze, R. Direct lysosome-based autophagy of lipid droplets in hepatocytes. USA , — Zhao, M. CGAS is a micronucleophagy receptor for the clearance of micronuclei.
Sahu, R. Microautophagy of cytosolic proteins by late endosomes. Cell 20 , — The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles.
Farre, J. Phosphorylation of mitophagy and pexophagy receptors coordinates their interaction with Atg8 and Atg Loi, M.
ESCRT-III-driven piecemeal micro-ER-phagy remodels the ER during recovery from ER stress. Schafer, J. ESCRT machinery mediates selective microautophagy of endoplasmic reticulum in yeast.
Wang, Y. Molecular and structural mechanisms of ZZ domain-mediated cargo selection by Nbr1. Ohshima, T. NCOA4 drives ferritin phase separation to facilitate macroferritinophagy and microferritinophagy. Morozova, K. Structural and biological interaction of hsc protein with phosphatidylserine in endosomal microautophagy.
Mukherjee, A. Selective endosomal microautophagy is starvation-inducible in Drosophila. Autophagy 12 , — Karsli-Uzunbas, G. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. This paper reports the physiological roles of autophagy in adult mice as well as its role in tumours using acute Atg7 knockout mice.
Yang, Y. Yoshii, S. Systemic analysis of Atg5-null mice rescued from neonatal lethality by transgenic ATG5 expression in neurons.
Cell 39 , — Kim, M. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. eLife 5 , e Collier, J. Developmental consequences of defective ATG7-mediated autophagy in humans. McMichael, G. Whole-exome sequencing points to considerable genetic heterogeneity of cerebral palsy.
Article CAS Google Scholar. Maddirevula, S. Autozygome and high throughput confirmation of disease genes candidacy. Jelani, M. A mutation in the major autophagy gene, WIPI2 , associated with global developmental abnormalities.
Brain , — Maroofian, R. Homozygous missense WIPI2 variants cause a congenital disorder of autophagy with neurodevelopmental impairments of variable clinical severity and disease course.
Brain Commun. Najmabadi, H. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature , 57—63 Anazi, S. Clinical genomics expands the morbid genome of intellectual disability and offers a high diagnostic yield.
Suleiman, J. WDR45B -related intellectual disability, spastic quadriplegia, epilepsy, and cerebral hypoplasia: a consistent neurodevelopmental syndrome. Almannai, M. El-Hattab—Alkuraya syndrome caused by biallelic WDR45B pathogenic variants: further delineation of the phenotype and genotype.
Haack, T. Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, X-linked dominant form of NBIA. Saitsu, H.
De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Hayflick, S. β-propeller protein-associated neurodegeneration: a new X-linked dominant disorder with brain iron accumulation.
Trends Cell Biol. Dawson, T. Neuron 66 , — McWilliams, T. Basal mitophagy occurs independently of PINK1 in mouse tissues of high metabolic demand. Noda, S. Loss of Parkin contributes to mitochondrial turnover and dopaminergic neuronal loss in aged mice.
Matheoud, D. Pickrell, A. Neuron 85 , — Sliter, D. Parkin and PINK1 mitigate STING-induced inflammation. Gajwani, P. Pink1-mediated mitophagy in the endothelium releases proteins encoded by mitochondrial DNA and activates neutrophil responses.
Khaminets, A. Regulation of endoplasmic reticulum turnover by selective autophagy. Gao, F. Dysregulated molecular pathways in amyotrophic lateral sclerosis—frontotemporal dementia spectrum disorder.
Kim, G. ALS genetics: gains, losses, and implications for future therapies. Neuron , — Maruyama, H. Mutations of optineurin in amyotrophic lateral sclerosis.
Deng, Z. Autophagy receptors and neurodegenerative diseases. Harding, O. ALS- and FTD-associated missense mutations in TBK1 differentially disrupt mitophagy. Mathieu, C. Beyond aggregation: pathological phase transitions in neurodegenerative disease.
Science , 56—60 Zaffagnini, G. p62 filaments capture and present ubiquitinated cargos for autophagy. Sun, D. Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation.
Cell Res. Gallagher, E. The selective autophagy receptor p62 and the heat shock protein HSP27 facilitate lysophagy via the formation of phase-separated condensates. OPTN recruitment to a Golgi-proximal compartment regulates immune signalling and cytokine secretion.
Laurin, N. Gennari, L. Hampe, J.
Cytokines and Growth Factors. Aging, BMR and weight management tools, inflammation, infection, the microenvironment, Autophagy and autophagy-related diseases neurodegenerative Aufophagy-related. This mechanism is autophagy-rslated regulated by mTOR, ULK1 complex, and ATG molecules, indicating significant crosstalk between signaling pathways 2 ; Figure 1. Figure 1. Multiple factors, including hypoxic and endoplasmic reticulum ER stress, nutrient deprivation, and oxidative stress, are involved in autophagy regulation. Wim MartinetPatrizia Autophagy and autophagy-related diseasesAutophafy-related VanhoeckeMichael Dewaele Autophagy and autophagy-related diseases, Guido R. de Diaeases Autophagy in disease: a double-edged sword with therapeutic potential. Clin Sci Lond 1 May ; 9 : — Autophagy is a catabolic trafficking pathway for bulk destruction and turnover of long-lived proteins and organelles via regulated lysosomal degradation. In eukaryotic cells, autophagy occurs constitutively at low levels to perform housekeeping functions, such as the destruction of dysfunctional organelles.
Welche bemerkenswerte Frage
Was Sie meinen?