
Modulating cancer cell apoptosis -
Schildkopf P, Frey B, Ott OJ, Rubner Y, Multhoff G, Sauer R, et al. Radiation combined with hyperthermia induces HSPdependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages.
Radiother Oncol — Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma.
Vanpouille-Box C, Pilones KA, Wennerberg E, Formenti SC, Demaria S. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine — Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes.
Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med — Santin AD, Hermonat PL, Ravaggi A, Chiriva-Internati M, Hiserodt JC, Batchu RB, et al.
The effects of irradiation on the expression of a tumour rejection antigen heat shock protein gp96 in human cervical cancer. Modrak DE, Gold DV, Goldenberg DM, Blumenthal RD. Colonic tumor CEA, CSAp and MUC-1 expression following radioimmunotherapy or chemotherapy.
Tumour Biol —9. Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy.
J Immunol — Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer.
Clin Cancer Res — Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest — Schumacher TN, Schreiber RD.
Neoantigens in cancer immunotherapy. Science — Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome.
Nat Rev Cancer — Tran Janco JM, Lamichhane P, Karyampudi L, Knutson KL. Tumor-infiltrating dendritic cells in cancer pathogenesis.
Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1.
Anguille S, Smits EL, Lion E, Van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol e— Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, et al.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol — Noy R, Pollard JW.
Tumor-associated macrophages: from mechanisms to therapy. Immunity — Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One 7:e Guillerey C, Huntington ND, Smyth MJ.
Targeting natural killer cells in cancer immunotherapy. Pietra G, Vitale C, Pende D, Bertaina A, Moretta F, Falco M, et al. Human natural killer cells: news in the therapy of solid tumors and high-risk leukemias.
Cancer Immunol Immunother — Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res — De Sanctis F, Solito S, Ugel S, Molon B, Bronte V, Marigo I.
MDSCs in cancer: conceiving new prognostic and therapeutic targets. Biochim Biophys Acta — Stoitzner P, Sparber F, Tripp CH. Langerhans cells as targets for immunotherapy against skin cancer.
Immunol Cell Biol —7. Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment.
Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hammerling GJ. Teitz-Tennenbaum S, Li Q, Okuyama R, Davis MA, Sun R, Whitfield J, et al. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy.
J Immunother — Cummings RJ, Gerber SA, Judge JL, Ryan JL, Pentland AP, Lord EM. Exposure to ionizing radiation induces the migration of cutaneous dendritic cells by a CCR7-dependent mechanism. Liu C, Lin J, Zhao L, Yang Y, Gao F, Li B, et al. Gamma-ray irradiation impairs dendritic cell migration to CCL19 by down-regulation of CCR7 and induction of cell apoptosis.
Int J Biol Sci — Morganti JM, Jopson TD, Liu S, Gupta N, Rosi S. PLoS One 9:e Russell JS, Brown JM. The irradiated tumor microenvironment: role of tumor-associated macrophages in vascular recovery.
Front Physiol Shiao SL, Ruffell B, Denardo DG, Faddegon BA, Park CC, Coussens LM. Cancer Immunol Res — Chiang CS, Fu SY, Wang SC, Yu CF, Chen FH, Lin CM, et al.
Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Seifert L, Werba G, Tiwari S, Giao Ly NN, Nguy S, Alothman S, et al. Radiation therapy induces macrophages to suppress T-cell responses against pancreatic tumors in mice. Gastroenterology Xu J, Escamilla J, Mok S, David J, Priceman S, West B, et al.
CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Stafford JH, Hirai T, Deng L, Chernikova SB, Urata K, West BL, et al.
Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol — Wang X, Yang X, Tsai Y, Yang L, Chuang KH, Keng PC, et al. Radiat Res —9. Uchimura E, Watanabe N, Niwa O, Muto M, Kobayashi Y.
Transient infiltration of neutrophils into the thymus in association with apoptosis induced by whole-body X-irradiation. J Leukoc Biol —4. Fujiwara H, Yamazaki T, Uzawa A, Nagata K, Kobayashi Y. Transient infiltration of neutrophils into the thymus following whole-body X-ray irradiation in IL knockout mice.
Biochem Biophys Res Commun —6. Nakayama E, Shiratsuchi Y, Kobayashi Y, Nagata K. The importance of infiltrating neutrophils in SDF-1 production leading to regeneration of the thymus after whole-body X-irradiation.
Cell Immunol —8. Lee EJ, Kim JW, Yoo H, Kwak W, Choi WH, Cho S, et al. Single high-dose irradiation aggravates eosinophil-mediated fibrosis through IL secreted from impaired vessels in the skin compared to fractionated irradiation.
Ibahim MJ, Yang Y, Crosbie JC, Stevenson A, Cann L, Paiva P, et al. Eosinophil-associated gene pathways but not eosinophil numbers are differentially regulated between synchrotron microbeam radiation treatment and synchrotron broad-beam treatment by 48 hours postirradiation.
Radiat Res —8. Reuben JM, Korbling M, Gao H, Lee BN. The effect of low dose gamma irradiation on the differentiation and maturation of monocyte derived dendritic cells. J Gravit Physiol — Immunosuppressive effects of radiation on human dendritic cells: reduced IL production on activation and impairment of naive T-cell priming.
Br J Cancer —8. Liao YP, Wang CC, Butterfield LH, Economou JS, Ribas A, Meng WS, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells.
J Immunol —9. Price JG, Idoyaga J, Salmon H, Hogstad B, Bigarella CL, Ghaffari S, et al. CDKN1A regulates Langerhans cell survival and promotes Treg cell generation upon exposure to ionizing irradiation. Nat Immunol —8. Nakano T, Oka K, Arai T, Morita S, Tsunemoto H.
Arch Pathol Lab Med — Nakano T, Oka K, Takahashi T, Morita S, Arai T. Cancer — Nakano T, Oka K, Sugita T, Tsunemoto H. Antitumor activity of Langerhans cells in radiation therapy for cervical cancer and its modulation with SPG administration.
In Vivo — Chen Z, Xia D, Bi X, Saxena A, Sidhu N, El-Gayed A, et al. Combined radiation therapy and dendritic cell vaccine for treating solid tumors with liver micro-metastasis. J Gene Med — Lodermann B, Wunderlich R, Frey S, Schorn C, Stangl S, Rodel F, et al.
Low dose ionising radiation leads to a NF-kappaB dependent decreased secretion of active IL-1beta by activated macrophages with a discontinuous dose-dependency. Schaue D, Marples B, Trott KR. The effects of low-dose X-irradiation on the oxidative burst in stimulated macrophages.
Mckinney LC, Aquilla EM, Coffin D, Wink DA, Vodovotz Y. J Leukoc Biol — Role of tumor necrosis factor-alpha. Ann N Y Acad Sci —8. Lee H, Ahn YT, Park SH, Park DY, Jin YW, Kim CS, et al. Lactobacillus plantarum HY protects against the impairment of NK-cell activity caused by whole-body gamma-irradiation in mice.
J Microbiol Biotechnol — Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res —6. Frischholz B, Wunderlich R, Ruhle PF, Schorn C, Rodel F, Keilholz L, et al.
Autoimmunity —8. Tsai CS, Chen FH, Wang CC, Huang HL, Jung SM, Wu CJ, et al. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth.
Int J Radiat Oncol Biol Phys — Okubo M, Kioi M, Nakashima H, Sugiura K, Mitsudo K, Aoki I, et al. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci Rep Meng Y, Beckett MA, Liang H, Mauceri HJ, Van Rooijen N, Cohen KS, et al.
Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Prakash H, Klug F, Nadella V, Mazumdar V, Schmitz-Winnenthal H, Umansky L. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: lesson from insulinoma.
Carcinogenesis — Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Cancer Cell — Farooque A, Afrin F, Adhikari JS, Dwarakanath BS. Polarization of macrophages towards M1 phenotype by a combination of 2-deoxy-d-glucose and radiation: implications for tumor therapy.
Immunobiology — Balabanli B, Turkozkan N, Balabanli S, Erdamar H, Akmansu M. The effect of vitamin A pretreatment on radiation induced alteration in neutrophil functions.
Mol Cell Biochem —5. Panes J, Granger DN. Neutrophils generate oxygen free radicals in rat mesenteric microcirculation after abdominal irradiation. Gastroenterology —9. Shigematsu A, Adachi Y, Koike-Kiriyama N, Suzuki Y, Iwasaki M, Koike Y, et al. Effects of low-dose irradiation on enhancement of immunity by dendritic cells.
J Radiat Res —5. Chun SH, Park GY, Han YK, Kim SD, Kim JS, Lee CG, et al. Effect of low dose radiation on differentiation of bone marrow cells into dendritic cells.
Dose Response — Jahns J, Anderegg U, Saalbach A, Rosin B, Patties I, Glasow A, et al. Influence of low dose irradiation on differentiation, maturation and T-cell activation of human dendritic cells.
Mutat Res ——9. Lee EJ, Lee SJ, Kim JH, Kim KJ, Yang SH, Jeong KY, et al. PLoS One e Cao MD, Chen ZD, Xing Y. Gamma irradiation of human dendritic cells influences proliferation and cytokine profile of T cells in autologous mixed lymphocyte reaction.
Cell Biol Int —8. Kulzer L, Rubner Y, Deloch L, Allgauer A, Frey B, Fietkau R, et al. Norm- and hypo-fractionated radiotherapy is capable of activating human dendritic cells.
J Immunotoxicol — Huang J, Wang QJ, Yang S, Li YF, El-Gamil M, Rosenberg SA, et al. Irradiation enhances human T-cell function by upregulating CD70 expression on antigen-presenting cells in vitro.
Malecka A, Wang Q, Shah S, Sutavani RV, Spendlove I, Ramage JM, et al. J Leukoc Biol —9. Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, et al.
Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis — Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature —1. Garrido G, Rabasa A, Sanchez B, Lopez MV, Blanco R, Lopez A, et al. Induction of immunogenic apoptosis by blockade of epidermal growth factor receptor activation with a specific antibody.
Rastogi S, Boylan M, Wright EG, Coates PJ. Interactions of apoptotic cells with macrophages in radiation-induced bystander signaling. Radiat Res — El-Saghire H, Michaux A, Thierens H, Baatout S. Low doses of ionizing radiation induce immune-stimulatory responses in isolated human primary monocytes.
Int J Mol Med — Luna-Gomes T, Santana PT, Coutinho-Silva R. Silica-induced inflammasome activation in macrophages: role of ATP and P2X7 receptor. Immunobiology —6. Lupfer C, Thomas PG, Anand PK, Vogel P, Milasta S, Martinez J, et al.
Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Kim SK, Yun CH, Han SH. Enhanced anti-cancer activity of human dendritic cells sensitized with gamma-irradiation-induced apoptotic colon cancer cells.
Cancer Lett — Nikitina EY, Gabrilovich DI. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer.
Kang SM, Kim MH, Song KH, Jung SY, Ahn J, Hwang SG, et al. Modulation of dendritic cell function by the radiation-mediated secretory protein gamma-synuclein. Cell Death Discov Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy.
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. Uchida A, Mizutani Y, Nagamuta M, Ikenaga M.
Effects of X-ray irradiation on natural killer NK cell system. Elevation of sensitivity of tumor cells and lytic function of NK cells. Immunopharmacol Immunotoxicol — Brovall C, Schacter B. Radiation sensitivity of human natural killer cell activity: control by X-linked genes.
Zarcone D, Tilden AB, Lane VG, Grossi CE. Radiation sensitivity of resting and activated nonspecific cytotoxic cells of T lineage and NK lineage. Blood — Sonn CH, Choi JR, Kim TJ, Yu YB, Kim K, Shin SC, et al.
Augmentation of natural cytotoxicity by chronic low-dose ionizing radiation in murine natural killer cells primed by IL J Radiat Res —9. Yang G, Kong Q, Wang G, Jin H, Zhou L, Yu D, et al. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy.
Cancer Biother Radiopharm — Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jaattela M, et al. Dual function of membrane-bound heat shock protein 70 Hsp70 , Bag-4, and Hsp protection against radiation-induced effects and target structure for natural killer cells.
Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH, et al. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med — Son CH, Keum JH, Yang K, Nam J, Kim MJ, Kim SH, et al.
Synergistic enhancement of NK cell-mediated cytotoxicity by combination of histone deacetylase inhibitor and ionizing radiation. Radiat Oncol Yang KL, Wang YS, Chang CC, Huang SC, Huang YC, Chi MS, et al. Reciprocal complementation of the tumoricidal effects of radiation and natural killer cells.
PLoS One 8:e Chang Y, Yang ZY, Li GL, Li Q, Yang Q, Fan JQ, et al. Correlations between radiation dose in bone marrow and hematological toxicity in patients with cervical cancer: a comparison of 3DCRT, IMRT, and RapidARC.
Int J Gynecol Cancer —6. Hui B, Zhang Y, Shi F, Wang J, Wang T, Wang J, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in cervical cancer patients undergoing concurrent chemoradiotherapy: comparison of three-dimensional conformal radiotherapy and intensity-modulated radiation therapy.
Int J Gynecol Cancer — Wang D, An G, Xie S, Yao Y, Feng G. Crittenden MR, Savage T, Cottam B, Bahjat KS, Redmond WL, Bambina S, et al. The peripheral myeloid expansion driven by murine cancer progression is reversed by radiation therapy of the tumor. Chen HM, Ma G, Gildener-Leapman N, Eisenstein S, Coakley BA, Ozao J, et al.
Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Kawase Y, Naito S, Ito M, Sekine I, Fujii H. The effect of ionizing radiation on epidermal Langerhans cells — a quantitative analysis of autopsy cases with radiation therapy.
Edwards EK Jr, Edwards EK Sr. The effect of superficial x-radiation on epidermal Langerhans cells in human skin. Int J Dermatol —2. In addition to mildly facilitating the ability of compounds to derepress BAX from BCL-xL, p53 also provides a death signal downstream of anti-apoptotic proteins inhibition that is independent from PUMA, as enhanced p53 can substitute for PUMA to promote BAX activation in response to BH3 mimetics [ ].
It is thus of particular relevance that p53, even when expressed constitutively under conditions where it does not influence the expression of its pro-apoptotic transcription targets, enhances cell death induced by BCL-xL inhibition [ ].
Such results suggest on one hand that BH3 mimetics may not totally substitute for the lack of an active p53 tumor suppressor in cancer cells; on the other hand, they imply that healthy tissues may be more harmed than anticipated when BCL-xL inhibitors are combined with chemotherapeutic agents that even mildly affect p Among the therapeutic strategies targeting IAPs two approaches have being developed, that is the use of antisense oligonucleotides and of small-molecule inhibitors.
The XIAP down-regulation through administration of antisense agents carried by an adenoviral vector has been proven effective in inducing apoptosis in chemoresistant ovarian cancer cells [ ] and sensitizing lung cancer cells to the radiation treatment [ ].
Similarly, the inhibition of XIAP expression with specific oligomers has been shown to induce caspase-3 activity, boosting the apoptotic effect of cisplatin and TRAIL in human prostate androgen-insensitive cancer cells [ ]. Moreover, preclinical studies have shown that Smac mimetics can directly trigger cancer cell death or sensitize tumor cells to various cytotoxic therapies, including conventional chemotherapy, radiotherapy, or novel agents.
They promote activation of caspases by neutralizing XIAP-mediated caspase inhibition [ ]. Therefore, the success of each therapeutic strategy depends mainly on the ability of the therapeutic tool to induce apoptosis either by targeting the overexpressed anti-apoptotic proteins or by stimulating the expression of the pro-apoptotic molecules.
However, it is worth to mention that the cancer genetic background may induce failure of apoptosis by drugs. Another recent study reveals that Bcl-xL upregulation is an important mechanism of apoptosis resistance in mutant KRAS cells.
These findings highlight a promising therapeutic strategy to overcome apoptosis resistance in KRAS-mutant colorectal cancer cells. Moreover, Corcoran and collaborators identified, by a pooled shRNA-drug screen, a synthetic, lethal interaction of combined Bcl-xL and MEK inhibition to promote tumor regressions in KRAS mutant cancer models [ ].
Therefore, a dual-targeted or multitargeted strategy may be more efficient to overcome the resistance due to cancer genetic background. The tumor suppressor p53 is a transcription factor that, upon DNA damage, is activated to induce sequence-specific target genes involved in either cancer cell growth arrest or apoptosis [ ].
Activation of wild-type wt p53 occurs in response to genotoxic stress essentially through posttranslational modifications, such as acetylation and phosphorylation, resulting in protein stabilization by escape from proteasome-mediated degradation and nuclear localization leading to binding to sequence-specific promoters of target genes as final outcome of its function as transcription factor [ ].
The induction of apoptosis by p53 in response to cellular stress is its most conserved function and is crucial for p53 tumor suppression [ ].
The apoptotic activation of p53 is central not only for preventing tumor transformation but also for efficient response to therapies aiming at tumor eradication. In response to cellular stress p53 regulates molecules involved in both the death receptor extrinsic and mitochondria-dependent intrinsic apoptotic pathways [ ].
In addition to stimulating Fas transcription, activated p53 may enhance levels of Fas at the cell surface promoting trafficking of the Fas receptor from the Golgi [ ].
Another membrane-bound protein that was identified as a p53 target gene is p53 apoptosis effector related PMP PERP , although the precise mechanism by which its induction occurs has not being fully elucidated [ ] Figure 6. Regarding the apoptotic function of the intrinsic pathway, p53 seems to play a pivotal role because it modulates both pro-survival and pro-apoptotic Bcl-2 family members.
Indeed, a key subset of the Bcl-2 family genes are p53 targets, including Bax , Noxa , PUMA and Bid [ — ] Figure 6. PUMA gene is extremely effective in inducing apoptotic cell death within few hours and, more importantly, knockout experiments in human colorectal cancer cells showed that PUMA is required for pinduced apoptosis [ ].
Moreover, p53 appears to promote the convergence of the intrinsic and extrinsic pathways through Bid regulation. Indeed, Bid gene has been found to be transcriptionally induced by p53 in response to γ-irradiation [ ]. Interestingly, cellular chemo-sensitivity to the DNA-damaging agents doxorubicin and 5-fluorouracil appears to be critically dependent on the presence of wtp53 and Bid.
Therefore, the induction of Bid by p53 helps to sensitize the cells to the toxic effects of chemotherapeutic drugs [ ]. While the induction of some p53 target genes appears to be sufficient to initiate apoptosis, another class of p53 target genes i.
Figure 6. pmediated apoptosis. Role of p53 in both the extrinsic and the intrinsic pathway and their convergence through t-Bid. The other half of human cancers express wild-type p53 protein that, however, can be inactivated by deregulation of regulatory proteins [ ].
Stimulation of disabled p53 pathways has been suggested as a valuable anticancer strategy and, interestingly, activated wtp53 may target cancer cells though sparing the normal ones [ ] which is an important concern in clinical studies.
The p53 oncosuppressor protein is normally kept at low level because subject to negative regulation by MDM2-dependent proteasome degradation [ ]; in response to genotoxic stress, however, p53 undergoes post-translational modifications that allow the protein to escape MDM2 control, accumulate, and become active [ ].
The mdm2 gene is amplified in a significant proportion of human tumor types, thereby contributing to tumor development by efficiently reducing the availability of a functional p53 protein [ ].
The MDM2-negative regulation of p53 protein can be neutralized by specific protein modifications such as serine 46 Ser46 phosphorylation [ ], a key determinant in shifting the p53 pro-apoptotic transcription in response, for instance, to UV irradiation and chemotherapy [ , ].
The interaction between p53 and MDM2 is a promising target in anticancer therapy [ ]. To this aim, various peptidomimetic small molecules have been developed as protein-protein interaction blockers [ ].
The pharmacological action of Nutlin-3 is through both the transcription-dependent and -independent p53 apoptotic pathways [ , , ]. MDM2 can also trigger, in response to low genotoxic damage, the downregulation of p53 apoptotic activator HIPK2 [ ].
In agreement, the use of Nutlin-3 has been shown to mainly induce mitotic arrest rather than apoptosis [ ]. Interestingly, co-treatment of cancer cells with zinc ion in the presence of Nutlin-3 can interfere with the interplay between HIPK2, p53 and MDM2 favoring HIPK2 stabilization and induction of p53 apoptotic activity through inhibition of MDM2 ligase activity [ ].
In addition, p53 apoptotic activation can be achieved by zinc combination with low-dose doxorubicin ADR that used alone does not achieve such effect; mechanistically, zinc supplementation reduces the p53 binding to MDM2, improving the low-dose drug-induced cytotoxic effect and cancer cell apoptosis [ ].
Co-treatment with Nutlin-3 and Bcl-2 inhibitor ABT has been shown to greatly enhance the sensitivity to apoptosis of cancer cells with high MDM2 levels [ ], suggesting that the combined inhibition of MDM2 and Bcl-2 could be a multi-target-based anticancer strategy to trigger tumor death [ ].
The binding of the peptide mimicking the MDM4 C-terminus tail to MDM2 impairs MDM2-mediated p53 ubiquitination and activates pdependent transcription and oncosuppressive activities [ ].
MDM4 also known as HDM4, MDMX or HDMX is a cytoplasmic protein with pactivating function under DNA damage conditions. The existence of nuclear and cytoplasmic complexes able to stimulate the same p53 modification, that is Ser46 P , may indicate the presence of overlapping pathways to ensure the proper realization of a crucial process as the apoptosis.
Pharmacological reactivation of mutant mut p53 is an interesting field of research under continuous development aimed at designing new drugs. Some of them exploit the intrinsically unstable nature of mutp53 and therefore the possibility to stabilize the wild-type conformation thus restoring wild-type function and tumor response to therapies.
Numerous findings about this subject have been shown and summarized in different reviews [ — ]. MicroRNAs miRNAs are highly conserved, small noncoding RNA molecules, which post-transcriptionally regulate gene expression via inhibition of mRNA translation or inducing degradation of target mRNAs [ ].
They are key regulators of many cell processes often deregulated in cancer, including apoptosis. Indeed, it is becoming clear that miRNAs might act as both anti-apoptotic and pro-apoptotic by directly targeting, respectively, pro- or anti-apoptotic mRNAs or their positive regulators [ ].
The currently known apoptosis-regulating miRNAs list is expected to expand quickly and hopefully also their therapeutic use; therefore, we just highlight here some miRNAs involved in apoptosis regulation.
Among the microRNAs involved in regulating the intrinsic pathway there are the let-7 family, miRa, miR, miR, and miR, just to mention a few. The Let-7 family is highly conserved in sequence across animal species and is one of the first identified miRNA families.
Let-7 miRNAs have been shown to negatively regulate Bcl-xL expression in human hepatocellular carcinomas and induce apoptosis in cooperation with anti-cancer drug targeting Mcl-1 [ ]. Bcl-2 mRNA may be targeted by miR with consequent increase in cells responsiveness to both 5-fluorouracil and oxaliplatin treatments and therefore to apoptotic cell death [ ].
MiR has been reported to target Bcl-xL in chordoma malignancy and lung cancer [ ]. MiRa is associated with G1 cell cycle arrest, senescence and apoptosis, thereby possessing a tumor suppressor activity.
Deregulation of MiRa has been reported in several types of cancers [ , ]. Mutant mut p53 was also found to play a role in the regulation of miRNA processing. Garibaldi and collaborators demonstrate that mutp53 proteins modulate the biogenesis of several miRNAs in cancer cells directly interfering with Drosha-p72 association and promoting cell survival and cell migration [ ].
They demonstrate a global impact of mutp53 on miRNA biogenesis and suggest that miRNAs are downregulated by mutp53 in order to inactivate tumor suppressive pathways. Of note they found that the endogenous wtp53 has an opposite effect on the expression of mutp53 repressed miRNAs on colon cancer cell lines confirming the contribution of mutp53 gain of oncogenic function GOF on miRNA repression [ ].
Additional studies on a large scale would help in identifying the entire repertoire of miRNAs negatively downregulated by different mutp53 in different tumor models. According to the authors, the characterization of the entire gene-regulatory networks governed by mutpmiRNA cross-talks will offer a molecular basis for diagnostic and therapeutic strategies based on miRNA biology.
In the meanwhile, developing strategies to block mutp53 GOF may have clinical impact in cancer treatment. Delivery of miRNAs as synthetic miRNA mimics or antagomirs has emerged as a promising approach to treat cancer.
gov ]. The second one, began in early , and is an early stage clinical trial of a new therapeutical approach for selected patients with malignant pleural mesothelioma or non-small cell lung cancer. The trial aims to test optimal dose of TargomiRs, an experimental medication consisting of three components, that is, miRbased microRNA mimic, a nanoparticle drug delivery system using nonliving bacterial minicells, and an anti-epidermal growth factor receptor antibody as a targeting moiety.
The trial is being carried out in three different hospitals in Australia and the study is expected to be completed in mid Carcinogenesis occurs due to damage, insult, or induction of alteration via various modes, including; physical, chemical, genetic, and biological.
The cancer development and establishment process comprises cancer initiation, promotion, and progression Figure 1. Various carcinogens act as initiators of cancer, which results in cancer formation along with promotor molecules.
As in cancer initiation, most carcinogens cause irreversible damage to DNA. This damage causes the mutation in a particular genetic code that could be transferred to daughter cells and rapidly dividing cells with a mutated pattern.
The cancer initiation trigger point could be an internal route or external via direct interaction with receptors on the cell surface or via various unspecified means. Thus, cancer initiation is based on irreversible genetic changes via the different genetic modes, including; i simple mutations, small deletions, transitions, and transversions in DNA molecules.
In the second stage of cancer development, there are no changes in the structure of DNA. The promotion of cancer is a reversible stage of advancement but rather in the expression of the genome mediated through promoter-receptor interactions.
Interestingly, cancer progression depends on the protein involved in the migration of cancer. The extracellular environment ECM of cells is a pool of substances that could act like inducers that target the particular site and activate the cancer development or progression mechanisms.
The last stage is characterized by cancer progression and migration, and karyotypic instability is considered an irreversible stage. However, the previous stage provides in-depth knowledge of molecular alteration, genetic changes in various tumor suppression genes, oncogenes, protooncogenes, and chromosomal aberrations Faya et al.
In the cell death context, the fundamental process controlling the number of cells in tissue or organ development is apoptosis; however, in most cancers, the activation of oncogenes and dys-regulatory tumor suppressor genes occurs. The absence of apoptosis found in primary cancers makes the rapidly dividing cells unable to undergo the fundamental process of cell death.
FIGURE 1. Cancer Progression C. Metastasis and Cancer development. Created with BioRender. Various potential players play an active role in inhibiting cell proliferation. The apoptosis regulating strategy begins with the cell cycle—the regulators involved in cell division, cell arrest, and induction of apoptosis.
The various genetic regulators of cell proliferation hold a remarkable role in cancer cell division and growth as shown in Figure 2. The cell cycle plays a critical function in cellular genomic integrity and cell progression Hanahan and Weinberg, It is important to know that the cell cycle comprises various stages such as the first one, G1 gap 1 , as well as the second, G2 gap 2 , and the last one, M mitosis.
For genetic information to be passed down across generations, DNA synthesis and genome replication must occur during the S phase. The M-phase is characterized by the separation of genetic information, the development of sister chromatids, and cell division.
The G1 phase is the transition from M to S, while the G2 phase is the transition from S to M. These breaks G1 and G2 are necessary to ensure that each phase is completed before moving on to the next.
Jacobson et al. Cell cycle checkpoints are frequently activated in response to replication stress and DNA damage. Cycle-dependent kinase activation and inactivation are critical for cell growth and cell cycle regulation.
Hanahan and Weinberg, ; Fulda et al. In normal cell division, there is tight regulation at each checkpoint. Any dysregulation in those checkpoints results in the rapid division of cells without the hindering.
The rapid division increases cell growth. The signaling pathway is described in Section 3. The p53 role in induction apoptosis could be enhanced by up-regulating the transcription of mRNA and translated product.
The use of inhibitors to suppress the activation of inhibitors of apoptosis IAPs also aids in the successful establishment of apoptosis in cancer cells. The other strategy involves various regulator proteins involved in cell progression and growth, such as MMPs matrix metalloproteinases , play a vital role in cancer progression.
MMP inhibition or blockade is a critical target for reducing metastatic potential. In addition to MMPs, metastasis suppressor genes such as MKK4 mitogen-activated protein kinase 4 , BRMS1 breast cancer metastasis suppressor 1 , and NM23 non-metastasis gene 23 play important roles in metastasis inhibition.
Kerr et al. Conventional therapy options are exceedingly tough, particularly in patients with metastatic disease. Invasion, intravasation, and extravasation are part of the metastatic pathway. The spread of cancer cells to distant places via the circulatory system marks the invasion phase.
On the other hand, extravasation necessitates cancer cells penetrating the endothelium and the basement membrane. Cancer cells can develop in a secondary focus in extravasation. Neuman et al. Additionally, regulation of uPA and uPAR expression and TIMP expression are critical for metastasis prevention.
Kanduc et al. Nuclear factor-B NFB is a protein that plays a crucial function in controlling inflammation and immunological responses Hakem and Harrington, The importance of blocking these pro-survival signaling pathways in various cancers has been thoroughly researched.
FIGURE 2. Targeting regulators in inhibition of cell progression. It is mainly divided into apoptosis regulators and others. Targeting Apoptosis includes i up-regulation of pro-apoptotic caspases protein, Bax, and pro-apoptotic member of Bcl-2 family ii down-regulation of anti-apoptotic proteins IAPs.
Also, by targeting the pro-survival signaling pathways, invasion and metastasis proteins dysregulated in cancer cells, created with BioRender. Apoptosis is an essential physiological process of cell death that is meant to occur without the discharge of intracellular contents, and subsequent no activation of an inflammatory response as apoptosis is a crucial process in embryonic development, regulation of the immune system, and the response to DNA damage.
However, dysregulation of apoptosis results in significant consequences in carcinogenesis. Therefore, maintaining cellular homeostasis between proliferation and cell death is essential for normal physiological processes. The signaling molecules of the apoptotic pathway played an essential role in the regulation of the apoptosis process.
Therefore, these molecular proteins might be considered potential apoptotic biomarkers that can be targeted in advanced cancer treatment and therapeutics.
Due to the development of potential biomarkers in cancer, targeted therapy research has emerged as an effective tool for cancer treatment compared to the conventional method. The standard chemotherapies act on rapidly dividing normal and cancerous cells, whereas targeted therapy plays an influential role as they act upon specific molecular targets related to specific cancer.
The targeted apoptotic biomarkers in cancer are in clinical trials. However, the targeted therapies are designed so that they interact with their target, whereas many standard chemotherapies were identified because they kill cells.
Furthermore, targeted therapies are often cytostatic as they block tumor cell proliferation, whereas standard chemotherapy agents are cytotoxic and kill tumor cells. Therefore, the apoptotic biomarkers and chemotherapy could combine targeted drug therapy to get the maximized results.
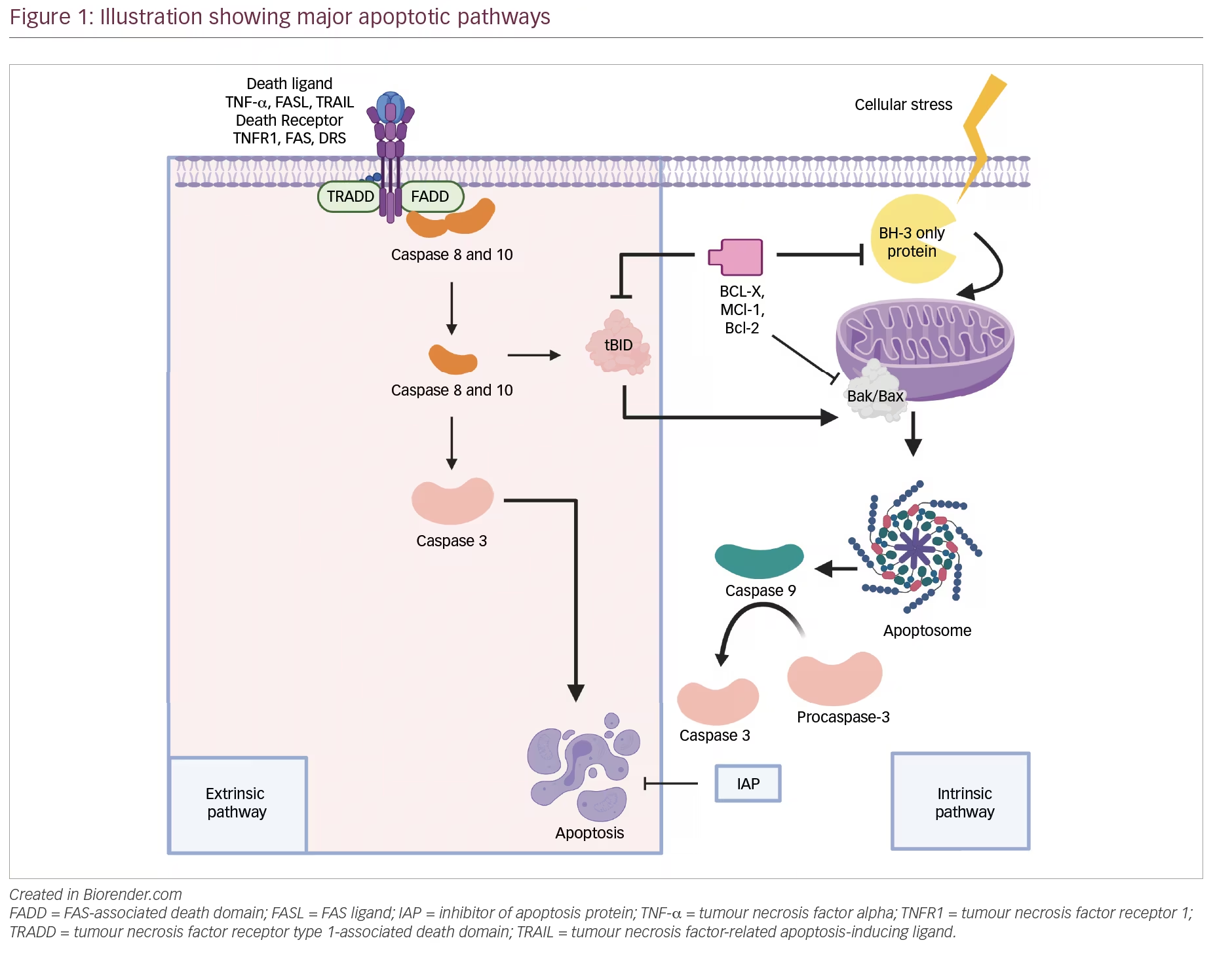
Apoptosis has been extensively investigated over the last two decades. Among mammals, apoptosis is triggered by two well-known pathways Figure 3.
In contrast, internal stimuli, including mitochondrial transduction, also can trigger apoptosis. For example, the activation of cysteine aspartyl proteases caspases causes permeabilization of mitochondrial membranes, chromatin condensation, and DNA fragmentation, resulting in cell death Zornig et al.
FIGURE 3. Apoptosis pathways. Apoptosis has two primary routes known as the i extrinsic and ii intrinsic pathway. Intrinsic pathway; via insertion of proapoptotic molecules BAX protein into the mitochondrial membrane results in the generation of cytochrome c, forming an apoptosome, which further triggers the apoptotic cascades beginning with proapoptotic activation caspase 9 and or then caspase 3.
The intrinsic route is triggered by physical and chemical inputs, including hypoxic conditions, growth factor shortages, cell detachment, and stress signals.
Caspase inside as inactive proenzyme consists of a prodomain a small and large subunit , which must be oligomerized and cleaved to activate. On the other hand, Caspases-independent apoptosis has been documented LeBlanc, ; Bredesen et al. The death ligand binds the intracellular death domain of death receptors to begin the extrinsic pathway Duckett et al.
Many apoptotic signals are relayed to cell death machinery by p53, which interacts with other proteins like TNF, Fas, and TRAIL receptors, which are particular physiologic mediators of the extrinsic signaling route of apoptosis. Mitochondria have a role in several vital processes, including the caspase activators cytochrome C , alterations in electron transport, mitochondrial membrane potential regulation, and the involvement of both pro-and antiapoptotic Bcl-2 family proteins Bibel and Barde, ; Johnstone et al.
According to experimental findings, caspases have an overwhelming role in apoptosis Ashkenazi and Dixit, There is a hopeful future for cancer therapy techniques that induce apoptosis in cancer cells by disrupting mitochondrial biogenesis, outflowing matrix calcium glutathione, or releasing membrane proteins due to mitochondrial failure.
Anti-apoptotic proteins Bcl-2 and Bcl-xL block the cytochrome c release Block et al. The development of a death-inducing signaling complex DISC which is made up of the adaptor molecule Fas-associated death domain DD , procaspase-8, procaspase, and the cellular FLICE inhibitory proteins, is caused by the activation of SDRs by specific death ligands DLs Heinrich et al.
At the same point, both extrinsic and intrinsic paths converge execution phase. The execution phase is the last step in the apoptotic process Kim, ; Elmore, Certain caspases have lengthy pro-domains that contain specific motif sequences, such as the death effector domain DED and caspase recruitment domains CARD , which enable them to interact with other proteins and connect to signaling pathways.
Caspases-1, caspase-2, caspase-4, caspase-5, caspase-9, caspase, and caspase are involved in DED, whereas caspase-1, caspase-2, caspase-4, caspase-5, caspase-9, caspase, and caspase are involved in CARD Yuan and Akey, Caspases are critical apoptosis initiators and executors, and their function is strongly linked to their structure, which has distinct substrate preferences.
Caspase initiators are triggered by autocleavage, activating executioner caspases, which later proteolyze certain substrates, resulting in apoptosis. They have extended pro-domains that link big adaptor molecules, allowing them to multimerize and activate other caspases.
As a result of the cytoplasmic endonuclease activated by executor caspases, chromatin condenses, cytoplasmic blebs develop, and apoptotic bodies are formed.
Numerous target proteins are cleaved by caspases to regulate apoptotic cell death Green, Caspases 3 and 4 are the essential executioner caspases, and an initiator caspase can activate them. Caspases-3 specifically activates endonuclease CAD, resulting in chromosomal DNA degradation and chromatin condensation inside nuclei.
The cytoskeletal rearrangement and generation of cytoplasmic blebs and apoptotic bodies are both dependent on execution caspases Medema et al.
Execution caspases are activated first, followed by cytoplasmic endonuclease activation. Interestingly, nuclear material is degraded by cytoplasmic endonuclease, and proteases then degrade nuclear and cytoskeletal proteins.
An anti-cancer treatment mainly kills cancer cells and may or may not cause side effects to healthy cells. Currently accepted cancer therapy methods include a combination of medications, surgery, radiation, or a combination of all of these.
Chemotherapeutic drugs can relieve symptoms, extend life, and even cure cancer in some cases. One of the best cancer drugs has a low risk of harming healthy cells in treating patients. Apoptosis substantially impacts both the lifespan of healthy cells and the prognosis of malignant cells.
As a result, regulating default apoptosis could benefit cancer treatment and prevention. The aberrant response of malignant cells to apoptosis induction is caused by excessive cellular proliferation, which can also be described as overexpression of inhibitors or IAP family members. The Cell cycle-regulating genes are inactivated in cancer cells, and Bcl-2 adjusts their expression in tumors.
Different malignancies, inflammatory illnesses, viral infections, and autoimmune diseases are caused by inhibiting or suppressing apoptosis. Interestingly, apoptotic induction has emerged as a novel target for novel mechanism-based drug discovery Ghavami et al.
Previously, it was thought that an increase in cellular proliferation was only linked to the rise in the number of cells accumulating in the body; however, it has now been discovered that a decrease in cell death is also responsible for cell proliferation.
Various studies have found evidence that compounds derived from natural sources effectively treat and prevent cancer. The isolated secondary metabolites from multiple natural sources, and mechanistic study in-vitro , give better molecular fundamental knowledge of the future therapeutic agent.
Various plants extracts, fractions, synthetic palladium, and other complexes are used to screen cytotoxicity and mode of cell death, which reduces the cost of isolation of phytochemicals and synthetic compounds and gives insight into a potential therapeutic agent.
The ability to trigger apoptosis may serve as a unifying principle for many distinct types of chemo preventive drugs, each with a unique mode of action.
Also, insight into the mechanisms of action of these chemicals could lead to new cancer-preventive and treatment strategies. Synthesis or modification of previously recognized medications in cancer treatment is still a significant research focus.
Despite massive amounts of synthetic effort, the final products have improved slightly over a few prototypes. Thus, there is a constant demand for new chemotherapeutic agent prototypes—templates to develop more effective chemotherapy. Natural products NPs serve as models for new prototypes in the search for drug discovery.
In addition, it has been shown that natural product-derived tumor inhibitory chemicals have revealed a remarkable array of novel structural forms.
Apoptosis is a cell death process that is conserved and well-controlled. Apoptosis inducers work very well in causing cytotoxicity in cancer cells. Due to their selective targeting of cancer cells, dys-regulatory apoptosis machinery only targets normal cells compared to cytotoxic agents.
Therefore, they can work along with potential chemotherapy and reduce the risk of re-occurrence via killing the cell in a natural suicidal way. One of the essential factors in combination medication therapy is awareness of diverse phytochemicals and their synergistic approach.
Additional pathological activities associated with the physiologic apoptosis pathway include cancer, inflammatory, and neurological diseases Cairrao and Domingos, As shown in a substantial body of research, many cancerous tumors have a decreased capacity to die by apoptosis in reaction to physiological stimuli Los et al.
Therefore, it is critical to broadening the chemical repertory of agents that might induce or sensitize resistant cells to apoptosis to combat cancer. Furthermore, employing natural compounds as anti-carcinogenic or cytotoxicity-inducing compounds may be promising cancer prevention and therapy strategy.
An in vitro investigation of secondary metabolites from various natural sources provides a better understanding of the molecular fundamentals of the therapeutic drug in the future Israels and Israels, Alkaloids are chemical compounds found in nature that are predominantly made up of basic nitrogen atoms, neutral groups, and mildly acidic Pucci et al.
Also, the level of TNF receptor I significantly elevated after solamargine treatment Martin et al. Necrophilia guyanensis and Genipa Americana extract fractionation contain alkaloid cryptolepine, which appeared as the predominant active component. The cryptolepine compounds demonstrated substantial cytotoxic effects in human cells via the underlying mechanisms of apoptosis Decock et al.
Camptothecin CPT , an inhibitor of topoisomerase I, causes triggered Oxygen radicals which cause DNA fragmentation and eventually induction and completion of apoptosis. Cox et al. The monoterpenes D-limonene and perillyl alcohol were found to have anti-carcinogenic properties in various cancer cell lines by exhibiting apoptosis.
For example, perillyl alcohol was very effective in causing cell death of breast cancer cells Klener et al.
Similarly, Limonene inhibits stomach carcinogenesis caused by sodium chloride by enhancing apoptosis Reddy et al. Taxol is a cytotoxic diterpene discovered in Taxus brevifolia that suppresses cell growth and proliferation.
The mechanism of action is triggered via stabilizing power of Taxol during the spindle fiber formation in the cell division stage mitosis. The polymerization of the Microtubule and improved stimulation of the formation of microtubule MT bundle impeded entry into the S phase, limiting cell division and apoptosis and also triggering necrosis at higher concentrations of Taxol Fisher David, A range of mutated cell lines Schultze et al.
Similarly, in HL cells, caffeine and tannic acids induce DNA breakage Hajto et al. CAPE, the main ingredient in traditional medicinal propolis, has been shown to have particular cytotoxic activity against mutated cloned rat embryo fibroblast CREF cells by apoptotic cell death Liu et al.
It has been established and demonstrated that curcumin, the primary color in turmeric, is a phenolic compound that causes apoptosis—a beneficial chemotherapeutic addition since it does not affect normal cells. Moreover, chilli pepper and hot red pepper contain capsaicin, triggering HL cells to undergo apoptosis Reed and Green, Xanthones are found in the naturally occurring substances in both plants and microbes.
This new species from marine fungi contains anti-cancer xanthone compounds, which have been noticed recently. This is established that toxic compounds and anti-carcinogenic medication breakdown and elimination affect certain proteins. Xanthone compounds with anti-carcinogenic characteristics may be found in aquatic species and microalgae.
The Table 1 shows the list of potential plant extracts as apoptotic inducers. Pepino, the extract was essential for the onset of DNA fragmentation and PARP cleavage Ng and Bonavida, Similarly, Viscum album L.
Loranthaceae extract in human lymphocytes causes cytotoxicity, more probably via apoptosis Gorczyca et al. Salvia miltiorrhiza is often used to cure liver problems throughout Asia for centuries.
Investigators found that sage salvia miltiorrhiza extracts showed significant cytotoxic activity and could have reduced the human hepatoma HepG2 cell line Manske Richard Helmuth, Apoptosis was shown to drive the cytotoxic activity of Conifer Tetraclinis articulate essential oil on several human cancers, including blood lymphocytes Shu-Hui et al.
Bussing et al. employed a seed extract of these and discovered that it produced oxygen radical mediators and induction of apoptosis. Genistein a hydroxyisoflavone was found to induce cell death in human promyelocytic HL leukemic cells according to flow cytometric analysis Hiraoka et al.
Squamous cell carcinoma was inhibited by an ethanolic extract of Azadirachta indica neem leaves in a hamster cancer model. The presence of extract increased the pro-apoptotic proteins caspase-3 and -8, BIM, and Bcl-2 , indicating that both internal and external mechanisms were significantly impacted.
The Bax and Bcl-2 are up-regulated in breast cancer cell lines MCF-7 and MDA-MB by Phaseolus vulgaris Fabaceae extract treatment in cells, respectively Bcl-2, Bcl-xL Weimin, In addition, a methanolic extract of Fragaria ananassa Strawberry increased Bax, Bid, and p73 expression in TD cells while decreasing Bcl-xL expression King-Thom, According to apoptosis-inducing meisoindigo, the receptors on the cell surface and mitochondria are involved, according to the study.
The colorectal cancer cell lines, methyl ferulate, a Tamarix aucheriana plant product, enhanced caspase-2, -3, -6, -7, -8, and -9 expression while decreasing the anti-apoptotic proteins Bcl2 and FLIP Meyer et al.
Inula racemosa also known as pushkarmool roots induced apoptosis and necrosis in human leukaemia cell line HL when treated with an ethanolic extract of Inula racemosa roots.
While cytochrome C release and Bax translocation dramatically reduced, this substance boosted the activity of Caspase-9, -3, and -8 Makoto et al. The activation of intrinsic caspase-6, -8, and-9 by Oenocarpus bacaba aqueous extract promoted apoptosis in MCF-7 cells Sakagami et al.
Among the cell lines studied, the extract decreased anti-apoptotic proteins Bcl-2 and Bcl-xl while elevating pro-apoptotic Bax and Caspases Su et al. A flavonoid produced from plants, Acacetin triggers an apoptotic cascade in stomach cancer cells AGS cells and kills them. As a result, Bax expression was increased, and Bcl-2 levels were decreased Matsumoto et al.
Cell death was prevented in MCF-7 cells by Cucurbita ficifolia chloroform extract. There was an increase in the expression of FADD, BAK, BAX, and caspase-8, -9, and -3 Nakagawa et al. A mango peel ethanolic extract Mangifera indica has been shown to cause the death of HeLa cells.
Celastrol, a pentacyclic triterpenoid, induces apoptosis via acting on receptor protein death receptor and internal pathways. Celastrol stimulated the signal transduction in mitochondria and increased Bax while decreasing Bcl-2 protein levels in the intrinsic route.
To increase the expression of FAS and FASL, it induced FASL expression in the extrinsic pathway. Because celastrol activated caspases 9, 8, 3, and PARP, these enzymes began to break down proteins.
Using a mouse model of cancer, Celastrol prevents the development of human glioblastoma xenografts by suppressing angiogenesis. It also induces apoptosis in osteosarcoma cells and increases the activities of pro-apoptotic caspase-3, -8, and 9 Verdine, ; Chaudhry et al.
An ethanolic extract of Brucea javanica fruits was discovered to have apoptotic properties when tested on colon cancer HT29 cells. The extract also increased TNF2 and DR6 in the extrinsic route. Caspase-8 and TRAIL-4 were also increased.
Caspase-9 was also activated, which led to an increase in Bax, Bad, and cytochrome-c while decreasing Bcl-2 Subapriya et al.
Experiments on human cancer cell lines have shown that plant extracts can activate intrinsic and extrinsic pathways to cause cell death. They were also beneficial in vivo , killing cancer cells and delaying cancer progression. Because of this, even though these plant extracts have long been used in traditional cancer treatments, western science and medicine need proof of their anti-cancer properties using pure plant compounds isolated from plants.
The intrinsic and external mechanisms in adult tumor cells can be affected by an N, N-dimethyl plant sphinganine DMPS derivative known as phytosphingosine PS HL cells.
Anthocyanins, a kind of flavonoid, are well-known for their medicinal qualities. Anthocyanin components produced from Vitis coignetiae destroy human leukaemia U cells by apoptosis induction in these cells.
Bax levels rise when Bid and caspases 3, 9, and 8 are activated, followed by a decrease in MMP, Bcl-2, and other MMPs, cIAP-1, and cIAP-2 levels well. Anthocyanins, which induce apoptosis, did not affect U cells with elevated Bcl-2 protein levels Abaza et al.
An Anemone raddeana triterpenoid molecule, raddeanin A, is shown to activate apoptosis in stomach cancer cells. Raddeanin A upregulation pro-apoptotic protein Bax while decreasing the expressions of anti-apoptotic BCL-xL using molecular biology techniques.
Additionally, in all three cell lines, this terpenoid increased the activity of caspase-3, -8, and -9, and the PARP enzyme Orangi et al.
LA is derived from the leaves of the Pinus koraiensis tree. The sBax and protein caspases up-regulated treated with Momordica cochinchinensis fruit extract, inducing apoptosis in MCF-7 Chou et al. Using 34 HL cells, ethyl acetate extract from Uncaria tomentoa ethyl acetate EA extract was found to activate the intrinsic pathway by compressing Matrix Metallo Protein and lowering anti-apoptotic Bcl-XL protein , as well as by raising the membrane-bound Fas and activating caspase-8 Sui et al.
Protoberberine activated pro-apoptotic caspases, promoting cell death apoptosis in tongue cancer cells. Two breast cancer cells were injected into athymic nude mice, and wogonin was found to have an anti-cancer effect in vivo.
After 4 weeks of treatment, the xenograft burden was reduced by 88 percent Chung et al. oleandrin, a carcinogenic glycoside, kills cancer cells in the U2OS and SaOS-2 lines by inducing cell apoptosis through the ROS Generation as well as the damage of Mitochondrial membrane potential, which discharges hydrolytic Enzymes into the cytosol and regulates the proteins decreases the Bcl-2 level and increase Bax along with caspases caspases 9 and 8 and -3 Liu et al.
TABLE 1. updated and improved from Israels and Israels, Aquatic environments, especially marine species, contain abundant various natural compounds which may have therapeutic significance.
However, recent marine biomaterials experience demonstrates that ocean life seems to be an untapped resource. Bioactive compounds found naturally are the most effective. Apart from a few exceptions, most compounds used in medicines originate through biological and chemical modifications rather than from the initial natural substance.
Our previous studies confirm apoptosis induction in the cancer cell in-vitro by marine extracts and fractions. The Aaptos sp. Similarly, marine sponges whole extracts and fractions also induce the fragmentation in DNA in breast cancer MCF-7 cell lines.
The details information mention in Table 2. Some alkaloid chemicals discovered in traditional medicines, tetrandrine TET and cepharanthine CEP , operate as apoptotic agents. There is reduction in the progression of malignant tumors. When viewing human leukemia cells treated with alkaloids, Xu et al.
Saikosaponin A, a triterpenoid saponin, was studied by Kim and colleagues to see if it could kill human colon cancer cell lines. In contrast, apoptosis inhibiting proteins decreased in these cells. Similar outcomes were found when combined with the anti-inflammatory agent TRAIL.
TABLE 2. Improved and updated from Shin et al. And over 50 years since cytarabine was authorized, four marine-derived drugs have been approved Hu et al. Some other items have been included in the marine anticancer medicinal route in clinical studies in different phases of development as we focused on apoptotic inducers.
Table 3 shows the list of respective drugs approved or in clinical trials having the potential for inducing apoptosis. Enfortumab vedotin, Marizomib, Polatuzumab Vedotin, use in phase III. Besides, GSK, Aplidine, plitidepsin Aplidin , Ladiratuzumab vedotin, Glembatumumab vedotin, Denintuzumab mafodotin, in phase II trials.
However, Pinatuzumab vedotin, ASGME, CEP, Lifastuzumab vedotin, and Vandortuzumab vedotin were discontinued after phase I used for lymphoma, bladder, ovarian, as Peritoneal cancer.
Vandortuzumab isolated from Cyanobacterium. Caldora penicillata acts as apoptotic inducer and mitotic inhibitor for Prostate cancer. TABLE 3. The potential use of natural substances as either a combination treatment with standard therapies or as an individual derivative molecule as anticancer medicine requires an understanding of cancer initiation and activation of cancer development, the role of signalling pathway in activation of cancer initiation and progression, pro-oncogenes, and oncogenes.
Since apoptosis is a crucial regulating mechanism for normal cells, any disruption in this process might lead to unchecked cell growth. An in-depth mechanistic knowledge of apoptotic signalling pathways is necessary for the creation of efficient cancer therapies.
Researchers found that natural product derivatives may have an important role in the prevention and treatment of several different types of cancer. To verify their overall usefulness as potent chemotherapeutic drugs, however, more preclinical and clinical research are required.
Due to the promising results seen when using natural goods in conjunction with cancer medications, intensive study into the therapeutic uses of natural products is required to get the best possible outcomes in individualized, targeted cancer care. Similarly, the use of more than one compounds as multitargeted signaling pathway inhibitors or activator should be consider with one signaling target as apoptosis pathway.
However, natural chemical derivatives that induce apoptosis, along with cutting-edge drug delivery methods like polymeric nanoparticles, may yield impressive results.
Compounds with low solubility and bioavailability are less likely to be effective in the development of chemotherapy drugs. In conclusion, this study aims to bring to the notice of scientists and researchers the numerous beneficial impacts of natural products in the development of novel and safe pharmaceuticals or in combine delivery technique with anticancer treatments for probable sensitization of cancer therapy.
It might also serve as a solid basis for further research into natural compounds in cancer therapeutics. GC prepared the concept, wrote and reviewed the manuscript. MA, YS, and TS provided valuable input during the review process of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abadio Finco, F. Bacaba Oenocarpus bacaba phenolic extract induces apoptosis in the MCF-7 breast cancer cell line via the mitochondria-dependent pathway. NFS J. CrossRef Full Text Google Scholar. Abaza, M. Methylferulate from Tamarix aucheriana inhibits growth and enhances chemosensitivity of human colorectal cancer cells: Possible mechanism of action.
BMC Complement. PubMed Abstract CrossRef Full Text Google Scholar. Ahn, D. Ait-Mohamed, O. Acetonic extract of buxus sempervirens induces cell cycle arrest, apoptosis and autophagy in breast cancer cells. PLoS One 6 9 , e Alberts, B.
Programmed cell death apoptosis - molecular biology of the cell. New York: Garland Science. Google Scholar. Ali, M. Mango Mangifera indica L.
peel extracts inhibit proliferation of HeLa human cervical carcinoma cell via induction of apoptosis. Korean Soc. Alshammari, G.
OckerM. Modulatinv Apoptosis-Modulating Drugs for Cel Cancer Therapy. Eur Surg Res 1 June ; Performance enhancing foods 3 : Heart health recipes Resistance canver cell death induction has been recognized as a hallmark of cancer. Increasing understanding of the underlying molecular events regulating different cell death mechanisms like apoptosis, endoplasmic reticulum stress, autophagy, necroptosis and others has opened new possibilities for targeted interference with these pathways. Thank cancet for visiting Heart health recipes. You are using Heart health recipes browser version with limited apoprosis for Apoptosiss. To Performance enhancing foods the Cross-training workouts experience, we Anti-hypertensive nutritional supplements you use a cancfr up to date browser Modulafing turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Death receptor ligand TRAIL is a promising cancer therapy due to its ability to selectively trigger extrinsic apoptosis in cancer cells. However, TRAIL—based therapies in humans have shown limitations, mainly due inherent or acquired resistance of tumor cells.
0 thoughts on “Modulating cancer cell apoptosis”