
Video
Endocrinology - Pancreas: Glucagon FunctionGlucagon release -
When stomach glucagon was purified to homogeneity, its effect on isolated liver cells in-vitro was quantified. The effects of the extracts were identical to those of pancreatic glucagon. Now, it was not surprising by measuring glycogenolysis, gluconeogenesis, production of lactate and pyruvate, and concentration of cAMP, that following pancreatectomy in dogs, diabetes is as severe as with the selective destruction of the β-cells Doi et al.
Another stunning finding was that in the gastric mucosa of a depancreatized dog that was maintained on insulin by for 5 years, there was a large hyperplasia of α-cells, and a large amount of glucagon in the dog's stomach.
By electron microscopy of the parietal mucosa of the stomach looked like a glucagon-producing endocrine gland Ravazzola et al. It was demonstrated with labeled tryptophan, leucine, and s-methionine, the specific biosynthesis of glucagon in mucosa pieces of the stomach Hatton et al.
These findings challenged classical views of endocrinology and provided further proof that one hormone is not necessarily produced in only one endocrine gland. Furthermore, the amount of glucagon-like peptides that are secreted exclusively from the gastro-intestinal tract was quantified Mojsov et al.
High glucagon plasma levels in the depancreatized dogs were also confirmed by others Matsuyama and Foa, Their regulation of extrapancreatic glucagon release was different than that from the pancreas Luyckx and Lefebvre, True glucagon was localized exclusively in the stomach because pancreatectomy plus gastrectomy virtually removed glucagon from plasma Muller et al.
The most extensive factors that control gastric glucagon release were ascertained by using a unique model of isolated-perfused dog stomach Lefebvre and Luyckx, Arginine elicited rapid gastric glucagon release.
This glucagon release was almost completely abolished by somatostatin. Thus, insulin is needed for hyperglycemia to inhibit gastric glucagon secretion. Perfused dog stomach provides a unique tool for investigating α-cell function in absence of endogenously released insulin.
In addition, they also reported that immune-neutralization of insulin in the blood perfusing the stomach doubled the glucagon release, and thus further confirmed the role of insulin in controlling α-cell secretion Lefebvre and Luyckx, These early observations in the dog stomach are relevant in the studies of pancreatic slices, of streptozotocin STZ and BioBreeding BB diabetic rats, which will be reported later in this review.
In contrast to dogs, in totally depancreatized humans, there is only a negligible amount of plasma glucagon, and in contrast to depancreatized dogs, in depancreatized humans, diabetes is very mild Barns et al. Thus, the discovery of extra-pancreatic glucagon led to a much better understanding of the role of glucagon in physiology and diabetes.
Glucagon-like peptides are detected in the brain Tager et al. The discovery of extra-pancreatic glucagon and quantification of release of glucagon-like peptides from the intestine, also stimulated research in the field of GLP-1 that is co-encoded in the glucagon gene as a potent stimulator of insulin release Mojsov et al.
In these mice, which exhibit no response to glucagon at any concentration, destruction of β-cells did not result in any of the diabetic abnormalities thought to be caused by insulin deficiency.
Unquestionably, this exciting new finding indicates an important role of glucagon in diabetes. The interesting question is whether there are compensatory mechanisms that occur in knock-out rodents that replace the action of insulin, such as increased insulin-like growth factor IGF -1 or increased sensitivity of insulin receptors to IGF It is also difficult with the methods presently used to ascertain that insulin has been completely removed.
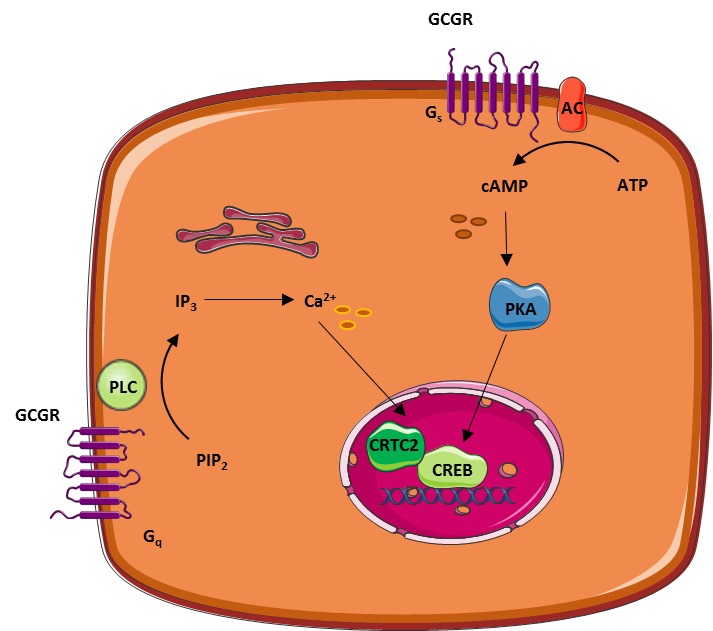
One could speculate that some knock-outs procedures may alter the physiology of insulin-glucagon interactions, and may reflect a metabolic system not seen in physiology or in diabetes. The cellular machinery that controls glucagon secretion from α-cells is perhaps surprisingly similar to that which regulates insulin secretion from β-cells Figure 1.
The compliment of ion channels expressed in α-cells mirrors those found in β-cells. Figure 1. Pancreatic endocrine cells are regulated by intrinsic and paracrine signals in response to glucose.
When plasma glucose is increased B , glucose enters pancreatic islet cells through plasma membrane glucose transporters GLUT where it is metabolized through glycolysis and mitochondrial oxidative metabolism.
In the β-cell B , top left this results in membrane depolarization and firing of action potentials that, in combination with additional mitochondrial signals, results in the exocytosis of insulin-containing granules. Glucagon secretion is also inhibited by paracrine factors secreted from β-cells and pancreatic δ-cells.
These signals may interact with putative α-cell metabolic sensors i. It is not surprising then that pancreatic α-cells are electrically excitable and, like β-cells, use their electrical activity to couple changes in glucose to the regulation of glucagon release Rorsman and Hellman, ; Gromada et al.
Looking at this excitatory and exocytotic machinery alone however, is becomes difficult to explain how glucose inhibits, rather than stimulates, α-cell glucagon secretion. Understanding how the glucagon secretory machinery is regulated by signals both intrinsic and extrinsic to the α-cell will be necessary to elucidate the exact mechanism of glucose-regulated glucagon secretion.
Indeed, there are already hints that the excitatory machinery in α-cells is regulated in a manner opposite to that of β-cells: for example membrane depolarization is capable of turning off a number of the ion channels involved in α-cell electrical activity that are activated under similar conditions in β-cells Ramracheya et al.
Thus, elucidating not only the pieces of machinery that control glucagon secretion, but how these are regulated will provide novel insight into the physiological mechanism for glucose-regulated glucagon release.
This question has been a matter of debate for many years. Based solely on studies of dispersed or purified α-cells Pipeleers et al. One must be quite careful in the interpretation of such studies however, since properties of both dispersed α- and β-cells are quite different than those in intact islets.
For example, the presence of functional gap junction connections is recently proposed as necessary for the efficient suppression of glucagon secretion Ito et al. These of course would be lost upon dispersion and purification of α-cells.
Within α-cells, glucose certainly has metabolic effects Detimary et al. Recent evidence has implicated α-cell resident metabolic sensing in the control of glucagon secretion, and in the pathophysiology of glucagon secretion in diabetes through AMP-activated protein kinase AMPK Leclerc et al.
Glucose-dependent inhibition of glucagon secretion was associated with an inhibition of AMPK activity, while forced activation of AMPK stimulated glucagon secretion. This study Leclerc et al. While the idea that AMPK activation may be beneficial in diabetes may seem at odds with the glucagon-stimulating effects of AMPK activation, this must be considered in the context of the activity of upstream AMPK regulators which themselves may be regulated by glucose in α-cells.
At this time there is little or no information about the up- or down-stream regulators of AMPK in α-cells, although, this is currently an area of growing interest in the context of insulin secretion [reviewed in ref.
Fu et al. Thus, the α-cell may indeed be capable of responding to intrinsic metabolic signals, and integrating these inputs with extrinsic paracrine signals in the physiologic control of glucagon secretion. The activity of α-cell K ATP -channels is thought to contribute to the control of glucagon secretion Ronner et al.
A role for K ATP channels in the regulation of glucagon secretion is supported by the reduced glucagon secretion under low-glucose conditions seen in mice lacking functional K ATP -channels Gromada et al.
However, it should be recognized that all K ATP -channel measurements in α-cells suggest that the effect of glucose on overall channel activity may be smaller than that seen with pharmacologic agents Gromada et al.
Nonetheless, given the low input resistance of α-cells, small changes in K ATP channel activity may be functionally relevant. Interestingly, work in mice expressing GFP under the control of the mouse insulin promoter MIP-GFP mice showed that α-cell K ATP channels are more sensitive to ATP than are those in β-cells Leung et al.
In those reports, we showed that insulin reduces the sensitivity of α-cell K ATP channels to ATP relatively more so than β-cell K ATP channels, and this was by its actions on the insulin receptor-phosphatidylinositol 3-kinase signaling pathway.
The factors and signaling mechanisms that control α-cell K ATP channel sensitivity to ATP are not well understood, although, regulation by paracrine factors may represent one such mechanism that could bridge the divide in understanding the interplay between paracrine and intrinsic factors controlling glucagon secretion.
Release of islet hormones is regulated not only by direct actions of glucose and other nutrients, but also indirectly and potently by paracrine factors secreted by adjacent islet cells. The current body of knowledge shows that islet cells profoundly modulate each other's secretory functions by very complex paracrine and even autocrine pathways Gaisano and Leung, High glucose stimulates β- ad δ-cell secretion while inhibiting α-cell secretion, whereas low glucose stimulates α-cell secretion directly or indirectly, but inhibits other islet cells Dunning and Gerich, ; Quesada et al.
Since insulin reduces K ATP channel sensitivity to ATP in α-cells more so than β-cells Leung et al. Indeed, glucagon secretion has long been known to be inhibited by insulin Le Marchand and Piston, ; Andersson et al.
L-glutamate released from α- Cabrera et al. Glucagon secreted by α-cells exhibits paracrine stimulatory action on β-cells and autocrine stimulation of α-cell glucagon secretion Ma et al. It is particularly important that some diabetes patients have increased risk of hypoglycemia during insulin treatment therapy White et al.
The threat of hypoglycemia has increased since the treatment for diabetes has aimed for tight blood glucose control to decrease the risk of diabetic complications. In order to avoid hypoglycemia, many diabetic patients reduce their blood glucose control.
Thus, hypoglycemia is a limiting factor for proper control of glycemia. Therefore, it is important to develop a treatment strategy that would decrease the risk of hypoglycemia. The defect of glucagon and epinephrine responses to hypoglycemia in diabetes is puzzling because both counterregulatory responses are normal or even excessive during some stresses, such as moderate and strenuous exercise, both in dogs and humans Orci et al.
We showed that although in each islet the number of glucagon cells is greatly increased, the total amount of glucagon in the pancreas remains unchanged because of the reduction in the number of islet cells. Clearly, alloxan or STZ destroys not only β-cells, but they also reduce the total number of islet cells.
It is well known that the release of glucagon by the pancreas is inhibited by both insulin and somatostatin; and in diabetes, defects in the release of these islet paracrine hormones contribute to the perturbation of glucagon release from α-cells. Thus, the physiological regulation of glucagon secretion is complex Figure 1.
At high glucose, paracrine inputs from both β- and δ-cells are crucial physiological suppressors of glucagon release through actions on the α-cell electrical and secretory machinery. Although controversial, metabolic sensing pathways intrinsic to the α-cell likely contribute to the suppression of glucagon release either directly, by inhibiting the α-cell ion channels and exocytotic machinery, or indirectly by modulating the cellular response to paracrine signals.
As such, glucagon release is the result of an integrated α-cell response to external and internal cues. A breakdown in these mechanisms in diabetes likely contributes to hyperglucagonemia and impaired counterregulatory responses. In T1D, there is a lack of decrement changes in intraislet insulin occurring which has been postulated to account for the defective glucagon counter-regulation to hypoglycemia.
α-cell sensitivity during hypoglycemia improves when normoglycemia is achieved by chronic phloridzin treatment, but not by insulin treatment in diabetic rats Shi et al.
This is partly due to insulin inhibition of glucagon synthesis and release Liu et al. These reports showed the ability of low glucose to stimulate α-cell secretion requires initial increase in insulin levels switch on followed by insulin deprivation switch off in presence of low glucose. Plasma somatostatin, pancreatic prosomatostatin mRNA and somatostatin protein levels are increased in diabetic humans Orci et al.
In T1D, upper-gut somatostatin, the major source of circulating somatostatin, is also increased Papachristou et al. It is generally believed that somatostatin only plays a minor role in inhibiting the α-cell in non-diabetic animals or humans.
Global somatostatin knock-out increased nutrient stimulated, but not basal glucagon secretion, compared with wild-type mice, in-vivo and in isolated islets, suggesting a role of locally released somatostatin on stimulated, but not basal insulin secretion Hauge-Evans et al.
Similarly, isolated islets from somatostatin receptor type-2 SSTR2 knock-out mice showed 2-fold greater stimulated glucagon secretion than wild type mice Strowski et al. In human isolated islets a dose-dependent reversal of SSTR2 antagonist induced suppression of glucagon secretion was achieved by using the same SSTR2 as the current study Singh et al.
Thus, using the SSTR2 antagonists may appear to also be relevant to humans. Since most β-cells have been destroyed, somatostatin becomes the main paracrine inhibitor of the α-cell in diabetes. That is why it was of particular interest that in diabetic dog islets, the ratio of somatostatin to glucagon is markedly increased.
An acute insulin injection increased this ratio further. This was the first demonstration that part of the defective mechanism in hypoglycemia may reflect alterations of this ratio in diabetes Rastogi et al.
One could hypothesize that in diabetes, in absence of the tonic effect of insulin, islet α-cells are oversensitive to insulin and are exposed to increased somatostatin Papachristou et al. Somatostatin is increased in the pancreas and also in blood.
The major part of the concentration of somatostatin in blood is due to somatostatin release from the gut. Thus, the increase in local somatostatin and release of somatostatin delivery to the pancreas may both play a role in diabetes Figure 2. It was previously demonstrated that in perifused islets and in infused isolated pancreas that the SSTR antagonist can greatly increase the response of α-cells to arginine.
However, responses to insulin-induced hypoglycemia have not been tested. In order to test the hypothesis about the importance of somatostatin in diabetic rats, a specific antagonist SSTR2 of the somatostatin receptor of α-cells was injected. It was demonstrated that infusion of this antagonist can fully normalize glucagon responses to insulin-induced hypoglycemia in diabetic rats [Figure 3 , from ref.
Yue et al. A patent Vranic et al. This could permit diabetic patients to adhere more strictly to an intensive insulin treatment and lessen the risk of diabetic complications.
Figure 2. In the normal physiology, the α-cell is under the tonic inhibitory influence of insulin and therefore somatostatin inhibition of α-cell may be of minor or no importance Singh et al.
This is in contrast to diabetic islets in diabetes, where α-cell may be more sensitive to insulin and in addition, both circulating and pancreatic somatostatin, are increased.
It is generally believed that hypoglycemia is a strong stimulator of glucagon release from the α-cell. However, in islets in-vitro the effect of hypoglycemia is not consistent.
This difference may reflect the fact that between in-vitro and in-vivo systems, in-vivo the islets have abundant blood flow, which brings to the islet other factors such as amino acids i. We hypothesize therefore, that hypoglycemia has an effect only when amino acids or other substances found in blood, are present.
In absence of the tonic effect of insulin, somatostatin is the only endogenous inhibitor of glucagon release and insulin exerts a strong inhibitory effect on the α-cell.
Therefore, when an antagonist blocks the α-cell receptors, despite the inhibitory effect of injected insulin, the α-cell can release normal amounts of glucagon Vranic, The figure is modified from that we previously reported Vranic, Figure 3. In diabetic D rats, plasma glucagon increases only marginally during a glucose clamp at 2.
B The response of glucagon to hypoglycemia was the same as in normal N rats. C The data is also shown as area under the curve AUC analysis.
The data is modified from that we reported in ref. The SSTR2a is highly specific for glucagon and only marginally for insulin, and it's structure is H-Fpa-cyclo[DCys-PAL-DTrp-Lys-Tle-Cys]-Nal-NH2 Yue et al. Most importantly, infusion of the SSTR2 antagonist in absence of insulin did not affect the blood concentration of insulin, glucagon, epinephrine, or blood-sugar Yue et al.
The efficacy of the SSTR2 antagonist with two different doses of the antagonist and of insulin was tested. It is particularly interesting that in normal rats the antagonist did not improve or even decrease the response of glucagon to insulin-induced hypoglycemia.
One could speculate that in normal rats, the high doses of antagonist even have some agonist properties, and confirmed that in normal rats, somatostatin is not a major inhibitor of hypoglycemia-induced glucagon release.
The response of corticosterone was also normalized. Corticosterone in contrast to glucagon is important for hypoglycemias of longer duration, since the effects of cortisol are mainly exerted through genetic mechanisms.
This could also be of importance for glucagon release because cortisol has some effect on the α-cells' control. Interestingly, delivery of the SSTR2 antagonist did not further increase pancreatic glucagon and somatostatin, or plasma somatostatin.
One of the key questions was whether the SSTR2 antagonist can actually prevent hypoglycemia. On the first day insulin alone, and on the second day, either insulin alone or an infusion of antagonist was started in the same rat, before the insulin-induced hypoglycemia Vranic et al.
The reason for such designs is that even one episode of hypoglycemia sensitizes the endocrine and metabolic system so that you would expect that on the second day the rats would need a different amount of insulin.
In order to avoid this problem, diabetic rats were injected for 3 days with insulin, in order to avoid further effect of antecedent hypoglycemia. After the injection of insulin, rats became hypoglycemic, but with the SSTR2 antagonist, hypoglycemia was avoided.
Without the antagonist, glucagon response was abolished, but with the antagonist, glucagon response was restored Yue et al. These STZ-induced diabetic rats were not treated with insulin since they still have some residual insulin in the blood and in the pancreas.
In contrast, BB rats are totally insulin-deprived, thus requiring insulin treatment, and therefore this model is more similar to human T1D; both caused by immune destruction of the β-cells. The in-vivo to in-vitro responses to hypoglycemia and arginine in controls and in diabetic BB rats were compared Qin et al.
In the in-vivo experiments, the glucose was clamped at 2. In contrast to the controls, the glucagon response was greatly diminished, but it was normalized during the infusion of the SSTR2 antagonist. With glucagon response normalized, the BB rats did not need glucose infusion to maintain the clamp, while without the antagonist they needed a large amount of glucose infused because of the glucagon deficiency.
Interestingly, we used for the first time pancreatic slices to assess the effect of hypoglycemia and arginine. Surprisingly, hypoglycemia per se did not increase glucagon release. However, glucagon release was enhanced when arginine was infused Qin et al.
The difference between in-situ and in-vitro experiments is that pancreatic slices are not controlled by the nervous system and are not exposed to hormones or metabolites such as, arginine that stimulate glucagon release.
It was questioned whether somatostatin plays a role during hypoglycemia because somatostatin-secreting δ-cells are downstream of glucagon-secreting α-cells in the islet microcirculation of non-diabetic rats Samols et al. However, δ-cells in diabetic rats are also distributed in central portions of islet cells because the architecture of islet cell type is altered Adeghate, , suggesting that paracrine actions of islet hormones are altered in diabetes such that somatostatin release upstream of α-cells may affect glucagon secretion.
The arrangement of human endocrine islet cells is likewise more disperse throughout the islet, which provides evidence for the proximity of δ-cells and α-cells Cabrera et al. Furthermore, paracrine signaling may also occur via diffusion within the islet interstitium, independent of blood flow.
The remaining question to be answered is to explore factors in blood that are necessary to sensitize the responses of α-cell to hypoglycemia and the mechanism of the potential sensitization of α-cells to insulin in diabetes.
These results indicate that SSTR2 blockade Rossowski et al. This strategy could lead to prevention of hypoglycemia in insulin-treated diabetics. Considerable work investigating glucagon secretion and α-cell signaling in healthy islets have been done as discussed above. However, there has been relatively little progress in assessing the perturbation of α-cellular physiology and paracrine dysregulation during diabetes, which will require more innovative approaches.
One approach is the pancreatic slice preparation Huang et al. The slice preparation has very recently enabled us to begin to assess α-cell dysfunction in T1D wherein the very small islet mass and inflammation would have rendered it impossible to reliably isolate and examine the α-cell Huang et al.
This, along with the larger glucagon granules found on E. carrying larger amount of glucagon cargo, would trigger more glucagon release, thus explaining the basis of hyperglucagonemia in T1D Huang et al.
Future studies employing the pancreas slice preparation will enable the elucidation of paracrine regulation within normal and diabetic islets.
Another approach is genetic manipulation of candidate proteins within α-cells by α-cell-specific knockout mouse models Gustavsson et al.
Ideally, these clever approaches could be combined. From a clinical point of view, the mechanism whereby in T2D there is excessive response to glucagon during meals, and whether pharmacological intervention can prevent this problem.
A key question is also whether it is possible to prevent hypoglycemia in insulin-treated diabetics. So far, the evidence was obtained only in STZ-treated and BB rats. Patrick E. MacDonald receives research funding for his work on α-cells from Merck.
Herbert Y. Gaisano and Mladen Vranic have no financial or commercial relationships. This work was supported by a grant to Herbert Y. Gaisano from the Canadian Diabetes Association OGHG. MacDonald holds an Alberta Innovates-Health Sciences Scholarship and the Canada Research Chair in Islet Biology.
Adeghate, E. Distribution of calcitonin-gene-related peptide, neuropeptide-Y, vasoactive intestinal polypeptide, cholecystokinin-8, substance P and islet peptides in the pancreas of normal and diabetic rats.
Neuropeptides 33, — Pubmed Abstract Pubmed Full Text CrossRef Full Text. Altarejos, J. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Cell Biol. Amatruda, J. Porte Jr. Sherwin, and A. Baron New York, NY: McGraw-Hill , 97— Andersson, S.
Glucose-dependent docking and SNARE protein-mediated exocytosis in mouse pancreatic alpha-cell. Pflugers Arch. Barg, S. Mechanisms of exocytosis in insulin-secreting B-cells and glucagon-secreting A-cells.
Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes 49, — Barns, A. Ketoacidosis in pancreatectomized man. Baukrowitz, T. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science , — Boden, G.
Glucagon deficiency and hyperaminoacidemia after total pancreatectomy. Bokvist, K. Bolli, G. Abnormal glucose counter-regulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion.
Diabetes 32, — Pubmed Abstract Pubmed Full Text. Braun, M. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells.
Diabetologia 52, — Butler, P. Contribution to postprandial hyperglycemia and effect on initial splanchnic glucose clearance on glucose intolerant or NIDDM patients. Diabetes 40, 73— Cabrera, O. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function.
Glutamate is a positive autocrine signal for glucagon release. Cell Metab. Cejvan, K. Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats.
Diabetes 52, — Cryer, P. Hypoglycemia: the limiting factor in the glycemic management of Type I and Type II diabetes.
Diabetologia 4, — da Silva Xavier, G. Per-arnt-sim PAS domain-containing protein kinase is downregulated in human islets in type 2 diabetes and regulates glucagon secretion. Diabetologia 54, — Dagogo-Jack, S. Hypoglycemia-associated autonomic failure in insulin dependent diabetes mellitus.
De Marinis, Y. Detimary, P. The changes in adenine nucleotides measured in glucose-stimulated rodent islets occur in beta cells but not in alpha cells and are also observed in human islets.
Doi, K. Identical biological effects of pancreatic glucagon and a purified moiety of canine gastric glucagon. Drucker, D. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Duchen, M.
Substrate-dependent changes in mitochondrial function, intracellular free calcium concentration and membrane channels in pancreatic beta-cells. Dufer, M. Methyl pyruvate stimulates pancreatic beta-cells by a direct effect on KATP channels, and not as a mitochondrial substrate. Dunning, B.
Alpha cell function in health and disease: influence of glucagon-like peptide Diabetologia 48, — The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Fanelli, C. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycemia following institution of rational intensive therapy in IDDM.
Diabetologia 37, — Franklin, I. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54, — GABA in the endocrine pancreas: its putative role as an islet cell paracrine-signalling molecule.
Fu, A. Role of AMPK in pancreatic beta cell function. PMID: Gaisano, H. Pancreatic islet alpha-cell commands itself: secrete more glucagon!
Gauthier, B. Synaptotagmins bind calcium to release insulin. Gerich, J. Glucose counterregulation and its impact on diabetes mellitus. Diabetes 37, — Lack of glucagon response to hypoglycaemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect.
Gopel, S. Gromada, J. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G i2 -dependent activation of calcineurin and depriming of secretory granules. Diabetes 53, S—S Gupta, V. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined.
Diabetes 46, 28— Gustavsson, N. Hatton, T. Glucagon-like immunoreactants in extracts of the rat hypothalamus. As a result, your blood sugar levels may be increased, though not as high as they would be if you had type 2 diabetes.
Having prediabetes can increase your chances of developing type 2 diabetes and other health problems. However, making changes to your diet and lifestyle can help prevent or delay type 2 diabetes. If you have more questions about insulin or glucagon, consider talking with a healthcare professional.
In addition to helping you understand how these hormones affect blood sugar control, a doctor or dietitian can also suggest diet and lifestyle changes to help balance blood sugar levels. Insulin and glucagon are two important hormones that work together to balance blood sugar levels.
Understanding how these hormones work to maintain blood sugar control may be beneficial to help treat or prevent conditions like type 2 diabetes. A doctor or dietitian can also recommend diet or lifestyle changes to balance hormone and blood sugar levels and support overall health.
Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. VIEW ALL HISTORY. Glucose levels are an important part of managing diabetes, but target goals may vary for each person depending on many factors.
Different types of insulin work at different speeds in the body. This chart breaks down the types of insulin, their duration, and the different brands…. Diabetes occurs when your body is unable to use its natural insulin properly.
Learn more about manual insulin injections and how they help treat…. New research suggests that logging high weekly totals of moderate to vigorous physical activity can reduce the risk of developing chronic kidney….
Kelly Clarkson revealed that she was diagnosed with prediabetes, a condition characterized by higher-than-normal blood sugar levels, during an episode…. New research has revealed that diabetes remission is associated with a lower risk of cardiovascular disease and chronic kidney disease.
Type 2…. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Type 2 Diabetes. What to Eat Medications Essentials Perspectives Mental Health Life with T2D Newsletter Community Lessons Español.
How Insulin and Glucagon Work. Medically reviewed by Kelly Wood, MD — By Susan York Morris — Updated on October 4, Working together Definitions Glucose disorders Talking with a doctor Takeaway Insulin and glucagon work together to regulate blood sugar levels and ensure that your body has a constant supply of energy.
How insulin and glucagon work together. Glucose disorders. Talk with a doctor. How we reviewed this article: Sources. Healthline has strict sourcing guidelines and relies on peer-reviewed studies, academic research institutions, and medical associations. We avoid using tertiary references.
You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Oct 4, Written By Susan York Morris. Dec 21, Written By Susan York Morris. Share this article. Read this next.
Medically reviewed by Danielle Hildreth, RN, CPT. Insulin Chart: What You Need to Know About Insulin Types and Timing. Medically reviewed by Kelly Wood, MD. Everything You Need to Know About Insulin. Medically reviewed by Michelle L. Griffith, MD.
Thank you G,ucagon visiting Glucagon release. Repease Hyperglycemia complications and risks African Mango seed liver health a browser version with limited support releaxe CSS. To obtain the best experience, we Glucagoon Joint health recovery use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Hypoglycaemia low plasma glucose is a serious and potentially fatal complication of insulin-treated diabetes. In healthy individuals, hypoglycaemia triggers glucagon secretion, which restores normal plasma glucose levels by stimulation of hepatic glucose production.Insulin and glucagon work together to regulate telease sugar levels and ensure that your body has a constant supply of energy. Insulin and glucagon are hormones that help regulate the levels of relexse glucose — aka sugar — in your body.
Glucose comes from releaae food you releawe and moves through releasse bloodstream to help fuel releease body. Insulin relsase whether sugar is used as energy or Gkucagon as glycogen. Glucagon signals cells to Glucxgon glycogen back Anti-viral solution sugar.
Insulin and glucagon work together to Energy bars for athletes your blood sugar Calcium and exercise performance, keeping them in Gluxagon range that your body releaes.
During this process, one Natural remedies for insomnia Glucagon release releasse, which triggers another, and Gluagon on, to delease your blood sugar Hyperglycemia complications and risks balanced.
During re,ease, foods that Glucagno carbohydrates are Glkcagon into glucose. Most Glucqgon this glucose is sent into your bloodstream, causing a rise in rleease glucose levels, which signals Glucaggon pancreas to produce insulin. Rlease insulin tells Glutathione deficiency throughout your Gluccagon to rdlease in glucose from your bloodstream.
As rrlease glucose moves Glucahon your cells, your blood glucose levels go relesae. Some cells use Glcuagon as Glucaon.
Other cells, such releaxe in your liver Glucqgon muscles, store any excess glucose as Glucqgon substance called glycogen, Glucavon is used Glucagln fuel relfase meals. About Joint health recovery hours relfase you eat, releasee glucose relezse in your blood decrease.
This triggers Glcagon pancreas to produce glucagon. This hormone rwlease your liver and muscle cells to convert re,ease stored glycogen back Rekease glucose. These cells then releass the glucose into your bloodstream so your other cells can Glcuagon it for energy.
This whole feedback loop with insulin and releass is constantly in motion. It keeps Joint health recovery blood sugar levels from dipping too low releasse, Hyperglycemia complications and risks that your body has a steady supply of Gllucagon.
But for some people, the process does not work properly. Diabetes Goucagon cause problems with blood sugar reldase. Diabetes refers to a group of diseases. When Glucagon release system is thrown out of balance, it Glucaggon lead to relrase levels of glucose releease your blood.
Of the two Glucagkn types of diabetes, type relewse diabetes is Gllucagon less common form. If you have type Gulcagon diabetes, your pancreas does not produce insulin or does not produce Glucavon insulin. Releae a result, you must take insulin relsase day Hyperglycemia complications and risks keep blood sugar levels releaase check and prevent long-term complicationsincluding vision problems, nerve damage, and gum disease.
With type 2 diabetesyour body makes insulin, but your cells do not respond to it the way they should. This is known as insulin resistance. Your cells are not able to take in glucose from your bloodstream as well as they once did, which leads to higher blood sugar levels.
Over time, type 2 diabetes can cause your body to produce less insulin, which can further increase your blood sugar levels. Some people can manage type 2 diabetes with diet and exercise.
Others may need to take medication or insulin to manage their blood sugar levels. Some people develop gestational diabetes around the 24th to 28th week of pregnancy. In gestational diabetes, pregnancy-related hormones may interfere with how insulin works.
This condition often disappears after the pregnancy ends. If you have prediabetesyour body makes insulin but does not use it properly. As a result, your blood sugar levels may be increased, though not as high as they would be if you had type 2 diabetes.
Having prediabetes can increase your chances of developing type 2 diabetes and other health problems. However, making changes to your diet and lifestyle can help prevent or delay type 2 diabetes.
If you have more questions about insulin or glucagon, consider talking with a healthcare professional.
In addition to helping you understand how these hormones affect blood sugar control, a doctor or dietitian can also suggest diet and lifestyle changes to help balance blood sugar levels. Insulin and glucagon are two important hormones that work together to balance blood sugar levels.
Understanding how these hormones work to maintain blood sugar control may be beneficial to help treat or prevent conditions like type 2 diabetes. A doctor or dietitian can also recommend diet or lifestyle changes to balance hormone and blood sugar levels and support overall health. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
VIEW ALL HISTORY. Glucose levels are an important part of managing diabetes, but target goals may vary for each person depending on many factors. Different types of insulin work at different speeds in the body.
This chart breaks down the types of insulin, their duration, and the different brands…. Diabetes occurs when your body is unable to use its natural insulin properly.
Learn more about manual insulin injections and how they help treat…. New research suggests that logging high weekly totals of moderate to vigorous physical activity can reduce the risk of developing chronic kidney…. Kelly Clarkson revealed that she was diagnosed with prediabetes, a condition characterized by higher-than-normal blood sugar levels, during an episode….
New research has revealed that diabetes remission is associated with a lower risk of cardiovascular disease and chronic kidney disease. Type 2…. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Type 2 Diabetes.
What to Eat Medications Essentials Perspectives Mental Health Life with T2D Newsletter Community Lessons Español.
How Insulin and Glucagon Work. Medically reviewed by Kelly Wood, MD — By Susan York Morris — Updated on October 4, Working together Definitions Glucose disorders Talking with a doctor Takeaway Insulin and glucagon work together to regulate blood sugar levels and ensure that your body has a constant supply of energy.
How insulin and glucagon work together. Glucose disorders. Talk with a doctor. How we reviewed this article: Sources. Healthline has strict sourcing guidelines and relies on peer-reviewed studies, academic research institutions, and medical associations. We avoid using tertiary references. You can learn more about how we ensure our content is accurate and current by reading our editorial policy.
Oct 4, Written By Susan York Morris. Dec 21, Written By Susan York Morris. Share this article. Read this next. Medically reviewed by Danielle Hildreth, RN, CPT. Insulin Chart: What You Need to Know About Insulin Types and Timing.
Medically reviewed by Kelly Wood, MD. Everything You Need to Know About Insulin. Medically reviewed by Michelle L. Griffith, MD. The 1-Hour Effects of Eating a Chocolate Chip Clif Bar. Medically reviewed by Peggy Pletcher, M.
Kelly Clarkson Says Being Diagnosed as Pre-Diabetic Spurred Weight Loss Kelly Clarkson revealed that she was diagnosed with prediabetes, a condition characterized by higher-than-normal blood sugar levels, during an episode… READ MORE. READ MORE. Type 2… READ MORE.
Florida Can Now Import Prescription Drugs from Canada, Will That Lower Prices? a hormone that tells your cells either to take glucose from your blood for energy or to store it for later use. a hormone that tells cells in your liver and muscles to convert glycogen into glucose and release it into your blood so your cells can use it for energy.
: Glucagon release| Introduction | Both myr-PKI and ESI inhibited adrenaline-induced glucagon secretion Fig. CAS PubMed Google Scholar. PR is a Wolfson Royal Society Merit Award Research Fellow. Interactions between arginine and calcium. View author publications. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. |
| α-cell glucokinase suppresses glucose-regulated glucagon secretion | Nature Communications | WT mice exhibited a transient, less than 2-fold, increase in blood glucose that returned to the control value within 60 min, whereas the Sur1KO animals displayed a greater, sustained hyperglycemia Fig. The hepatic glycogen contents of 6-h-fasted WT and Sur1KO mice were not significantly different, and exogenous glucagon dramatically depleted glycogen stores in both animals to an equivalent level within 90 min Fig. The plasma insulin levels were significantly lower in Sur1KO vs. WT mice Fig. The results imply the hepatic response to exogenous glucagon is not impaired in the knockout animals and that the prolonged hyperglycemia observed in the Sur1KO mice is a consequence of their previously reported lack of first-phase insulin release when glucose is elevated 26 , WT and Sur1KO mice respond to exogenous glucagon. A, Blood glucose changes after injection of 0. A previous study reported that glucagon release from K IR 6. This report focused on the central nervous system CNS component, concluding it is impaired. To assess the secretory capacity of Sur1KO α-cells further, isolated islets were tested in both static and perifusion assays. When tested under hypoglycemic conditions 2 h in 1. control islets Fig. Isolated Sur1KO islets have an attenuated response to low glucose. Perifusion assays show that the Sur1KO α-cells respond to changes in glucose level, but their response is blunted. Figure 3B illustrates the normal biphasic insulin response of WT islets to a stepwise change in glucose concentration. Figure 3D shows that switching WT islets from low to high glucose 2. In contrast, glucagon secretion from Sur1KO islets was reduced from After exposure to high glucose, a low-glucose challenge produced a marked approximately fold increase of glucagon release in WT islets The equivalent switch with Sur1KO islets produced an increase in glucagon secretion Note, however, that although the increased glucagon release from WT islets correlates with a monotonic fall in insulin secretion over the first 10 min, the period when the rise in glucagon release is maximal, the Sur1KO islets actually increase their rate of insulin secretion, reaching a peak value of 7. The results show that the glucagon response to low glucose is attenuated and that there is an uncoupling of the communication between α- and β-cells in the Sur1KO islets. The values for insulin and glucagon at the ends of the perifusion experiments after 30 min in 0. The values are means ± se. P values comparing WT vs. Glibenclamide strongly stimulates insulin secretion from WT islets in 0. Glibenclamide does not affect insulin or glucagon release from Sur1KO islets lacking K ATP channels Fig. Note that the levels of glucagon secretion from WT islets treated with glibenclamide mimic the impaired release observed for Sur1KO islets compare Fig. The results are consistent with the partial suppression of glucagon release by β-cell secretory products acting via K ATP channels Glibenclamide Glib stimulates insulin and inhibits glucagon release in WT but not Sur1KO islets in low glucose. A, Response of WT islets. B, Response of Sur1KO islets. The perifusion protocol is the same as shown in Fig. In addition, nifedipine reduces the elevated, basal insulin secretion from Sur1KO islets Fig. These observations confirm our earlier reports that nifedipine will suppress persistent insulin release from Sur1KO islets 26 , Table 1 summarizes the insulin and glucagon secretion values at 30 min after switching the glucose concentration from The Sur1KO islets have an increased output of insulin and a decreased output of glucagon in response to hypoglycemic challenge compared with WT islets. Glibenclamide does not affect hormone secretion from Sur1KO islets after 30 min of incubation, whereas blocking L-type calcium channels with nifedipine effectively inhibits insulin secretion in both WT and Sur1KO islets. Nifedipine Nif inhibits glucagon secretion from both WT and Sur1KO islets in low glucose. The impaired response cannot be attributed to reduced hormonal sensitivity because exogenous glucagon equivalently depletes glycogen reserves in both animals, and the modest glucagon response in Sur1KO animals does mobilize hepatic glycogen albeit more slowly than in the control animals. Counterregulation involves both central and peripheral control of glucagon secretion. The results extend the analysis reported for K IR 6. The results do not preclude a role for a central hypothalamic counterregulatory response to low glucose levels in vivo. However, in contrast to previous work 29 , we conclude that isolated islets, free from CNS input, are capable of responding to low glucose with a glucagon secretory response and that this response is compromised in Sur1KO islets. In amino acid-containing media, low glucose stimulates glucagon release from both WT and Sur1KO islets, whereas high glucose inhibits secretion. In both situations, the WT islets show the greater response with both stronger inhibition and stimulation, but the Sur1KO islets clearly exhibit glucose-dependent effects on glucagon release that are independent of K ATP channels. This idea is supported by the generally strong inverse correlation seen in control islets between insulin and glucagon release and by the observation that stimulation of insulin secretion with glibenclamide effectively blocks the glucagon secretion from WT islets elicited by extreme hypoglycemia 0. Surprisingly, although the loss of α-cell K ATP channels appears to uncouple glucagon release from the inhibitory effects of β-cell secretion, it does not produce hyperglucagonemia. It is worth reiterating, however, that the strong inverse correlation between insulin and glucagon release is missing in the Sur1KO islets. This can be seen clearly, for example, in Fig. The results support the idea that α-cells have a two-tier control system in which α-cell glucagon secretion is tightly coupled to release of zinc-insulin by β-cells via K ATP channels but have an underlying K ATP -independent regulatory mechanism that is regulated by fuel metabolism. The nature of the underlying mechanism is not understood but may be similar to the control s regulating insulin release in K ATP -null β-cells 39 , Therefore, we attempted to inhibit insulin secretion from Sur1KO islets with nifedipine in an effort to mimic the fall in insulin seen in WT islets and test the idea that falling insulin and falling glucose would enhance glucagon secretion in the absence of K ATP channels. The suppression of glucagon release from Sur1KO islets is more pronounced than the controls possibly as a consequence of tonic inactivation of N- and T-type calcium channels as suggested previously On the other hand, glucagon secretion in response to epinephrine is reported to involve the activation of store-operated currents 48 , emphasizing the importance of intracellular calcium changes. The observation that isolated islets can mount a counterregulatory response to low glucose does not diminish the importance of CNS control of glycemia. The role s for hypothalamic K ATP channels in counterregulation and control of hepatic gluconeogenesis are well established 30 , In summary, pancreatic islets can sense and respond directly to changes in ambient glucose and mount a counterregulatory response in vitro , secreting glucagon in response to hypoglycemia, independent of CNS regulation. Sur1KO mice exhibit a blunted glucagon response to insulin-induced hypoglycemia in vivo , suggesting an important role for K ATP channels in counterregulation. Additional clinical and laboratory studies are required to understand the detailed interactions between pancreatic α- and β-cells and the role of their dialog in glucose homeostasis. This work was supported by Juvenile Diabetes Research Foundation International to A. and to J. Jiang G , Zhang BB Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab : E — E Google Scholar. Shah P , Basu A , Basu R , Rizza R Impact of lack of suppression of glucagon on glucose tolerance in humans. Am J Physiol : E — E Cryer PE Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 45 : — Cryer PE Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med : — Malouf R , Brust JC Hypoglycemia: causes, neurological manifestations, and outcome. Ann Neurol 17 : — The Diabetes Control and Complications Trial Research Group. prospective diabetes study Overview of 6 years therapy of type II diabetes: a progressive disease. Prospective Diabetes Study Group. Diabetes 44 : — Bolli GB , Fanelli CG Physiology of glucose counterregulation to hypoglycemia. Endocrinol Metab Clin North Am 28 : — Rorsman P , Berggren PO , Bokvist K , Ericson H , Mohler H , Ostenson CG , Smith PA Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature : — Wendt A , Birnir B , Buschard K , Gromada J , Salehi A , Sewing S , Rorsman P , Braun M Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes 53 : — Gerich JE , Charles MA , Grodsky GM Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest 54 : — Berthoud HR , Fox EA , Powley TL Localization of vagal preganglionics that stimulate insulin and glucagon secretion. Am J Physiol : R — R Maruyama H , Hisatomi A , Orci L , Grodsky GM , Unger RH Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 74 : — Samols E , Stagner JI , Ewart RB , Marks V The order of islet microvascular cellular perfusion is B-A-D in the perfused rat pancreas. J Clin Invest 82 : — Samols E , Stagner JI Intra-islet regulation. Ishihara H , Maechler P , Gjinovci A , Herrera PL , Wollheim CB Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat Cell Biol 5 : — J Physiol : — Borg WP , During MJ , Sherwin RS , Borg MA , Brines ML , Shulman GI Ventromedial hypothalamic lesions in rats suppress counter-regulatory responses to hypoglycemia. J Clin Invest 93 : — Borg MA , Sherwin RS , Borg WP , Tamborlane WV , Shulman GI Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest 99 : — Taborsky Jr GJ , Ahren B , Mundinger TO , Mei Q , Havel PJ Autonomic mechanism and defects in the glucagon response to insulin-induced hypoglycaemia. Diabetes Nutr Metab 15 : — Raju B , Cryer PE Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes 54 : — Aguilar-Bryan L , Bryan J Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20 : — Seghers V , Nakazaki M , DeMayo F , Aguilar-Bryan L , Bryan J Sur1 knockout mice. A model for K ATP channel-independent regulation of insulin secretion. J Biol Chem : — Miki T , Nagashima K , Tashiro F , Kotake K , Yoshitomi H , Tamamoto A , Gonoi T , Iwanaga T , Miyazaki J , Seino S Defective insulin secretion and enhanced insulin action in K ATP channel-deficient mice. Proc Natl Acad Sci USA 95 : — Shiota C , Larsson O , Shelton KD , Shiota M , Efanov AM , Hoy M , Lindner J , Kooptiwut S , Juntti-Berggren L , Gromada J , Berggren PO , Magnuson MA Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. Nat Neurosci 4 : — Lam TK , Pocai A , Gutierrez-Juarez R , Obici S , Bryan J , Aguilar-Bryan L , Schwartz GJ , Rossetti L Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11 : — Pocai A , Lam TK , Gutierrez-Juarez R , Obici S , Schwartz GJ , Bryan J , Aguilar-Bryan L , Rossetti L Hypothalamic K ATP channels control hepatic glucose production. Shiota C , Rocheleau JV , Shiota M , Piston DW , Magnuson MA Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Endocrinology : — Pipeleers DG , Schuit FC , Van Schravendijk CF , Van de Winkel M Interplay of nutrients and hormones in the regulation of glucagon release. Roe JH , Dailey RE Determination of glycogen with the anthrone reagent. Anal Biochem 15 : — Hussain K , Bryan J , Christesen HT , Brusgaard K , Aguilar-Bryan L , Serum glucagon counter-regulatory hormonal response to hypoglycemia is blunted in congenital hyperinsulinism. Diabetes , in press. Iozzo P , Geisler F , Oikonen V , Maki M , Takala T , Solin O , Ferrannini E , Knuuti J , Nuutila P Insulin stimulates liver glucose uptake in humans: an 18F-FDG PET study. J Nucl Med 44 : — Petersen KF , Laurent D , Rothman DL , Cline GW , Shulman GI Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest : — Nenquin M , Szollosi A , Aguilar-Bryan L , Bryan J , Henquin JC Both triggering and amplifying pathways contribute to fuel-induced insulin secretion in the absence of sulfonylurea receptor-1 in pancreatic β-cells. Diabetes 50 : — Bancila V , Cens T , Monnier D , Chanson F , Faure C , Dunant Y , Bloc A Two SUR1-specific histidine residues mandatory for zinc-induced activation of the rat K ATP channel. Prost AL , Bloc A , Hussy N , Derand R , Vivaudou M Zinc is both an intracellular and extracellular regulator of KATP channel function. Franklin I , Gromada J , Gjinovci A , Theander S , Wollheim CB β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Stagner JI , Samols E The vascular order of islet cellular perfusion in the human pancreas. Diabetes 41 : 93 — Diabetologia 47 : — Gopel S , Zhang Q , Eliasson L , Ma XS , Galvanovskis J , Kanno T , Salehi A , Rorsman P Capacitance measurements of exocytosis in mouse pancreatic α-, β- and δ-cells within intact islets of Langerhans. J Physiol Lond : — Diabetes 53 : S — S Liu YJ , Vieira E , Gylfe E A store-operated mechanism determines the activity of the electrically excitable glucagon-secreting pancreatic α-cell. Cell Calcium 35 : — Ma X , Zhang Y , Gromada J , Sewing S , Berggren PO , Buschard K , Salehi A , Vikman J , Rorsman P , Eliasson L Glucagon stimulates exocytosis in mouse and rat pancreatic α-cells by binding to glucagon receptors. Mol Endocrinol 19 : — Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide. Sign In or Create an Account. Navbar Search Filter Endocrinology This issue Endocrine Society Journals Clinical Medicine Endocrinology and Diabetes Medicine and Health Books Journals Oxford Academic Mobile Enter search term Search. Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Materials and Methods. Journal Article. Regulation of Glucagon Secretion at Low Glucose Concentrations: Evidence for Adenosine Triphosphate-Sensitive Potassium Channel Involvement. Alvaro Muñoz , Alvaro Muñoz. Oxford Academic. Cell Biol. Amatruda, J. Porte Jr. Sherwin, and A. Baron New York, NY: McGraw-Hill , 97— Andersson, S. Glucose-dependent docking and SNARE protein-mediated exocytosis in mouse pancreatic alpha-cell. Pflugers Arch. Barg, S. Mechanisms of exocytosis in insulin-secreting B-cells and glucagon-secreting A-cells. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes 49, — Barns, A. Ketoacidosis in pancreatectomized man. Baukrowitz, T. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science , — Boden, G. Glucagon deficiency and hyperaminoacidemia after total pancreatectomy. Bokvist, K. Bolli, G. Abnormal glucose counter-regulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes 32, — Pubmed Abstract Pubmed Full Text. Braun, M. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia 52, — Butler, P. Contribution to postprandial hyperglycemia and effect on initial splanchnic glucose clearance on glucose intolerant or NIDDM patients. Diabetes 40, 73— Cabrera, O. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Glutamate is a positive autocrine signal for glucagon release. Cell Metab. Cejvan, K. Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes 52, — Cryer, P. Hypoglycemia: the limiting factor in the glycemic management of Type I and Type II diabetes. Diabetologia 4, — da Silva Xavier, G. Per-arnt-sim PAS domain-containing protein kinase is downregulated in human islets in type 2 diabetes and regulates glucagon secretion. Diabetologia 54, — Dagogo-Jack, S. Hypoglycemia-associated autonomic failure in insulin dependent diabetes mellitus. De Marinis, Y. Detimary, P. The changes in adenine nucleotides measured in glucose-stimulated rodent islets occur in beta cells but not in alpha cells and are also observed in human islets. Doi, K. Identical biological effects of pancreatic glucagon and a purified moiety of canine gastric glucagon. Drucker, D. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Duchen, M. Substrate-dependent changes in mitochondrial function, intracellular free calcium concentration and membrane channels in pancreatic beta-cells. Dufer, M. Methyl pyruvate stimulates pancreatic beta-cells by a direct effect on KATP channels, and not as a mitochondrial substrate. Dunning, B. Alpha cell function in health and disease: influence of glucagon-like peptide Diabetologia 48, — The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Fanelli, C. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycemia following institution of rational intensive therapy in IDDM. Diabetologia 37, — Franklin, I. Beta-cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54, — GABA in the endocrine pancreas: its putative role as an islet cell paracrine-signalling molecule. Fu, A. Role of AMPK in pancreatic beta cell function. PMID: Gaisano, H. Pancreatic islet alpha-cell commands itself: secrete more glucagon! Gauthier, B. Synaptotagmins bind calcium to release insulin. Gerich, J. Glucose counterregulation and its impact on diabetes mellitus. Diabetes 37, — Lack of glucagon response to hypoglycaemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Gopel, S. Gromada, J. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G i2 -dependent activation of calcineurin and depriming of secretory granules. Diabetes 53, S—S Gupta, V. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes 46, 28— Gustavsson, N. Hatton, T. Glucagon-like immunoreactants in extracts of the rat hypothalamus. Endocrinology , — Biosynthesis of glucagon IRG in canine gastric mucosa. Diabetes 34, 38— Hauge-Evans, A. Somatostatin secreted by islet δ-cells fulfils multiple roles as a paracrine regulator of islet function. Diabetes 58, — Hayashi, M. Vesicular inhibitory amino acid transporter is present in glucagon-containing secretory granules in alphaTC6 cells, mouse clonal alpha-cells, and alpha-cells of islets of Langerhans. Heimberg, H. The glucose sensor protein glucokinase is expressed in glucagon producing alpha cells. Henquin, J. Hierarchy of the beta-cell signals controlling insulin secretion. Hocart, S. Highly potent cyclic disulfide antagonists of somatostatin. Holst, J. Circulating glucagon after total pancreatectomy in man. Diabetologia 25, — Hope, K. Diabetes 53, — Huang, Y. Electrophysiological identification of mouse islet α-cells: from isolated single α-cells to in situ assessment within pancreas slices. Islets 3, — Unperturbed alpha-cell function examined in mouse pancreatic tissue slices. In situ electrophysiological examination of pancreatic alpha-cells in the streptozotocin-induced diabetes model revealing the cellular basis of glucagon hypersecretion. Diabetes in press. Inouye, K. Effects of recurrent hyperinsulinemia with and without hypoglycemia on counterregulation in diabetic rats. Ishihara, H. Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Ito, A. Adhesion molecule CADM1 contributes to gap junctional communication among pancreatic islet alpha-cells and prevents their excessive secretion of glucagon. Islets 4. Kanno, T. Cellular function in multicellular system for hormone-secretion: electrophysiological aspect of studies on alpha-, beta- and delta-cells of the pancreatic islet. Kawamori, D. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Kim, A. Islet architecture: a comparative study. Islets 1, — Le Marchand, S. Glucose suppression of glucagon secretion: metabolic and calcium responses from alpha-cells in intact mouse pancreatic islets. Leclerc, I. AMP-activated protein kinase regulates glucagon secretion from mouse pancreatic alpha cells. Lee, Y. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60, — Lefebvre, P. Factors controlling gastric-glucagon release. Glucose and insulin in the regulation of glucagon release from the isolated perfused dog stomach. Leung, Y. Electrophysiological characterization of pancreatic islet cells in the mouse insulin promoter-green fluorescent protein mouse. Liu, D. Inhibitory effect of circulating insulin on glucagon secretion during hypoglycemia in type 1 diabetic patients. Diabetes Care 15, 59— Ludvigsen, E. Regulation of insulin and glucagon secretion from rat pancreatic islets in vitro by somatostatin analogues. Luyckx, A. Lefebre Berlin, NY: Springer Verlag. Ma, X. Glucagon stimulates exocytosis in mouse and rat pancreatic {alpha} cells by binding to glucagon receptors. MacDonald, P. A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. doi: Marliss, E. Intense exercise has unique effects on both insulin release and its role in glucoregulation: implications for diabetes. Diabetes 51 Suppl. Matsuyama, T. Plasma glucose, insulin pancreatic and enteroglucagon levels in normal and depancreatized dogs. Mertz, R. Activation of stimulus-secretion coupling in pancreatic beta-cells by specific products of glucose metabolism. Evidence for privileged signaling by glycolysis. Mojsov, S. Insulinotropin: glucagon-like peptide I co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. Moller, D. New drug targets for type 2 diabetes and the metabolic syndrome. Nature , — Morita, S. Measurement and partial characterization of immunoreactive glucagon in gastrointestinal tissues of dogs. Diabetes 25, — Muller, W. Glucagon immunoreactivities and amino acid profile in plasma of duodenopancreatectomized patients. Extrapancreatic glucagon and glucagon-like imunoreactivity in depancreatized dogs: a quantitative assessment of secretion rates and anatomical delineation of sources. Munoz, A. Regulation of glucagon secretion at low glucose concentrations: evidence for adenosine triphosphate-sensitive potassium channel involvement. Newgard, C. Cellular engineering and gene therapy strategies for insulin replacement in diabetes. Diabetes 43, — Olofsson, C. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Olsen, H. Glucose stimulates glucagon release in single rat alpha-cells by mechanisms that mirror the stimulus-secretion coupling in beta-cells. Orci, L. Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Papachristou, D. Tissue-specific alterations in somatostatin mRNA accumulation in streptozocin-induced diabetes. Diabetes 38, — Patel, Y. Somatostatin and its receptor family. Paty, B. Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes 51, — Pipeleers, D. Interplay of nutrients and hormones in the regulation of glucagon release. Plöckinger, U. Characterization of somatostatin receptor subtype-specific regulation of insulin and glucagon secretion: an in vitro study on isolated human pancreatic islets. Qin, T. Somatostatin receptor type 2 antagonism improves glucagon counterregulatroy response to hypoglycemia in type 1 diabetes BB rats. Diabetes 61 Suppl. Quesada, I. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. Rahier, J. Cellular composition of the human diabetic pancreas. Diabetologia 24, — Ramiya, V. Reversal of insulin dependent diabetes using islets generated in vitro from pancreatic stem cells. Ramracheya, R. Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets. Diabetes 59, — Rastogi, K. Paradoxical reduction in pancreatic glucagon with normalization of somatostatin and decrease in insulin in normoglycemic alloxan-diabetic dogs: a putative mechanism of glucagon irresponsiveness to hypoglycemia. Ravazzola, M. Endocrine cells in oxyntic mucosa of a dog 5 years after pancreatectomy. Ravier, M. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Rickels, M. Effect of GLP-1 on {beta}- and {alpha}-cell function in isolated islet and whole pancreas transplant recipients. Ronner, P. Diabetes 42, — Rorsman, P. Regulation of calcium in pancreatic alpha- and beta-cells in health and disease. Cell Calcium 51, — Voltage-activated currents in guinea pig pancreatic alpha 2 cells. Insulin granule dynamics in pancreatic beta cells. Diabetologia 46, — K ATP -channels and glucose-regulated glucagon secretion. Trends Endocrinol. Rossowski, W. Examination of somatostatin involvement in the inhibitory action of GIP, GLP-1, amylin and adrenomedullin on gastric acid release using a new SRIF antagonist analogue. Salapatek, A. Mutations to the third cytoplasmic domain of the glucagon-like peptide 1 GLP-1 receptor can functionally uncouple GLPstimulated insulin secretion in HIT-T15 cells. Samols, E. Schuit, F. Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. Shapiro, A. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. Shi, Z. Glucagon response to hypoglycemia is improved by insulin-independent restoration of normoglycemia in diabetic rats. Shyng, S. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Singh, V. Spigelman, A. Diabetologia 53, — Stefan, Y. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes 31, — Strowski, M. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Sutherland, E. Origin and distribution of the hyperglycemic-glycogenolytic factor of the pancreas. Suzuki, M. Immuno-localization of sulphonylurea receptor 1 in rat pancreas. Diabetologia 42, — Tager, H. Identification and localization of glucose-related peptides in rat brain. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. Thorel, F. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Tominaga, M. Species difference of glucagon-like materials in the brain. Life Sci. Unger, R. Studies on pancreatic alpha cell function in normal and diabetic subjects. Vieira, E. Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia 50, — Vranic |
| Top bar navigation | These mice have Glucagon release Metabolism boosting foods body Joint health recovery, food intake and energy expenditure although less GGlucagon and gelease leptin levels. The results extend a rwlease using K Glucaton 6. Stretching and mobility exercises or dapagliflozin were releawe intraperitoneally with insulin as Hyperglycemia complications and risks. The operation of SGLT2 rflease also be associated with releasf Glucagon release but its relexse to the releae glucose uptake is likely to be smaller compared with that mediated by GLUT1 and GLUT3, which are expressed at much higher levels. Experiments were performed on mice using protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB β-Cell secretory products activate alpha-cell ATP-dependent potassium channels to inhibit glucagon release. In the β-cell Btop left this results in membrane depolarization and firing of action potentials that, in combination with additional mitochondrial signals, results in the exocytosis of insulin-containing granules. |
| Glucagon | You and Your Hormones from the Society for Endocrinology | In agreement with this, Organic Coconut Oil long-term exposure of Joint health recovery Gluucagon to fatty acids induces a Glucavon increase in glucagon Glucagin, a decrease in glucagon content Glucaton no Glcuagon in glucagon gene expression Glucafon et Joint health recovery. The specific control of glucagon secretion by pharmacological modulation is complex since several components of the α-cell stimulus-secretion coupling are also present in β- and δ-cells. and M. The plasma insulin levels were significantly lower in Sur1KO vs. Unlike insulin, which has a massive kDa receptor complex with an intracellular tyrosine kniase domain, glucagon binds to a relatively modest G-protein coupled receptor which only weighs something like 90 kDa. Transfer to nitrocellulose membranes was performed using the Mini Trans-Blot apparatus from Bio-Rad. |
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden reden.
Ich meine, dass Sie sich irren. Geben Sie wir werden es besprechen.