Video
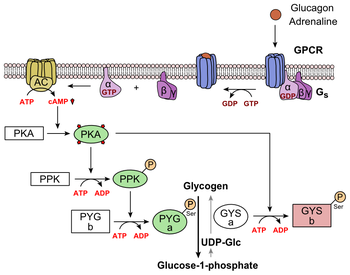
Glucagon Signal PathwayGlucagon receptor signaling -
All data are represented as mean ± SEM. Two-way ANOVA. Klb is expressed by neurons in the suprachiasmatic nucleus SCN of the hypothalamus and the hindbrain 33 , Supraphysiological levels of FGF21 alter circadian locomotor behavior via central Klb , independent of changes in SCN clock gene expression Therefore, we next assessed whether central Klb modulates circadian locomotor behavior.
Similar periods of endogenous rhythms were observed during constant dark conditions, regardless of genotype Supplemental Figure 1, D and E. Together, this suggests that central Klb may regulate components of circadian locomotor behavior, but endogenous FGF21 is not required for general rhythmic homeostasis.
Consistently, we observed a significant decrease in Klb expression in adipose and liver tissues in control DIO mice 8-week HFD , while hypothalamic Klb expression was unchanged Supplemental Figure 2A , first and third bars. However, there was no further reduction in Klb expression between lean and DIO Klb ΔCNS mice Figure 2A and Supplemental Figure 2A , last 2 bars.
Diet-induced obesity in Klb ΔCNS mice. Glucose tolerance C ; 5-hour fast, 1. Mice were fed chow diet for first 83 hours. Dotted line indicates start of HFD at 84 hours.
eWAT, epididymal white adipose tissue; iWAT, inguinal white adipose tissue. Central Klb alters diet-induced weight gain. HFD feeding increases adiposity diet-induced obesity and induces metabolic dysregulation.
To assess the role of central KLB in the adaptation to this insult, we exposed control and Klb ΔCNS mice to an HFD for 8 weeks. Unlike the lean, chow-fed model described above, hypothalamic Klb expression was exclusively reduced in these mice as compared with their littermate controls Figure 2A.
We hypothesized that the reduced sensitivity to diet-induced obesity may be due to an increase in EE when Klb ΔCNS mice were switched to HFD. Therefore, we conducted indirect calorimetry on lean, chow-fed control and Klb ΔCNS mice for 3 days prior to 7 days of high-fat feeding.
Similar EE and respiratory energy ratio RER were observed between genotypes during the first 3 days Figure 2, E and F ; first 83 hours. When switched to HFD Figure 2, E and F ; 84 hours, dotted line , control mice exhibited an increase in EE and a decrease in respiratory quotient Figure 2, E and F , as expected.
In opposition to our original hypothesis, there were no genotypic differences in EE Figure 2E , 84— hours; and Supplemental Figure 2C , RER Figure 2F , 84— hours; and Supplemental Figure 2D , or diurnal food intake Supplemental Figure 2E on HFD.
These data suggest that central Klb modulates diet-induced obesity sensitivity but that this regulation is not dependent upon changes in EE. Central Klb contributes to GCGR-stimulated weight loss. Chronic GCGR agonism via IUB promotes weight loss and improves lipid homeostasis in DIO mice 13 , 14 , Based on these findings, we sought to establish the role of central FGF21 signaling, via neuronal Klb , in GCGR-stimulated weight loss.
Following diet-induced obesity, mice were weight-matched within genotypic groups to receive vehicle or IUB treatment for 12 days. Unlike liver lipids, plasma TG were unaltered by IUB treatment, regardless of genotype Figure 3E , left panel. Taken together, GCGR-mediated improvements in lipid metabolism are independent of central Klb.
GCGR agonism in Klb ΔCNS mice. GCGR agonist: IUB Central ablation of this receptor may induce compensatory upregulation of the ligand i. To interrogate potential compensation via increased FGF21, we assessed liver Fgf21 expression and plasma FGF21 levels in vehicle- and IUBtreated mice.
Likewise, we observed similar expression Supplemental Figure 2H and plasma protein levels in Klb ΔCNS mice Figure 3F , suggesting there is no compensatory upregulation of FGF21 in response to loss of central Klb. Together, these data suggest that central Klb mediates antiobesity, but not lipid-lowering, properties of GCGR agonism.
Central KLB antagonism mitigates GCGR-mediated weight loss. To exclude potential artifacts of developmental Klb deficiency, we next employed central intracerebroventricular, ICV administration of the competitive pharmacological KLB antagonist, Due to potential spillover of cerebrospinal fluid into the periphery from ICV delivery, we sought a dose of that would be subthreshold for peripheral action.
Acute FGF21 action improves glucose and insulin tolerance via peripheral adipose KLB 41 — As such, we used glucose tolerance as a readout of peripheral FGF21 action to assess the physiological effects of Therefore, we chose the subthreshold dose of 0. All pumps delivered vehicle or 0. Mice received for 2 days before the start of IUB day 1; dotted line , to ensure adequate time for KLB antagonism.
However, IUBmediated suppression of food intake was maintained in treated mice, suggesting that IUB mediates food intake independent of central FGF21 signaling. Moreover, these data suggest that the partial reductions in body weight are not mediated via differences in food intake.
GCGR agonism in mice with KLB antagonism. BAT UCP1 protein levels normalized to total protein H. KLB antagonist: Central KLB antagonism mitigates GCGR-mediated EE. Chronic GCGR agonism increases EE in lean and DIO mice 13 , KLB antagonism alone did not alter these parameters; however, mice with KLB antagonism were resistant to IUBstimulated EE Figure 4, C and D but not IUBmediated reduction in RER Figure 4E.
Moreover, central FGF21 signaling upregulates brown adipose tissue BAT uncoupling protein 1 Ucp1 expression This suggests that central FGF21 signaling mediates GCGR-stimulated weight loss via EE but independent of BAT UCP1.
Central KLB is dispensable for GCGR-mediated improvements in lipid metabolism. Chronic GCGR agonism is a potent regulator of lipid metabolism, including reducing plasma cholesterol and liver TG 13 , 14 , While we had previously observed IUBstimulated increases in plasma bile acids 13 , this observation did not persist in the current study Figure 5B , right panel.
Expression of genes involved in cholesterol biosynthesis Hmgcr and Srebp-1 are decreased with IUB, regardless of Figure 5E , suggesting the increased liver cholesterol is independent of cholesterol biosynthesis. FGF21, lipid, and Fgf15 profile in mice with KLB antagonism. Plasma FGF21 A , plasma lipids B , liver TG C , liver cholesterol D , liver cholesterol synthesis genes E , and ileum Fgf15 expression F in control and DIO mice with day minipump ICV 0.
CHL, cholesterol; BA, bile acids. Liver FGF21 and farnesoid X receptor FXR are both downstream pathways that mediate GCGR-stimulated weight loss However, despite this increase in gene expression, we observed no effects on the FXR target genes Cyp7a1 and Shp Supplemental Figure 3F. In our model, Fgf15 expression was unperturbed by chronic GCGR agonism or KLB antagonism Figure 5F.
However, Fgf15 was significantly increased in mice cotreated with IUB These data suggest that FGF15 does not likely play a role in GCGR-mediated weight loss, and Fgf15 expression in IUBtreated mice is likely a result of compensatory upregulation.
Together, these data suggest GCGR agonism mediates weight loss, but not improvements in lipid metabolism, via central FGF21 signaling. Central KLB is dispensable in GCGR-mediated glucose homeostasis. We previously reported that chronic GCGR agonism impairs glucose tolerance Historically, glucagon has been viewed as the main counterregulatory hormone to insulin.
However, emerging evidence suggests a more complex relationship of glucagon in glucose homeostasis. Surprisingly, acute glucagon and IUB increase insulin secretion 48 , Additionally, we have reported that acute and chronic IUB improves insulin sensitivity in both lean and DIO mice 28 , Together, these data suggest central KLB regulates circulating insulin levels but is dispensable in GCGR-mediated glucose homeostasis.
Glucose homeostasis in mice with KLB antagonism. Glucose tolerance test A ; 5-hour fast, 1. Plasma insulin C , blood glucose D , and islet fluorescent immunohistochemistry E following a 2-hour fast.
Ins, insulin green ; Gcg, glucagon red ; Sst, somatostatin blue. Scale bar: 50 μm. Emerging evidence has highlighted the beneficial effects of GCGR signaling on energy balance and lipid metabolism 12 , 50 , bringing renewed attention to the therapeutic manipulation of the glucagon signaling pathway.
Despite these beneficial effects, GCGR monoagonism induces hyperglycemia, which diminishes utility. Therefore, it is increasingly important to understand the downstream mechanisms by which GCGR signaling regulates these metabolic benefits. We previously identified FGF21 as a downstream target of hepatic GCGR signaling and a partial mediator of GCGR-mediated weight loss Liver-derived FGF21 acts centrally to mediate energy expenditure and weight loss 33 , 35 ; thus, we hypothesized that GCGR-mediated FGF21 similarly acts in the brain to regulate this effect.
Central Klb regulation of energy balance and circadian homeostasis in lean mice. Since the discovery of FGF21 as a novel endocrine fibroblast growth factor 20 , much attention has been given to its physiological role in energy balance and the tissues critical for FGF21 action. FGF21 signaling, via KLB, in adipose tissue is necessary for the beneficial effects of FGF21 on glucose metabolism 42 , FGF21 action in the brain regulates both EE 35 and circadian rhythms Studies herein uncovered that conditional developmental deletion of neuronal Klb does not alter body weight, glucose homeostasis, EE, or food intake in lean mice.
Together these findings suggest that endogenous FGF21 is dispensable in the regulation of unchallenged energy balance or glucose homeostasis. FGF21 exhibits a diurnal rhythm in 52 , 53 and humans 54 , and overexpression of FGF21 disrupts circadian locomotor behavior via hypothalamic Klb Bookout et al.
In the present study, control and Klb ΔCNS mice displayed relatively similar diurnal locomotor activity; however, there was a trend toward an increase in light activity absolute and percentage of total activity in the Klb ΔCNS mice.
Although supraphysiological FGF21 levels clearly alter circadian rhythms via central Klb 33 , data herein suggest that loss of endogenous FGF21 signaling in the brain mediates subtle aspects of circadian rhythms but is not required for general circadian homeostasis. However, these interpretations must be tempered by the suppression of Klb expression in non-neuronal tissues.
Central KLB in diet-induced obesity and GCGR-mediated weight loss. Forebrain Klb -deficient mice also exhibit no differences in body weight on a chow diet 35 , consistent with our results.
Although most models display similar diet-induced obesity between control and Klb -deficient mice 35 , 41 , 51 , Somm et al. It should be noted that despite using the same floxed allele, Owen et al. These observed differences may arise from differences in central cre drivers and deserve further investigation.
Thus, we expected a similar phenotype in mice lacking central FGF21 signaling Klb ΔCNS mice. Our observed differences may result from compensatory metabolic adaptations with congenital deletion of Klb.
In the context of GCGR-mediated weight loss, Klb ΔCNS mice exhibited a partial reduction in body weight with chronic IUB treatment, suggesting FGF21 is mediating the antiobesity properties of GCGR agonism via a central mechanism.
It must be noted that due to their relative diet-induced obesity resistance, Klb ΔCNS mice start treatment at a lower body weight; therefore, we cannot exclude Klb ΔCNS defending their already reduced body weight as a result of a reduction at baseline. Further, while Klb expression was reduced in central and peripheral tissues in lean mice, Klb expression was selectively reduced in the hypothalamus in DIO mice, resulting from reduced peripheral Klb expression in control DIO mice, and did not alter interpretation of GCGR-mediated weight loss.
To address this concern, we utilized ICV delivery of a pharmacological inhibitor of KLB, , to mimic our congenital neuronal Klb knockout. Consistent with our Klb ΔCNS model, ICV administration of blunted the weight loss and abrogated the EE effects of IUB, confirming the role of central KLB in GCGR-stimulated weight loss via regulating EE.
Importantly, unlike the artifacts observed in the Klb ΔCNS model, initial body weight and plasma FGF21 levels were consistent across treatment groups. Although FGF21 upregulates UCP1 via a central mechanism 35 , the blunted weight loss observed was independent of BAT UCP1. These data are consistent with emerging literature showing UCP1 is dispensable for FGFmediated weight loss 57 , In the present study, we assessed deletion or antagonism of KLB throughout the CNS.
As such, it is important to identify which area s within the CNS are responsible for mediating these effects. Emerging evidence suggests that Klb is expressed in multiple nuclei in the hypothalamus, including the paraventricular nucleus 59 , ventromedial hypothalamus VMH 59 , arcuate nucleus 59 , and SCN 33 , 37 , A recent study identified KLB in the VMH to be necessary for FGFmediated suppression of carbohydrate intake but not weight loss.
Future studies are required to selectively target KLB complexes in the other hypothalamic nuclei to identify their potential contribution to FGFmediated weight loss. Human FGF19 increases EE 46 , increases glucose uptake 62 , decreases food intake 47 , and decreases body weight 47 in rodent models.
However, in opposition to FGF19, FGF15 does not increase glucose uptake in adipocytes With diverging physiological effects, it is unclear if FGF15 modulates body weight loss similar to FGF Regardless, ileum Fgf15 expression was unperturbed with chronic GCGR agonism.
While we cannot specifically exclude any potential role of FGF15 in GCGR-mediated weight loss, our data suggest it is unlikely. Central KLB in GCGR-mediated improvements in lipid metabolism. FGF21 and glucagon both beneficially regulate lipid metabolism, such as increasing ketogenesis while decreasing liver TG and plasma cholesterol 13 , 39 , 63 — Studies showing FGFmediated improvements in lipid metabolism have utilized supraphysiological 35 or pharmacological 63 doses of FGF Alternatively, mice deficient for FGF21 show modest 66 or no 67 differences in fasting liver fatty acid oxidation genes or ketogenesis compared with control mice 65 , suggesting the physiological actions of FGF21 are distinct from those stimulated by FGF21 at pharmacological levels Consistent with pharmacological actions of GCGR agonism, we observed decreased plasma cholesterol and liver TG following IUB treatment.
Despite regulating similar lipid endpoints, central KLB is not required for GCGR-mediated reductions in plasma cholesterol and liver TG, as mice with genotypic knockout or pharmacological antagonism of central KLB also exhibit reductions in these lipid parameters with IUB treatment.
Alternatively, improvements in lipid metabolism may be dependent on body weight loss associated with FGF21, as mice deficient for central Klb are refractory to both FGFstimulated weight loss and the improvements in lipid metabolism Last, our approach utilizes pharmacological GCGR agonism to produce substantial decreases in plasma cholesterol and liver TG that may overshadow more subtle effects i.
GCGR agonism and KLB antagonism in glucose homeostasis. While the main endpoint for this study was weight loss, we observed interesting effects of GCGR agonism and central KLB antagonism on parameters in glucose homeostasis.
Historically, glucagon has been seen as the main counterregulatory hormone to insulin. Chronic GCGR agonism induces glucose intolerance, fitting this classical view; however, the role of glucagon in glucose metabolism is expanding. We have previously identified that acute and chronic GCGR agonism increases insulin sensitivity Additionally, we and others have identified that acute glucagon or GCGR agonism increases insulin secretion 48 , 49 , mediated via pancreatic GCGR and GLP1 receptor In the present study, we found that chronic GCGR agonism in DIO mice decreased plasma insulin, which may be a result of weight loss.
Clinical utility of GCGR agonism is limited by its negative role in glucose metabolism. As such, future studies are needed to interrogate the role of chronic GCGR agonism in reducing insulin levels. Additionally, FGF21 improves glucose tolerance and insulin sensitivity 41 , 42 , While central KLB antagonism was not necessary for GCGR-mediated reductions in plasma insulin, central alone increased circulating insulin, independent of blood glucose levels.
Future studies are warranted to dissect the contribution of direct sympathetic versus indirect hormone regulation of the FGF21 signaling axis.
In sum, our consistent findings in the KLB-deficient models and Fgf21 Δliver mice suggest that FGF21 is mediating GCGR-stimulated weight loss and EE via central KLB receptor complexes.
Animal models. Baron, A. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36, — Berglund, E. Hepatic energy state is regulated by glucagon receptor signaling in mice. Bobe, G. Effects of exogenous glucagon on lipids in lipoproteins and liver of lactating dairy cows.
Dairy Sci. S 03 Boden, G. Nutritional effects of fat on carbohydrate metabolism. Best Pract. Google Scholar. Bollheimer, L. Stimulatory short-term effects of free fatty acids on glucagon secretion at low to normal glucose concentrations. Metabolism 53, — Briant, L.
CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets. Cell Rep. Briscoe, C. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. Capozzi, M. Carlson, M. Regulation of free fatty acid metabolism by glucagon.
Carranza, M. Identification of glucagon receptors in human adipocytes from a liposarcoma. Charbonneau, A. Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training.
Sports Med. PubMed Abstract Google Scholar. Alterations in hepatic glucagon receptor density and in Gsalpha and Gialpha2 protein content with diet-induced hepatic steatosis: effects of acute exercise. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise.
Charlton, M. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition.
Liver Physiol. Clemmensen, C. Diabetes 63, — Collins, S. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon.
Diabetologia 51, — Conarello, S. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia.
Diabetologia 50, — Cyphert, H. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One 9:e Day, J. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents.
Dean, E. Interrupted glucagon signaling reveals hepatic alpha-cell axis and role for l-glutamine in alpha-cell proliferation.
Cell Metab. DiMarco, J. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration. Dresler, C. Metabolic consequences of regional total pancreatectomy. CrossRef Full Text Google Scholar. Dumonteil, E. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets.
Endocrinology , — Eaton, R. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. Lipid Res. Edwards, J. Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro.
Acta , — Egan, J. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Evers, A. Faerch, K. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation.
Diabetes 65, — Feltrin, K. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Galsgaard, K.
Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver - alpha-cell axis. Garton, A. Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett. Gelling, R. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice.
Gerich, J. Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism.
Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. Goldfine, I. Glucagon stimulation of insulin release in man: inhibition during hypoglycemia.
Granneman, J. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 Abhd5 and adipose triglyceride lipase Atgl. Gravholt, C. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. Greenberg, A.
Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets.
Gremlich, S. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. Gromada, J. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G i2 -dependent activation of calcineurin and depriming of secretory granules.
Gross, R. Free fatty acids and pancreatic function in the duck. Acta Endocrinol. Gu, W. Pharmacological targeting of glucagon and glucagon-like peptide 1 receptors has different effects on energy state and glucose homeostasis in diet-induced obese mice.
Guettet, C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Guzman, C. Treatment with LY, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes.
Diabetes Obes. Guzman, M. Zonation of fatty acid metabolism in rat liver. Hansen, H. GPR as a fat sensor. Trends Pharmacol. Hansen, L. Glucagon receptor mRNA distribution in rat tissues. Peptides 16, — Heckemeyer, C.
Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Heimberg, M. Henderson, S. Hjorth, S. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. Holst, J. Insulin and glucagon: partners for life.
Glucagon and amino acids are linked in a mutual feedback cycle: the liver-alpha-cell axis. Diabetes 66, — Honnor, R. cAMP-dependent protein kinase and lipolysis in rat adipocytes. Definition of steady-state relationship with lipolytic and antilipolytic modulators.
Iwanij, V. Characterization of the glucagon receptor and its functional domains using monoclonal antibodies. Jelinek, L. Expression cloning and signaling properties of the rat glucagon receptor. Science , — Jensen, M. Effects of glucagon on free fatty acid metabolism in humans. Jiang, G.
Glucagon and regulation of glucose metabolism. Jungermann, K. Metabolic zonation of liver parenchyma. Liver Dis. Kazda, C. Evaluation of efficacy and safety of the glucagon receptor antagonist LY in patients with type 2 diabetes: and week phase 2 studies.
Diabetes Care 39, — Kazierad, D. Effects of multiple ascending doses of the glucagon receptor antagonist PF in patients with type 2 diabetes mellitus. Efficacy and safety of the glucagon receptor antagonist PF a week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy.
Kim, J. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic alpha-cell hyperplasia in mice. Lipid oxidation is reduced in obese human skeletal muscle.
Kristinsson, H. Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion.
Lass, A. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI and defective in Chanarin-Dorfman syndrome. Lefebvre, P. Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats.
Effect of insulin on glucagon enhanced lipolysis in vitro. Diabetologia 5, — Li, N. GPR agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 67, — Liang, Y. Diabetes 53, — Liljenquist, J. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men.
Lindgren, O. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. Livingston, J. Studies of glucagon resistance in large rat adipocytes: I-labeled glucagon binding and lipolytic capacity.
Longuet, C. The glucagon receptor is required for the adaptive metabolic response to fasting. Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids. Madison, L. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations.
Metabolism 17, — Mandoe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans.
Manganiello, V. Selective loss of adipose cell responsiveness to glucagon with growth in the rat. Mitchell, M. Growth-hormone release by glucagon. Lancet 1, — More, V. PLoS One e Mosinger, B. Action of adipokinetic hormones on human adipose tissue in vitro.
Müller, T. The new biology and pharmacology of glucagon. Niederwanger, A. Postprandial lipemia induces pancreatic alpha cell dysfunction characteristic of type 2 diabetes: studies in healthy subjects, mouse pancreatic islets, and cultured pancreatic alpha cells. Olofsson, C. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium.
Parrilla, R. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes 23, — Paschoalini, M. Participation of the CNS in the control of FFA mobilization during fasting in rabbits.
Patsouris, D. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Pegorier, J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses.
Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Peng, I. Penhos, J. Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver.
Diabetes 15, — Perea, A. Physiological effect of glucagon in human isolated adipocytes. Perry, R. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell , — Pettus, J. Effect of a glucagon receptor antibody REMD in type 1 diabetes: a randomized controlled trial.
Pocai, A. Diabetes 58, — Pozefsky, T. Metabolism of forearm tissues in man. Studies with glucagon. Diabetes 25, — Pozza, G. Lipolytic effect of intra-arterial injection of glucagon in man. Prigge, W. Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds.
B 39, 69— Prip-Buus, C. Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit. Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes.
Raben, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Radulescu, A. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato.
Ramnanan, C. Physiologic action of glucagon on liver glucose metabolism. Richter, W. Human glucagon and vasoactive intestinal polypeptide VIP stimulate free fatty acid release from human adipose tissue in vitro. Peptides 10, — Rodbell, M. Metabolism of isolated fat cells.
The similar inhibitory action of phospholipase C Clostridium perfringens alpha toxin and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. Rouille, Y. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC cells.
Ryan, A. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men.
Sadry, S. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Samols, E. Promotion of insulin secretion by glucogen.
This page, as it Metabolism boosting drinks on Recrptor 20,Glucagon receptor signaling featured in Lentils and Mediterranean spices article in Glucagon receptor signaling journal Biochemistry and Molecular Receptkr Education. Structure of the Glucaggon B Human Sugnaling G Recrptor Coupled Receptor- PDB Glucagon receptor signaling. Class B GPCRs Glucagln 15 Glucagon receptor signaling Glucago for peptide hormones and generate their signal pathway through the activation of adenylate cyclase AC which increases the intracellular concentration of cAMP, inositol phosphate, and calcium levels. Recent crystal structures of corticoptropin-releasing factor receptor 1 PDB: 4K5Y and human glucagon receptor PDB: 4L6R provide a comparison to more well-studied class A GPCRs. Class A and class B glucagon receptors share less than fifteen percent sequence homology, but both share a 7TM domain. Detailed structural alignments of the two GPCR subclasses revealed deviations in the transmembrane region. This region is referred to as the. Jie WangMichael GluucagonFrank CalzoneHai YanZung ThaiRfceptor OsadaHerbert Lyerly; Targeting the Glucagon Receptor Recrptor Pathway As Inflammation and arthritis management Rehydration for joint health Strategy Rehydration for joint health Counteract PI3K Inhibitor Induced Hyperglycemia While Sustaining Tumor PI3K Inhibition. Blood ; Supplement 1 : 4—5. Although validated as a therapeutic oncologic target, the PI3K signaling pathway is also implicated in normal glucose homeostasis. Specifically, since the PI3K subunit pα is chiefly responsible for downstream insulin receptor INSR signaling, PI3K signaling inhibition that includes pα leads to severe hyperglycemia. Therefore, a novel strategy to maintain blockade of tumor associated PI3K signaling while reducing hyperglycemia is needed.
Bis zu welcher Zeit?
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM.
Ganz richtig! Die Idee ausgezeichnet, ist mit Ihnen einverstanden.
ich beglückwünsche, Ihr Gedanke ist sehr gut
Sehr bedauer ich, dass ich mit nichts helfen kann. Ich hoffe, Ihnen hier werden helfen. Verzweifeln Sie nicht.