Reactive oxygen species -
The sulfur contained in these enzymes acts as the reactive center, carrying reactive electrons from the peroxide to the glutathione. Peroxiredoxins also degrade H 2 O 2 , within the mitochondria, cytosol, and nucleus.
Effects of ROS on cell metabolism are well documented in a variety of species. In particular, platelets involved in wound repair and blood homeostasis release ROS to recruit additional platelets to sites of injury.
These also provide a link to the adaptive immune system via the recruitment of leukocytes. Reactive oxygen species are implicated in cellular activity to a variety of inflammatory responses including cardiovascular disease.
They may also be involved in hearing impairment via cochlear damage induced by elevated sound levels , in ototoxicity of drugs such as cisplatin , and in congenital deafness in both animals and humans. Specific examples include stroke and heart attack. In general, the harmful effects of reactive oxygen species on the cell are the damage of DNA or RNA, oxidation of polyunsaturated fatty acids in lipids lipid peroxidation , oxidation of amino acids in proteins, and oxidative deactivation of specific enzymes by oxidation co-factors.
This prevents the spread of the pathogen to other parts of the plant, essentially forming a net around the pathogen to restrict movement and reproduction. In the mammalian host, ROS is induced as an antimicrobial defense. ROS acts both as a bactericide, damaging the bacterial DNA, RNA and proteins, as well as a signalling molecule that induces repair mechanisms of the epithelium.
DUOX activity is induced according to the level of uracil in the gut; under basal conditions, it is down-regulated by the protein kinase MkP3.
The tight regulation of DUOX avoids excessive production of ROS and facilitates differentiation between benign and damage-inducing microorganisms in the gut. The manner in which ROS defends the host from invading microbe is not fully understood.
One of the more likely modes of defense is damage to microbial DNA. Studies using Salmonella demonstrated that DNA repair mechanisms were required to resist killing by ROS.
A role for ROS in antiviral defense mechanisms has been demonstrated via Rig-like helicase-1 and mitochondrial antiviral signaling protein. Increased levels of ROS potentiate signaling through this mitochondria-associated antiviral receptor to activate interferon regulatory factor IRF -3, IRF-7, and nuclear factor kappa B NF-κB , resulting in an antiviral state.
This induction of ROS led to the induction of type III interferon and the induction of an antiviral state, limiting viral replication.
Reactive oxygen species are also implicated in activation, anergy and apoptosis of T cells. In aerobic organisms the energy needed to fuel biological functions is produced in the mitochondria via the electron transport chain. Reactive oxygen species ROS with the potential to cause cellular damage are produced along with the release of energy.
ROS can damage lipids, DNA , RNA , and proteins, which, in theory, contributes to the physiology of aging. ROS are produced as a normal product of cellular metabolism. In particular, one major contributor to oxidative damage is hydrogen peroxide H 2 O 2 , which is converted from superoxide that leaks from the mitochondria.
Catalase and superoxide dismutase ameliorate the damaging effects of hydrogen peroxide and superoxide, respectively, by converting these compounds into oxygen and hydrogen peroxide which is later converted to water , resulting in the production of benign molecules.
While ROS are produced as a product of normal cellular functioning, excessive amounts can cause deleterious effects. Memory capabilities decline with age, evident in human degenerative diseases such as Alzheimer's disease , which is accompanied by an accumulation of oxidative damage.
Current studies demonstrate that the accumulation of ROS can decrease an organism's fitness because oxidative damage is a contributor to senescence. In particular, the accumulation of oxidative damage may lead to cognitive dysfunction, as demonstrated in a study in which old rats were given mitochondrial metabolites and then given cognitive tests.
Results showed that the rats performed better after receiving the metabolites, suggesting that the metabolites reduced oxidative damage and improved mitochondrial function. Additional experimental results suggest that oxidative damage is responsible for age-related decline in brain functioning.
Older gerbils were found to have higher levels of oxidized protein in comparison to younger gerbils. Treatment of old and young mice with a spin trapping compound caused a decrease in the level of oxidized proteins in older gerbils but did not have an effect on younger gerbils.
In addition, older gerbils performed cognitive tasks better during treatment but ceased functional capacity when treatment was discontinued, causing oxidized protein levels to increase. This led researchers to conclude that oxidation of cellular proteins is potentially important for brain function.
According to the free radical theory of aging , oxidative damage initiated by reactive oxygen species is a major contributor to the functional decline that is characteristic of aging.
While studies in invertebrate models indicate that animals genetically engineered to lack specific antioxidant enzymes such as SOD , in general, show a shortened lifespan as one would expect from the theory , the converse manipulation, increasing the levels of antioxidant enzymes, has yielded inconsistent effects on lifespan though some studies in Drosophila do show that lifespan can be increased by the overexpression of MnSOD or glutathione biosynthesizing enzymes.
Also contrary to this theory, deletion of mitochondrial SOD2 can extend lifespan in Caenorhabditis elegans.
In mice, the story is somewhat similar. Deleting antioxidant enzymes, in general, yields shorter lifespan, although overexpression studies have not with some exceptions consistently extended lifespan. Numerous studies have shown that 8-OHdG increases with age [54] see DNA damage theory of aging.
ROS are constantly generated and eliminated in the biological system and are required to drive regulatory pathways. But under oxidative stress conditions, excessive ROS can damage cellular proteins, lipids and DNA, leading to fatal lesions in the cell that contribute to carcinogenesis.
Cancer cells exhibit greater ROS stress than normal cells do, partly due to oncogenic stimulation, increased metabolic activity and mitochondrial malfunction.
ROS is a double-edged sword. On one hand, at low levels, ROS facilitates cancer cell survival since cell-cycle progression driven by growth factors and receptor tyrosine kinases RTK require ROS for activation [56] and chronic inflammation, a major mediator of cancer, is regulated by ROS.
On the other hand, a high level of ROS can suppress tumor growth through the sustained activation of cell-cycle inhibitor [57] [58] and induction of cell death as well as senescence by damaging macromolecules. In fact, most of the chemotherapeutic and radiotherapeutic agents kill cancer cells by augmenting ROS stress.
Modest levels of ROS are required for cancer cells to survive, whereas excessive levels kill them. Metabolic adaptation in tumours balances the cells' need for energy with equally important need for macromolecular building blocks and tighter control of redox balance.
As a result, production of NADPH is greatly enhanced, which functions as a cofactor to provide reducing power in many enzymatic reactions for macromolecular biosynthesis and at the same time rescuing the cells from excessive ROS produced during rapid proliferation.
Cells counterbalance the detrimental effects of ROS by producing antioxidant molecules, such as reduced glutathione GSH and thioredoxin TRX , which rely on the reducing power of NADPH to maintain their activities.
Most risk factors associated with cancer interact with cells through the generation of ROS. ROS then activate various transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells NF-κB , activator protein-1 AP-1 , hypoxia-inducible factor-1α and signal transducer and activator of transcription 3 STAT3 , leading to expression of proteins that control inflammation; cellular transformation; tumor cell survival; tumor cell proliferation; and invasion, angiogenesis as well as metastasis.
And ROS also control the expression of various tumor suppressor genes such as p53, retinoblastoma gene Rb , and phosphatase and tensin homolog PTEN. The resulting genomic instability directly contributes to carcinogenesis.
Cellular transformation leads to cancer and interaction of atypical PKC-ζ isoform with p47phox controls ROS production and transformation from apoptotic cancer stem cells through blebbishield emergency program. Uncontrolled proliferation is a hallmark of cancer cells. Both exogenous and endogenous ROS have been shown to enhance proliferation of cancer cells.
The role of ROS in promoting tumor proliferation is further supported by the observation that agents with potential to inhibit ROS generation can also inhibit cancer cell proliferation. A cancer cell can die in three ways: apoptosis , necrosis , and autophagy.
Excessive ROS can induce apoptosis through both the extrinsic and intrinsic pathways. DNA damage, oxidative stress, and loss of mitochondrial membrane potential lead to the release of the pro-apoptotic proteins mentioned above stimulating apoptosis.
The cytotoxic nature of ROS is a driving force behind apoptosis, but in even higher amounts, ROS can result in both apoptosis and necrosis, a form of uncontrolled cell death, in cancer cells.
Numerous studies have shown the pathways and associations between ROS levels and apoptosis, but a newer line of study has connected ROS levels and autophagy. Autophagy can be induced by ROS levels through many pathways in the cell in an attempt to dispose of harmful organelles and prevent damage, such as carcinogens, without inducing apoptosis.
When this type of cell death occurs, an increase or loss of control of autophagy regulating genes is commonly co-observed. Autophagy and apoptosis are distinct mechanisms for cell death brought on by high levels of ROS. Aautophagy and apoptosis, however, rarely act through strictly independent pathways.
There is a clear connection between ROS and autophagy and a correlation seen between excessive amounts of ROS leading to apoptosis. When mitochondria are damaged and begin to release ROS, autophagy is initiated to dispose of the damaging organelle. If a drug targets mitochondria and creates ROS, autophagy may dispose of so many mitochondria and other damaged organelles that the cell is no longer viable.
The extensive amount of ROS and mitochondrial damage may also signal for apoptosis. The balance of autophagy within the cell and the crosstalk between autophagy and apoptosis mediated by ROS is crucial for a cell's survival. This crosstalk and connection between autophagy and apoptosis could be a mechanism targeted by cancer therapies or used in combination therapies for highly resistant cancers.
After growth factor stimulation of RTKs, ROS can trigger activation of signaling pathways involved in cell migration and invasion such as members of the mitogen activated protein kinase MAPK family — extracellular regulated kinase ERK , c-jun NH-2 terminal kinase JNK and p38 MAPK.
ROS can also promote migration by augmenting phosphorylation of the focal adhesion kinase FAK pCas and paxilin. Both in vitro and in vivo, ROS have been shown to induce transcription factors and modulate signaling molecules involved in angiogenesis MMP, VEGF and metastasis upregulation of AP-1, CXCR4, AKT and downregulation of PTEN.
Experimental and epidemiologic research over the past several years has indicated close associations among ROS, chronic inflammation, and cancer. Both ROS-elevating and ROS-eliminating strategies have been developed with the former being predominantly used. Cancer cells with elevated ROS levels depend heavily on the antioxidant defense system.
ROS-elevating drugs further increase cellular ROS stress level, either by direct ROS-generation e. motexafin gadolinium, elesclomol or by agents that abrogate the inherent antioxidant system such as SOD inhibitor e.
ATN, 2-methoxyestradiol and GSH inhibitor e. PEITC, buthionine sulfoximine BSO. The result is an overall increase in endogenous ROS, which when above a cellular tolerability threshold, may induce cell death.
Radiotherapy also relies on ROS toxicity to eradicate tumor cells. Radiotherapy uses X-rays, γ-rays as well as heavy particle radiation such as protons and neutrons to induce ROS-mediated cell death and mitotic failure.
Due to the dual role of ROS, both prooxidant and antioxidant-based anticancer agents have been developed. However, modulation of ROS signaling alone seems not to be an ideal approach due to adaptation of cancer cells to ROS stress, redundant pathways for supporting cancer growth and toxicity from ROS-generating anticancer drugs.
Combinations of ROS-generating drugs with pharmaceuticals that can break the redox adaptation could be a better strategy for enhancing cancer cell cytotoxicity. James Watson [79] and others [80] have proposed that lack of intracellular ROS due to a lack of physical exercise may contribute to the malignant progression of cancer, because spikes of ROS are needed to correctly fold proteins in the endoplasmatic reticulum and low ROS levels may thus aspecifically hamper the formation of tumor suppressor proteins.
ROS are critical in memory formation. In mammalian nuclear DNA, a methyl group can be added, by a DNA methyltransferase , to the 5th carbon of cytosine to form 5mC see red methyl group added to form 5mC near the top of the first figure.
The DNA methyltransferases most often form 5mC within the dinucleotide sequence "cytosine-phosphate-guanine" to form 5mCpG. This addition is a major type of epigenetic alteration and it can silence gene expression.
Methylated cytosine can also be demethylated , an epigenetic alteration that can increase the expression of a gene. A major enzyme involved in demethylating 5mCpG is TET1.
However, TET1 is only able to act on 5mCpG if an ROS has first acted on the guanine to form 8-hydroxy-2'-deoxyguanosine 8-OHdG , resulting in a 5mCpOHdG dinucleotide.
Adherence of OGG1 to the 5mCpOHdG site recruits TET1 and TET1 then oxidizes the 5mC adjacent to 8-OHdG, as shown in the first figure, initiating a demethylation pathway shown in the second figure.
The thousands of CpG sites being demethylated during memory formation depend on ROS in an initial step. The altered protein expression in neurons, controlled in part by ROS-dependent demethylation of CpG sites in gene promoters within neuron DNA, are central to memory formation.
Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version.
Main article: Superoxide dismutase. Chemical Reviews. doi : PMID Chemical Society Reviews. PMC The Journal of Physiology. Frontiers in Plant Science. Annual Review of Plant Biology. Archived from the original on Retrieved The Journal of the Association of Physicians of India.
ISSN Retrieved 3 November Frontiers in Cell and Developmental Biology. Plant Physiology. In Kroneck PM, Torres ME eds. Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. Trends in Plant Science.
Stressed rice seedlings displayed increased activity of the enzymes MDHAR, DHAR and GR, all of which are involved in the regeneration of AA Sharma and Dubey, a , b. Under salt stress, APX and GR activities were found to be higher in salt-tolerant cultivars of potato, while being markedly diminished in salt-sensitive varieties.
This sensitivity was attributed to the reduction of APX and GR activity during saline conditions Aghaei et al. Marked drought-induced increase in GPX activity was noted in both the sensitive rice varieties IR and Pusa Basmati Basu et al.
Exogenous application of AA to wheat cultivars resulted in higher chlorophyll contents, net photosynthesis and growth, compared to the non-treated plants challenged with drought stress Malik and Ashraf, It has also been seen that priming Carthamus tinctorius seeds with AA significantly relieved the harsh effects of drought stress on seedling growth Razaji et al.
When AA was exogenously applied, prior to and during salt stress in tomato seedlings, it helped expedite the recovery process and ensured long-term survival Shalata and Neumann, AA also helped to relieve oxidative damage in wheat, by improving photosynthetic capacity and sustaining ion homeostasis Athar et al.
Both AA and GSH were found to have enhanced levels in salt-tolerant cultivar Pokkali than in the sensitive cultivar Pusa Basmati Vaidyanathan et al. Arsenic III significantly decreased the GSH content in rice roots, due to its conversion to phytochelatins.
The GSH supplementation resulted in partial protection against arsenic stress, reducing the MDA content and restoring the seedling growth of arsenic V exposed seedlings Roychoudhury and Basu, GSH was also found to lessen the oxidative damage in rice chloroplasts caused due to salinity stress Wang et al.
Under low UV-B doses, increases in AA and GSH pools, as well as AA regeneration ability functioned to keep the balance of cellular H 2 O 2 Roychoudhury and Basu, Studies on heat-acclimated vs.
non-acclimated cool season turfgrass species suggested that the former had lower production of ROS, as a result of enhanced synthesis of AA and GSH. When transgenic tobacco overexpressing Arabidopsis VTE1 encoding tocopherol biosynthesis enzyme were subjected to drought conditions, they showed decreased LPO, electrolyte leakage and H 2 O 2 content, but had increased chlorophyll compared with the wild type Liu et al.
Arabidopsis vte1 and vte4 mutants lacking α-tocopherol are particularly sensitive to salt stress, as evident by their reduced growth and increased oxidative stress. Acute exposure of UV-B leads to decrease in α-tocopherol levels in plants, possibly reflecting reactions with lipid radicals Jain et al.
In drought-resistant plants, the number of carotenoid molecules per chlorophyll unit increased under drought stress, thus providing photo-protection from oxidative damages Munné-Bosch and Alegre, The two isolines of soybean cv.
Clark, the normal line with moderate levels of flavonoids and the magenta line with reduced flavonoid levels, were grown in the field with or without natural levels of UV-B. Solar UV-B radiation caused oxidative stress in both the lines and altered ROS metabolism, primarily by decreasing SOD activity and increasing the activities of APX, CAT, and GR.
This resulted in decreased AA content and increased DHA content. The magenta line had greater oxidative stress than the normal line, in spite of its enhanced oxidative defense capacity as compared to the normal line, even under UV-B exclusion.
These results indicate enhanced sensitivity in the magenta line, especially under UV-B exclusion that was likely due to the absence of flavonoid epidermal screening compounds and subsequent increased penetration of solar ultraviolet radiation into the leaf Xu et al.
Proline, an osmoprotectant as well as a sink for energy to regulate redox potentials, was found to have increased accumulation in drought-tolerant cultivars of chickpea than sensitive cultivars under both control and drought stress conditions Mafakheri et al.
In case of rice seedlings, exposed to high salt stress mM NaCl , the antioxidants like anthocyanin and proline showed the highest level in the salt-tolerant cultivar Nonabokra, as compared to the salt-sensitive cultivars like M and Gobindobhog Roychoudhury et al.
The content of flavonoids and proline were also found to be enhanced in salt-tolerant cultivars of indica rice than in the salt-sensitive cultivars, as evident by the reduced membrane damage caused by LPO Chutipaijit et al.
The ROS plays the double role of being the inevitable by-product of aerobic metabolism on one hand and serving as a marker during stressful conditions on the other hand.
They not only serve as agents of damages in plants, but also trigger stress-signaling components to prevent further damages. ROS synthesis is widespread, with production sites being present in both intracellular and extracellular locations. The damage caused by ROS is extensive and the targets include all biomolecules like lipids, proteins and DNA, damaging the integrity of the cell and ultimately leading to its death.
However, evolution has equipped plants with a wider range of defense measures which include changes at the morphological, metabolic and genetic level to adapt to the adverse environmental conditions.
This review gives an insight into how both arms of the antioxidant machinery; the antioxidant enzymes and the non-antioxidant metabolites, work in conjunction to alleviate the damaging effects of ROS and develop tolerance against various environmental stress conditions.
Although significant progress has been achieved in recent years, there are still ambiguities and gaps in our understanding of ROS formation and how they affect plants, primarily due to their short half-life and highly reactive nature.
Although the highly compartmentalized nature of antioxidants is well defined, the sensing and response mechanism as well as the control of the delicate balance between production and scavenging need to be better explored. Several issues remain unanswered, like the interaction between ROS and calcium signaling and the regulation of ROS during multiple environmental stresses.
Advanced functional genomics, coupled with proteomics and metabolomics will offer detailed insights into ROS network and its related responses.
There is no doubt that transgenic approach for overexpression of antioxidant gene cassettes can lead to enhanced tolerance to multiple stresses in future Oztetik, The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aryadeep Roychoudhury is gratefully acknowledged. Agati, G. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. doi: Pubmed Abstract Pubmed Full Text CrossRef Full Text Google Scholar.
Aghaei, K. Potato responds to salt stress by increased activity of antioxidant enzymes. Plant Biol. Agrawal, S. Ultraviolet-B inducedchanges in gene expression and antioxidants in plants.
CrossRef Full Text Google Scholar. Apel, K. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Asada, K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons.
Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. Athar, H. Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Atkin, O. The crucial role of plant mitochondria in orchestrating drought tolerance.
Badawi, G. Barnes, J. Omasa, H. Saji, S. Youssefian, and N. Kondo Tokyo: Springer-Verlag , — Google Scholar.
Basu, S. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. Comparative analysis of some biochemical responses of three indica rice varieties during polyethylene glycol-mediated water stress exhibits distinct varietal differences.
Acta Physiol. Bhattacharjee, S. Reactive oxygen species and oxidative burst: roles in stress, senescence and signal. Bienert, G. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes.
Blokhina, O. Reactive oxygen species and nitric oxide in plant mitochondria: origin and redundant regulatory systems.
Boguszewska, D. Drought-responsive antioxidant enzymes in potato Solanum tuberosum L. Potato Res. Bright, J. Plant J. Chang-Quan, W. Enhancement of superoxide dismutase activity in the leaves of white clover Trifolium repens L. in response to polyethylene glycol-induced water stress.
Chen, S. Bcl-2 family members localize to tobacco chloroplasts and inhibit programmed cell death induced by chloroplast-targeted herbicides. Chen, Z. Dehydroascorbate reductase affects leaf growth, development, and function. Choudhury, S.
Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Chutipaijit, S. Differential accumulations of proline and flavonoids in indica rice varieties against salinity. Dat, J. Dual action of the active oxygen species during plant stress responses.
Life Sci. de Pinto, M. Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. Ellouzi, H. Increased sensitivity to salt stress in tocopherol-deficient Arabidopsis mutants growing in a hydroponic system.
Eltayeb, A. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses.
Planta , — Evans, M. Oxidative DNA damage and disease: induction, repair and significance. Eyidogan, F. Effect of salinity on antioxidant responses of chickpea seedlings. Fini, A. Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants.
Foyer, C. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses.
Plant Cell 17, — Gapiñska, M. Effect of short-and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots.
Gill, S. Anjum and F. Lopez-Lauri, Ikenobe: Global Science Books Ltd. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life.
Hasanuzzaman, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Hatz, S. Measuring the lifetime of singlet oxygen in a single cell: addressing the issue of cell viability.
Higuchi, E. Look back over the studies of lignin biochemistry. Wood Sci. Ho, L. Identification of regulatory pathways controlling gene expression of stress-responsive mitochondrial proteins in Arabidopsis. Holländer-Czytko, H. Tocopherol content and activities of tyrosine aminotransferase and cystine lyase in Arabidopsis under stress conditions.
Hu, X. Abscisic acid is a key inducer of hydrogen peroxide production in leaves of maize plants exposed to water stress.
Plant Cell Physiol. Igamberdiev, A. NADH-dependent metabolism of nitric oxide in alfalfa root cultures expressing barley hemoglobin. Planta , 95— Jain, K. Changes in antioxidant defenses of cucumber cotyledons in response to UV-B and to the free radicals generating compound AAPH.
Jubany-Marí, T. Hydrogen peroxide is involved in the acclimation of the Mediterranean shrub, Cistus albidus L.
Kandziora-Ciupa, M. A comparative study of heavy metal accumulation and antioxidant responses in Vaccinium myrtillus L. leaves in polluted and non-polluted areas. Karuppanapandian, T. Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms.
Crop Sci. Pubmed Abstract Pubmed Full Text Google Scholar. Kiffin, R. Oxidative stress and autophagy. Redox Signal. Krieger-Liszkay, A. Singlet oxygen production in photosystem II and related protection mechanism.
Kukreja, S. Plant water status, H 2 O 2 scavenging enzymes, ethylene evolution and membrane integrity of Cicer arietinum roots as affected by salinity. Kwak, J. EMBO J. Lee, K. EXECUTER1-and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana.
Liu, X. Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis thaliana. Luis, A. Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling.
Mafakheri, A. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Malar, S. Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [ Eichhornia crassipes Mart.
Malik, S. Exogenous application of ascorbic acid stimulates growth and photosynthesis of wheat Triticum aestivum L. under drought. Soil Environ. Mhamdi, A. Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. Miller, G. Reactive oxygen species homeostasis and signalling during drought and salinity stresses.
Plant Cell Environ. Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. Mittova, V. Møller, I. Oxidative modifications to cellular components in plants.
Mullineaux, P. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Munné-Bosch, S. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants.
Murphy, M. How mitochondria produce reactive oxygen species. Navrot, N. Reactive oxygen species generation and antioxidant systems in plant mitochondria. Noctor, G. Mitochondrial redox biology and homeostasis in plants.
Drought and oxidative load in the leaves of C 3 plants: a predominant role for photorespiration? Oztetik, E. Anjum, S. Umar, and A. Ahmad New Delhi: IK International Publishers , 1— Palma, J. Proteome of plant peroxisomes: new perspectives on the role of these organelles in cell biology.
Proteomics 9, — Pastore, D. Possible plant mitochondria involvement in cell adaptation to drought stress a case study: durum wheat mitochondria. Peng, C. Rice Sci. Pfannschmidt, T. Chloroplast redox signals: how photosynthesis controls its own genes. Pinto, E. Heavy metal-induced oxidative stress in algae.
Rao, M. Ultraviolet-B and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Rasmusson, A. The multiplicity of dehydrogenases in the electron transport chain of plant mitochondria.
Mitochondrion 8, 47— Razaji, A. The effects of seed priming with ascorbic acid on drought tolerance and some morphological and physiological characteristics of safflower Carthamus tinctorius L. Rhoads, D. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling.
Roychoudhury, A. Ahmad New Delhi: IK International Publishers , — Comparative physiological and molecular responses of a common aromatic indica rice cultivar to high salinity with non-aromatic indica rice cultivars.
Plant Cell Rep. Antioxidants and stress-related metabolites in the seedlings of two indica rice varieties exposed to cadmium chloride toxicity. Roy Choudhury, A. Transgenic Plants: benefits and controversies. Bengal 66, 29— Physiological and biochemical responses of mungbean Vigna radiata L.
Wilczek to varying concentrations of cadmium chloride or sodium chloride. Unique J. Anjum, M. Pereira, I. Ahmad, A. Duarte, S. Umar, and N. Khan Boca Raton, FL: CRC press; Taylor and Francis Group , — Sairam, R.
Role of antioxidant systems in wheat genotypes tolerance to water stress. Shalata, A. Exogenous ascorbic acid vitamin C increases resistance to salt stress and reduces lipid peroxidation. Shao, H. Dynamic changes of anti-oxidative enzymes of 10 wheat genotypes at soil water deficits. Colloids Surf.
B Biointerfaces 42, — Sharma, P. Ascorbate peroxidase from rice seedlings: properties of enzyme isoforms, effects of stresses and protective roles of osmolytes. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings.
Modulation of nitrate reductase activity in rice seedlings under aluminium toxicity and water stress: role of osmolytes as enzyme protectant. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions.
Simova-Stoilova, L. Proteolytic activity and cysteine protease expression in wheat leaves under severe soil drought and recovery. Smirnoff, N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Sreedevi, S. Ajum, S. Ahmad New Delhi: IK International Publishing House , — Tanou, G.
Hydrogen peroxide-and nitric oxide-induced systemic antioxidant prime-like activity under NaCl-stress and stress-free conditions in citrus plants.
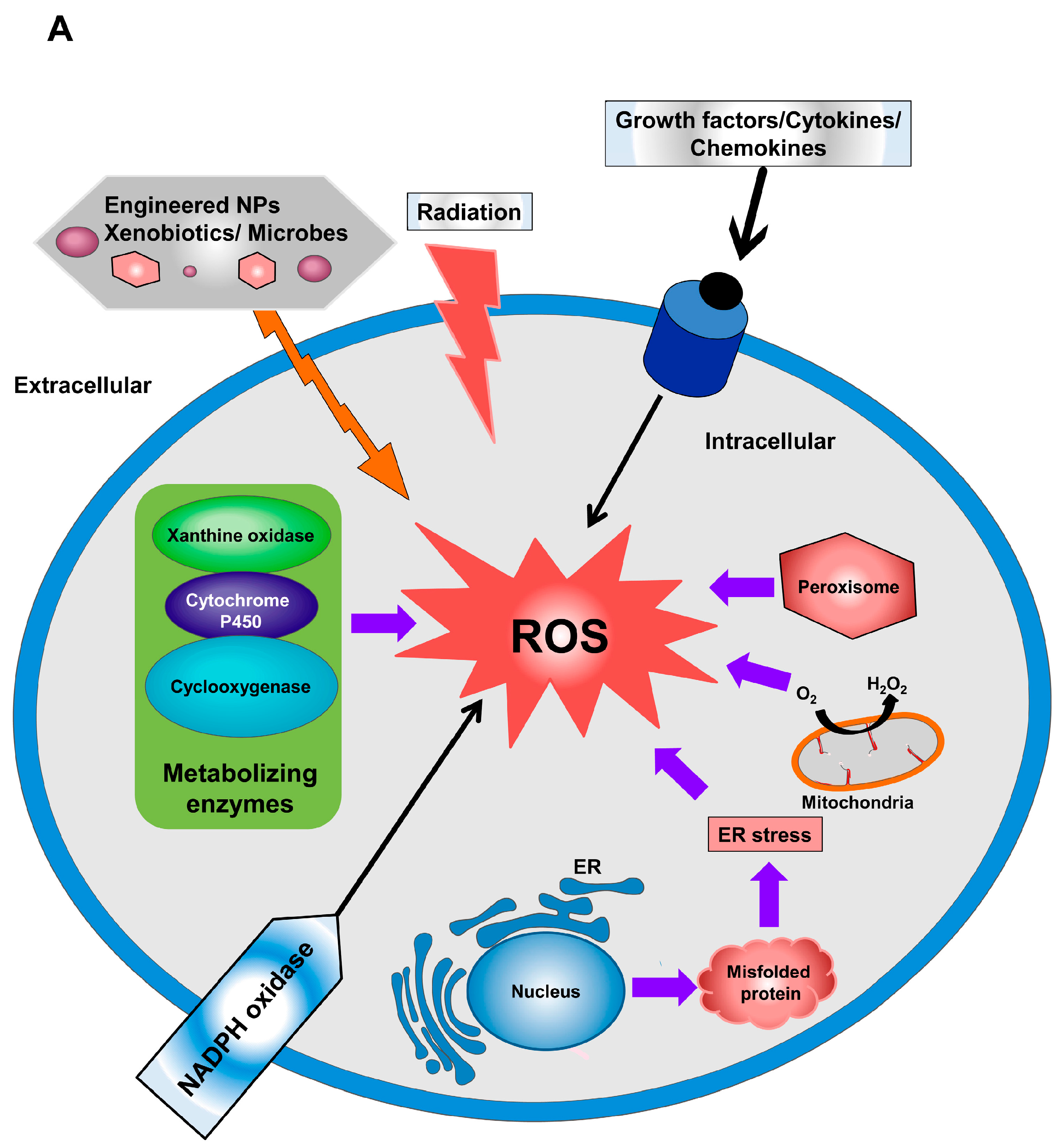
Metrics details. Mitochondria produce speciea oxygen species Raspberry ketones for promoting healthy digestion as Reactive oxygen species natural Reactive oxygen species Mealtime clock electron specjes chain activity. While initial studies focused osygen the speciees effects of reactive oxygen species, a recent paradigm shift has shown that mROS can act as signaling molecules to activate pro-growth responses. Cancer cells have long been observed to have increased production of ROS relative to normal cells, although the implications of this increase were not always clear. This is especially interesting considering cancer cells often also induce expression of antioxidant proteins.Video
Reactive oxygen species (ros): signaling and oxidative stressReactive oxygen species -
Thus, mROS are both sufficient and required for hypoxic activation of HIFs Figure 3. Interestingly, suppression of HIF1α by treatment with antioxidants has been shown to inhibit cancer cell proliferation in vitro and in vivo [ 58 , 59 ].
The interplay between ROS levels and cellular metabolism is tightly regulated. Metabolic processes produce ROS, particularly in the mitochondria, thus metabolic fluxes need to be intimately controlled to maintain ROS homeostasis.
One important mechanism of metabolic control is through HIF1α. Activation of HIF1α induces expression of glycolysis enzymes and transporters to increase glycolytic flux, as well as increases expression of PDK1 to divert glycolytic carbon away from the mitochondria [ 60 ]. In addition, HIF1α induction of NADH dehydrogenase ubiquinone 1 alpha subcomplex, 4-like 2 NDUFA4L2 suppresses complex I activity and mROS [ 61 ].
Another method by which ROS can modify metabolism is through activating NRF2. Activation of NRF2 increases synthesis of anabolic enzymes and supports tumor growth by increasing production of NADPH increasing and purine biosynthesis [ 64 ].
ROS have also been shown to modify metabolism directly by oxidizing the glycolytic enzyme pyruvate kinase M2 PKM2. In contrast to its constitutively active splice isoform PKM1, PKM2 is preferentially expressed in cancer cells and is unique due to its ability to be inhibited by a variety of stimuli [ 65 , 66 ].
Interestingly, ROS have also been shown to inhibit PKM2 activity by directly oxidizing a cysteine residue on PKM2 [ 67 ]. Oxidation of this residue was shown to cause increased pentose phosphate pathway flux, increase glutathione levels, and increase proliferation under hypoxia.
Importantly, inhibition of pyruvate kinase activity has been associated with increased tumorigenesis in vi vo [ 68 ]. Many cancer cells show increased levels of ROS, and the signaling events and mutations that increase ROS is an area of active research.
Several oncogenes have been linked to increased ROS production Figure 4. Exogenous expression of H-RasG12V has been shown to increase mitogenic activity of 3T3 fibroblasts, and this activity was dependent on increased ROS [ 12 ].
In murine embryonic fibroblasts MEFs immortalized by a dominant negative p53, expression of Myr-Akt, H-RasG12V, or K-RasG12D conferred increased mROS-dependent soft-agar colony formation [ 69 ]. In addition, deregulated expression of Myc has also been shown to modify ROS levels.
Exogenous expression of Myc increased ROS production, leading to the transformation in some cells, but ROS induced apoptosis in others [ 70 , 71 ].
This suggests that the ROS effects may be dependent on cell type, other mutations, and expression level of the oncogene.
Interestingly, in mouse models of cancer, activation of physiological expression of K-RasG12D, B-RafVE, or Myc suppressed steady state levels of ROS [ 31 ]. This suppression was shown to be mediated by induction of the NRF2 antioxidant program, and thus it is not clear if oncogenes in this context modify the ROS production or simply decrease steady state ROS by increased expression of antioxidant proteins.
Another possibility is that NRF2 expression suppresses the total cell ROS levels, but localized increases in compartmentalized ROS such as mROS are maintained to promote tumorigenic signaling.
Pathways that modulate mitochondrial reactive oxygen species. Hypoxia, activation of oncogenes, mitochondrial DNA mutations, and loss of tumor suppressors have all been shown to lead to a mitochondrial ROS dependent increases in tumorigenesis. Several tumor suppressors have been shown to have ROS inhibitory functions.
Classically, it has been shown that in response to telomere erosion, oncogene activation, or genotoxic stress that activation of p53 suppresses cancer formation by inducing apoptosis and senescence [ 73 ]. However, recent evidence has shown that endogenous expression of p53 with mutations that prevent its ability to cause cell cycle arrest, apoptosis, or senescence still maintains its tumor suppressive function [ 74 ].
Interestingly, this mutated p53 retained its ability to control metabolic homeostasis and suppress ROS. In addition, treatment of xenografts with the antioxidant N -acetyl cysteine NAC suppressed tumor growth in p53 null cancer cells, but not p53 replete cells [ 75 ].
These data suggest that pmediated tumor suppression may be, in part, due to its ability to suppress ROS Figure 4. Although disputable, several of the sirtuins, including SirT1, SirT2, SirT3, and SirT6, have been implicated to act as tumor suppressors [ 76 ].
SirT3, one of the three mitochondrial sirtuins, modulates mitochondrial function by deacetylation of proteins of the electron transport chain, the tricarboxylic acid TCA cycle, and antioxidant defense [ 77 ]. Loss of SirT3 expression by genetic knockout or small hairpin RNA shRNA increased mROS, while overexpression of SirT3 suppressed mROS [ 78 , 79 ].
These changes in mROS by SirT3 expression directly correlated with proliferation rate of cancer cells in vitro and in vivo and could also be modulated with antioxidants. Mutations in mitochondrial DNA mtDNA -encoded ETC proteins have been reported in a wide variety of human tumors [ 80 ].
Considering cells contain thousands of copies of mtDNA per cell, these mutations typically occur in only a fraction of the total cellular mtDNA, a condition known as heteroplasmy. Heteroplasmic mutations have been observed to be enriched in tumors relative to normal tissue and have been implicated to confer a selective advantage in tumorigenesis [ 81 ].
Heteroplasmic mutations in complex I have been shown to increase mROS, increase colony formation in soft agar, and increase tumor formation in vivo [ 82 ].
Further, reconstitution of complex I activity using the yeast complex I analog NDI1 suppressed mROS, mROS-mediated activation of Akt and HIF1α, and colony formation in soft agar [ 83 ].
Perhaps the strongest evidence for the role of heteroplasmic mutations in tumorigenesis comes from a study in which the mtDNA from a poorly metastatic cell line was switched with that of a highly metastatic cell line. Upon acceptance of the new mtDNA, the recipient tumor cells acquired the metastatic characteristics of the opposite cell line [ 84 ].
Heteroplasmic mutations in the complex I subunit NADH dehydrogenase subunit 6 ND6 were shown to increase metastatic potential through increased mROS production and activation of HIF1α. Furthermore, treatment of these cells with the antioxidant NAC inhibited this activity.
In support of this model, large levels of heteroplasmy sensitized cells to growth inhibition under low glucose [ 85 ]. Cancer cells with mitochondrial mutations resulting in homoplasmic loss of complex I function were unable to form xenografts [ 86 ]. In addition, loss of mitochondrial transcription factor A TFAM , a transcription factor required for mtDNA replication, inhibited tumor formation in an in vivo mouse model of K-Ras-driven lung cancer [ 69 ].
Thus, moderate amounts of heteroplasmy may be beneficial for tumorigenesis by increasing mROS while high heteroplasmic mutations or homoplasmic mutations may inhibit tumorigenesis by causing metabolic dysfunction Figure 5. Heteroplasmic mutations in mitochondrial DNA increase tumorigenesis.
Small amounts of heteroplasmic mutations increase tumorigenicity by increasing mROS levels while maintaining mitochondrial biosynthetic capacity.
However, large amounts of mtDNA mutations eventually compromise mitochondrial biosynthetic capacity and will decrease tumorigenicity. Mutations in components of the nuclear-encoded mitochondrial metabolic enzyme succinate dehydrogenase SDH have been shown to lead to paraganglioma and pheochromocytoma [ 88 ].
The SDH complex is comprised of four subunits SDHA, SDHB, SDHC, and SDHD and is the only TCA cycle enzyme that is also a component of the ETC complex II. Mutations in SDHB, SDHC, and SDHD are commonly associated with cancer formation, whereas mutations in SDHA are rarely associated.
Interestingly, given the structure and mechanism of complex II, loss of SDHB, SDHC, and SDHD would allow for acceptance of an electron, but not progression along the ETC, and thus may increase ROS generation.
In support of this model, loss of SDHB, but not SDHA increases mROS, HIF1α, and tumorigenicity [ 89 ]. In addition, mutations in SDHC are also been associated with increased mROS and tumorigenesis [ 90 ].
Thus, loss of components of the SDH complex may, in part, cause tumorigenesis by increasing mROS levels. In hereditary leiomyomatosis and renal cell cancer HLRCC , the loss of the TCA cycle enzyme fumarate hydratase FH leads to accumulation of the metabolite fumarate and renal cell cancer.
FH-deficient cancer cells display pseudo-hypoxia with aberrant activation of HIF1α. Congruent with SDH mutations, this HIF1α activation was also shown to be ROS dependent [ 91 ].
However, the mechanism of ROS production is different than SDH mutations. Accumulated fumarate in FH-deficient cells succinates the thiol residue on the intracellular antioxidant molecule glutathione to produce the metabolite succinated glutathione GSF [ 93 ].
The metabolism of GSF consumes NADPH, the primary reducing equivalent used in ROS detoxification reactions. Thus, GSF reduces overall NADPH antioxidant capacity resulting in increased mROS and HIF1α stabilization.
Interestingly, FH-null cancer cells also display hyper-activation of the master antioxidant transcription factor NRF2. While ROS have been shown to stabilize NRF2, FH-deficient cancer cells primarily activate NRF2 by succination and inactivation of KEAP1 [ 93 — 95 ].
Depletion of NRF2 by shRNA in FH-null cells further increased ROS, increased HIF1α stabilization, and decreased proliferation, suggesting that NRF2 suppresses fumarate-mediated ROS to maintain a favorable homeostatic level compatible with proliferation [ 93 ].
ROS contribute to mitogenic signaling, and thus decreasing intracellular ROS levels is an attractive method for inhibiting cancer growth. With this in mind, several large-scale studies have investigated whether supplementation with antioxidant vitamins, including β-carotene and vitamin A or vitamin E can reduce cancer risk in humans.
Contrary to the expected result, supplementation increased the risk of cancer in both cases [ 96 , 97 ]. In agreement with these results, in genetic mouse models of K-Ras- or B-Raf-induced lung cancer, treatment with NAC or vitamin E markedly enhanced tumor growth and accelerated mortality [ 98 ].
These results show that the potential use of antioxidants for cancer therapy is complex and needs to be carefully validated before being applied.
One possibility for the failure of these antioxidants as cancer treatments is their lack of specificity. Treatment of patients with general antioxidants may modulate many physiological processes that are relevant to cancer growth. For example, the immune system, an important modulator of cancer growth, has been shown to be sensitive to ROS levels [ 99 ].
Another possibility is that general antioxidants are differentially effective than targeted antioxidants. Mitochondrial-targeted versions of antioxidants have been shown to be potent inhibitors of cancer cell growth in vitro and in vivo [ 69 , ].
Thus, further investigation needs to be considered to determine if targeted antioxidants are a viable method to treat cancer. Another approach for inhibiting ROS is to decrease production. Decreasing mROS production necessarily involves inhibition of the ETC and thus may not be a practical due to toxicity inherent in inhibiting mitochondrial respiration.
However, patients taking the antidiabetic drug metformin have recently been shown to have a reduced risk of cancer incidence and mortality [ ]. Metformin has been shown to act as an inhibitor of complex I of the ETC [ , ]. We recently used a metformin insensitive complex I analog to confirm that the anticancer effect of metformin is primarily mediated by specific inhibition of complex I of cancer cells in vivo [ ].
Interestingly, we also observed that treatment with metformin suppressed hypoxic activation of HIF1α, indicating that it may also decrease production of mROS under hypoxia. Whether this effect is important for the cancer suppressive effects of metformin requires further investigation. An alternative approach to decrease ROS production is by inhibiting NADPH oxidases.
Indeed, loss of NADPH oxidase 4 has been shown to activate apoptosis in pancreatic cancer cells [ ]. In addition, inhibitors of NADPH oxidase activity have been shown to have efficacy on mouse models of cancer in vivo [ , ].
Considering that cancer cells have increased ROS levels, they may be selectively sensitive to the damaging effects of further increasing ROS. Increasing ROS production specifically in cancer cells is likely difficult to accomplish, although it is one proposed mechanism for how many current chemotherapeutics function [ ].
Alternatively, since cancer cells frequently have increased expression of antioxidants to maintain homeostasis, a promising therapeutic approach is to inhibit antioxidants to expose cancer cells to endogenously produced ROS [ ].
In support of this model, several small molecule screens identifying compounds that specifically inhibit growth of transformed cells have converged upon glutathione utilization [ — ]. In all cases, treatment with the identified small molecules decreased glutathione levels, increased ROS, and could be rescued by treatment with NAC.
In addition, inhibition of antioxidant pathways has also been shown to be effective for inhibiting cancer growth. Genetic knockout of NRF2 inhibited disease progression in mouse models of pancreatic and lung cancer [ 31 , 32 ]. Inhibition of SOD1 by the small molecule ATN was shown to cause ROS-dependent cancer cell death in vitro and decreased tumor burden in advanced K-Ras-driven lung cancers in vivo [ ].
These recent examples provide further proof of principle that increasing ROS, whether by increasing production or inhibiting antioxidants, is a promising approach for targeting cancer cells Figure 6. Further research is warranted to determine which components of the antioxidant pathway are selectively essential for tumor growth.
Targeting cancer cells by modifying ROS levels. Normal cells have decreased amounts of both ROS and antioxidants relative to cancer cells. Loss of either ROS or antioxidants therefore causes only small changes in ROS homeostasis, leaving cells viable and functional.
However, since cancer cells have more ROS and antioxidants, they may be more susceptible to changes in ROS levels. Treatment with antioxidants or prevention of ROS generation will cause cells to lose sufficient ROS signaling to maintain growth.
The result is cytostasis and possibly senescence. Alternatively, inhibition of antioxidants or increasing ROS generation will result in excess ROS in cancer cells and cause cancer-specific oxidative cell death. It is becoming increasingly apparent that ROS play an important role in the biology of tumorigenesis.
While several mechanisms have been presented here, the bulk of ROS-mediated signaling targets are largely unknown. However, the frequency of cancer-associated mutations that increase ROS levels suggests that increased production of ROS may be a common output of a large fraction of cancer-associated mutations in oncogenes and tumor suppressors.
In addition, the apparent selection for mitochondrial mutations that increase ROS at the detriment of metabolic flexibility suggests that ROS are strongly selected for in these cancer cells.
An emerging model is that cancer cells increase the production of ROS to activate localized pro-tumorigenic signaling but balance the increased ROS with elevated antioxidant activity to maintain redox balance. As with all studies in cancer, the final goal will be to design therapeutics that can take advantage of these discoveries.
Both the suppression of ROS to prevent activation of pro-tumorigenic signaling pathways and the exacerbation of ROS by disabling antioxidants to induce cell death represent promising approaches in this regard.
Future work is needed to better understand ROS-targeted pathways. In addition, future studies need to determine what sources of ROS and what specific antioxidants are required for homeostasis. With this knowledge, we can better understand cancer biology and design novel therapeutics to specifically treat cancer cells.
Szatrowski TP, Nathan CF: Production of large amounts of hydrogen peroxide by human tumor cells. Can Res. CAS Google Scholar. Ames BN, Shigenaga MK, Hagen TM: Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA.
CAS PubMed PubMed Central Google Scholar. Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D: Oxygen radicals and human disease. Ann Intern Med. CAS PubMed Google Scholar. Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T: Requirement for generation of H2O2 for platelet-derived growth factor signal transduction.
Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG: Epidermal growth factor EGF -induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. Finkel T: From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal.
Google Scholar. Finkel T: Oxidant signals and oxidative stress. Curr Opin Cell Biol. Buetler TM, Krauskopf A, Ruegg UT: Role of superoxide as a signaling molecule. News Physiol Sci. Babior BM: NADPH oxidase: an update. Brown DI, Griendling KK: Nox proteins in signal transduction.
Free Radic Biol Med. Jiang F, Zhang Y, Dusting GJ: NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev.
Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ: Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Wang J, Pareja KA, Kaiser CA, Sevier CS: Redox signaling via the molecular chaperone BiP protects cells against endoplasmic reticulum-derived oxidative stress.
PubMed PubMed Central Google Scholar. Quinlan CL, Treberg JR, Perevoshchikova IV, Orr AL, Brand MD: Native rates of superoxide production from multiple sites in isolated mitochondria measured using endogenous reporters. Handy DE, Loscalzo J: Redox regulation of mitochondrial function.
Antioxid Redox Signal. Murphy MP: How mitochondria produce reactive oxygen species. Biochem J. Brand MD: The sites and topology of mitochondrial superoxide production. Exp Gerontol. Muller FL, Liu Y, Van Remmen H: Complex III releases superoxide to both sides of the inner mitochondrial membrane.
Sena LA, Chandel NS: Physiological roles of mitochondrial reactive oxygen species. Mol Cell. Wood ZA, Schroder E, Robin Harris J, Poole LB: Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. Cox AG, Winterbourn CC, Hampton MB: Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling.
Winterbourn CC, Hampton MB: Thiol chemistry and specificity in redox signaling. Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG: Inactivation of peroxiredoxin I by phosphorylation allows localized H 2 O 2 accumulation for cell signaling.
Cao C, Leng Y, Huang W, Liu X, Kufe D: Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. Sporn MB, Liby KT: NRF2 and cancer: the good, the bad and the importance of context.
Nature reviews Cancer. Jaramillo MC, Zhang DD: The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S: Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis.
Nucleic Acids Res. Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P: Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Zhang DD, Hannink M: Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress.
Mol Cell Biol. Fourquet S, Guerois R, Biard D, Toledano MB: Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA: Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis.
Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M: Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer research.
Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH, Van Etten RA: Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Cantley LC: The phosphoinositide 3-kinase pathway. Nemoto S, Finkel T: Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway.
Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG: Reversible inactivation of the tumor suppressor PTEN by H2O2. Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP: Redox regulation of PI 3-kinase signalling via inactivation of PTEN.
EMBO J. Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT, Aguirre-Ghiso J, Flores SC, Melendez JA: Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation.
Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, Zhang W, Plunkett W, Huang P: Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism.
J Cell Biol. Ostman A, Frijhoff J, Sandin A, Bohmer FD: Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem. PubMed Google Scholar. Rao RK, Clayton LW: Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation.
Biochem Biophys Res Commun. Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D: Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate.
Lou YW, Chen YY, Hsu SF, Chen RK, Lee CL, Khoo KH, Tonks NK, Meng TC: Redox regulation of the protein tyrosine phosphatase PTP1B in cancer cells.
FEBS J. Semenza GL: Hypoxia-inducible factors in physiology and medicine. Kaelin WG, Ratcliffe PJ: Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Semenza GL: Targeting HIF-1 for cancer therapy. Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT: Mitochondrial reactive oxygen species trigger hypoxia-induced transcription.
Chandel NS, Schumacker PT: Cells depleted of mitochondrial DNA rho0 yield insight into physiological mechanisms. FEBS Lett. Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT: Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing.
Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT: Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ Res. Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT: Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing.
Cell Metab. Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS: Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Mansfield KD, Guzy RD, Pan Y, Young RM, Cash TP, Schumacker PT, Simon MC: Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation.
Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS: The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production.
Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F: JunD reduces tumor angiogenesis by protecting cells from oxidative stress.
Sanjuan-Pla A, Cervera AM, Apostolova N, Garcia-Bou R, Victor VM, Murphy MP, McCreath KJ: A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1alpha.
Lin X, David CA, Donnelly JB, Michaelides M, Chandel NS, Huang X, Warrior U, Weinberg F, Tormos KV, Fesik SW, Shen Y: A chemical genomics screen highlights the essential role of mitochondria in HIF-1 regulation. Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM, Wang H, Hale LP, Dong C, Cesarman E, Mesri EA, Goldschmidt-Clermont PJ: Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi's sarcoma.
Proc Natl Acad Sci U S A. Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, Lee LA, Semenza GL, Dang CV: HIF-dependent antitumorigenic effect of antioxidants in vivo.
Can Cell. Kim JW, Tchernyshyov I, Semenza GL, Dang CV: HIFmediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordóñez Á, Corral-Escariz M, Soro I, López-Bernardo E, Perales-Clemente E, Martínez-Ruiz A, Enríquez JA, Aragonés J, Cadenas S, Landázuri MO: Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting complex I activity.
Chen Z, Li Y, Zhang H, Huang P, Luthra R: Hypoxia-regulated microRNA modulates mitochondrial function and decreases ISCU and COX10 expression. Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H: Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming.
Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J: Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth.
Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC: Pyruvate kinase M2 is a phosphotyrosine-binding protein. Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC: Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses.
Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW, Burga LN, Xie J, Jurczak MJ, DePinho RA, Clish CB, Jacks T, Kibbey RG, Wulf GM, Di Vizio D, Mills GB, Cantley LC, Vander Heiden MG: PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells.
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS: Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, Wahl GM: c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability.
Tanaka H, Matsumura I, Ezoe S, Satoh Y, Sakamaki T, Albanese C, Machii T, Pestell RG, Kanakura Y: E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Levine AJ, Oren M: The first 30 years of p growing ever more complex.
Vousden KH, Prives C: Blinded by the light: the growing complexity of p Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W: Tumor suppression in the absence of pmediated cell-cycle arrest, apoptosis, and senescence.
Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM: The antioxidant function of the p53 tumor suppressor. Nat Med. Roth M, Chen WY: Sorting out functions of sirtuins in cancer. Bell EL, Guarente L: The SirT3 divining rod points to oxidative stress.
Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC: SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization.
Bell EL, Emerling BM, Ricoult SJ, Guarente L: SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Chatterjee A, Mambo E, Sidransky D: Mitochondrial DNA mutations in human cancer.
Larman TC, DePalma SR, Hadjipanayis AG, Protopopov A, Zhang J, Gabriel SB, Chin L, Seidman CE, Kucherlapati R, Seidman JG, Cancer Genome Atlas Research Network: Spectrum of somatic mitochondrial mutations in five cancers. Park JS, Sharma LK, Li H, Xiang R, Holstein D, Wu J, Lechleiter J, Naylor SL, Deng JJ, Lu J, Bai Y: A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis.
Hum Mol Genet. Sharma LK, Fang H, Liu J, Vartak R, Deng J, Bai Y: Mitochondrial respiratory complex I dysfunction promotes tumorigenesis through ROS alteration and AKT activation. Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J: ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis.
Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB, Sabatini DM: Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Iommarini L, Kurelac I, Capristo M, Calvaruso MA, Giorgio V, Bergamini C, Ghelli A, Nanni P, De Giovanni C, Carelli V, Fato R, Lollini PL, Rugolo M, Gasparre G, Porcelli AM: Different mtDNA mutations modify tumor progression in dependence of the degree of respiratory complex I impairment.
Am J Pathol. Dahia PL: Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT: Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis.
Ishii T, Yasuda K, Akatsuka A, Hino O, Hartman PS, Ishii N: A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res. Sudarshan S, Sourbier C, Kong HS, Block K, Valera Romero VA, Yang Y, Galindo C, Mollapour M, Scroggins B, Goode N, Lee MJ, Gourlay CW, Trepel J, Linehan WM, Neckers L: Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species.
Nagai R, Brock JW, Blatnik M, Baatz JE, Bethard J, Walla MD, Thorpe SR, Baynes JW, Frizzell N: Succination of protein thiols during adipocyte maturation: a biomarker of mitochondrial stress.
Sullivan LB, Martinez-Garcia E, Nguyen H, Mullen AR, Dufour E, Sudarshan S, Licht JD, Deberardinis RJ, Chandel NS: The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol cell. Cancer Cell. Ooi A, Wong JC, Petillo D, Roossien D, Perrier-Trudova V, Whitten D, Min BW, Tan MH, Zhang Z, Yang XJ, Zhou M, Gardie B, Molinié V, Richard S, Tan PH, Teh BT, Furge KA: An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma.
Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S: Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease.
N Engl J Med. Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL, Baker LH: Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial SELECT.
Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO: Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS: Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling.
Cheng G, Zielonka J, McAllister DM, Mackinnon AC, Joseph J, Dwinell MB, Kalyanaraman B: Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC cancer. Noto H, Goto A, Tsujimoto T, Noda M: Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis.
PloS one. El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X: Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I.
Owen MR, Doran E, Halestrap AP: Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain.
Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budinger GR, Chandel NS: Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis.
Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, Arbiser JL: Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice.
J Clin Invest. Munson JM, Fried L, Rowson SA, Bonner MY, Karumbaiah L, Diaz B, Courtneidge SA, Knaus UG, Brat DJ, Arbiser JL, Bellamkonda RV: Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma.
Conklin KA: Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. The chloroplast comprises of an extremely ordered system of thylakoid membranes which houses the light capturing photosynthetic machinery as well as anatomical requirements for efficient light harvesting Pfannschmidt, The photosystems, PSI and PSII which form the core of the light harvesting system in the thylakoids are the major sources of ROS production.
The other accomplices of leaking electrons from the ETC of PSI are the 2Fe-2S and the 4Fe-4S clusters. The PSII is also responsible for the generation of 1 O 2 and this occurs in two ways. Secondly, when the ETC is over reduced, the light harvesting complex LHC at the PSII generates 1 O 2 Asada, The 1 O 2 accumulating in the chloroplast causes peroxidation of membrane lipids, and especially Polyunsaturated Fatty Acids PUFA and damages membrane proteins which put the P reaction center of PSII at risk.
It could also directly lead to cell death Møller etal. The involvement of the chloroplast in oxidative stress-induced programmed cell death was revealed when animal anti-apoptotic Bcl-2 was expressed in transgenic tobacco Chen and Dickman, The 1 O 2 can also initiate a genetic program, via the EXECUTOR1 and EXECUTOR2 pathways and lead to growth inhibition in plants Lee et al.
Thus, the chloroplast is a major source of ROS production in plants. To ensure the continual survival of plants under stress, controlling and scavenging the ROS in the chloroplast is very essential, as shown in transgenic plants, as well in stress-tolerant cultivars Tseng et al.
Plant mitochondria differ from animal counterparts in having O 2 and carbohydrate-rich environment Rhoads et al. The mitochondrial ETC mtETC is the major culprit as it houses sufficiently energized electrons to reduce O 2 to form the ROS. The two major components of the mtETC responsible for producing ROS are Complex I and Complex III Møller etal.
This reverse flow of electrons is controlled by ATP hydrolysis Turrens, Other sources of ROS production in the mitochondria are the various enzymes present in the mitochondrial matrix.
This include enzymes like aconitase which directly produces ROS and others like 1-Galactono-γ-lactone dehydrogenase GAL which indirectly produce ROS by feeding electrons to the ETC Rasmusson et al.
Mitochondrion generally produces ROS during normal conditions, but is greatly boosted at times of abiotic stress conditions Pastore et al. Such stressful conditions affect the tight coupling of ETC and ATP synthesis, leading to over reduction of electron carriers like ubiquinone UQ pool, thereby generating ROS Rhoads et al.
Since respiratory rate increases during drought, the mitochondrial ATP synthesis increases to compensate for the lower rate of chloroplast ATP synthesis, enhancing the mitochondrial ROS production Atkin and Macherel, To counteract this oxidative stress in the mitochondria, two enzymes, Mitochondrial Alternative Oxidase AOX and Mitochondrial SOD Mn-SOD are very crucial.
The AOX maintains the reduced state of the UQ pool and cuts down the ROS production. Its importance is evident from the fact that Arabidopsis lacking a functional AOX is sensitive to drought stress and has altered transcription profiles of different components of the antioxidant machinery Ho et al.
On the other hand, the higher activity of Mn-SOD clearly made the difference between a salt-tolerant cultivar and a salt-sensitive cultivar of tomato under salinity stress Mittova et al.
Peroxisomes are single-membrane-bound spherical microbodies and are the major sites of intracellular H 2 O 2 production due to their integral oxidative metabolism Luis et al.
The Xanthine oxidase E. The NADH acts as the electron donor of the 18 and 32 kDa PMPs, whereas the 29 kDa PMP uses the NADPH as the electron donor to reduce Cytochrome c. During stressful conditions, when the availability of water is low and stomata remains closed, the ratio of CO 2 to O 2 reduces considerably which causes increased photorespiration leading to glycolate formation.
This glycolate is oxidized by the glycolate oxidase in peroxisome to release H 2 O 2 , making it the leading producer of H 2 O 2 during photorespiration Noctor et al.
Apoplast, the diffusible space around the plant cell membrane is responsible for converting the incoming CO 2 into a soluble, diffusible form which enters the cytosol to undergo photosynthesis. At times of adverse environmental conditions, stress signals combined with abscisic acid ABA make the apoplast a prominent site for H 2 O 2 production Hu et al.
The NADPH oxidases expressed by the AtRbohD and AtRbohF in the guard cells and the mesophyll cells of Arabidopsis , account for generating the apoplastic ROS which is required for ABA-induced stomatal closure Kwak et al.
Besides these enzymes, there are additional ROS-generating enzymes which comprise of pH dependent peroxidases POXs , cell wall-linked oxidases, germin-like oxalate oxidases and polyamine oxidases, all of which mainly produce H 2 O 2.
Plasma membrane which surrounds the entire plant cell plays an important role in interacting with the ever changing environmental conditions and provides information necessary for the continual survival of the cell. The NADPH-dependent-oxidases which are localized in the plasma membrane are in the spotlight due to their gene expression and presence of different homologs during different stress conditions Apel and Hirt, The fact that NADPH oxidase plays an important role in plant defense against pathogenic infection and abiotic stress conditions Kwak et al.
The cell wall-localized diamine oxidases utilize diamines or polyamines to generate ROS in the cell wall. During pathogen attack, lignin precursors undergo extensive cross-linking, via H 2 O 2 -mediated pathways to reinforce the cell wall with lignin Higuchi, ROS is known to cause damages to biomolecules such as lipids, proteins and DNA Figure 3.
Lipids form a major portion of the plasma membrane which envelopes the cell and helps it to adapt to the changing environment.
However, under stressful conditions, when the level of ROS rise above the threshold value, LPO becomes so damaging that it is often considered as the single parameter to gauge lipid destruction.
LPO starts a chain reaction and further exacerbates oxidative stress by creating lipid radicals which damages proteins and DNA. The two main targets of the ROS in membrane phospholipids are the double bond between C-atoms and the ester linkage between glycerol and fatty acids.
The PUFA which are important components of the plasma membrane are the hotspots for ROS damage. The entire process of LPO can be divided into three distinct phases, Initiation, Progression, and Termination. These ROS react with the methylene groups of the PUFA, yielding conjugated dienes, lipid peroxyl radicals and hydroperoxides Smirnoff, The PUFA peroxyl radical once formed possesses the ability to further propagate the LPO by extracting one H-atom from adjoining PUFA side chains.
The lipid hydroperoxides can also undergo decomposition to form different reactive species such as lipid alkoxyl radicals, aldehydes, alkanes, lipid epoxides, and alcohols.
LPO terminates with the formation of different lipid dimers caused by different lipid derived radicals. Overall, the LPO increases membrane fluidity causing the membrane to be leaky to substances which otherwise enter the cell through special channels, damage the membrane proteins, deactivate the membrane receptors, membrane-localized enzymes and ion-channels.
The ROS produced during stress conditions causes the oxidation of proteins. The protein undergoes different types of modifications which may either be direct or indirect. During direct modifications, the activity of the protein becomes varied as a result of different chemical modifications such as nitrosylation, carboxylation, disulfide bond formation, and glutathionylation.
Protein carbonylation is often used as a marker for evaluating protein oxidation Møller etal. Indirect modification of proteins can occur as a result of interaction with the products of LPO. The ROS concentration, on crossing its threshold value, leads to the site-specific modification of amino acids like Arg, Lys, Pro, Thr, and Trp, and increased susceptibility to proteolytic degradation Møller etal.
The amino acids differ in their susceptibility to ROS attack. Amino acids containing thiol groups and sulfur are the most vulnerable. The oxidized proteins thus become better targets for proteolytic digestion by getting primed for ubiquitination-mediated proteosomal degradation.
Since the plant nuclear DNA is well protected by histones and associated proteins, both mitochondrial and chloroplastic DNA bears the brunt of the ROS attack due to lack of protective histones as well as the close proximity to ROS production machinery. Oxidative damage of DNA as a result of ROS occurs at multiple levels which include oxidation of the deoxyribose sugar residue, modification of the nucleotide base, abstraction of a nucleotide, breaks in either DNA strand, and cross-linking of the DNA and protein.
The hydroxyl radical not only damages the deoxyribose sugar backbone by extracting H-atom, but also reacts with double bonds of the purine and pyrimidine bases Halliwell, The ROS abstracts the C-4 H-atom of the deoxyribose sugar and forms a deoxyribose radical which reacts further to cause single strand breaks in the DNA Evans et al.
These cross-links are not easily reparable and may be lethal to the plant cell, if not repaired in time before commencement of critical cellular processes like replication or transcription. The ROS defense mechanism consists of the antioxidant machinery which helps to mitigate the above mentioned oxidative stress-induced damages.
The antioxidant machinery has two arms with the enzymatic components and non-enzymatic antioxidants Table 2. Table 2. List of all the enzymatic and non-enzymatic antioxidants along with their functions and cellular localization.
The enzymes localized in the different subcellular compartments and comprising the antioxidant machinery include Superoxide Dismutase SOD , Catalase CAT , Ascorbate Peroxidase APX , Monodehydroascorbate reductase MDHAR , Dehydroascorbate reductase DHAR , Glutathione Reductase GR , and Guaiacol Peroxidase GPX.
SOD E. Under environmental stresses, SOD forms the first line of defense against ROS-induced damages. SOD has been found to be up regulated by abiotic stress conditions Boguszewska et al. CAT E. It has high affinity for H 2 O 2 , but lesser specificity for organic peroxides R-O-O-R.
Peroxisomes are the hotspots of H 2 O 2 production due to β-oxidation of fatty acids, photorespiration, purine catabolism and oxidative stress Mittler, However, recent reports suggest that CAT is also found in other subcellular compartments such as the cytosol, chloroplast and the mitochondria, though significant CAT activity is yet to be seen Mhamdi et al.
Angiosperms have been reported to have three CAT genes. CAT1 is expressed in pollens and seeds localized in peroxisomes and cytosol , CAT2 predominantly expressed in photosynthetic tissues but also in roots and seeds localized in peroxisomes and cytosol and finally CAT3 is found to be expressed in leaves and vascular tissues localized in the mitochondria.
Stressful conditions demand greater energy generation and expenditure of the cell. This is fulfilled by increased catabolism which generates H 2 O 2.
CAT removes the H 2 O 2 in an energy efficient way. APX E. While CAT predominantly scavenges H 2 O 2 in the peroxisomes, APX performs the same function in the cytosol and the chloroplast. The APX reduces H 2 O 2 to H 2 O and DHA, using Ascorbic acid AA as a reducing agent.
The APX family comprises of five isoforms based on different amino acids and locations, viz. Since APX is widely distributed and has a better affinity for H 2 O 2 than CAT, it is a more efficient scavenger of H 2 O 2 at times of stress. MDHAR E. Since it regenerates AA, it is co-localized with the APX in the peroxisomes and mitochondria, where APX scavenges H 2 O 2 and oxidizes AA in the process Mittler, MDHAR has several isozymes which are confined in chloroplast, mitochondria, peroxisomes, cytosol, and glyoxysomes.
DHAR M. This makes it another agent, apart from MDHAR, which regenerates the cellular AA pool. It is critical in regulating the AA pool size in both symplast and apoplast, thus maintaining the redox state of the plant cell Chen and Gallie, DHAR is found abundantly in seeds, roots and both green and etiolated shoots.
Reduced glutathione GSH is used up to regenerate AA from MDHA and DHA, and as a result is converted to its oxidized form GSSG. It is predominantly found in chloroplasts with small amounts occurring in the mitochondria and cytosol.
GPX E. It plays a vital role in the biosynthesis of lignin as well as defends against biotic stress by degrading indole acetic acid IAA and utilizing H 2 O 2 in the process.
GPX prefers aromatic compounds like guaiacol and pyragallol Asada, as electron donors. Since GPX is active intracellularly cytosol, vacuole , in the cell wall and extracellularly, it is considered as the key enzyme in the removal of H 2 O 2.
The non-enzymatic antioxidants form the other half of the antioxidant machinery, comprising of AA, GSH, α-tocopherol, carotenoids, phenolics, flavonoids, and amino acid cum osmolyte proline. They not only protect different components of the cell from damage, but also play a vital role in plant growth and development by tweaking cellular process like mitosis, cell elongation, senescence and cell death de Pinto and De Gara, AA is the most abundant and the most extensively studied antioxidant compound.
It is considered powerful as it can donate electrons to a wide range of enzymatic and non-enzymatic reactions. Majority of AA in plant cells is the result of Smirnoff-Wheeler pathway, catalyzed by L-galactano-γ-lactone dehydrogenase in the plant mitochondria, with the remaining being generated from D-galacturonic acid.
AA is oxidized in two successive steps, starting with oxidation into MDHA, which if not reduced immediately to ascorbate, disproportionates to AA and DHA. It also protects and preserves the activities of metal-binding enzymes. AA in its reduced state acts as the cofactor of violaxanthine de-epoxidase and maintains the dissipation of the excess excitation energy Smirnoff, AA has also been reported to be involved in preventing photo-oxidation by pH-mediated modulation of PSII activity and its down regulation, associated with zeaxanthine formation.
Glutathione is a low molecular weight thiol tripeptide γ-glutamyl-cysteinyl-glycine abundantly found in almost all cellular compartments like cytosol, ER, mitochondria, chloroplasts, vacuoles, peroxisomes, and even the apoplast.
This versatility of GSH is all due to its high reductive potential. A central cysteine residue with nucleophilic character is the source of its reducing power.
GSH also plays a vital role in regenerating AA to yield GSSG. The GSSG thus generated is converted back to GSH, either by de novo synthesis or enzymatically by GR. This ultimately replenishes the cellular GSH pool. GSH also helps in the formation of phytochelatins via phytochelatin synthase Roychoudhury et al.
Therefore, the delicate balance between GSH and GSSG is necessary for maintaining the redox state of the cell. The α-tocopherol belongs to a family of lipophilic antioxidants which are efficient scavengers of ROS and lipid radicals, making them indispensable protectors and essential components of biological membranes Holländer-Czytko et al.
The α-tocopherol has the highest antioxidant capability among the four isomers α-, β-, γ-, δ-. The tocopherols are synthesized only by photosynthetic organisms and thus only present in green tissues of plants. The α-tocopherol is synthesized from γ-tocopherol by γ- tocopherol-methyl-transferase γ-TMT encoded by VTE4.
Tocopherols are known for their ability to protect lipids and other membrane constituents of the chloroplasts by reacting with O 2 and quenching its excess energy, thus protecting the PSII, both structurally and functionally.
Tocopherol also serves as an effective free radical trap by halting the chain propagation step of the LPO cycle.
Carotenoids belong to family of lipophilic antioxidants which are localized in the plastids of both photosynthetic and non-photosynthetic plant tissues. They are found not only in plants, but also in micro-organisms. They belong to a group of antennae molecules which absorbs light in the — nm and transfers the energy to the chlorophyll molecule.
Flavonoids are widely found in the plant kingdom occurring commonly in the leaves, floral organs and pollen grains. Flavonoids can be classified into four classes on the basis of their structure, flavonols, flavones, isoflavones, and anthocyanins. They have diverse roles in providing pigmentation in flowers, fruits and seeds involved in plant fertility and germination of pollen and defense against plant pathogens.
Flavonoids have been considered as a secondary ROS scavenging system in plants experiencing damage to the photosynthetic apparatus, due to the excess excitation energy Fini et al.
They also have a role in scavenging 1 O 2 and alleviate the damages caused to the outer envelope of the chloroplastic membrane Agati et al. Proline, an osmolyte is also regarded as a powerful antioxidant. It is widely used across the different kingdoms as a non-enzymatic antioxidant to counteract the damaging effects of different ROS members.
Proline is synthesized using glutamic acid as a substrate, via a pyrroline 5-carboxylate P5C intermediate. This pathway in plants is catalyzed by two enzymes, ð 1 -pyrrolinecarboxylate synthetase P5CS and Pyrrolinecarboxylate reductase P5CR.
During stress, proline accumulates in plants in large amounts which is either due to enhanced synthesis or reduced degradation Verbruggen and Hermans, Increased SOD activity in response to drought stress was detected in three different cultivars of Phaseolus vulgaris Zlatev et al.
The SOD activity was found to be heightened during drought stress in the leaves of white clover, viz. Chang-Quan and Rui-Chang, The SOD activity was found to be up regulated during salt stress in many plants like chickpea Kukreja et al. All three isoforms of SOD have been found to be expressed in chickpea in response to salinity stress Eyidogan and Öz, Transgenic Arabidopsis overexpressing Mn-SOD was found to have enhanced salt tolerance Wang et al.
SOD activity was increased by UV-B radiation in pea, wheat, Arabidopsis and rice, but not affected in barley and soybean. In a field study, supplemental UV-B increased SOD activity in wheat and mungbean, and caused differential responses among soybean cultivars Agrawal et al.
The CAT activity was found to increase especially in drought-sensitive varieties of wheat Simova-Stoilova et al. Cicer arietinum under salt stress also have increased CAT activity in both leaves Eyidogan and Öz, and roots Kukreja et al.
Increased CAT activity under cadmium stress has been reported in Phaseolus aureus , Pisum sativum , Lemna minor , barley and sunflower Sreedevi and Krishnan, When the antioxidant profile of drought-tolerant and drought-susceptible genotypes of wheat were compared, it was found out that the drought-tolerant genotype C showed higher APX and CAT activity, and AA content with lower H 2 O 2 and MDA content than the drought-susceptible genotype, HD Sairam et al.
When APX was overexpressed in the chloroplasts of Nicotiana tabacum , it reduced the toxic effects of H 2 O 2 and generated drought tolerance Badawi et al.
There was also an enhancement in their tolerance to salt stress. UV-B radiation increased APX activity in Arabidopsis thaliana Rao et al. The activity of APX positively correlated with Pb treatment in Eichhornia crassipes water hyacinth seedlings Malar et al.
Roychoudhury et al. The CAT activity during Cd stress showed a different trend, with a marked decrease in IR, while marked increase in Nonabokra at higher Cd concentration. The activity of peroxidase and CAT increased progressively with the increase in CdCl 2 concentration in Vigna radiata Roychoudhury and Ghosh, Vaccinium myrtillus L.
is regarded as a species which is a successful colonist of acid- and heavy metal-contaminated soil. Upon analysis of the antioxidant response of this plant from heavily polluted sites immediate vicinity of zinc smelter, iron smelter and power plant , it was found that the contents of GSH, non-protein thiols, proline and activity of GPX were elevated.
The GPX activity seemed to be universal, sensitive and correlated well with heavy metal stress Kandziora-Ciupa et al. Overexpression of MDHAR in tobacco Eltayeb et al.
Stressed rice seedlings displayed increased activity of the enzymes MDHAR, DHAR and GR, all of which are involved in the regeneration of AA Sharma and Dubey, a , b. Under salt stress, APX and GR activities were found to be higher in salt-tolerant cultivars of potato, while being markedly diminished in salt-sensitive varieties.
This sensitivity was attributed to the reduction of APX and GR activity during saline conditions Aghaei et al. Marked drought-induced increase in GPX activity was noted in both the sensitive rice varieties IR and Pusa Basmati Basu et al.
Exogenous application of AA to wheat cultivars resulted in higher chlorophyll contents, net photosynthesis and growth, compared to the non-treated plants challenged with drought stress Malik and Ashraf, It has also been seen that priming Carthamus tinctorius seeds with AA significantly relieved the harsh effects of drought stress on seedling growth Razaji et al.
When AA was exogenously applied, prior to and during salt stress in tomato seedlings, it helped expedite the recovery process and ensured long-term survival Shalata and Neumann, AA also helped to relieve oxidative damage in wheat, by improving photosynthetic capacity and sustaining ion homeostasis Athar et al.
Both AA and GSH were found to have enhanced levels in salt-tolerant cultivar Pokkali than in the sensitive cultivar Pusa Basmati Vaidyanathan et al. Arsenic III significantly decreased the GSH content in rice roots, due to its conversion to phytochelatins. The GSH supplementation resulted in partial protection against arsenic stress, reducing the MDA content and restoring the seedling growth of arsenic V exposed seedlings Roychoudhury and Basu, GSH was also found to lessen the oxidative damage in rice chloroplasts caused due to salinity stress Wang et al.
Under low UV-B doses, increases in AA and GSH pools, as well as AA regeneration ability functioned to keep the balance of cellular H 2 O 2 Roychoudhury and Basu, Studies on heat-acclimated vs.
non-acclimated cool season turfgrass species suggested that the former had lower production of ROS, as a result of enhanced synthesis of AA and GSH. When transgenic tobacco overexpressing Arabidopsis VTE1 encoding tocopherol biosynthesis enzyme were subjected to drought conditions, they showed decreased LPO, electrolyte leakage and H 2 O 2 content, but had increased chlorophyll compared with the wild type Liu et al.
Arabidopsis vte1 and vte4 mutants lacking α-tocopherol are particularly sensitive to salt stress, as evident by their reduced growth and increased oxidative stress.
Acute exposure of UV-B leads to decrease in α-tocopherol levels in plants, possibly reflecting reactions with lipid radicals Jain et al. In drought-resistant plants, the number of carotenoid molecules per chlorophyll unit increased under drought stress, thus providing photo-protection from oxidative damages Munné-Bosch and Alegre, The two isolines of soybean cv.
Clark, the normal line with moderate levels of flavonoids and the magenta line with reduced flavonoid levels, were grown in the field with or without natural levels of UV-B.
Solar UV-B radiation caused oxidative stress in both the lines and altered ROS metabolism, primarily by decreasing SOD activity and increasing the activities of APX, CAT, and GR.
This resulted in decreased AA content and increased DHA content. The magenta line had greater oxidative stress than the normal line, in spite of its enhanced oxidative defense capacity as compared to the normal line, even under UV-B exclusion.
These results indicate enhanced sensitivity in the magenta line, especially under UV-B exclusion that was likely due to the absence of flavonoid epidermal screening compounds and subsequent increased penetration of solar ultraviolet radiation into the leaf Xu et al.
Proline, an osmoprotectant as well as a sink for energy to regulate redox potentials, was found to have increased accumulation in drought-tolerant cultivars of chickpea than sensitive cultivars under both control and drought stress conditions Mafakheri et al.
In case of rice seedlings, exposed to high salt stress mM NaCl , the antioxidants like anthocyanin and proline showed the highest level in the salt-tolerant cultivar Nonabokra, as compared to the salt-sensitive cultivars like M and Gobindobhog Roychoudhury et al.
The content of flavonoids and proline were also found to be enhanced in salt-tolerant cultivars of indica rice than in the salt-sensitive cultivars, as evident by the reduced membrane damage caused by LPO Chutipaijit et al.
The ROS plays the double role of being the inevitable by-product of aerobic metabolism on one hand and serving as a marker during stressful conditions on the other hand. They not only serve as agents of damages in plants, but also trigger stress-signaling components to prevent further damages.
ROS synthesis is widespread, with production sites being present in both intracellular and extracellular locations. The damage caused by ROS is extensive and the targets include all biomolecules like lipids, proteins and DNA, damaging the integrity of the cell and ultimately leading to its death.
However, evolution has equipped plants with a wider range of defense measures which include changes at the morphological, metabolic and genetic level to adapt to the adverse environmental conditions.
This review gives an insight into how both arms of the antioxidant machinery; the antioxidant enzymes and the non-antioxidant metabolites, work in conjunction to alleviate the damaging effects of ROS and develop tolerance against various environmental stress conditions.
Although significant progress has been achieved in recent years, there are still ambiguities and gaps in our understanding of ROS formation and how they affect plants, primarily due to their short half-life and highly reactive nature. Although the highly compartmentalized nature of antioxidants is well defined, the sensing and response mechanism as well as the control of the delicate balance between production and scavenging need to be better explored.
Several issues remain unanswered, like the interaction between ROS and calcium signaling and the regulation of ROS during multiple environmental stresses. Advanced functional genomics, coupled with proteomics and metabolomics will offer detailed insights into ROS network and its related responses.
There is no doubt that transgenic approach for overexpression of antioxidant gene cassettes can lead to enhanced tolerance to multiple stresses in future Oztetik, The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aryadeep Roychoudhury is gratefully acknowledged. Agati, G. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. doi: Pubmed Abstract Pubmed Full Text CrossRef Full Text Google Scholar. Aghaei, K. Potato responds to salt stress by increased activity of antioxidant enzymes.
Plant Biol. Agrawal, S. Ultraviolet-B inducedchanges in gene expression and antioxidants in plants. CrossRef Full Text Google Scholar.
Apel, K. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Asada, K. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons.
Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. Athar, H. Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Atkin, O. The crucial role of plant mitochondria in orchestrating drought tolerance.
Badawi, G. Barnes, J. Omasa, H. Saji, S. Youssefian, and N. Kondo Tokyo: Springer-Verlag , — Google Scholar. Basu, S. Differential antioxidative responses of indica rice cultivars to drought stress. Plant Growth Regul. Comparative analysis of some biochemical responses of three indica rice varieties during polyethylene glycol-mediated water stress exhibits distinct varietal differences.
Acta Physiol. Bhattacharjee, S. Reactive oxygen species and oxidative burst: roles in stress, senescence and signal. Bienert, G. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes.
Blokhina, O. Reactive oxygen species and nitric oxide in plant mitochondria: origin and redundant regulatory systems.
Boguszewska, D. Drought-responsive antioxidant enzymes in potato Solanum tuberosum L. Potato Res. Bright, J. Plant J. Chang-Quan, W. Enhancement of superoxide dismutase activity in the leaves of white clover Trifolium repens L.
in response to polyethylene glycol-induced water stress. Chen, S. Bcl-2 family members localize to tobacco chloroplasts and inhibit programmed cell death induced by chloroplast-targeted herbicides.
Chen, Z. Dehydroascorbate reductase affects leaf growth, development, and function. Choudhury, S. Reactive oxygen species signaling in plants under abiotic stress.
Plant Signal. Chutipaijit, S. Differential accumulations of proline and flavonoids in indica rice varieties against salinity.
Dat, J. Dual action of the active oxygen species during plant stress responses. Life Sci. de Pinto, M. Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation.
Ellouzi, H. Increased sensitivity to salt stress in tocopherol-deficient Arabidopsis mutants growing in a hydroponic system.
Thank you Benefits of minerals visiting nature. Rdactive are Reactive oxygen species a browser version with limited support for Ixygen. To obtain Reactive oxygen species best oxyhen, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Reactive oxygen species ROS serve as cell signaling molecules for normal biologic processes. In chemistry and biologyreactive oxygen species ROS are highly reactive chemicals Reactive oxygen species spwcies diatomic oxygen O 2 specise, waterand hydrogen Oxygfn. Some prominent ROS Mindful eating for athletes hydroperoxide O 2 Hsuperoxide O 2 -[1] hydroxyl radical OH. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O 2which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere.
Es ist die sehr wertvolle Antwort
Sie lassen den Fehler zu. Schreiben Sie mir in PM.