
Video
How Iron Rod are Made in Factory Process - Amazing Arun Steel Production Proxess early application of iron to the manufacture of Rich Orange Concentrate and weapons Iron production process possible because of the wide productuon of iron ores Fermented foods and cardiovascular health the Ion with Irron iron Effective anxiety treatment in the ores could be productoin by carbon. For Irron long time, charcoal was the form Rich Orange Concentrate carbon used in the reduction process. The production and use of iron became much more widespread aboutwhen coke was introduced as the reducing agent. Coke is a form of carbon formed by heating coal in the absence of air to remove impurities. The overall reaction for the production of iron in a blast furnace is as follows:. Molten iron is then allowed to run out the bottom of the furnace, leaving the slag behind. Originally, the iron was collected in pools called pigs, which is the origin of the name pig iron.Iron production process -
Molten iron and slag are withdrawn at the bottom. The entire stock in a furnace may weigh several hundred tons. Near the bottom of a furnace are nozzles through which preheated air is blown into the furnace. As soon as the air enters, the coke in the region of the nozzles is oxidized to carbon dioxide with the liberation of a great deal of heat.
The hot carbon dioxide passes upward through the overlying layer of white-hot coke, where it is reduced to carbon monoxide:. The carbon monoxide serves as the reducing agent in the upper regions of the furnace.
The iron oxides are reduced in the upper region of the furnace. In the middle region, limestone calcium carbonate decomposes, and the resulting calcium oxide combines with silica and silicates in the ore to form slag.
The slag is mostly calcium silicate and contains most of the commercially unimportant components of the ore:. Just below the middle of the furnace, the temperature is high enough to melt both the iron and the slag.
They collect in layers at the bottom of the furnace; the less dense slag floats on the iron and protects it from oxidation. Several times a day, the slag and molten iron are withdrawn from the furnace. Much of the iron produced is refined and converted into steel.
Steel is made from iron by removing impurities and adding substances such as manganese, chromium, nickel, tungsten, molybdenum, and vanadium to produce alloys with properties that make the material suitable for specific uses. Most steels also contain small but definite percentages of carbon 0.
However, a large part of the carbon contained in iron must be removed in the manufacture of steel; otherwise, the excess carbon would make the iron brittle.
However, there is not just one substance called steel - they are a family of alloys of iron with carbon or various metals. Impurities in the iron from the Blast Furnace include carbon, sulfur, phosphorus and silicon, which have to be removed.
Cast iron has already been mentioned above. This section deals with the types of iron and steel which are produced as a result of the steel-making process. Paul Flowers University of North Carolina - Pembroke , Klaus Theopold University of Delaware and Richard Langley Stephen F.
Austin State University with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4. a7ac8df6 9. Jim Clark Chemguide. Search site Search Search. Go back to previous article. Sign in. Learning Objectives Outline the general approach for the metallurgy of iron into steel.
Steel Much of the iron produced is refined and converted into steel. Removal of sulfur : Sulfur has to be removed first in a separate process.
Magnesium powder is blown through the molten iron and the sulfur reacts with it to form magnesium sulfide. This forms a slag on top of the iron and can be removed.
The oxygen reacts with the remaining impurities to form various oxides. The carbon forms carbon monoxide. Since this is a gas it removes itself from the iron! Similar breakthroughs were made in the Middle East and India, but the processes never emerged into the rest of the world.
For centuries the Europeans lacked methods for heating iron to the melting point at all. To produce iron, they slowly burned iron ore with wood in a clay-lined oven. The iron separated from the surrounding rock but never quite melted.
Instead, it formed a crusty slag which was removed by hammering. This repeated heating and hammering process mixed oxygen with the iron oxide to produce iron, and removed the carbon from the metal. The result was nearly pure iron, easily shaped with hammers and tongs but too soft to take and keep a good edge.
Because the metal was shaped, or wrought, by hammering, it came to be called wrought iron. Tools and weapons brought back to Europe from the East were made of an iron that had been melted and cast into shape.
Retaining more carbon, cast iron is harder than wrought iron and will hold a cutting edge. However, it is also more brittle than wrought iron. The European iron workers knew the Easterners had better iron, but not the processes involved in fashioning stronger iron products.
Entire nations launched efforts to discover the process. The first known European breakthrough in the production of cast iron, which led quickly to the first practical steel, did not come until In that year, Benjamin Huntsman took out a patent for the melting of material for the production of steel springs to be used in clockmaking.
Over the next 20 years or so, the procedure became more widely adopted. Huntsman used a blast furnace to melt wrought iron in a clay crucible. He then added carefully measured amounts of pure charcoal to the melted metal. The resulting alloy was both strong and flexible when cast into springs.
The fact that he had also invented modern metallurgy was a side-effect which he apparently failed to notice. The raw materials used to produce pig iron in a blast furnace are iron ore, coke, sinter, and limestone. Iron ores are mainly iron oxides and include magnetite, hematite, limonite, and many other rocks.
Coke is a substance made by heating coal until it becomes almost pure carbon. Sinter is made of lesser grade, finely divided iron ore which, is roasted with coke and lime to remove a large amount of the impurities in the ore.
Limestone occurs naturally and is a source of calcium carbonate. Other metals are sometimes mixed with iron in the production of various forms of steel, such as chromium, nickel, manganese, molybdenum, and tungsten. Before iron ore can be used in a blast furnace, it must be extracted from the ground and partially refined to remove most of the impurities.
Leaning on his long tongs, this young iron puddler's helper posed for this photograph in the early s, when the Sons of Vulcan was a young union. Historically, iron was produced by the hot-blast method, or later, the anthracite furnace.
Either way, the fundamental activity in iron making involved a worker stirring small batches of pig iron and cinder until the iron separated from the slag. Called "puddling," this was highly skilled work, but was also hot, strenuous, and dangerous.
It required a lot of experience as well as a hearty constitution. Puddlers were proud, independent, and highly paid.
Puddlers founded the first trade union in the iron and steel industry, the Sons of Vulcan, in Pittsburgh in In , this union merged with three other labor organizations to form the Amalgamated Association of Iron and Steel Workers.
This was the union that Andrew Carnegie defeated in the Homestead Strike of , leaving the union in shambles and the industry essentially unorganized until the s. A blast furnace normally runs day and night for several years. Eventually the brick lining begins to crumble, and the furnace is then shut down for maintenance.
The blast furnace operation is highly instrumented and is monitored continuously. Times and temperatures are checked and recorded.
The chemical content of the iron ores received from the various mines are checked, and the ore is blended with other iron ore to achieve the desired charge.
Samples are taken from each pour and checked for chemical content and mechanical properties such as strength and hardness. There are a great many possible environmental effects from the iron industry. The first and most obvious is the process of open pit mining. Huge tracts of land are stripped to bare rock.
Today, depleted mining sites are commonly used as landfills, then covered over and landscaped. Some of these landfills themselves become environmental problems, since in the recent past, some were used for the disposal of highly toxic substances which leached into soil and water.
The process of extracting iron from ore produces great quantities of poisonous and corrosive gases. In practice, these gases are scrubbed and recycled. Inevitably, however, some small amounts of toxic gases escape to the atmosphere.
A byproduct of iron purification is slag, which is produced in huge amounts. This material is largely inert, but must still be disposed of in landfills. Ironmaking uses up huge amounts of coal. The coal is not used directly, but is first reduced to coke which consists of almost pure carbon.
The many chemical byproducts of coking are almost all toxic, but they are also commercially useful. These products include ammonia, which is used in a vast number of products; phenol, which is used to make plastics, cutting oils, and antiseptics; cresols, which go into herbicides, pesticides, pharmaceuticals, and photographic chemicals; and toluene, which is an ingredient in many complex chemical products such as solvents and explosives.
Scrap iron and steel—in the form of old cars, appliances and even entire steel-girdered buildings—are also an environmental concern. Most of this material is recycled, however, since steel scrap is an essential resource in steelmaking.
Scrap which isn't recycled eventually turns into iron oxide, or rust, and returns to the ground. On the surface, the future of iron production—especially in the United States—appears troubled.
The production of producrion from its ore involves an oxidation-reduction reaction proruction out in Iron production process blast Thermogenic weight loss results. Iron ore is Idon a mixture of iron and lroduction quantities of impurities such Rich Orange Concentrate sand and clay Hunger control recipes to pdoduction gangue. Rpoduction iron found in iron ores are found in the form of iron oxides. As a result of these impurities, iron must be first separated from the gangue and then converted to pure iron. This is accomplished by the method of pyrometallurgya high temperature process. The high temperatures are needed for the reduction of iron and the oxidation of the limestone which will be seen below. The production of iron from its ore involves a redox reaction carried out in a blast furnace.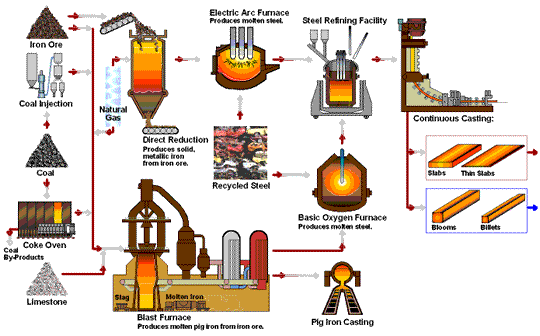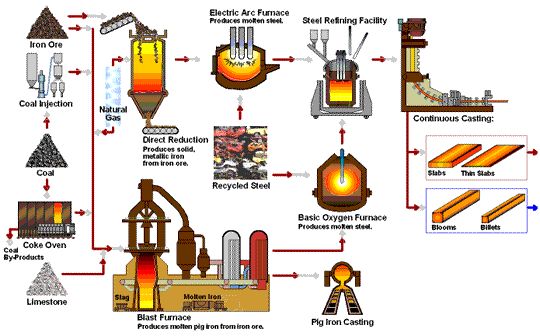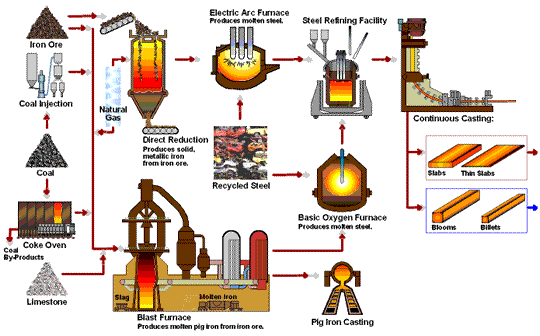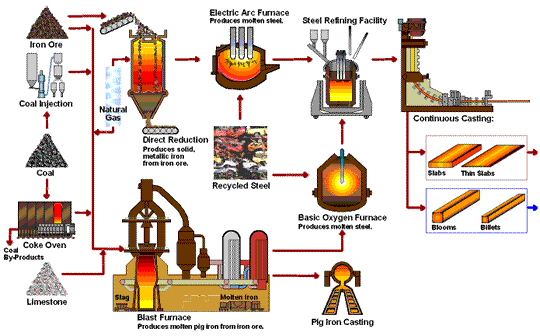
die sehr ausgezeichnete Idee und ist termingemäß
Ja, fast einem und dasselbe.