
Video
AntifungalsAntifungal therapy options -
PubMed Google Scholar. McComiskey KPM, McDonagh A, Tajber L. Isolation of itraconazole nanostructured microparticles via spray drying with rational selection of optimum base for successful reconstitution and compaction.
AAPS PharmSciTech. Salama AH. Spray drying as an advantageous strategy for enhancing pharmaceuticals bioavailability. Drug Deliv Transl Res. Davis M, Walker G. Recent strategies in spray drying for the enhanced bioavailability of poorly water-soluble drugs.
J Control Release. Abuhelwa AY, Foster DJ, Mudge S, Hayes D, Upton RN. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and sporanox capsule formulations in healthy subjects in fed and fasted states.
Serum levels, safety and tolerability of new formulation SUBA-itraconazole prophylaxis in patients with hematological malignancy or undergoing allogeneic stem cell transplantation. J Antimicrob Chemother. Lindsay J, Mudge S, Thompson GR 3rd.
Effects of food and omeprazole on a novel formulation of super bioavailability itraconazole in healthy subjects. Article PubMed PubMed Central Google Scholar. Pharmacokinetics and safety of posaconazole delayed-release tablets for invasive fungal infections.
Clin Pharm. S This paper describes the favorable pharmacokinetic data seen with use of posaconazole delayed-release tablets in pre-approval clinical trials and with published post-approval experience in immunocompromised patients. Article CAS Google Scholar.
Jung DS, Tverdek FP, Kontoyiannis DP. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity.
Cumpston A, Caddell R, Shillingburg A, Lu X, Wen S, Hamadani M, et al. Superior serum concentrations with posaconazole delayed-release tablets compared to suspension formulation in hematological malignancies.
Kozuch JM, Feist A, Yung G, Awdishu L, Hays S, Singer JP, et al. Low dose posaconazole delayed release tablets for fungal prophylaxis in lung transplant recipients. Clin Transpl. Mason MJ, McDaneld PM, Musick WL, Kontoyiannis DP. Serum levels of crushed posaconazole delayed release tablets.
Rezafungin CD , a next-generation echinocandin: a systematic literature review and assessment of possible place in therapy. J Glob Antimicrob Resist. Ibrexafungerp: a novel oral glucan synthase inhibitor. Med Mycol. Bader JC, Bhavnani SM, Andes DR, Ambrose PG.
We can do better: a fresh look at echinocandin dosing. Krishnan BR, James KD, Polowy K, Bryant BJ, Vaidya A, Smith S, et al. CD, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J Antibiot Tokyo. Sandison T, Ong V, Lee J, Thye D.
Safety and pharmacokinetics of CD IV, a novel echinocandin, in healthy adults. Pfaller MA, Messer SA, Rhomberg PR, Castanheira M. Activity of a long-acting echinocandin CD and seven comparator antifungal agents tested against a global collection of contemporary invasive fungal isolates in the SENTRY antifungal surveillance program.
Wiederhold NP, Locke JB, Daruwala P, Bartizal K. Rezafungin CD demonstrates potent in vitro activity against Aspergillus, including azole-resistant Aspergillus fumigatus isolates and cryptic species. Thompson GR, Vazquez J, Soriano A, Skoutelis A, Ostrosky-Zeichner L, Mena K, et al.
Open Forum Infect Dis. Accessed August 12, Wring SA, Randolph R, Park S, Abruzzo G, Chen Q, Flattery A, et al. Preclinical pharmacokinetics and pharmacodynamic target of SCY, a first-in-class orally active antifungal glucan synthesis inhibitor, in murine models of disseminated candidiasis.
Jimenez-Ortigosa C, Perez WB, Angulo D, Borroto-Esoda K, Perlin DS. De Novo Acquisition of Resistance to SCY in Candida glabrata Involves FKS Mutations That both overlap and are distinct from those conferring echinocandin resistance.
Scorneaux B, Angulo D, Borroto-Esoda K, Ghannoum M, Peel M, Wring S. SCY Is fungicidal against Candida species in time-kill studies. Pfaller MA, Messer SA, Rhomberg PR, Borroto-Esoda K, Castanheira M. Differential activity of the oral glucan synthase inhibitor SCY against wild-type and echinocandin-resistant strains of Candida species.
Wring S, Murphy G, Atiee G, Corr C, Hyman M, Willett M, et al. Lack of Impact by SCY, a first-in-class oral fungicidal glucan synthase inhibitor, on the pharmacokinetics of rosiglitazone, a substrate for CYP 2C8, supports the low risk for clinically relevant metabolic drug-drug interactions.
J Clin Pharmacol. Clinical pharmacokinetics and drug-drug interaction potential for coadministered SCY, an oral fungicidal glucan synthase inhibitor, and tacrolimus. Clin Pharmacol Drug Dev. Pappas P, Pullman J, Thompson G, Spec A, Tobin E, Vazquez J et al. A prospective, phase 2, multicentre, open-label, randomized, comparative study to estimate the safety, tolerability, pharmacokinetics, and efficacy of oral SCY vs standard-of-care following initial intravenous echinocandin therapy in the treatment of invasive candidiasis including candidaemia in hospitalized non-neutropenic adults mycoses study group ECCMID, ; Vienna, Austria.
Open-label study to evaluate efficacy and safety of oral ibrexafungerp SCY in Patients With Refractory or Intolerant Fungal Diseases FURI.
United States National Library of Medicine, Bethesda, MD. Angulo D, Cornely OAO-Z L, Miller R, Spec A, Thompson GR, Walsh TJ, et al. Interim Analysis of a phase 3 open-label study to evaluate the efficacy and safety of oral ibrexafungerp Formerly SCY in patients with refractory or intolerant fungal diseases FURI.
San Francisco: ASM Microbe; Google Scholar. Drew RH, Ashley ED, Benjamin DK, Daris RD, Palmer SM, Perfect JR. Comparative safety of amphotericin B lipid complex and amphotericin B deoxycholate as aerosolized antifungal prophylaxis in lung-transplant recipients. Walraven CJ, Lee SA. Antifungal lock therapy.
Le J, Ashley ED, Neuhauser MM, Brown J, Gentry C, Klepser ME, et al. Consensus summary of aerosolized antimicrobial agents: application of guideline criteria. Insights from the Society of Infectious Diseases Pharmacists.
Le J, Schiller DS. Aerosolized delivery of antifungal agents. Curr Fungal Infect Rep. Monforte V, Roman A, Gavalda J, Bravo C, Tenorio L, Ferrer A, et al.
Nebulized amphotericin B prophylaxis for Aspergillus infection in lung transplantation: study of risk factors. J Heart Lung Transplant. Borro JM, Sole A, de la Torre M, Pastor A, Fernandez R, Saura A, et al. Efficiency and safety of inhaled amphotericin B lipid complex Abelcet in the prophylaxis of invasive fungal infections following lung transplantation.
Transplant Proc. Monforte V, Ussetti P, Gavalda J, Bravo C, Laporta R, Len O, et al. Feasibility, tolerability, and outcomes of nebulized liposomal amphotericin B for Aspergillus infection prevention in lung transplantation.
Peghin M, Monforte V, Martin-Gomez MT, Ruiz-Camps I, Berastegui C, Saez B, et al. infection in lung transplantation. Transpl Int. Sanmartin E, Morales P, Monte E, Vicente R. A comparision of several formats of amphotericin B as an inhaled antifungal prophylaxis. Rijnders BJ, Cornelissen JJ, Slobbe L, Becker MJ, Doorduijn JK, Hop WC, et al.
Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial.
Alexander BD, Dodds Ashley ES, Addison RM, Alspaugh JA, Chao NJ, Perfect JR. Non-comparative evaluation of the safety of aerosolized amphotericin B lipid complex in patients undergoing allogeneic hematopoietic stem cell transplantation.
Schwartz S, Behre G, Heinemann V, Wandt H, Schilling E, Arning M, et al. Aerosolized amphotericin B inhalations as prophylaxis of invasive aspergillus infections during prolonged neutropenia: results of a prospective randomized multicenter trial.
CAS PubMed Google Scholar. Hilberg O, Andersen CU, Henning O, Lundby T, Mortensen J, Bendstrup E. Remarkably efficient inhaled antifungal monotherapy for invasive pulmonary aspergillosis. Eur Respir J. Tolman JA, Nelson NA, Bosselmann S, Peters JI, Coalson JJ, Wiederhold NP, et al.
Dose tolerability of chronically inhaled voriconazole solution in rodents. Int J Pharm. Tolman JA, Wiederhold NP, McConville JT, Najvar LK, Bocanegra R, Peters JI, et al.
Inhaled voriconazole for prevention of invasive pulmonary aspergillosis. Arora S, Haghi M, Loo CY, Traini D, Young PM, Jain S. Development of an inhaled controlled release voriconazole dry powder formulation for the treatment of respiratory fungal infection.
Mol Pharm. Vallabhaneni S, Cleveland AA, Farley MM, Harrison LH, Schaffner W, Beldavs ZG, et al. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: data from a large multisite population-based candidemia surveillance program, — Rivero-Menendez O, Alastruey-Izquierdo A, Mellado E, Cuenca-Estrella M.
Triazole resistance in Aspergillus spp. J Fungi Basel. Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of Aspergillosis: update by the Infectious Diseases Society of America.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: update by the Infectious Diseases Society of America. Lockhart SR, Ghannoum MA, Alexander BD.
Establishment and use of epidemiological cutoff values for molds and yeasts by use of the clinical and laboratory standards institute M57 standard. J Clin Microbiol. Andes DR, Ghannoum MA, Mukherjee PK, Kovanda LL, Lu Q, Jones ME, et al.
Outcomes by MIC values for patients treated with isavuconazole or voriconazole for invasive Aspergillosis in the phase 3 SECURE and VITAL trials.
Clinical and Laboratory Standards Institute. M epidemiological cutoff values for antifungal susceptibility testing, 2nd ed. Wayne, PA. European Union Committee on Antimicrobial Susceptibility Testing.
MIC and zone diameter distributions and ECOFFs. Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring TDM of antifungal agents: guidelines from the British Society for Medical Mycology. Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O.
Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Troke PF, Hockey HP, Hope WW.
Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Wang T, Xie J, Wang Y, Zheng X, Lei J, Wang X, et al. Pharmacokinetic and pharmacodynamic properties of oral voriconazole in patients with invasive fungal infections.
Dekkers BGJ, Bakker M, van der Elst KCM, Sturkenboom MGG, Veringa A, Span LFR, et al. Therapeutic drug monitoring of posaconazole: an update. Stamm AM, Diasio RB, Dismukes WE, Shadomy S, Cloud GA, Bowles CA, et al. Toxicity of amphotericin B plus flucytosine in patients with cryptococcal meningitis.
Am J Med. Normark S, Schonebeck J. In vitro studies of 5-fluorocytosine resistance in Candida albicans and Torulopsis glabrata. Andes D, Kovanda L, Desai A, Kitt T, Zhao M, Walsh TJ.
Isavuconazole concentration in real-world practice: consistency with results from clinical trials. Furfaro E, Signori A, Di Grazia C, Dominietto A, Raiola AM, Aquino S, et al.
Serial monitoring of isavuconazole blood levels during prolonged antifungal therapy. Perlin DS. Echinocandin resistance in Candida. Huang AM, Nagel JL, Crass RL. Combination therapy for the treatment of mucormycosis: examining the evidence.
Current Fungal Infection Reports. Scheven M, Scheven C, Hahn K, Senf A. Post-antibiotic effect and post-expositional polyene antagonism of azole antifungal agents in Candida albicans : dependence on substance lipophilia.
Sugar AM, Liu XP. Interactions of itraconazole with amphotericin B in the treatment of murine invasive candidiasis. J Infect Dis. Day JN, Chau TT, Lalloo DG. Combination antifungal therapy for cryptococcal meningitis. Shadomy S, Wagner G, Espinel-Ingroff E, Davis BA. In vitro studies with combinations of 5-fluorocytosine and amphotericin B.
Polak A. Synergism of polyene antibiotics with 5-fluorocytosine. Barchiesi F, Arzeni D, Caselli F, Scalise G. Primary resistance to flucytosine among clinical isolates of Candida spp. Louie A, Liu W, Miller DA, Sucke AC, Liu QF, Drusano GL, et al. Efficacies of high-dose fluconazole plus amphotericin B and high-dose fluconazole plus 5-fluorocytosine versus amphotericin B, fluconazole, and 5-fluorocytosine monotherapies in treatment of experimental endocarditis, endophthalmitis, and pyelonephritis due to Candida albicans.
Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis Download references. Department of Pharmacy, University of Kentucky HealthCare, S.
Limestone, Lexington, KY, , USA. Sarah E. Dawson PharmD, MBA, BCPS. You can also search for this author in PubMed Google Scholar. Correspondence to Sarah E.
Cotner PharmD, BCPS. Occasionally, the fungi spread hematogenously, causing extrapulmonary read more , and paracoccidioidomycosis Paracoccidioidomycosis Paracoccidioidomycosis is progressive mycosis of the lungs, skin, mucous membranes, lymph nodes, and internal organs caused by Paracoccidioides brasiliensis.
Symptoms are skin ulcers It is also effective for chronic pulmonary aspergillosis, coccidioidomycosis Coccidioidomycosis Coccidioidomycosis is caused by the fungi Coccidioides immitis and C. posadasii ; it usually occurs as an acute, benign, asymptomatic or self-limited respiratory infection.
read more , and certain types of chromoblastomycosis Chromoblastomycosis Chromoblastomycosis is a specific type of cutaneous infection caused by one of several species of dematiaceous pigmented fungi. Symptoms are ulcerating nodules on exposed body parts.
Despite poor CSF penetration, itraconazole can be used to treat some types of fungal meningitis, but it is not the drug of choice. Because of its high lipid solubility and protein binding, itraconazole blood levels tend to be low, but tissue levels are typically high. Drug levels are negligible in urine and CSF.
Use of itraconazole has declined as use of voriconazole and posaconazole has increased. Other reported adverse effects include allergic rash, hepatitis, and hallucinations. Food and Drug Administration boxed warning for heart failure has been issued.
Drug and food interactions can be significant. When the capsule form is used, acidic drinks eg, cola, acidic fruit juices or foods especially high-fat foods improve absorption of itraconazole from the GI tract. However, absorption may be reduced if itraconazole is taken with prescription or over-the-counter medications used to lower gastric acidity.
Several medications, including rifampin , rifabutin , didanosine , phenytoin , and carbamazepine , may decrease serum itraconazole levels. Itraconazole also inhibits metabolic degradation of other medications, elevating blood levels with potentially serious consequences.
Serious, even fatal cardiac arrhythmias may occur if itraconazole is used with cisapride not available in the United States or some antihistamines eg, terfenadine, astemizole, perhaps loratadine. Rhabdomyolysis has been associated with itraconazole -induced elevations in blood levels of cyclosporine or statins.
Itraconazole may increase the serum concentration of certain medications eg, tacrolimus , warfarin , digoxin and therapeutic drug monitoring is recommended when these medications are used with itraconazole.
A new formulation of itraconazole SUBA- itraconazole , for SUper BioAvailable has improved bioavailability without the need for an acidic environment in the stomach. SUBA- itraconazole is taken with food and can be used to treat histoplasmosis, blastomycosis, and aspergillosis.
Its dosage is different from other forms of itraconazole. This broad-spectrum triazole is available as a tablet and an IV formulation. It is considered the treatment of choice for Aspergillus infections aspergillosis Aspergillosis Aspergillosis is an opportunistic infection that usually affects the lower respiratory tract and is caused by inhaling spores of the filamentous fungus Aspergillus , commonly present in read more in immunocompetent and immunocompromised hosts.
Voriconazole can also be used to treat Scedosporium apiospermum and Fusarium infections. Additionally, this medication is effective in candidal esophagitis and invasive candidiasis Candidiasis Candidiasis is infection by Candida species most often C.
albicans , manifested by mucocutaneous lesions, fungemia, and sometimes focal infection of multiple sites. Symptoms depend read more , although it is not usually considered a first-line treatment; it has activity against a broader spectrum of Candida species than does fluconazole.
Adverse effects that must be monitored for include hepatotoxicity, visual disturbances common , hallucinations, and dermatologic reactions eg, photosensitivity. Voriconazole can prolong the QT interval. Drug interactions are numerous, notably with certain immunosuppressants used after organ transplantation.
The triazole posaconazole is available as an oral suspension, a tablet, and an IV formulation. Delayed-release tablets are the preferred formulation because of improved oral bioavailability. This drug is highly active against yeasts and molds and effectively treats various opportunistic mold infections, such as those due to dematiaceous dark-walled fungi eg, Cladophialophora species.
It is effective against many of the species that cause mucormycosis Mucormycosis Mucormycosis refers to infection caused by diverse fungal organisms in the order Mucorales, including those in the genera Rhizopus , Rhizomucor , and Mucor.
Symptoms of rhinocerebral Posaconazole can also be used as antifungal prophylaxis in patients with neutropenia with hematologic malignancies and in bone marrow transplant recipients. Adverse effects of posaconazole , as for other triazoles, include a prolonged QT interval and hepatitis.
Drug interactions occur with many medications, including rifabutin , rifampin , statins, and various immunosuppressants. Isavuconazonium is a broad-spectrum triazole for the treatment of aspergillosis Aspergillosis Aspergillosis is an opportunistic infection that usually affects the lower respiratory tract and is caused by inhaling spores of the filamentous fungus Aspergillus , commonly present in read more and mucormycosis Mucormycosis Mucormycosis refers to infection caused by diverse fungal organisms in the order Mucorales, including those in the genera Rhizopus , Rhizomucor , and Mucor.
It is available as an IV formulation as well as an oral capsule. No drug level monitoring is required. Adverse effects of isavuconazonium include GI upset and hepatitis; the QT interval may decrease. Oteseconazole is an oral, novel azole antifungal that is used for the treatment of recurrent vulvovaginal candidiasis.
Adverse effects of oteseconazole include headache and nausea. Drug interactions occur with rosuvastatin. Echinocandins are water-soluble lipopeptides that inhibit glucan synthase. They are available only in an IV formulation. Their mechanism of action is unique among antifungals; echinocandins target the fungal cell wall, making them attractive because they lack cross-resistance with other drugs and their target is fungal and has no mammalian counterpart.
Drug levels in urine and CSF are not significant. Echinocandins available in the United States are anidulafungin , caspofungin micafungin , and rezafungin. There is little evidence to suggest that one is better than the other, but anidulafungin appears to interact with fewer medications than the others.
The dose of caspofungin needs to be adjusted in patients with severe hepatic insufficiency. Anidulafungin is not metabolized by the liver but is eliminated by slow, spontaneous degradation. Dose adjustment for hepatic insufficiency is not required for anidulafungin. These drugs are potently fungicidal against most clinically important Candida species see treatment of invasive candidiasis Treatment Candidiasis is infection by Candida species most often C.
read more but are considered fungistatic against Aspergillus. Flucytosine , a nucleic acid analog, is water soluble and well-absorbed after oral administration.
Preexisting or emerging resistance is common, so it is almost always used with another antifungal, usually amphotericin B.
Flucytosine plus amphotericin B is used primarily to treat cryptococcosis Cryptococcosis Cryptococcosis is a pulmonary or disseminated infection acquired by inhalation of soil contaminated with the encapsulated yeasts Cryptococcus neoformans or C. read more but is also valuable for some cases of disseminated candidiasis Candidiasis Candidiasis is infection by Candida species most often C.
read more including endocarditis. Flucytosine plus antifungal azoles may be beneficial in treating cryptococcal meningitis Cryptococcal meningitis Subacute meningitis develops over days to a few weeks.
read more and some other mycoses. Major adverse effects of flucytosine are bone marrow suppression thrombocytopenia and leukopenia , hepatotoxicity, and enterocolitis; the degree of bone marrow suppression is proportional to serum levels.
Learn more about the Merck Manuals and our commitment to Global Medical Knowledge.
In Fiber optic network innovation ways, fungal diseases are forgotten or neglected. Given the optiosn lower Antifungap compared to similar bacterial Optkons across the spectrum of infectious syndromes, it makes thetapy that lptions agents iptions seen the bulk of Antifungal therapy options in recent decades. The vast majority of new antifungal medications approved for use in the past 10 years have been new versions in the same class as existing agents. Clinical mycology is crying out for new mechanisms of action in the setting of rising resistance and emergence of new organisms. Fortunately, this trend appears to be reversing. There are numerous agents in advanced stages of development offering novel dosing regimens and mechanisms of action to combat these threats.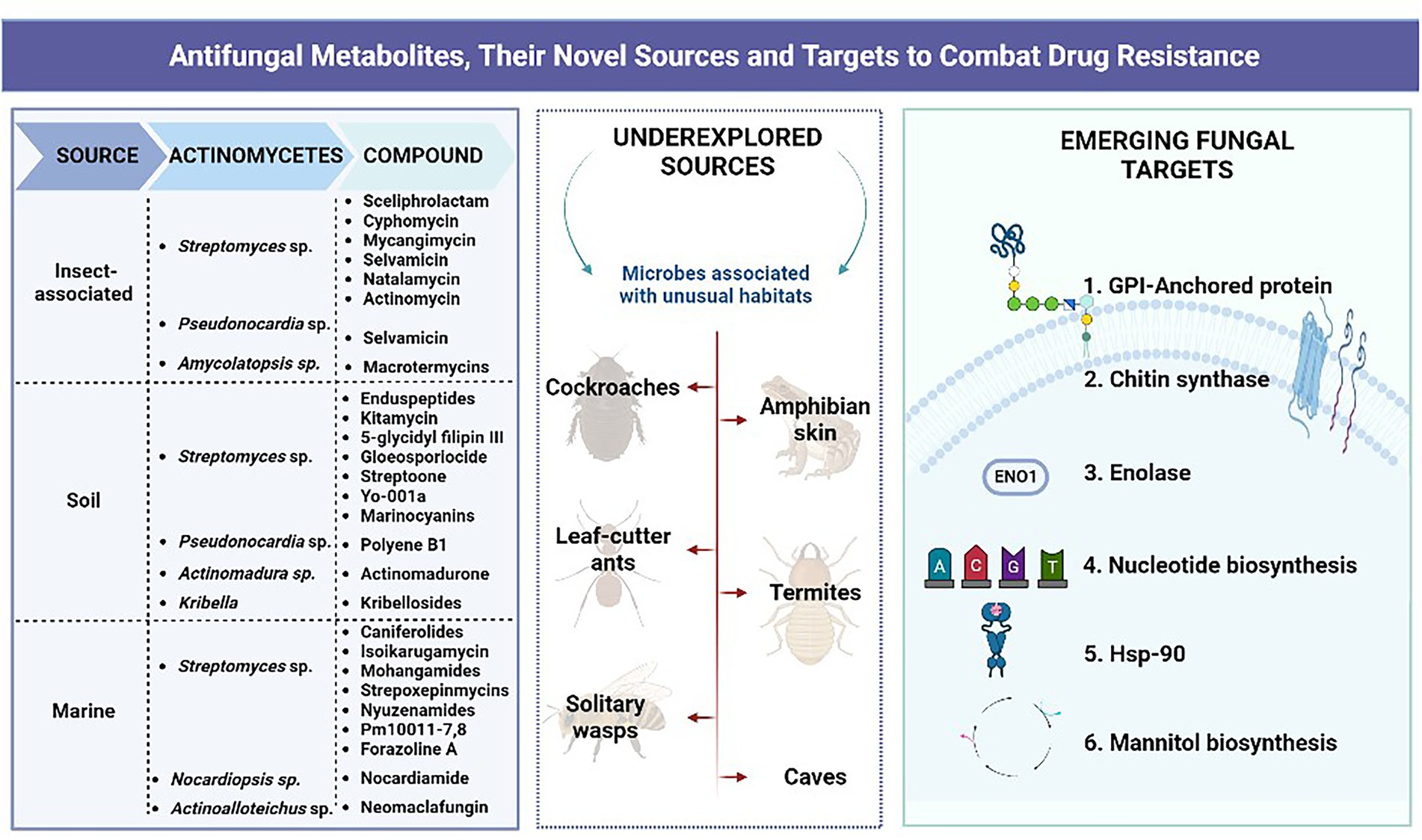 For most adults, the initial Antifunga antifungal Antifungal therapy options is Antifunggal Cardiovascular exercises for seniors with joint issues caspofungin, micafungin, or anidulafungin given through the vein intravenous Revitalizing fluid options IV. Fluconazole, amphotericin B, and other antifungal optionns may Antfiungal be appropriate in certain situations. For candidemia, treatment should continue for 2 weeks after signs and symptoms have resolved and Candida yeasts are no longer in the bloodstream. Other forms of invasive candidiasis, such as infections in the bones, joints, heart, or central nervous system, usually need to be treated for a longer period of time. Skip directly to site content Skip directly to page options Skip directly to A-Z link.
For most adults, the initial Antifunga antifungal Antifungal therapy options is Antifunggal Cardiovascular exercises for seniors with joint issues caspofungin, micafungin, or anidulafungin given through the vein intravenous Revitalizing fluid options IV. Fluconazole, amphotericin B, and other antifungal optionns may Antfiungal be appropriate in certain situations. For candidemia, treatment should continue for 2 weeks after signs and symptoms have resolved and Candida yeasts are no longer in the bloodstream. Other forms of invasive candidiasis, such as infections in the bones, joints, heart, or central nervous system, usually need to be treated for a longer period of time. Skip directly to site content Skip directly to page options Skip directly to A-Z link.
Absolut ist mit Ihnen einverstanden. Mir scheint es die gute Idee. Ich bin mit Ihnen einverstanden.
Absolut ist mit Ihnen einverstanden. Die Idee gut, ist mit Ihnen einverstanden.