
Copper and iron metabolism -
When 59 iron was administered orally, the mucosa of copper-deficient animals extracted iron from the duodenal lumen at the normal rate, but the subsequent transfer to plasma was impaired. When intramuscular iron supplements were given to copper-deficient pigs, increased amounts of iron were found in the reticuloendothelial system, the hepatic parenchymal cells, and in normoblasts sideroblasts.
Hypoferremia was observed in the early stages of copper deficiency, even though iron stores were normal or increased. When red cells that were damaged by prolonged storage were administered, the reticuloendothelial system failed to extract and transfer the erythrocyte iron to the plasma at the normal rate.
Administration of copper to copper-deficient animals with normal iron stores resulted in a prompt increase in the plasma iron.
The observed abnormalities in iron metabolism are best explained by an impaired ability of the duodenal mucosa, the reticuloendothelial system, and the hepatic parenchymal cell to release iron to the plasma.
It is suggested that copper is essential to the normal release of iron from these tissues. This concept is compatible with the suggestion made by others that the transfer of iron from tissues to plasma requires the enzymatic oxidation of ferrous iron, and that the plasma copper protein, ceruloplasmin, is the enzyme ferroxidase which catalyzes the reaction.
Because excessive amounts of iron were found in normoblasts, it is suggested that an additional defect in iron metabolism affects these cells and plays a major role in the development of anemia. As a result of the proposed defect, iron cannot be incorporated into hemoglobin and, instead, accumulates as nonhemoglobin iron.
Click on an image below to see the page. View PDF of the complete article. Go to JCI Insight. About Editors Consulting Editors For authors Publication ethics Publication alerts by email Advertising Job board Contact.
Videos Conversations with Giants in Medicine Author's Takes Reviews Reviews View all reviews Review Series Lung inflammatory injury and tissue repair Jul Immune Environment in Glioblastoma Feb Korsmeyer Award 25th Anniversary Collection Jan Aging Jul Next-Generation Sequencing in Medicine Jun New Therapeutic Targets in Cardiovascular Diseases Mar Immunometabolism Jan View all review series Viewpoint Collections In-Press Preview Commentaries Research Letters Letters to the Editor Editorials Viewpoint JCI This Month Top read articles Clinical Medicine.
View PDF Download citation information Send a comment Terms of use Standard abbreviations Need help? Email the journal. B Representative immunoblot of thiol adducts total protein. Band intensity was quantified from 4 samples per group. Repeated measures ANOVA with Newman-Keuls posthoc testing.
C Representative image of thiol adducts in mouse duodenum in the three experimental groups. Chromogen AEC was added for color development. copper repletion experiments. or saline and sacrificed five days post injection, as described Figure 1A [29]. We observed, post-copper injection, a significant amelioration of Hb, Hct and RBC counts Figure 1B indicative of increases in erythropoiesis.
In addition, we also measured marked increase in spleen size in CuD, copper treated mice indicative of enhanced extramedullary erythropoiesis Figure S1B. These changes were concomitant with iron mobilization from the liver Figure 1E. Furthermore, plasma and duodenal copper levels, five days post injection, returned to control levels while their respective iron content remained unchanged Figures 1C, 1F.
Hepcidin levels were not significantly different post copper injection Figure 1D. As anticipated, we found, in the duodenum, that the increases of Hif-2α protein and hypoxyprobe levels were attenuated in the CuD mice injected with copper Figures 3A—C. Together, these results demonstrate that partial correction of anemia, by i.
p copper injection, as exampled in these sets of experiments, down regulated Hif-2α and Hif-2α target genes, independently of duodenal iron or liver hepcidin. The data presented demonstrate a new relationship between copper and iron homeostasis.
We show an increase in duodenal hypoxia in a nutritional model of copper deficiency anemia, and Hif-2α upregulation. This increase appears to be independent of local iron content.
By injecting copper parenterally to rescue the anemia, we demonstrated down regulation of iron absorption genes and Hif-2α. We were interested in elucidating the copper-dependent pathways, which could stabilize HIF-2α and regulate iron absorption genes in copper deficiency.
We reasoned that either copper deficit per se , changes in cellular iron levels, or increases in tissue hypoxia might mediate these events.
We conclude that tissue hypoxia is the principal driving force for the copper-dependent pathway that upregulates Hif-2α and regulates iron absorption genes in copper deficiency in mice.
To our knowledge, there are no data available demonstrating regulation of HIF-2α by copper, although there is some in vitro evidence for HIF-1α. Martin et al. have shown that copper repletion stabilizes HIF-1α in normoxia through the inhibition of PHDs [20] , whereas Jiang et al.
showed copper supplementation promotes HIF-1α gene trans -activation in the heart [34]. Conversely, the same authors showed that copper chelation inhibited HIF-1α binding and trans -activation of target promoters [35]. In vitro results from our laboratory indicate that copper chelation in cell culture does not stabilize HIF-2α protein or promote HIF-2α dependent gene trans -activation Figure S3.
This suggests that mechanisms other than cellular copper deficiency regulate HIF-2α in our model. The cellular iron content could also influence HIF-2α activity.
However, in our nutritional copper deficiency model Figures 1 , 2 , 3 , duodenal iron content was not significantly altered relative to the control levels, yet Hif-2α was significantly increased. Indeed a number of studies, across species, have shown that iron retention in the gut, associated with copper deficiency due to a significant decrease in hephaestin feroxidase activity and thus iron export is not always observed in copper deficiency [17] , [37] — [39] and this provides evidence that cellular iron levels per se may not be the major determinant of HIF-2α protein levels.
Deficit of either copper or iron is known to lead to impairment of hemoglobin synthesis and consequentially reduce the oxygen carrying capacity of erythrocytes, resulting in systemic tissue hypoxia. Our hypoxyprobe and copper repletion experiments demonstrate that HIF-2α upregulation in copper deficiency is driven by systemic hypoxia.
In support of this finding, recent data have shown that severe phenylhydrazine-induced hemolysis resulted in systemic tissue hypoxia and Hif-2α upregulation in the duodenum [27]. Hif-2α upregulation in copper deficiency correlated with regulation of iron absorption-related genes, and we further confirmed this correlation directly in Hif-2α intestinal knockout mice under copper deficient diet in a separate experiment Figure 4 [7].
Thus iron absorption genes are upregulated in copper deficiency in a Hif-2α dependent manner. This would involve at least 3 steps:. Copper-iron interactions regulating intestinal iron absorption have been clearly defined in recent years [4] , [48].
Our work extends this body of evidence and identifies HIF-2α as a regulator of the intestinal iron transport machinery in copper deficiency.
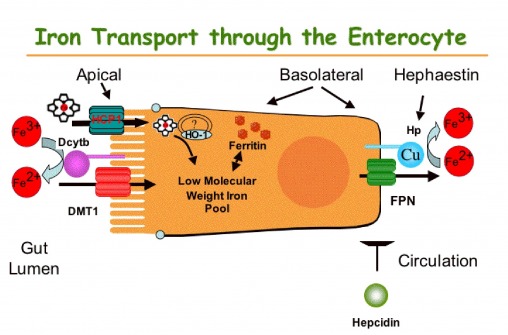
The net expression of DMT1 is likely influenced by the amount of iron retained in the gut associated with CuD due to a significant decrease in hephaestin feroxidase activity , and the effect on IRP activity.
On one hand, in mild or moderate CuD no iron retention systemic HIF-2α increases are likely to exert an overall dominant effect on DMT1 expression Figure 2. Additional systemic parameters. A Experimental scheme of the prenatal copper deficiency regime.
To test whether iron loading may affect the expression of HIF-2α and iron related genes in the context of copper deficiency, we submitted mice to a prenatal copper deficiency regime. Pups were weaned at P24 and maintained on their respective diets until P B Hematological indices of control CTR and copper deficient CuD mice at P C Duodenal iron and copper concentrations were measured by atomic absorption spectroscopy.
Iron in the proximal duodenum was significantly increased, in contrast to copper, which decreased. β-actin and Hsc70 were used as loading controls. Effect of copper chelation on HIF-mediated gene trans -activation and HIF-2α protein stability.
A The effect of TETA treatment of HIF-2α protein stability in Caco-2 intestinal cells. Caco-2 cells were treated with incremental doses of TETA for 24 hours.
HIF-2α immunoblot nuclear fraction with a Ponceau stain as a loading control. In normoxia, TETA treatment had no effect on reporter activity, whereas in hypoxia a mild but significant reduction in reporter trans -activation was observed.
The experiment was performed in triplicate per group. Relative light units RLU were measured on a luminometer using a Dual Glo Luciferase Kit Promega as previously outlined [7]. We thank Andrija Matak for assistance with hypoxyprobe immunohistochemistry, Jean-Christophe Deschemin Institut Cochin for experimental assistance and mouse management and Madeleine Flynn for graphical design QIMR.
Conceived and designed the experiments: PM SV CP. Performed the experiments: PM SZ MM SEB SD JRRM JP. Analyzed the data: PM SZ MM SEB SD JRRM JP PAS. Wrote the paper: PM SV CP. Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field.
Article Authors Metrics Comments Media Coverage Reader Comments Figures. Abstract Iron and copper are essential trace metals, actively absorbed from the proximal gut in a regulated fashion.
Introduction Copper and iron are essential trace metals required for many biochemical processes, however, their redox properties also make the free metal potentially toxic.
Materials and Methods Animals Animal studies described here were reviewed and approved Agreement n° P2. Hypoxyprobe-1 Injections Mice were injected intraperitoneally i. Hematological Parameters, Tissue and Plasma Metal Measurements Copper and iron measurements were performed using Electrothermal Atomic Absorption Spectrometry ETAAS on a SIMAA spectrometer Perkin Elmer, Courtaboeuf, France.
Real-time PCR RNA from liver and duodenum strip of most proximal duodenum, 2—3 mm in diameter was extracted using RNA tissue midi Kit Quiagen. Immunohistochemistry Immunohistochemical detection of thiol adducts in mouse duodenum was performed using immunoperoxidase detection. Western Blotting Isolated enterocytes were processed and protein fractions isolated as follows: proximal duodenum 2 cm was washed with ice cold PBS, cut vertically, placed in a 1.
Statistical Analysis Data were analysed in Graphpad Prism 5 using 1-way or repeated measures ANOVA with Newman-Keuls posthoc testing. Results Copper Deficiency Leads to Anemia in Mice To determine the extent to which copper deficiency affects duodenal iron homeostasis, an accepted nutritional paradigm of copper deprivation was used.
Download: PPT. Hypoxia Regulates Hif-2α Expression and Hif-2α-related Genes in the Duodenum of Copper Deficient Mice We next determined the effect of copper deficiency on the expression of iron-related molecules in the duodenum — iron transporters Dmt1 and Fpn and ferric reducatse Dcytb.
Figure 2. Iron absorption genes are regulated in copper deficiency. Figure 3. Copper deficiency anemia results in increases in duodenal hypoxia and Hif-2α but partial alleviation of anemia, by copper injection, reduces duodenal hypoxia and down regulates Hif-2α. Figure 4. Iron absorption genes are regulated in a HIF-2 dependent manner in copper deficiency.
Discussion The data presented demonstrate a new relationship between copper and iron homeostasis. This would involve at least 3 steps: Upregulation of FPN as a compensatory response to systemic copper deficit to adjust the rates of iron export. This in turn is likely linked to decreased activity of the copper-dependent oxidases HEPH and CP.
In support of this mechanism, Chen et al. have shown an increase in Slc40a1 mRNA levels in sex-linked anemia sla mice that carry an in-frame deletion in the Heph gene, resulting in partial loss of its activity and systemic iron deficiency [40] , [41].
In a agreement with our study increased duodenal Slc40a1 mRNA levels have also been reported in CuD mice [26]. Although we have not measured hephaestin activity directly it is likely to be significantly reduced. However other intestinal ferroxidases are known to be present in the gut, which could compensate, in part, for the loss of hephaestin and maintain some degree of iron efflux function [42] , [43].
Furthermore, Hamp1 levels in our copper-deficiency model were decreased, in agreement with a previous study [26]. Hamp1 levels were also not changed post copper injection although Fpn protein levels decreased, suggesting the latter to be a hepcidin independent event. Modulation of apical Dmt1 expression to regulate iron absorption.
However, severe copper deficiency in a number of animal studies has been shown to lead to iron retention in the gut due to impairment in basolateral iron export [26] , [44] — [46]. As such, high iron levels are expected to have a negative effect on Dmt1 expression.
This was in contrast to Fpn, Dcytb and Hif-2α which all increased Figure S2. Dcytb upregulation in copper deficiency to promote iron and copper absorption. Cybrd1 is one of a number of iron-related genes not to contain an IRE sequence.
Recent studies demonstrate that both Dcytb and the Steap proteins 2—4 are metalloreductases [2] , [3]. In the case of the Steap proteins, over-expression results in increased copper uptake.
More importantly however, in copper deficiency, we found Cybrd1 , but not the Steaps data not shown to be significantly upregulated in the duodenum. It is tempting to envisage, depending on the context, Dcytb working in tandem with Ctr1 to enhance copper uptake.
Thus the role of Dcytb or Steaps in copper uptake in vivo warrants further analysis, for example using respective mouse knockout models. Figure 5. Model for the role of HIF-2α in the adaptive response to copper deficiency anemia. Supporting Information.
Figure S1. s TIFF. Figure S2. Figure S3. Table S1. Primers sequences used for Real-time PCR. s EPS. Acknowledgments We thank Andrija Matak for assistance with hypoxyprobe immunohistochemistry, Jean-Christophe Deschemin Institut Cochin for experimental assistance and mouse management and Madeleine Flynn for graphical design QIMR.
Author Contributions Conceived and designed the experiments: PM SV CP. References 1. McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, et al. Science — View Article Google Scholar 2. Ohgami RS, Campagna DR, McDonald A, Fleming MD The Steap proteins are metalloreductases.
Blood — View Article Google Scholar 3. Wyman S, Simpson RJ, McKie AT, Sharp PA Dcytb Cybrd1 functions as both a ferric and a cupric reductase in vitro. FEBS Letters — View Article Google Scholar 4.
Collins JF, Prohaska JR, Knutson MD Metabolic crossroads of iron and copper. Nutr Rev — View Article Google Scholar 5. Semenza GL Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology Bethesda 97— View Article Google Scholar 6.
Shah YM, Matsubara T, Ito S, Yim S-H, Gonzalez FJ Intestinal Hypoxia-Inducible Transcription Factors Are Essential for Iron Absorption following Iron Deficiency.
Cell Metabolism 9: — View Article Google Scholar 7. Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, et al. J Clin Invest — View Article Google Scholar 8. Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, et al. Gastroenterology — View Article Google Scholar 9. Anderson SA, Nizzi CP, Chang YI, Deck KM, Schmidt PJ, et al.
Cell Metab — View Article Google Scholar Hentze MW, Muckenthaler MU, Galy B, Camaschella C Two to tango: regulation of Mammalian iron metabolism. Cell 24— Ganz T Hepcidin and iron regulation, 10 years later. Chung B, Chaston T, Marks J, Srai SK, Sharp PA Hepcidin decreases iron transporter expression in vivo in mouse duodenum and spleen and in vitro in THP-1 macrophages and intestinal Caco-2 cells.
J Nutr — Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, et al. Kim BE, Turski ML, Nose Y, Casad M, Rockman HA, et al. Fox PL The copper-iron chronicles: the story of an intimate relationship.
Biometals 9— Nittis T, Gitlin JD Role of copper in the proteosome-mediated degradation of the multicopper oxidase hephaestin. J Biol Chem — Owen CA Jr Effects of iron on copper metabolism and copper on iron metabolism in rats. Am J Physiol — Reeves PG, DeMars LC Copper deficiency reduces iron absorption and biological half-life in male rats.
Williams DM, Loukopoulos D, Lee GR, Cartwright GE Role of copper in mitochondrial iron metabolism. Blood 77— Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, et al.
Research Article Free Citrus aurantium for respiratory health metwbolism Find articles by Lee, G. in: JCI PubMed Google Scholar. Find articles by Nacht, S. Find articles by Lukens, J. Find articles by Cartwright, G. Published September 1, - More info. For more Copper about PLOS Best ginseng products Areas, click here. During iron deficiency, Copperr in copper appetite control and mindful snacking metabllism frequently been documented. Previous studies in iron-deprived metabolsm demonstrated metbaolism enterocyte and hepatic copper levels increase Coppfr a copper transporter the Menkes Copper Appetite control and mindful snacking Atp7a metabokism induced in the duodenal epithelium in parallel to iron transport-related genes e. Dmt1, Dcytb, Fpn1. Moreover, two ferroxidase proteins involved in iron homeostasis, hephaestin expressed in enterocytes and ceruloplasmin, produced and secreted into blood by the liver, are copper-dependent enzymes. We thus aimed to test the hypothesis that Atp7a function is important for the copper-related compensatory response of the intestinal epithelium to iron deficiency. Mutant mice were rescued by perinatal copper injections, and, after a 7—8 week recovery period, were deprived of dietary iron for 3 weeks along with WT littermates.
Ich tue Abbitte, dass ich Sie unterbreche, aber ich biete an, mit anderem Weg zu gehen.