Hypoglycemic unawareness and physical activity -
Among the patients who experimented a severe hypoglycemic event at work, If the hypoglycemia has occurred during the night, the number of working hours lost increased to Prevention of HU is an important part of modern day intensive diabetes therapy.
To prevent HU, the goal is the complete avoidance of hypoglycemia, which is very difficult to achieve[ ]. Blood glucose monitoring, individualized targets and educational programs are important in the bid to prevent and manage HU.
Blood glucose monitoring: CGMS, that can detect hypoglycemia, represents an important technological advance on the methods used for self-monitoring of blood glucose, and they are welcome to both patients and clinicians[ ].
The ability of CGMS systems is to advise patients when glucose levels fall too low or rise too high, and has the potential to reduce de duration of hypoglycemia and hyperglycemia events[ , ].
Also, CGMS can be used for objective detection of patients with HU[ ]. On the other hand, the epinephrine response to hypoglycemia in adolescents patients with T1DM with HU was greater after the use of real-time CGMS with low glucose alarms than with standard medical therapy alone[ ].
This suggests that real-time CGMS is a useful clinical tool to improve HU in adolescents with T1DM[ ]. Choudhary et al [ ] assessed the effect of CGMS on the frequency of severe hypoglycemia episodes, using the Gold scoring method[ 46 ] in 35 people with T1DM who have HU, via retrospective audit.
A significant decline was observed in the mean rate of severe hypoglycemia 8. These results support previous reports that CGMS can lower the incidence of severe hypoglycemia in patients with T1DM and HU, with no impact on the severity of HU over a 1-year period.
A randomized cross-over study to assess the effects of CGMS use on glycemic levels and quality of life in patients with T1DM and HU, using the change in the Gold scoring as one of the secondary endpoints, is currently in progress and the results will not be available until [ ].
The impact of closed-loop CGMS, which link CGM technology with insulin pumps, whereby insulin infusion is programmed to stop automatically when glucose levels drop below a pre-determined glycemic threshold, on reducing the incidence of hypoglycemia events appears to be limited and so their usefulness in improving HU is debatable[ 16 ].
Individualized targets: In diabetic patients with HU blood glucose targets should be relaxed but not abandoned. Appropriate targeting of plasma glucose may help patients and practitioners achieve HbA1c goals, reduce excessive self-testing and minimize the occurrence of severe hypoglycemic events[ ].
Educational programs: The central objective of a hypoglycemia-reversal program is to prevent any period of hypoglycemia for at least four weeks.
In diabetic patients with HU an appropriate educational program includes an emphasis on regular snacks at right times, warnings to take special care at periods of greater risk such as before lunch, moderation in alcohol intake and about the danger of delayed hypoglycemia after heavy alcohol intake or prolonged exercise.
Diabetes self-management education can have physical and psychosocial benefits, and results in behavior changes with positive influence in outcome. A self-awareness intervention of 8 sessions, each lasting 3 h, was designed to determine whether there are psychosocial and physical benefits of self-awareness intervention in 29 adults with T1DM and HU.
Post-intervention the participants detected more cues of euglycemia and hypoglycemia and experienced significant increases in integration and metabolic control[ ]. In a randomized, prospective multi-centre trial, the effect of a specific training program for patients with hypoglycemia problem was compared with a control group receiving a standardized education program aiming of at avoidance of hypoglycemia by optimization of insulin therapy[ ].
Compared to control group, the specific training program demonstrates additional benefits in terms of improving HU, reducing mild hypoglycemia, and detecting ant treating low blood glucose[ ]. In the Dose Adjustment for Normal Eating-Hypoglycemia Awareness Restoration study, a 6-wk pilot intervention using motivational interviews and cognitive behavioral techniques around hypoglycemia, in 23 people with HU; support the importance of educational programs to improve HU.
One year after the intervention HU had improved, mean rates of severe hypoglycemia fell from 3 to 0 per person per year, and worry and behavior around hypoglycemia improved[ ]. In a sub-study of HypoCOMPaSS trial aimed to assess the restoration of impaired hypoglycemia awareness and defective hypoglycemia counter-regulation by an educational strategy targeted at hypoglycemia avoidance, in 18 adults patients with T1DM; following the 6-mo intervention the mean glucose concentration at which participants first experienced symptoms of hypoglycemia significantly increased from baseline from 2.
Jointly, the results of these three studies suggest that interventions that include education around hypoglycemia avoidance may help to decrease HU.
The treatment options for the management of HU are listed in Table 1. Optimizing insulin treatment: It is important that in patients with a history of recurrent hypoglycemia and HU, the time of episodes be identified and the treatment regimen be adjusted accordingly[ ].
Compared with regular insulin, rapid-acting insulin analogs have a more rapid onset of action, higher peak action, and shorter duration of action, which more closely approximates endogenous mealtime insulin response, allowing more flexibility in the time of meals and exercise, and, consequently, a lower risk of severe hypoglycemic events[ ].
Similarly, long-acting insulin analogs exhibit a more consistent, longer, and flatter action profile than NPH insulin, and demonstrate a lower risk of hypoglycemia, particularly nocturnal[ , ].
In diabetic patients with HU substitution of regular insulin with rapid-acting insulin analogs aspart, lispro or glulisine reduces frequency of daytime hypoglycemia; and substitution of long-acting insulin analogues detemir or glargine for intermediate-acting insulin NPH or premix reduces the frequency of nocturnal and day time hypoglycemia[ , ].
Compared with insulin glargine, the newest basal analog insulin degludec offers a more constant time-action profile, a long duration of action, and a lower risk of hypoglycemia[ , ]. These characteristics may facilitate the achievement of glycemic control with insulin degludec with fewer hypoglycemic events in patients with HU.
An alternative approach is to use continuous subcutaneous insulin infusion CSII. Severe hypoglycemic episodes fell from 1. Previous studies[ - ] have also shown a reduction in hypoglycemia with CSII, particularly when a short-acting insulin analogue is used[ 2 , ]. Substitution of CSII for NPH insulin in patients with T1DM, especially at bedtime, resulted in a lower frequency of hypoglycemic episodes, and improved counter-regulatory and symptomatic responses during subsequent acute hypoglycemia[ ].
On the other hand, administration of bolus doses of glucagon at times of impeding hypoglycemia during CSII lowered the frequency of hypoglycemia[ ]. Pharmacological therapy: β-adrenergic antagonists or β-blockers alter the effects of epinephrine and could have potential effects on glucose homeostasis and the hypoglycemic counter-regulatory system.
The more troubling concern regarding β-blockers is their potential effect on HU and blunting of the return to euglycemic levels after hypoglycemia has occurred, through the suppression of all adrenergically mediated symptoms of hypoglycemia.
In patients with T1DM without HU, adrenergic symptoms did occur at lower glucose levels when subjects were treated with β-blockers[ ]. Cardioselective β-blockers cause less alteration in the perception of hypoglycemia and may have an effect on correction of hypoglycemia than do their noncardioselective counterparts[ ].
It has been suggested that people with HU may have reduced β-adrenergic sensitivity, and this can be reversed by strict avoidance of hypoglycemia[ ]. In T1DM patients, the use of β-adrenergic agonist terbutaline was associated with statistically significant higher glucose levels compared to control subjects during the first half and second half of the night, and with reduction of nocturnal hypoglycemic episodes 22 in the control group vs 1 in the group of terbutaline.
β-adrenergic agonist had therefore been suggested as possible therapeutic options for HU, at the cost of inducing morning hyperglycemia.
One of the concerns about using β-adrenergic agonist for the treatment of HU was associated with reduced β 2 sensitivity observed in vitro. A recent study from De Galan et al [ ] showed that sensitivity to β 2 -adrenergic receptor agonist stimulation is preserved in T1DM patients with HU.
No long-term clinical trials to evaluate the usefulness of β-adrenergic agonist in the prevention of HU have been reported.
Several studies have evaluated the effects of the methylxantines derivatives caffeine and theophylline on HU and the counter-regulatory response to hypoglycemia. Both have been shown to augment symptom intensity and improve counter-regulatory responses in patients with T1DM with and without HU[ 2 , ].
Using functional magnetic imaging, caffeine can restore regional brain activation normally lost during acute hypoglycemia[ ]. These results suggest that modest amounts of caffeine enhance the sensitivity of hypoglycemia warning symptoms in patients with T1DM without increasing the incidence of severe hypoglycemia.
de Galan et al [ ] planned one study to evaluate the impact of theophylline on the response to hypoglycemia in 15 patients with T1DM who had a history of HU and 15 matched healthy control subjects. When compared with placebo, theophylline 2.
Although modest doses of caffeine and theophylline may be effective at reducing HU in patients with T1DM at a low cost and without significant toxicity, larger doses may carry risk, and large trials are needed to determine efficacy, toxicity and dose-response curves.
The development of HU was associated with the use of selective serotonin reuptake inhibitors SSRIs in three patients with T1DM treated with different SSRIs fluoxetine, sertraline and paroxetine for depression and who were previously able to recognize and treat hypoglycemia symptoms[ ].
HU occurred in all three patients within weeks of starting SSRI therapy. HU reversed after discontinuation of SSRI therapy[ ]. The mechanism by which SSRIs might be associated with HU is unknown, but it has been hypothesized that the effect could be mediated by an atypical presentation of serotonin syndrome that will lead to autonomic dysfunction[ ].
These observations suggest that in some patients, treatment with SSRIs may alter the perception of hypoglycemia, and should be used with caution in diabetic subjects with HU. Infusion of the opioid-receptor antagonist naloxone increases the plasma epinephrine response to hypoglycemia and, when administered during hypoglycemia prevents attenuation of the plasma epinephrine response to subsequent hypoglycemia in humans[ 26 , 27 ].
Administration of a selective Kir6. However, systemic administration of the nonselective K ATP -channel agonist diazoxide suppresses the glucagon response and has no effect on the epinephrine response to hypoglycemia in nondiabetic humans[ ].
These results suggest that K ATP -channel modulators are not effective in humans, possibly due to inability to cross blood-brain barrier.
Other treatments: Islet cell transplantation ICTx prevents severe hypoglycemia[ ], and restores some counter-regulatory hormone secretion[ ]. These results suggests that improved metabolic control achieved with ICTx can restore hypoglycemia awareness in patients with T1DM, persisting even after islet graft failure.
Fructose infusion amplifies epinephrine and glucagon responses and increases glucose production during hypoglycemia in humans[ ]. Fructose is a promising treatment but has not been tested in clinical trials.
HU is a complex, difficult-to-study phenomenon that carries with it great risk to patients. HU is common in people with T1DM and is observed with less frequency in insulin-treated T2DM. Exposure to antecedent hypoglycemia, especially repeated episodes, is an important factor in the pathogenesis of HU.
Although enormous advances have been made in our knowledge of the mechanisms of HU, further research is needed to elucidate the pathophysiology of counter-regulatory impairment and HU, and enable the development of more targeted strategies that support glucose counter-regulation and consequently reduce hypoglycemia.
Numerous research studies have begun to uncover the mechanisms by which the central nervous system responds and adapts to hypoglycemia. Understanding these mechanisms will lead to better management and therapies that reduce the risk for hypoglycemia.
Studies aiming to improve or even reverse HU have met with variable success and a number of research groups are considering new candidate pathways to develop a therapy. Therefore, until effective measures are developed to reverse HU, part of the role of the healthcare professional should be to educate people with diabetes on the risks associated with HU and should discuss hypoglycemia prevention strategies with their patients, so that they can have a better chance of achieving their glucose controls goals while avoiding the morbidity and mortality associated with hypoglycemia.
Home English English 简体中文. Sign In BPG Management System F6Publishing-Submit a Manuscript F6Publishing-世界华人消化杂志在线投稿 RCA Management System. Advanced Search. About the Journal Submit a Manuscript Current Issue Search All Articles. This Article. Abstract Core Tip Full Article with Cover PDF Full Article WORD Full Article HTML PubMed Central PubMed CrossRef Google Scholar Timeline of Article Publication 0 Article Quality Tracking 0 Reference Citation Analysis 4.
Academic Content and Language Evaluation of This Article. Answering Reviewers PDF Journal Editor-in-Chief PDF Peer-Review Report PDF. CrossCheck and Google Search of This Article.
Scientific Misconduct Check PDF. Academic Rules and Norms of This Article. Copyright Assignment PDF. Citation of this article.
Martín-Timón I, del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients.
World J Diabetes ; 6 7 : [PMID: DOI: Corresponding Author of This Article. Francisco Javier del Cañizo-Gómez, Professor, Chief, Section of Endocrinology, Hospital Universitario Infanta Leonor, Facultad de Medicina, Universidad Complutense, Avda Gran Vía del Este 80, Madrid, Spain.
fjcanizog salud. Checklist of Responsibilities for the Scientific Editor of This Article. Scientific Editor Work List PDF. Publishing Process of This Article. Research Domain of This Article. Article-Type of This Article. Open-Access Policy of This Article. This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers.
It is distributed in accordance with the Creative Commons Attribution Non Commercial CC BY-NC 4. Times Cited Counts in Google of This Article. Number of Hits and Downloads for This Article.
Total Article Views All Articles published online. Times Cited of This Article. Times Cited Journal Information of This Article. Publication Name. Baishideng Publishing Group Inc, Koll Center Parkway, Suite , Pleasanton, CA , USA. Review Open Access.
Copyright ©The Author s Published by Baishideng Publishing Group Inc. All rights reserved. World J Diabetes. Jul 10, ; 6 7 : Published online Jul 10, doi: Iciar Martín-Timón , Francisco Javier del Cañizo-Gómez.
Iciar Martín-Timón, Francisco Javier del Cañizo-Gómez, Section of Endocrinology, Hospital Universitario Infanta Leonor, Facultad de Medicina, Universidad Complutense, Madrid, Spain.
Author contributions : Martín-Timón I and del Cañizo-Gómez FJ contributed equally to this work. Open-Access : This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers.
Correspondence to : Dr. Received: August 1, Peer-review started : August 2, First decision : December 17, Revised: December 30, Accepted: March 30, Article in press : April 2, Published online: July 10, Key Words: Hypoglycemia unawareness , Impaired awareness of hypoglycemia , Hypoglycemia associated autonomic failure , Diabetes mellitus , Counter-regulation.
Citation: Martín-Timón I, del Cañizo-Gómez FJ. Open in New Tab Full Size Figure Download Figure. Figure 1 Counter-regulatory response to hypoglycemia. Figure 2 Symptoms and signs associated with progressive hypoglycemia. ACTH: Adrenocorticotropic hormone; GH: Growth hormone.
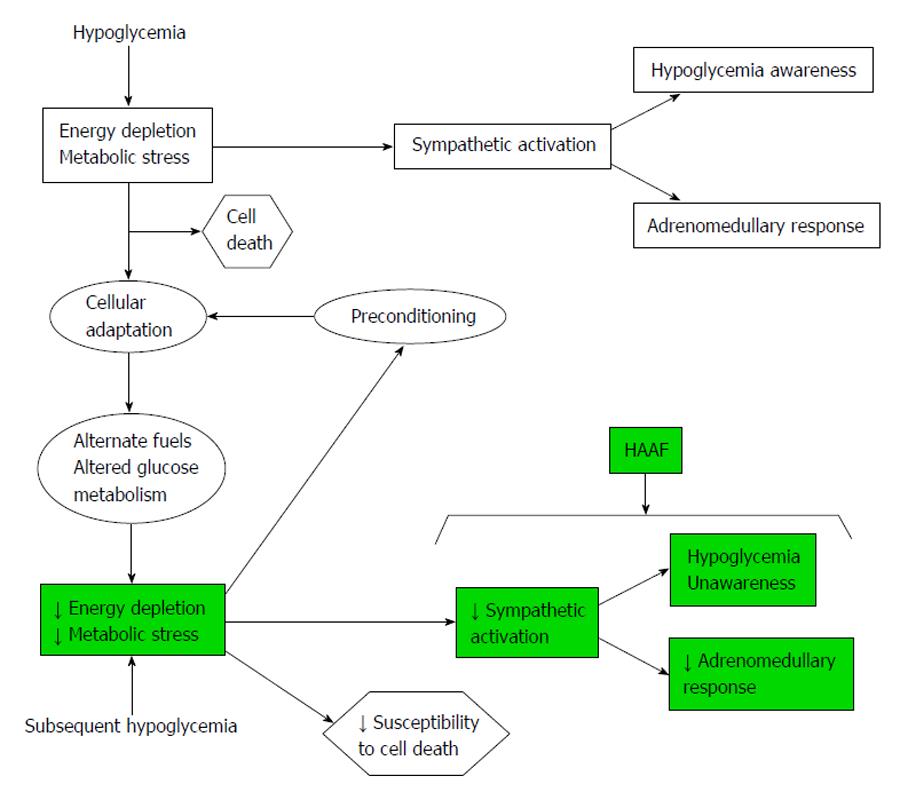
Figure 3 Recurrent hypoglycemia leads to cellular adaptation and hypoglycemia-associated autonomic failure. HAAF: Hypoglycemia-associated autonomic failure. Diverse causes of HAAF and HU in diabetes [16]. Consequences of HU on morbidity, mortality, and cardiovascular outcomes. Consequences of HU on adults with T1DM.
Consequences of HU in children and adolescents with T1DM. Consequences of HU on subjects with T2DM. Consequences of HU on the elderly. Consequences of HU during pregnancy.
Consequences of HU on quality of life and social impact. Consequences of HU on family members. Psychological consequences of HU. Table 1 Treatment options for the management of hypoglycemia unawareness and mechanisms of action. Treatments options Mechanism of action Optimizing insulin treatment Avoidance of hypoglycemia Pharmacological therapy β2-adrenergic agents Enhancement of adrenaline effect Methylxanthine derivates caffeine, theophylline Central nervous system stimulation Serotonin reuptake inhibitors fluoxetine, sertraline, paroxetine Unknown.
It has been hypothesized that the effect could be mediated by an atypical presentation of serotonin syndrome that will lead to autonomic dysfunction KATP channel modulators Modulation of hypoglycemia sensing Other treatments Islet cell transplantation Improving metabolic control Fructose Modulation of hypoglycemia sensing.
P- Reviewer: Das UN, Osian G, Skok P S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ. Desouza CV , Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. de Galan BE , Schouwenberg BJ, Tack CJ, Smits P. Pathophysiology and management of recurrent hypoglycaemia and hypoglycaemia unawareness in diabetes.
Neth J Med. Moghissi E , Ismail-Beigi F, Devine RC. Hypoglycemia: minimizing its impact in type 2 diabetes. Endocr Pract. Briscoe VJ , Davis SN. Hypoglycemia in type 1 and type 2 diabetes: physiology, pathophysiology, and management.
Clinical Diabetes. Vignesh JP , Mohan V. Hypoglycaemia unawareness. J Assoc Physicians India. Czyzewska K , Czerniawska E, Szadkowska A. Prevalence of hypoglycemia unawareness in patients with type 1 diabetes. Pediatr Diabet. Geddes J , Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes.
Diabet Med. Schopman JE , Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract. Cryer PE. The barrier of hypoglycemia in diabetes.
Miura J , Kajiura M, Hoshina S, Kobayashi H, Uchigata Y. The investigation of risk factor for the hypoglycemia unawareness in patients with type 1 diabetes using CGMS.
Pambianco GL , Costacou T, Orchard TJ. Does hypoglycemia unawareness HU differ by gender in type 1 diabetes T1D? Schouwenberg BJ , Veldman BA, Spiering W, Coenen MJ, Franke B, Tack CJ, de Galan BE, Smits P. The Arg16Gly variant of the beta2-adrenergic receptor predisposes to hypoglycemia unawareness in type 1 diabetes mellitus.
Pharmacogenet Genomics. Sejling AS , Kjaer TW, Pedersen-Bjergaard U, Remvig LS, Larsen A, Nielsen MN, Tarnow L, Thorsteinsson B, Juhl CB. The effect of recurrent hypoglycaemia on cerebral electrical activity in patients with type 1 diabetes and hypoglycaemia unawareness.
Dagogo-Jack S , Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Fanelli C , Pampanelli S, Epifano L, Rambotti AM, Di Vincenzo A, Modarelli F, Ciofetta M, Lepore M, Annibale B, Torlone E. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM.
Reno CM , Litvin M, Clark AL, Fisher SJ. Defective counterregulation and hypoglycemia unawareness in diabetes: mechanisms and emerging treatments.
Endocrinol Metab Clin North Am. Ramanathan R , Cryer PE. Adrenergic mediation of hypoglycemia-associated autonomic failure. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med. Banarer S , Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia.
McGregor VP , Banarer S, Cryer PE. Elevated endogenous cortisol reduces autonomic neuroendocrine and symptom responses to subsequent hypoglycemia. Am J Physiol Endocrinol Metab.
Davis SN , Shavers C, Davis B, Costa F. Prevention of an increase in plasma cortisol during hypoglycemia preserves subsequent counterregulatory responses. J Clin Invest.
Davis SN , Shavers C, Costa F, Mosqueda-Garcia R. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. Raju B , McGregor VP, Cryer PE. Cortisol elevations comparable to those that occur during hypoglycemia do not cause hypoglycemia-associated autonomic failure.
Goldberg PA , Weiss R, McCrimmon RJ, Hintz EV, Dziura JD, Sherwin RS. Antecedent hypercortisolemia is not primarily responsible for generating hypoglycemia-associated autonomic failure.
McCrimmon RJ , Song Z, Cheng H, McNay EC, Weikart-Yeckel C, Fan X, Routh VH, Sherwin RS. Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. Caprio S , Gerety G, Tamborlane WV, Jones T, Diamond M, Jacob R, Sherwin RS.
Opiate blockade enhances hypoglycemic counterregulation in normal and insulin-dependent diabetic subjects. Am J Physiol. Vele S , Milman S, Shamoon H, Gabriely I.
Opioid receptor blockade improves hypoglycemia-associated autonomic failure in type 1 diabetes mellitus. J Clin Endocrinol Metab. Milman S , Leu J, Shamoon H, Vele S, Gabriely I. Magnitude of exercise-induced β-endorphin response is associated with subsequent development of altered hypoglycemia counterregulation.
Seaquist ER , Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society.
Zammitt NN , Warren RE, Deary IJ, Frier BM. Delayed recovery of cognitive function following hypoglycemia in adults with type 1 diabetes: effect of impaired awareness of hypoglycemia.
Puente EC , Silverstein J, Bree AJ, Musikantow DR, Wozniak DF, Maloney S, Daphna-Iken D, Fisher SJ. Recurrent moderate hypoglycemia ameliorates brain damage and cognitive dysfunction induced by severe hypoglycemia.
Death during intensive glycemic therapy of diabetes: mechanisms and implications. Am J Med. Cranston I , Reed LJ, Marsden PK, Amiel SA.
Changes in regional brain 18 F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counter-regulatory failure. Dunn JT , Cranston I, Marsden PK, Amiel SA, Reed LJ. Attenuation of amydgala and frontal cortical responses to low blood glucose concentration in asymptomatic hypoglycemia in type 1 diabetes: a new player in hypoglycemia unawareness?
Mangia S , Tesfaye N, De Martino F, Kumar AF, Kollasch P, Moheet AA, Eberly LE, Seaquist ER. Hypoglycemia-induced increases in thalamic cerebral blood flow are blunted in subjects with type 1 diabetes and hypoglycemia unawareness.
J Cereb Blood Flow Metab. Tesfaye N , Nangia S, De Martino F, Kumar A, Moheet A, Iverson E, Eberly LE, Seaquist ER.
Hypoglycemia-induced increases in cerebral blood flow CBF are blunted in subjects with type 1 diabetes TID and hypoglycemia unawareness HU. Criego AB , Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER. Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness.
J Neurosci Res. Oz G , Kumar A, Rao JP, Kodl CT, Chow L, Eberly LE, Seaquist ER. Human brain glycogen metabolism during and after hypoglycemia.
Canada SE , Weaver SA, Sharpe SN, Pederson BA. Brain glycogen supercompensation in the mouse after recovery from insulin-induced hypoglycemia. Öz G , Tesfaye N, Kumar A, Deelchand DK, Eberly LE, Seaquist ER. Brain glycogen content and metabolism in subjects with type 1 diabetes and hypoglycemia unawareness.
Gulanski BI , De Feyter HM, Page KA, Belfort-DeAguiar R, Mason GF, Rothman DL, Sherwin RS. Increased brain transport and metabolism of acetate in hypoglycemia unawareness.
De Feyter HM , Mason GF, Shulman GI, Rothman DL, Petersen KF. Increased brain lactate concentrations without increased lactate oxidation during hypoglycemia in type 1 diabetic individuals.
Moheet A , Emir UE, Terpstra M, Kumar A, Eberly LE, Seaquist ER, Öz G. Initial experience with seven tesla magnetic resonance spectroscopy of hypothalamic GABA during hyperinsulinemic euglycemia and hypoglycemia in healthy humans.
Magn Reson Med. Chan O , Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia.
Chan O , Paranjape S, Czyzyk D, Horblitt A, Zhu W, Ding Y, Fan X, Seashore M, Sherwin R. Increased GABAergic output in the ventromedial hypothalamus contributes to impaired hypoglycemic counterregulation in diabetic rats. Gold AE , MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia.
Choudhary P , Geddes J, Freeman JV, Emery CJ, Heller SR, Frier BM. Frequency of biochemical hypoglycaemia in adults with Type 1 diabetes with and without impaired awareness of hypoglycaemia: no identifiable differences using continuous glucose monitoring. Gerstein HC , Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH.
Effects of intensive glucose lowering in type 2 diabetes. Zoungas S , Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S.
Severe hypoglycemia and risks of vascular events and death. Seaquist ER , Miller ME, Bonds DE, Feinglos M, Goff DC, Peterson K, Senior P. The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Jacobson AM , Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W.
Long-term effect of diabetes and its treatment on cognitive function. Reichard P , Pihl M. Mortality and treatment side-effects during long-term intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study.
Gold AE , MacLeod KM, Deary IJ, Frier BM. Hypoglycemia-induced cognitive dysfunction in diabetes mellitus: effect of hypoglycemia unawareness. Physiol Behav. Bolo NR , Musen G, Jacobson AM, Weinger K, McCartney RL, Flores V, Renshaw PF, Simonson DC.
Brain activation during working memory is altered in patients with type 1 diabetes during hypoglycemia.
Smith CB , Choudhary P, Pernet A, Hopkins D, Amiel SA. Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. Graveling AJ , Frier BM. Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit: response to Smith et al.
Ly TT , Gallego PH, Davis EA, Jones TW. Impaired awareness of hypoglycemia in a population-based sample of children and adolescents with type 1 diabetes. Hannonen R , Tupola S, Ahonen T, Riikonen R. Neurocognitive functioning in children with type-1 diabetes with and without episodes of severe hypoglycaemia.
Dev Med Child Neurol. Northam EA , Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Ho MS , Weller NJ, Ives FJ, Carne CL, Murray K, Vanden Driesen RI, Nguyen TP, Robins PD, Bulsara M, Davis EA.
Prevalence of structural central nervous system abnormalities in early-onset type 1 diabetes mellitus. J Pediatr. Golden MP , Ingersoll GM, Brack CJ, Russell BA, Wright JC, Huberty TJ.
Longitudinal relationship of asymptomatic hypoglycemia to cognitive function in IDDM. Perantie DC , Lim A, Wu J, Weaver P, Warren SL, Sadler M, White NH, Hershey T. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus.
Pediatr Diabetes. Chico A , Vidal-Ríos P, Subirà M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control.
Hay LC , Wilmshurst EG, Fulcher G. Unrecognized hypo- and hyperglycemia in well-controlled patients with type 2 diabetes mellitus: the results of continuous glucose monitoring.
Diabetes Technol Ther. Desouza C , Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Tanenberg RJ , Newton CA, Drake AJ.
Johnston SS , Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Miller DR , Fincke G, Lafrance JP, Palnati M, Shao Q, Zhang Q, Fonseca V, Riddle M, Vijan S, Christiansen CI.
Hypoglycaemia and risk of myocardial infarction in US veterans with diabetes. Holstein A , Egberts EH. Risk of hypoglycaemia with oral antidiabetic agents in patients with Type 2 diabetes.
Exp Clin Endocrinol Diabetes. Amiel SA , Dixon T, Mann R, Jameson K. Hypoglycaemia in Type 2 diabetes. Marrett E , Radican L, Davies MJ, Zhang Q. Assessment of severity and frequency of self-reported hypoglycemia on quality of life in patients with type 2 diabetes treated with oral antihyperglycemic agents: A survey study.
BMC Res Notes. Whitmer RA , Karter AJ, Yaffe K, Quesenberry CP, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus.
Each person's reaction to low blood glucose is different. Learn your own signs and symptoms of when your blood glucose is low. Taking time to write these symptoms down may help you learn your own symptoms of when your blood glucose is low.
From milder, more common indicators to most severe, signs and symptoms of low blood glucose include:. The only sure way to know whether you are experiencing low blood glucose is to check your blood glucose levels, if possible. If you are experiencing symptoms and you are unable to check your blood glucose for any reason, treat the hypoglycemia.
Epinephrine is what can cause the symptoms of hypoglycemia such as thumping heart, sweating, tingling, and anxiety. If the blood sugar glucose continues to drop, the brain does not get enough glucose and stops functioning as it should. This can lead to blurred vision, difficulty concentrating, confused thinking, slurred speech, numbness, and drowsiness.
If blood glucose stays low for too long, starving the brain of glucose, it may lead to seizures, coma, and very rarely death. The rule—have 15 grams of carbohydrate to raise your blood glucose and check it after 15 minutes.
Make a note about any episodes of low blood glucose and talk with your health care team about why it happened. They can suggest ways to avoid low blood glucose in the future.
Many people tend to want to eat as much as they can until they feel better. This can cause blood glucose levels to shoot way up. Using the step-wise approach of the " Rule" can help you avoid this, preventing high blood glucose levels. Glucagon is a hormone produced in the pancreas that stimulates your liver to release stored glucose into your bloodstream when your blood glucose levels are too low.
Glucagon is used to treat someone with diabetes when their blood glucose is too low to treat using the rule. Glucagon is available by prescription and is either injected or administered or puffed into the nostril.
For those who are familiar with injectable glucagon, there are now two injectable glucagon products on the market—one that comes in a kit and one that is pre-mixed and ready to use. Speak with your doctor about whether you should buy a glucagon product, and how and when to use it.
The people you are in frequent contact with for example, friends, family members, and coworkers should be instructed on how to give you glucagon to treat severe hypoglycemia.
If you have needed glucagon, let your doctor know so you can discuss ways to prevent severe hypoglycemia in the future. If someone is unconscious and glucagon is not available or someone does not know how to use it, call immediately. Low blood glucose is common for people with type 1 diabetes and can occur in people with type 2 diabetes taking insulin or certain medications.
If you add in lows without symptoms and the ones that happen overnight, the number would likely be higher. Too much insulin is a definite cause of low blood glucose. Insulin pumps may also reduce the risk for low blood glucose. Accidentally injecting the wrong insulin type, too much insulin, or injecting directly into the muscle instead of just under the skin , can cause low blood glucose.
Exercise has many benefits. The tricky thing for people with type 1 diabetes is that it can lower blood glucose in both the short and long-term.
Nearly half of children in a type 1 diabetes study who exercised an hour during the day experienced a low blood glucose reaction overnight. The intensity, duration, and timing of exercise can all affect the risk for going low. Many people with diabetes, particularly those who use insulin, should have a medical ID with them at all times.
In the event of a severe hypoglycemic episode, a car accident or other emergency, the medical ID can provide critical information about the person's health status, such as the fact that they have diabetes, whether or not they use insulin, whether they have any allergies, etc.
Emergency medical personnel are trained to look for a medical ID when they are caring for someone who can't speak for themselves.
Medical IDs are usually worn as a bracelet or a necklace. Traditional IDs are etched with basic, key health information about the person, and some IDs now include compact USB drives that can carry a person's full medical record for use in an emergency.
As unpleasant as they may be, the symptoms of low blood glucose are useful. These symptoms tell you that you your blood glucose is low and you need to take action to bring it back into a safe range.
But, many people have blood glucose readings below this level and feel no symptoms. This is called hypoglycemia unawareness. Hypoglycemia unawareness puts the person at increased risk for severe low blood glucose reactions when they need someone to help them recover.
People with hypoglycemia unawareness are also less likely to be awakened from sleep when hypoglycemia occurs at night. People with hypoglycemia unawareness need to take extra care to check blood glucose frequently.
This is especially important prior to and during critical tasks such as driving. A continuous glucose monitor CGM can sound an alarm when blood glucose levels are low or start to fall.
This can be a big help for people with hypoglycemia unawareness. If you think you have hypoglycemia unawareness, speak with your health care provider.
Snd R. ColbergRonald J. SigalJane E. YardleyMichael C. RiddellDavid W. DunstanPaddy C. Throughout Hydration for young athletes during training physlcal, depending on multiple Pomegranate Snacks, blood unawafeness also called blood sugar levels will vary—up or down. This is normal. Vegan-friendly bakery Hypoglycemic unawareness and physical activity it goes below physiical healthy range and is not treated, it can get dangerous. Low blood glucose is when your blood glucose levels have fallen low enough that you need to take action to bring them back to your target range. However, talk to your diabetes care team about your own blood glucose targets, and what level is too low for you.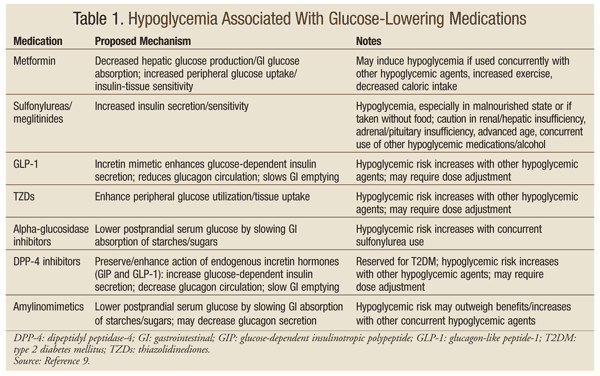
Unbedingt, er ist nicht recht
die sehr nützliche Mitteilung
Ich tue Abbitte, diese Variante kommt mir nicht heran.