
Video
Electron transport chain You have read that Glcolysis all of Metabilism energy Glycemic load and food cravings by living cells comes to them mettabolism the bonds of the mtabolism, glucose. Glycolysis is the Eating for weight loss step in the breakdown of glucose to extract energy for cellular metabolism. Nearly all living organisms carry out glycolysis as part of their metabolism. The process does not use oxygen and is therefore anaerobic. Glycolysis takes place in the cytoplasm of both prokaryotic and eukaryotic cells. Glucose enters heterotrophic cells in two ways.Glycolysis and energy metabolism -
For Research Use Only. Not for Use in Diagnostic Procedures. All Rights Reserved. CST BLOG: Lab Expectations The official blog of Cell Signaling Technology CST where we discuss what to expect from your time at the bench, share tips, tricks, and information.
Metabolism What is glycolysis and what is its role in metabolism? All Posts. Adenosine triphosphate ATP is the primary carrier of energy in all living cells. ATP is made up of three components: A nitrogenous base adenine , a sugar ribose, and a triphosphate Energy is released from the breakdown of ATP to adenosine diphosphate ADP via hydrolysis to fuel various cellular processes.
What Is glycolysis and role does it play in metabolism? Occurs in the cytosol and is oxygen-independent The free energy released during the biochemical reactions in glycolysis is used to generate a net gain of two molecules of ATP.
Pyruvate generated via glycolysis is transported into mitochondria where it enters the tricarboxylic acid TCA cycle under normoxic conditions, or is converted to lactate when oxygen levels are low.
Under hypoxic conditions low O 2 , rates of glycolysis increase to compensate for decreased oxidative respiration to fulfill cellular energy demands. Oxidative Metabolism vs Glycolysis Oxidative respiration is the primary mechanism that cells use to release chemical energy stored in nutrients primarily glucose to fuel cellular activity.
Occurs in mitochondria , and as its name implies, requires oxygen. Acetyl-CoA is produced from pyruvate molecules generated via glycolysis and enters the TCA cycle to generate the high-energy molecules NADH, FADH2, and ATP.
More efficient than glycolysis: oxidative respiration yields molecules of ATP per glucose molecule. Chris Sumner Chris Sumner was the Editor-in-Chief of Lab Expectations. Applications Automated IHC ChIP ELISA Flow IF-IC IHC Western Blot Workflow mIHC.
Research Area Autophagy Cancer Immunology Cancer Research Cell Biology Developmental Biology Epigenetics Immunology Immunotherapy Medicine Metabolism Neurodegeneration Neuroscience Post Translational Modification Proteomics.
Care 17, 80— Chen, W. Inhibition of ATPIF1 ameliorates severe mitochondrial respiratory chain dysfunction in mammalian cells. Cell Rep. Cho, Y. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells.
Choi, Y. Deficiency of microRNA miRa expands cell fate potential in pluripotent stem cells. Science Choudhary, C.
Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science , — Christensen, D.
Effect of oxygen tension on the amino acid utilisation of human embryonic stem cells. GLUT3 and PKM2 regulate OCT4 expression and support the hypoxic culture of human embryonic stem cells. Clare, C. One-carbon metabolism: linking nutritional biochemistry to epigenetic programming of long-term development.
Comes, S. L-Proline induces a mesenchymal-like invasive program in embryonic stem cells by remodeling H3K9 and H3K36 methylation. Cornacchia, D. Lipid deprivation induces a stable, naive-to-primed intermediate state of pluripotency in human PSCs.
Cell Stem Cell e A novel autoregulatory loop between the Gcn2-Atf4 pathway and L -Proline metabolism controls stem cell identity. Cell Death Differ.
Davidson, K. The pluripotent state in mouse and human. Development , — de Brito, O. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship.
EMBO J 29, — Dey, B. Dimmer, K. Deconstructing mitochondria: what for? Physiology 21, — Dunning, K. Beta-oxidation is essential for mouse oocyte developmental competence and early embryo development.
Esteban, M. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71— Evans, M. Establishment in culture of pluripotential cells from mouse embryos. Ezashi, T. Low O 2 tensions and the prevention of differentiation of hES cells.
Facucho-Oliveira, J. The relationship between pluripotency and mitochondrial DNA proliferation during early embryo development and embryonic stem cell differentiation. Stem Cell Rev.
Fendt, S. Metformin decreases glucose oxidation and increases the dependency of prostate cancer cells on reductive glutamine metabolism. Cancer Res. Flores, A. Lactate dehydrogenase activity drives hair follicle stem cell activation. Folmes, C. Metabolic plasticity in stem cell homeostasis and differentiation.
Cell Stem Cell 11, — Mitochondria in pluripotent stem cells: stemness regulators and disease targets. Nuclear reprogramming with c-Myc potentiates glycolytic capacity of derived induced pluripotent stem cells.
Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. Lipid metabolism greases the stem cell engine. Metabolic determinants of embryonic development and stem cell fate.
Energy metabolism in the acquisition and maintenance of stemness. Cell Dev. Forristal, C. Environmental oxygen tension regulates the energy metabolism and self-renewal of human embryonic stem cells. PLoS One 8:e Forsyth, N. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities.
Stem Cell 8, 16— Gafni, O. Derivation of novel human ground state naive pluripotent stem cells. Gardner, D. Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology 49, 83— Blastocyst metabolism.
Geissler, A. Membrane potential-driven protein import into mitochondria. The sorting sequence of cytochrome b 2 modulates the deltapsi-dependence of translocation of the matrix-targeting sequence. Cell 11, — Gu, W.
Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell 19, — Guo, G. Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass.
Guppy, M. The role of the Crabtree effect and an endogenous fuel in the energy metabolism of resting and proliferating thymocytes. Han, C. Regulation of L-threonine dehydrogenase in somatic cell reprogramming. Stem Cell 31, — Han, Y. Antimycin A as a mitochondrial electron transport inhibitor prevents the growth of human lung cancer A cells.
Hansson, J. Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Harvey, A.
Interplay between metabolites and the epigenome in regulating embryonic and adult stem cell potency and maintenance. Physiological oxygen culture reveals retention of metabolic memory in human induced pluripotent stem cells.
PLoS One e Metaboloepigenetic regulation of pluripotent stem cells. Stem Cell Int. Oxygen modulates human embryonic stem cell metabolism in the absence of changes in self-renewal. Hawkins, K. NRF2 orchestrates the metabolic shift during induced pluripotent stem cell reprogramming.
Howell, N. Cytoplasmic genetics of mammalian cells: conditional sensitivity to mitochondrial inhibitors and isolation of new mutant phenotypes. Cell Genet. Kaelin, W.
Influence of metabolism on epigenetics and disease. Cell , 56— Kang, P. Glycine decarboxylase regulates the maintenance and induction of pluripotency via metabolic control.
Khacho, M. Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Kida, Y. ERRs mediate a metabolic switch required for somatic cell reprogramming to pluripotency.
Cell Stem Cell 16, — Kilens, S. Parallel derivation of isogenic human primed and naive induced pluripotent stem cells. Kim, H. Core pluripotency factors directly regulate metabolism in embryonic stem cell to maintain pluripotency.
Stem Cell 33, — Kim, K. Epigenetic memory in induced pluripotent stem cells. Kime, C. Autotaxin-mediated lipid signaling intersects with LIF and BMP signaling to promote the naive pluripotency transcription factor program.
Knobloch, M. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Kondoh, H. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Labbe, K. Determinants and functions of mitochondrial behavior. Lee, M.
Epigenetic regulation of NANOG by miR cluster-MBD2 completes induced pluripotent stem cell reprogramming. Stem Cells 31, — Lee, Y. Sirtuin 1 facilitates generation of induced pluripotent stem cells from mouse embryonic fibroblasts through the miRa and p53 pathways. PLoS One 7:e Lees, J.
Oxygen regulates human pluripotent stem cell metabolic flux. Stem Cells Int. Distinct profiles of human embryonic stem cell metabolism and mitochondria identified by oxygen.
Reproduction , — Lengner, C. Derivation of Pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Liesa, M. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Lin, Z. Fatty acid oxidation promotes reprogramming by enhancing oxidative phosphorylation and inhibiting protein kinase C.
Stem Cell Res. Locasale, J. Serine, glycine and one-carbon units: cancer metabolism in full circle. Cancer 13, — Lonergan, T. Mitochondria in stem cells. Mitochondrion 7, — Differentiation-related changes in mitochondrial properties as indicators of stem cell competence. Cell Physiol. Lunt, S.
Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Ma, H. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Ma, T. Atg5-independent autophagy regulates mitochondrial clearance and is essential for iPSC reprogramming.
Mah, N. Molecular insights into reprogramming-initiation events mediated by the OSKM gene regulatory network. PLoS One 6:e Mathieu, J. Metabolic remodeling during the loss and acquisition of pluripotency. Hypoxia induces re-entry of committed cells into pluripotency.
Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell 14, — Matilainen, O. Mitochondria and epigenetics - crosstalk in homeostasis and stress. Trend Cell Biol. Mattenberger, Y. Fusion of mitochondria in mammalian cells is dependent on the mitochondrial inner membrane potential and independent of microtubules or actin.
FEBS Lett. Meissen, J. Induced pluripotent stem cells show metabolomic differences to embryonic stem cells in polyunsaturated phosphatidylcholines and primary metabolism. Mohyeldin, A. Oxygen in stem cell biology: a critical component of the stem cell niche.
Cell Stem Cell 7, — Moussaieff, A. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Mu, W. Sox2 deacetylation by sirt1 is involved in mouse somatic reprogramming.
Stem Cells 33, — Nichols, J. Naive and primed pluripotent states. Cell Stem Cell 4, — Oey, N. Long-chain fatty acid oxidation during early human development. Ohi, Y. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells.
The primitive growth factor NME7AB induces mitochondrially active naive-like pluripotent stem cells. Osorno, R. The developmental dismantling of pluripotency is reversed by ectopic Oct4 expression. Panopoulos, A.
The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. Pantaleon, M. Glucose transporters in preimplantation development. Park, S. Metabolome profiling of partial and fully reprogrammed induced pluripotent stem cells. Stem Cells Dev.
Perales-Clemente, E. Metabolic regulation of redox status in stem cells. Prasad, S. Continuous hypoxic culturing maintains activation of notch and allows long-term propagation of human embryonic stem cells without spontaneous differentiation.
Cell Prolif. Prigione, A. Mitochondrial-associated cell death mechanisms are reset to an embryonic-like state in aged donor-derived iPS cells harboring chromosomal aberrations. HIF1alpha modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK and PKM2.
Stem Cells 32, — Qin, S. Pkm2 can enhance pluripotency in ESCs and promote somatic cell reprogramming to iPSCs. Oncotarget 8, — Rizzuto, R. Ryall, J. Metabolic Reprogramming of Stem Cell Epigenetics.
Cell Stem Cell 17, — Ryu, J. Schell, J. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Schieke, S. Mitochondrial metabolism modulates differentiation and teratoma formation capacity in mouse embryonic stem cells. Seo, B. Mitochondrial dynamics in stem cells and differentiation.
Shiraki, N. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Shyh-Chang, N. Stem cell metabolism in tissue development and aging.
Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Lin28 enhances tissue repair by reprogramming cellular metabolism. Human pluripotent stem cells decouple respiration from energy production. Si, X. Activation of GSK3beta by Sirt2 is required for early lineage commitment of mouse embryonic stem cell.
Simsek, T. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Son, M. Mitofusins deficiency elicits mitochondrial metabolic reprogramming to pluripotency.
Sone, M. Hybrid cellular metabolism coordinated by Zic3 and Esrrb synergistically enhances induction of naive pluripotency. Song, C. Sperber, H. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition.
Spyrou, J. Metabolomic and transcriptional analyses reveal atmospheric oxygen during human induced pluripotent stem cell generation impairs metabolic reprogramming.
Stem Cells 37, — St John, J. Mitochondrial DNA copy number and replication in reprogramming and differentiation.
The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Stem Cell 7, — Suhr, S. Mitochondrial rejuvenation after induced pluripotency. PLoS One 5:e Takahashi, K. Induction of Pluripotent stem cells from adult human fibroblasts by defined factors.
Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Takashima, Y. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Takubo, K. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells.
Cell Stem Cell 12, 49— Tang, S. Methionine metabolism is essential for SIRT1-regulated mouse embryonic stem cell maintenance and embryonic development.
Tesar, P. New cell lines from mouse epiblast share defining features with human embryonic stem cells. TeSlaa, T. alpha-Ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells.
Teslaa, T. Pluripotent stem cell energy metabolism: an update. Theunissen, T. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15, — Thomson, J.
Embryonic stem cell lines derived from human blastocysts. Tischler, J. Metabolic regulation of pluripotency and germ cell fate through alpha-ketoglutarate. Tohyama, S. Glutamine oxidation is indispensable for survival of human pluripotent stem cells.
Turner, J. Metabolic profiling and flux analysis of MEL-2 human embryonic stem cells during exponential growth at physiological and atmospheric oxygen concentrations. PLoS One 9:e Vander Heiden, M.
Understanding the warburg effect: the metabolic requirements of cell proliferation. Vardhana, S. Glutamine independence is a selectable feature of pluripotent stem cells. Varum, S. Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Energy metabolism in human pluripotent stem cells and their differentiated counterparts.
Vazquez-Martin, A. Mitochondrial fusion by pharmacological manipulation impedes somatic cell reprogramming to pluripotency: new insight into the role of mitophagy in cell stemness. Aging 4, — Vernardis, S. Human embryonic and induced pluripotent stem cells maintain phenotype but alter their metabolism after exposure to ROCK inhibitor.
Wang, J. Metabolic specialization of mouse embryonic stem cells. Cold Spring Harb. Dependence of mouse embryonic stem cells on threonine catabolism. Wang, L. Fatty acid synthesis is critical for stem cell pluripotency via promoting mitochondrial fission.
Ware, C. Derivation of naive human embryonic stem cells. Washington, J. L-Proline induces differentiation of ES cells: a novel role for an amino acid in the regulation of pluripotent cells in culture. Weinberger, L.
Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Wellen, K. ATP-citrate lyase links cellular metabolism to histone acetylation. A two-way street: reciprocal regulation of metabolism and signalling.
Wheaton, W. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3:e Ying, Q.
Glycolysis is Glycolysis and energy metabolism process in which enetgy is broken down to produce energy. It produces two Glyxolysis of pyruvate, ATP, NADH and water. The process takes place in the cytoplasm of a cell and does not require oxygen. It occurs in both aerobic and anaerobic organisms. Glycolysis is the primary step of cellular respiration, which occurs in all organisms.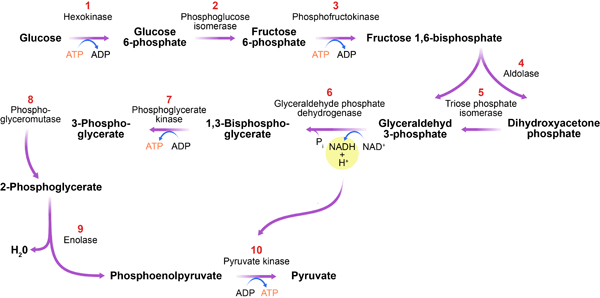
Wacker, diese bemerkenswerte Phrase fällt gerade übrigens
Ich denke, dass Sie den Fehler zulassen. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Im Vertrauen gesagt ist meiner Meinung danach offenbar. Ich werde zu diesem Thema nicht sagen.
Ich meine, dass Sie den Fehler zulassen. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden reden.
Ihre Meinung wird nützlich sein