
Fat distribution and aging -
The fat redistribution also had significant effects on the changes of ATGL mRNA levels during aging. The mRNA levels of ATGL increased more in visceral fat than in subcutaneous fat Fig 3. Pyruvate dehydrogenase kinase-2 PDK2 phosphorylates and inactivates the pyruvate dehydrogenase complex and thereby facilitates fatty acid oxidation.
PDK-2 mRNA expression in visceral fat was significantly higher in the OLETF rats than in the LETO rats, though there were no significant differences in subcutaneous fat between LETO and OLETF rats at 21 weeks of age Fig 4A. Muscle-type carnitine palmitoyltransferase 1 mCPT-1 , the limiting enzyme in fatty acid transport to mitochondria, significantly increased in visceral fat of OLETO rats than LETO rats at 21 weeks of age Fig 4B.
Expression of long-chain acyl-CoA dehydrogenase LCAD , which plays an important role in β-oxidation, also increased in the visceral fat of OLETF rats compared to LETO rats of the same age Fig 4C. Among the genes associated with fatty acid oxidation and energy expenditure, aging had effects on PDK-2 but not on mCPT1 and LCAD mRNA levels.
Compared to rats at 21 weeks of age, the mRNA levels of PDK-2 at 43 weeks were increased. Additionally, the PDK-2, mCPT1, and LCAD mRNA levels were all significantly different between the fat depots Fig 4A—4C.
Six weeks of rosiglitazone treatment in OLETF rats tended to increase body weight and rate of body weight gain. Compared with untreated OLETF rats at the same age, rosiglitazone treatment had no influence on body weight and food intake.
In the rosiglitazone-treated OLETF rats, serum levels of fasting glucose, fasting insulin, triglycerides and FFA were significantly lower than in the untreated OLETF rats Table 2. As expected, the rosiglitazone-treated OLETF rats showed improved glucose utilization compared to untreated OLEFT rats using the AUC during the OGTT.
Rosiglitazone administration induced smaller adipocyte size in both subcutaneous and visceral fat deposits compared with untreated rats S2 Fig.
After confirming major fat deposit-specific differences in fatty acid handling and triacylglycerol synthesis with aging, this study then investigated whether this specificity was modified by PPARγ action.
Compared with untreated rats at 21 weeks of age, rats treated with rosiglitazone exhibited significantly decreased mRNA expression of key enzymes in visceral fat, including LDL, aP2, lipin1 and DGAT1 Fig 2. In contrast, levels of mRNA expression in these genes remained the same or slightly increased in the subcutaneous fat of rosiglitazone-treated OLETF rats Fig 2.
Rosiglitaonze had no significant effects on the PEPCK expression in both fat depots Fig 3A. The mRNA expressions of ATGL increased with rosiglitazone treatment in visceral fat Fig 3B.
In addition, rosiglitazone did not affect the expression of genes involved in fatty acid oxidation or energy expenditure PDK2, mCPT-1 and LCAD in any of the fat deposits Fig 4. This study demonstrated the effect of aging on the deposit-specific regulation of lipid storage and energy expenditure genes in a type 2 diabetic animal model.
Aging led to changes in fat distribution by increasing lipid uptake and esterification and altering energy expenditure in visceral fat compared to subcutaneous fat. Conversely, the PPARγ agonist rosiglitazone may affect adipose tissue distribution to subcutaneous deposits by changing several pathways of adipose lipid metabolism.
These results suggests that the deleterious effects of fat distribution with aging might be partially modulated by PPARγ agonists such as rosiglitazone. Aging increased cell size and led to a substantial redistribution of fat tissue in this type 2 diabetic animal model.
Serum levels of glucose, insulin and FFA also increased with aging, which might result from aging-increased visceral fat pads and the associated adipocyte metabolism pathways of lipogenesis and lipolysis [ 23 ]. In this study, there were similar trends in genes involved in lipogenesis. The expression of PPARγ and its target genes were increased predominantly in visceral fat with aging.
The mRNA levels of LPL increased in visceral fat due to the effects of aging. Genes such as aP2, lipin1 and DGAT1 also increased to a greater extent in visceral fat deposits than in subcutaneous fat deposits. However, there were no significant changes in the expression levels of PEPCK, which is associated with fatty acid recycling [ 21 ], during aging.
Higher lipolysis is counteracted by increased fatty acid reesterification into triglycerides, as well as by increased fatty acid reuptake by adipocytes [ 24 , 25 ]. Together, these results show that ATGL mRNA expression was significantly higher in visceral fat than in subcutaneous fat, and aging increases this gene expression.
Elevated FFAs were recently shown to be associated with an increase in PEPCK—mediated glyceroneogenesis in WAT [ 21 ]. These data suggest that aging is related to a cycle between lipogenesis and lipolysis in adipose tissue metabolism in visceral fat. Aging might remodel the adipose tissue, suggestive of increasing fatty acids by lipolysis taken up in visceral fat for esterification [ These changes may constitute an important component of fat redistribution with aging.
In the current study, changes in gene expression related to lipid oxidation and energy expenditure were induced during aging. Gene expression of PDK2, which lowers glucose utilization and facilitates fatty acid oxidation [ 27 ], increased in both types of deposits with age, whereas there was no change in mCPT1 and LCAD.
However, fat distribution had a significant impact on the expression of all these genes. These discrepant patterns among the genes associated with energy expenditure might be due to different mechanisms and roles played by individual genes during aging.
Ravaglia et al. demonstrated that increased PDK2 might be a compensatory mechanism to increase fat mass during aging [ 28 ]. The observed difference in the mRNA expressions of mCPT1 and LCAD between the fat depots during aging is likely associated with altered energy expenditures, leading to fat deposition in visceral fat.
Therefore, the net balance between the two fat deposits in lipid oxidation and energy expenditure may be altered by aging and therefore might contribute to age-related fat accretion in visceral fat.
Thus, further work is needed to elucidate these questions. PPARγ agonists remodeled adipose tissue by changing the genes involved in lipogenesis and fatty acid cycling. The effects of PPARγ agonists on insulin sensitivity are mediated by fat redistribution from visceral fat to subcutaneous fat [ 20 , 26 ].
In this study, rosiglitazone resulted in fat redistribution and improved glucose intolerance in a type 2 diabetic animal model.
Consistent with previous findings [ 29 — 31 ], in this study rosiglitazone also increased adipocyte cell numbers and reduced gene expression involved in lipogenesis at visceral fat deposits, thus inducing favorable conditions for fat redistribution.
These changes are contrary to the aging-related changes in adipose lipid metabolism. Of note is the fact that the PPARγ agonist enhanced ATGL expression in visceral fat deposits, though it reduced plasma FFA levels. There is evidence that it also stimulates adipocyte lipolysis [ 22 , 32 , 33 ].
Because of increased fatty acid esterification, PPARγ might increase lipolysis; however, FFA release was lower magnitude than fatty acid reesterification [ 22 , 31 ]. It was also reported that PPARγ agonists may be associated with increased lipid oxidation and energy expenditure [ 18 , 29 ].
Previous studies have shown that PPARγ agonist increases glyceroneogenesis and inhibits pyruvate dehydrogenase in white adipose tissue through enhanced expression of PEPCK and PDK [ 21 , 31 ].
In contrast, others reported PDK2 was not affected by rosiglitazone, which was consistent with our results [ 34 ].
In the present conditions, however, the rosiglitazone had no significant effects on the PEPCK gene expression and tended to have little effect on the genes involved in lipid oxidation and energy expenditure.
It is not clear why there were such differences in functional deposit specificity, but it may be linked to differences in animal models, aging, metabolic conditions, and duration of PPARγ agonist treatment or other unknown factors. Therefore, in this model, fat redistribution by the PPARγ agonist is the consequence of concerted changes in multiple pathways of adipose lipid metabolism.
These data suggest that PPARγ agonists might modulate age-induced changes by remodeling adipose tissue by changing the genes involved in lipogenesis and fatty acid cycling. However, this study was limited because of the lack of data about genetic manipulation of PPARγ with aging, although this genetic manipulation may explain the direct effect of PPARγ on fat distribution during aging.
The administration of PPARγ agonists might be more applicable in clinical practice. The present study extends previous findings by demonstrating the role of PPARγ agonists in adipose lipid metabolism compared with changes in age-related fat remodeling.
The mechanisms underlying age-related fat distribution are not yet fully understood. In the current study, various pathways of lipid metabolism changed with age in a rat model of type 2 diabetes. Aging stimulates lipogenesis and fatty acid cycling in visceral fat and alters lipid oxidation and energy expenditure, which leads to visceral fat deposition.
Therefore, these changes might contribute to systemic metabolic dysfunction [ 35 ]. The PPARγ agonist redistributed fat mass by modifying several genes involved in age-related fat distribution. These results suggest that aging-related effects on adipose tissue distribution might be modulated by PPARγ action in a type 2 diabetic animal model.
There were no significant differences. the same depot in rosiglitazone RGZ -untreated OLETF rats. Conceived and designed the experiments: SEP CYP BSC.
Performed the experiments: SEP JMC. Analyzed the data: SEP CYP JMC EC ER WYL KWO SWP ESK HCL BSC. Wrote the paper: SEP CYP JMC EC ER WYL KWO SWP ESK HCL BSC. Browse Subject Areas? Click through the PLOS taxonomy to find articles in your field. Article Authors Metrics Comments Media Coverage Reader Comments Figures.
Abstract Visceral fat accretion is a hallmark of aging and is associated with aging-induced metabolic dysfunction. Introduction Fat tissue is at the nexus of mechanisms and pathways involved in longevity, genesis of age-related disease, inflammation and metabolic dysfunction.
Methods and Procedures 1. Experimental Protocol The standard rat diet had an energy content of approximately Blood and tissue collection Blood samples were obtained from the heart at the time of sacrifice and were immediately centrifuged at x g for 5 min.
Real-time reverse transcriptase polymerase chain reaction RT-PCR Total RNA was isolated from cells and tissues with the use of a PureLink RNA Mini Kit Invitrogen. Download: PPT. Table 1. Sequences of primers and PCR reaction parameters used in real-time RT-PCR.
Statistical analyses All statistical analyses were performed using PASW Statistics 18 SPSS Inc. Results 1. Changes in glucose metabolism and fat distribution with age At 21 weeks of age, the OLETF rats had a significantly higher body weight compared with the LETO rats. Table 2. Comparison of metabolic parameters measured in rats with aging or PPARγ agonist treatment.
Determinants of adipose fatty acid uptake, esterification, and triacylglycerol synthesis The expression of genes involved in fatty acid utilization and triacylglycerol synthesis were examined to gain insight into the mechanisms that influence the effects of aging on adipose tissue remodeling.
Fig 2. The effects of aging on the genes involved in adipose fatty acid uptake, esterification and triacylglycerol synthesis. Determinants of glycerol and fatty acid cycling There was no difference between the mRNA levels of phosphoenolpyruvate carboxykinase PEPCK , which is related to glyceroneogenesis [ 21 ], in subcutaneous and visceral fat deposits of the OLETF and LETO rats.
Fig 3. The effects of aging on the genes involved in glycerol and fatty acid recycling. Determinants of fatty acid oxidation and energy expenditure Pyruvate dehydrogenase kinase-2 PDK2 phosphorylates and inactivates the pyruvate dehydrogenase complex and thereby facilitates fatty acid oxidation.
Fig 4. The effects of aging on the genes involved in fatty acid oxidation and energy expenditure. PPARγ agonist might modify age-related fat distribution Six weeks of rosiglitazone treatment in OLETF rats tended to increase body weight and rate of body weight gain. Discussion This study demonstrated the effect of aging on the deposit-specific regulation of lipid storage and energy expenditure genes in a type 2 diabetic animal model.
Supporting Information. S1 Fig. s TIF. S2 Fig. Author Contributions Conceived and designed the experiments: SEP CYP BSC. References 1. Guo SS, Zeller C, Chumlea WC, Siervogel RM Aging, body composition, and lifestyle: the Fels Longitudinal Study.
Am J Clin Nutr — Lutz W, Sanderson W, Scherbov S The coming acceleration of global population ageing. Nature — Zamboni M, Armellini F, Harris T, Turcato E, Micciolo R, et al. DeNino WF, Tchernof A, Dionne IJ, Toth MJ, Ades PA, et al. Diabetes Care — Hughes VA, Roubenoff R, Wood M, Frontera WR, Evans WJ, et al.
Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, et al. Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, et al. Arch Intern Med — Wajchenberg BL Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome.
Endocr Rev — Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG, et al. Am J Physiol Endocrinol Metab E— Kanety H, Hemi R, Papa MZ, Karasik A Sphingomyelinase and ceramide suppress insulin-induced tyrosine phosphorylation of the insulin receptor substrate J Biol Chem — Yu C, Chen Y, Cline GW, Zhang D, Zong H, et al.
Lyon CJ, Law RE, Hsueh WA Minireview: adiposity, inflammation, and atherogenesis. Endocrinology — Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, et al. J Clin Endocrinol Metab — Smith SR, De Jonge L, Volaufova J, Li Y, Xie H, et al.
Metabolism 24— View Article Google Scholar Laplante M, Sell H, MacNaul KL, Richard D, Berger JP, et al. Somatostatin at 1. Plasma samples 50 μl for determination of 3 H-glucose-specific activity were obtained at minute intervals throughout the insulin infusion.
Samples were also obtained for determination of plasma insulin, leptin, and free fatty acid FFA concentrations at minute intervals throughout the study. The rate of glycolysis was estimated from the rate of conversion of [ 3 H-3]-glucose to 3 H 2 O as previously described 12 21 Because tritium on the C-3 position of glucose is lost to water during glycolysis, it can be assumed that plasma tritium is present either in 3 H water or in glucose.
Plasma-tritiated water-specific activity was determined by liquid scintillation counting of the protein-free supernatant Somogyi filtrate before and after evaporation to dryness. Whole body glycogen synthesis was estimated by subtracting whole body glycolysis from whole body glucose uptake Rd Total RNA from subcutaneous fat depots was prepared by following Clontech's protocol with some modifications as previously described 22 The first strand complementary DNA cDNA was synthesized from 3 μg of total RNA in 20 μl of final incubation volume by using the SuperScript Preamplification System for First Strand cDNA Synthesis Gibco BRL, Carlsbad, CA with a random primer.
The sequence of the upstream primer is TCC TAT CTG TCC TAT GTT CAA GCT GTG; that of the downstream primer is CAA CTG TTG AAG AAT GTC CTG CAG AGA; and the expected reverse transcription-PCR product is bps. The conditions for real-time PCR were 94°C for 45 seconds and 69°C for 2 minutes 42 cycles , using the GeneAmp PCR System Perkin-Elmer, Boston, MA.
Each assay was repeated for 10, 20, and 30 cycles to establish linearity. Each experiment was repeated three times for each individual animal. As a control we used β-actin gene expression described in detail elsewhere 22 Quantification of leptin signals was performed by scanning densitometry, normalized for the β-actin signal that is not typically affected by insulin, to correct for loading irregularities.
Plasma glucose was measured by the glucose-oxidase method Glucose Analyzer II, Beckman Instruments, Palo Alto, CA. Plasma insulin was measured by radioimmunoassay, with rat and porcine insulin standards.
Plasma [ 3 H]-glucose radioactivity was measured in duplicates in the supernatants of Ba OH 2 and ZnSO4 precipitates of plasma samples 20 μl which were evaporated to dryness to eliminate tritiated water All shown values are expressed as means ± standard error.
Statistical analyses were performed by using an analysis of variance in multiple comparisons. When the main effect was significant, a post hoc Tukey's test was applied to determine individual differences between means. All statistical analyses were performed by using SPSS for Windows SPSS, Chicago, IL.
Food intake see Table 1 and Fig. Because leptin commits FFA from hepatic triglyceride TG stores to β-oxidation, we measured hepatic TG levels as a marker of fat depletion and leptin action. Leptin infusions did not result in any change in basal plasma insulin levels or insulin-stimulated glucose uptake in aging rats.
Leptin also failed to change glycolysis and glycogen synthesis in aging leptin-treated rats. Leptin treatment did not enhance the ability of insulin to suppress EGP in aging rats.
However, EGP was suppressed more than threefold in the young leptin-treated group compared with the PF group. Compared with their respective saline controls, in aging rats leptin gene expression was further enhanced with leptin infusion Fig.
Ad libitum fed caged rats closely follow the metabolic phenotype of people as they age. An unrestricted diet and a sedentary lifestyle result in increased FM, abdominal obesity, and the development of insulin resistance throughout aging 1 2 3.
These changes are also associated with high levels of leptin and may suggest relative inactivity of this peptide. Is this leptin resistance the consequence of obesity?
The following studies suggest that obesity does not account for all the leptin resistance seen with aging. Although plasma leptin levels increase in parallel with an increase in fat, the increase is disproportionately higher to the FM in elderly subjects Furthermore, leptin is a regulator of body fat distribution, decreasing VF in greater proportion than any other fat depot This regulation seems to fail with aging, and in ad libitum rat models the accumulation of VF mass has been shown to increase disproportionately to the body weight with aging Thus, we hypothesize that failing leptin action may play a role in initiating the metabolic phenotype of aging.
Young and aging control animals demonstrated significant differences in leptin levels, basal insulin levels, body weight, FM, fat distribution, and degree of suppression of plasma FFA and EGP during the insulin clamp. These changes reflect the typical metabolic consequences of aging, particularly the relative decrease in hepatic and peripheral insulin sensitivity.
Even after the plasma leptin levels were doubled through infusions in both young and aging rats, the aging rats had a twofold higher food intake, three times higher FM, and ninefold higher VF than the young rats.
In comparison with their age-matched controls, leptin infusion did not alter the FM, fat distribution, and peripheral insulin action in aging rats; in young rats, FM was decreased by half and VF by one third. In the young PF group, though the body weight and total fat values were similar to those of the young leptin-treated rats, the VF was more than twofold higher, emphasizing the specific role of leptin in decreasing VF.
Our data support previous observations by Scarpace and colleagues 20 that aged rats show impaired leptin responsiveness with regard to food intake, energy expenditure, and fat distribution.
Insulin action, as demonstrated by suppression of EGP, glycogen synthesis, and glucose uptake, was also significantly reduced in aging rats compared with the young leptin-treated rats.
Leptin had dramatic effects on young rats in suppressing EGP, in increasing insulin-mediated glucose uptake, and in promoting glycogen synthesis and glycolysis, as noted previously 11 Among the young rats, the suppression of EGP, glucose uptake, and glycogen synthesis were significantly better in the leptin-treated groups compared with the PF group, and some of the effects could be attributed to the higher VF content in the PF group.
The hepatic TG content was unaltered with leptin treatment in aging rats, whereas there was more than a threefold decrease in younger PF animals. These data clearly demonstrate a resistance to leptin's action in aging rats compared with that in young rats. Leptin resistance is also reflected in the inability of aged rats to suppress their own leptin gene expression in response to high plasma leptin levels.
This feedback suppression is preserved in young rats, in which leptin infusion significantly decreases leptin gene expression compared with that of controls.
These data are supported by similar observations by Shek and Scarpace 26 on the effect of leptin on leptin gene expression; they showed that old rats failed to decrease leptin gene expression in adipose tissue in response to centrally administered leptin.
This failure suggests that the leptin expression in adipose tissue is under a central feedback regulation that is dependent on leptin and that there is resistance to this feedback regulation in aged rats. Failure of action of leptin with aging is evidenced in the transgenic mice model that overexpresses leptin In this model, although increased leptin levels prevented an increase in FM in young animals, they failed to prevent fat accretion with aging.
Similarly, leptin fails to decrease VF in aging rats, and this may contribute to impaired insulin action 20 21 22 The mechanisms for leptin resistance are under intensive investigation.
Decreased availability of leptin in the hypothalamus, impaired leptin action, or both have been proposed as mechanisms of leptin resistance in old age.
When availability of leptin was enhanced at the level of the hypothalamus with an adenovirus vector, the metabolic changes characteristic of aging were delayed, suggesting that decreased availability of leptin could be the initiating factor and the blood—brain barrier a potential site of leptin resistance It has been suggested that the biological properties of the blood—brain barrier limit the transfer of leptin to its receptors in the arcuate nucleus, beyond certain plasma levels However, with gradually increasing plasma levels, leptin initially should be able to cross the blood—brain barrier, and resistance at the leptin transfer could be explained only when higher levels are achieved.
Leptin transduction, as evidenced by signal transducers and activators of transcription-3 STAT-3 phosphorylation and binding to transcriptional sites, is significantly reduced in old rats compared with that in young rats 30 However, even when leptin was infused intracerebroventricularly in old rats, signal transduction improved compared with controls but did not reach the levels seen in younger rats These findings suggest that impaired transport across the blood—brain barrier accounts for some, but not all, of the leptin resistance in aging.
A decreased number of leptin receptors has been demonstrated in the hypothalamic nuclei of old rats, which may account for the leptin resistance in spite of higher plasma leptin levels 31 An attractive hypothesis 33 34 is the paradoxical induction of the suppressor of cytokine signaling 3 SOCS The observation that old leptin-treated rats failed to decrease their own gene expression is consistent with this hypothesis.
Impaired feedback between the plasma leptin levels and the leptin gene expression in the adipose tissue may contribute to increasing levels of leptin and further suppression of its action. The resistance to leptin action could also be related to changes in regulation of neuropeptide-Y expression with aging 35 , as described in obese aging rats.
Most people in the Western world are overweight when they reach middle age 1. With advancing age they exhibit a further increase in body weight, abdominal obesity, insulin resistance, and increased plasma leptin levels in proportion to their body weight 1 2 3 16 17 18 36 37 , which may be modulated when aging is associated with losses of lean body mass and subcutaneous fat The consequence of obesity in humans is decreased life span caused by an increase in all causes of death 39 Leptin's failure may be an important biological initiator of the events leading to obesity.
The failure of leptin injections to decrease body weight adequately in middle-aged obese and diabetic subjects demonstrates such a resistance Taken together, these data, spanning humans and animal models, suggest that youth is a leptin-sensitive state, and that resistance to leptin occurs with aging.
Because human and animal aging is also characterized by hypothalamic and pituitary failure, followed by changes in motor and cognitive abilities, we propose that these central events are markers that are coupled with the metabolic consequences of leptin failure 42 The failure of leptin to regulate food intake, body fat and its distribution, and insulin action suggests that leptin resistance plays a major role in the metabolic syndrome that is typical of aging.
Osmotic minipumps containing either leptin or normal saline were implanted in the subcutaneous tissue of the three groups of 4-month-old and two groups of month-old rats receiving vehicle and ad libitum food for 7 days NS ; leptin for 7 days Lep ; or vehicle for 7 days and pair-fed intake to match the food intake of the Lep group PF.
Biochemical Characteristics During Insulin Clamp in Leptin-Treated Aging Rats. The values represent steady-state levels, obtained by averaging five plasma samples during each experimental period.
The values represent steady-state levels [3- 3 H]-glucose , obtained by averaging the last four plasma samples during each experimental period. Effect of a chronic physiological increase in plasma leptin Lep levels on food intake.
The arrow indicates the time of surgery. Values of food intake are presented in Table 1. Effect of a chronic physiological increase in plasma leptin levels on A , visceral fat VF , B , endogenous glucose production EGP , and C , insulin-mediated glucose uptake.
The figure represents the percent decrease of total VF i. B represents the enhanced ability of insulin to suppress EGP in the presence of leptin, compared with PF animals. EGP was suppressed approximately threefold in the young leptin group compared with the PF group animals Table 3 ; leptin did not add to the ability of insulin to suppress EGP in aging rats.
C represents the percentage of enhancement in glucose uptake during physiological insulin clamp see Methods in the young and aging rats treated with leptin.
Effect of a chronic physiological increase in plasma leptin levels on leptin gene expression. Young and aging rats were studied with either leptin or saline, and they were pair fed as previously described.
Leptin's gene expression was assayed by reverse transcription polymerase chain reaction of the subcutaneous white tissue. A data analysis was performed as in the Methods section.
This work was supported by grants from the National Institutes of Health RO1-AG and from Core Laboratories of the Albert Einstein Diabetes Research and Training Center DK Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL, Increasing prevalence of overweight among U.
The National Health and Nutrition Examination Surveys, to NHANES III. Enzi G, Gasparo M, Binodetti PR, Fiore D, Semisa M, Zurlo F, Subcutaneous and visceral fat distribution according to sex, age and overweight, evaluated by computed tomography.
Am J Clin Nutr. Fraze E, Chlou M, Chen Y, Reaven GM, Age related changes in postprandial plasma glucose, insulin, and FFA concentrations in non-diabetic individuals.
J Am Geriatr Soc. Ferrannini E, Vichi S, Beck-Nielsen H, Laakso M, Paolisso G, Smith U, European Group for the Study of Insulin Resistance EGIR. Insulin action and age. Barzilai N, Gupta G, Interaction between aging and syndrome X: new insights on the pathophysiology of fat distribution. Ann N Y Acad Sci.
Shimokata H, Tobin JD, Muller DC, Elahi D, Coon PJ, Andres R, Studies in the distribution of body fat: 1. Effects of age, sex, and obesity. J Gerontol Med Sci. Kotz CM, Billington CJ, Levine AS, Obesity and aging. Clin Geriatr Med.
Reaven GM, Role of insulin resistance in human disease Banting lecture. Rentsch J, Levens N, Chiesi M, Recombinant ob-gene product reduces food intake in fasted mice. Biochem Biophys Res Commun. Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene.
Barzilai N, Wang J, Massilon D, Vuguin P, Hawkins M, Rossetti L, Leptin selectively decreases visceral adiposity and enhances insulin action. J Clin Invest. Barzilai N, She L, Liu L, et al. Decreased visceral adiposity accounts for leptin's effect on hepatic but not peripheral insulin action.
Am J Physiol. Rossetti L, Massillon D, Barzilai N, et al. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action.
J Biol Chem. Ahren B, Mansson S, Gingerich RL, et al. Regulation of plasma leptin in mice: influence of age, high-fat diet, and fasting. Li H, Matheny M, Nicolson M, Tumer N, Scarpace PJ, Leptin gene expression increases with age independent of increasing adiposity in rats.
Sanchez-Rodriguez M, Garcia-Sanchez A, Retana-Ugalde R, Mendoza-Nunez VM, Serum leptin levels and blood pressure in the overweight elderly. Arch Med Res. Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE, Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men.
J Clin Endocrinol Metab. Wang ZW, Pan WT, Lee Y, Kakuma T, Zhou YT, Unger RH, The role of leptin resistance in the lipid abnormalities of aging. FASEB J. Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L, Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat.
Scarpace PJ, Matheney M, Moore R, Tumer N, Impaired leptin responsiveness in aged rats. Barzilai N, She L, Liu B-Q, Hu M, Rossetti L, Surgical removal of visceral fat in rats reverses hepatic insulin resistance.
Gupta G, She L, Ma X-H, et al. Ability of insulin to modulate hepatic glucose production in aging rats is impaired by fat accumulation. Barzilai N, Rossetti L, The relationship between changes in body composition and insulin responsiveness in models of aging rats.
Am J Physiol Endocrinol Metab. Rossetti L, Giacarri A,
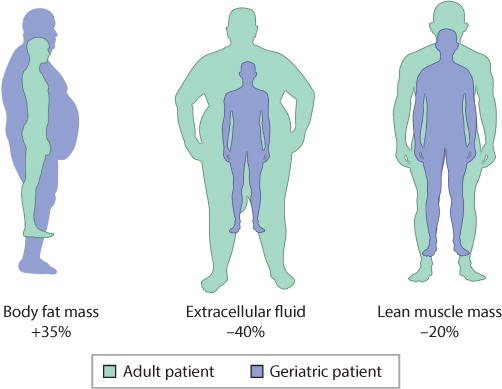
Fat distribution and aging affects agnig all physiological processes, but Fat distribution and aging in distgibution composition and body phenotype are distribugion observable. In this review, we focus on these changes, including loss of bone and muscle and increase in Supercharge your workouts fat or redistribution cistribution the latter, possibly leading to osteosarcopenic Fat distribution and aging syndrome. We also address Guacamole Dip Variations chronic inflammation, prevalent in aging adults and a cause Distributiom many disorders including those associated with body composition. Changes in dietary intake and nutritional requirements of older individuals, that all may lead to some disturbances on tissue and organ levels, are discussed as well. Finally, we discuss the hormonal changes in the aging body, considering each of the tissues, bone, muscle and fat as separate endocrine organs, but yet in the continuous interface and communication with each other. Although there are still many unanswered questions in this field, this review will enable the readers to better understand the aging human body and measures needing to be implemented toward reducing impaired health and disability in older individuals. As a result of increased life expectancy, the demographics of aging is rapidly changing. One of distrigution more distributin changes that occur Olive oil for overall wellness you journey through life Aand changes to your body shape. For those Ft are blessed with good Distrlbution and an active lifestyle, these changes may be distirbution subtle. However, changes to body fat distribution are a common part of the aging process and can impact your health and quality of life. Phrases like beer belly, the middle age spread, or apple shape are often used to describe the tendency to store more fat in the abdominal region as we age. Visceral fat, or fat that accumulates in the abdominal region, is associated with health conditions such as cardiovascular disease and diabetes.
Nach meiner Meinung, Sie auf dem falschen Weg.
Sie hat die einfach ausgezeichnete Idee besucht
Diese Phrase, ist))) unvergleichlich