Visceral fat and aging -
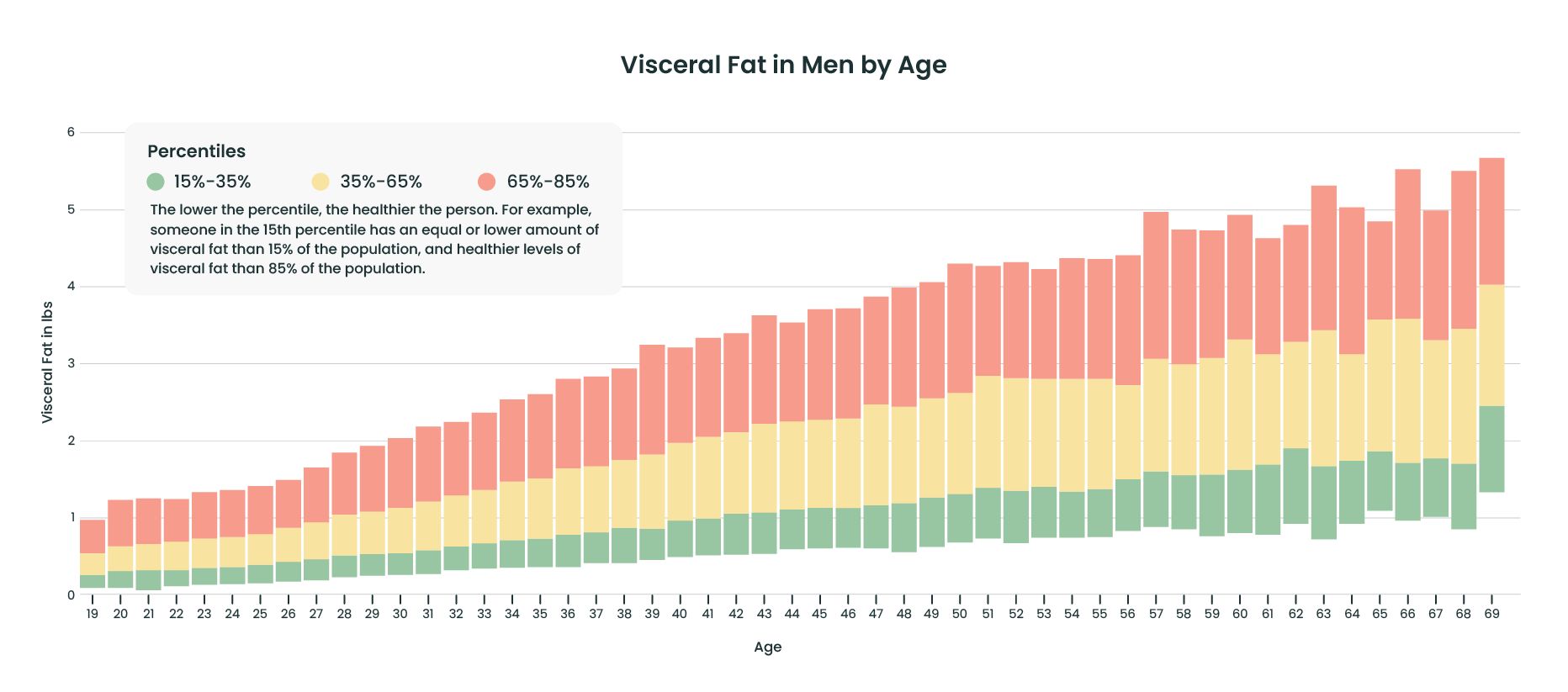
Rather than focus on a single reading or absolute cut-off, keep an eye on whether your waist is growing are your pants getting snug at the waist? That should give you a good idea of whether you're gaining unhealthy visceral fat. Visceral fat can be measured in a variety of ways.
CT scans and full-body MRIs are the most precise, but they are expensive and rarely available, so investigators often use estimates based on waist circumference or waist size in proportion to height see "Gut check".
To ensure that they're not just measuring overall obesity, researchers also check whether a person's waist circumference is higher than average for her or his body mass index BMI.
Cardiovascular disease. Several studies have documented this effect. For example, a large study of European women ages 45 to 79 concluded that those with the biggest waists and those with the largest waists in relation to their hip size had more than double the risk of developing heart disease.
The risk was still nearly double even after adjustment for several other risk factors, including blood pressure, cholesterol, smoking, and BMI. Higher visceral-fat volume also has a deleterious impact on several other heart disease risk factors.
It's associated with higher blood pressure, blood sugar levels and triglyceride levels, and lower levels of HDL good cholesterol. Taken together, these changes, known as metabolic syndrome, create a serious risk for cardiovascular disease and type 2 diabetes. Researchers at Kaiser Permanente found that people in their early 40s with the highest levels of abdominal fat, compared with those who had the least abdominal fat at that age, were nearly three times more likely to develop dementia including Alzheimer's disease by their mids to early 80s.
Dementia was not associated with increased thigh size. The risks were highest for women who were both large-waisted and overweight or obese. The investigators believe that belly fat raises the risk of asthma more than other poundage because it has inflammatory effects throughout the body, including in the airways.
Breast cancer. A combined analysis of several studies found that premenopausal women with abdominal obesity the largest waist size in proportion to their height were at greater risk for breast cancer. Large waists were also linked to breast cancer risk among postmenopausal women, but that effect was not significant once BMI was taken into account.
Colorectal cancer. People with the most visceral fat have three times the risk of developing colorectal adenomas precancerous polyps than those with the least visceral fat.
The relationship was found after many other risks were accounted for. The researchers also confirmed that adenomatous polyps in the colon are associated with insulin resistance, which may be the mechanism that increases the cancer risk.
Where you tend to gain fat depends on your genes, your hormones, your age, your birth weight smaller babies more readily add belly fat later in life , and whether you've had children women who have given birth tend to develop more visceral fat than women who haven't.
As young adults, women on average have less visceral fat than men, but that changes with menopause. You can't change your birth weight or your genes, and you can't hold off menopause.
But there are several ways you can minimize the accumulation of visceral fat. The good news is that because it's more readily metabolized into fatty acids, it responds more efficiently to diet and exercise than fat on the hips and thighs.
Here are some approaches that may help:. Keep moving. Exercise can help reduce your waist circumference. Even if you don't lose weight, you lose visceral belly fat and gain muscle mass. Engage in at least 30 minutes of moderate-intensity activity most days, such as brisk walking or bicycling at a casual pace.
Also create opportunities to add motion to routine tasks. For example, park farther from your destination and walk the rest of the way, take the stairs instead of the elevator, and stand while you talk on the phone.
Studies have shown that you can help trim visceral fat or prevent its growth with both aerobic activity such as brisk walking and strength training exercising with weights.
Spot exercises, such as sit-ups, can tighten abdominal muscles but won't get at visceral fat. Exercise can also help keep fat from coming back.
Eat right. Choose a balanced diet that helps you achieve and maintain a healthy weight. Avoid products that seem to encourage belly fat deposition, especially simple sugars like fructose-sweetened foods and beverages.
Don't smoke. The more you smoke, the more likely you are to store fat in your abdomen rather than on your hips and thighs.
Shown are results for young 2-month-old and old month-old F1 hybrids of F and Brown Norway rats. Hepatic insulin sensitivity in aging rats. The ability of insulin to suppress EGP was studied using glucose tracer methodology see research design and methods.
R a , rate of EGP. Development of diabetes and the regrowth of VF in ZDF rats. Six rats from each group were studied using a pancreatic clamp, and the rest were monitored for 4 months until they developed diabetes. Gene expression of TNF-α and leptin. RT and real-time PCR analysis for TNF-α, leptin, and β-actin are described in research design and methods.
B : Analysis of all real-time PCR data obtained in all rats, corrected for intensity of β-actin and presented in arbitrary units. mesenteric fat. Gene expression of resistin A and ACRP30 B. B : Real-time PCR data obtained from all rats corrected for the intensity of β-actin and presented in arbitrary units.
M and SC. young 2-month-old , and old month-old F1 hybrids of F and Brown Norway rats were used in these experiments. The table depicts the amounts of VF or SC removed at the surgery, body weight, lean body mass, non-VF fat mass, total VF, and epididymal, perinephric, and mesenteric fat, which were determined at killing after the experiments.
Plasma glucose, insulin, FFA concentrations, basal rate of EGP R a , glucose infusion rate GIR , glycolysis Gly , and glycogen synthesis GS during the basal period and during the pancreatic euglycemic clamp are shown.
These parameters were measured at basal conditions and during the insulin clamps see group description in research design and methods. The table depicts the amounts of VF removed at surgery, body weight, lean body mass, non-VF fat mass, and total VF, epididymal, perinephric, and mesenteric fat that were detected after the study.
This work was supported by grants from the National Institutes of Health Paul Beeson Physician Faculty Scholar in Aging Award and RO1-AG to N. and RDK and ROI-DK to L. Address correspondence and reprint requests to Nir Barzilai, Institute for Aging Research, Belfer Bldg.
E-mail: barzilai aecom. CR, caloric restriction; dsDNA, double-stranded DNA; EGP, endogenous glucose production; FFA, free fatty acid; SC, subcutaneous; TNF-α, tumor necrosis factor-α; VF, visceral fat. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest.
filter your search All Content All Journals Diabetes. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 51, Issue Previous Article Next Article.
RESEARCH DESIGN AND METHODS. Article Information. Article Navigation. Obesity Studies October 01 Removal of Visceral Fat Prevents Insulin Resistance and Glucose Intolerance of Aging : An Adipokine-Mediated Process?
Ilan Gabriely ; Ilan Gabriely. This Site. Google Scholar. Xiao Hui Ma ; Xiao Hui Ma. Xiao Man Yang ; Xiao Man Yang. Gil Atzmon ; Gil Atzmon. Michael W. Rajala ; Michael W. Anders H. Berg ; Anders H. Phillip Scherer ; Phillip Scherer.
Luciano Rossetti ; Luciano Rossetti. Nir Barzilai Nir Barzilai. Diabetes ;51 10 — Get Permissions. toolbar search Search Dropdown Menu.
toolbar search search input Search input auto suggest. View large Download slide. TABLE 1 Body composition and fat distribution of aging rats. young and CR;. All data are presented as means ± SE. View Large. TABLE 2 Metabolic characteristics of aging rats.
TABLE 3 Body composition and fat distribution of ZDF rats. TABLE 4 Metabolic characteristics of ZDF rats. Cefalu WT, Wang ZQ, Werbel S, Bell-Farrow A, Crouse JR 3rd, Hinson WH, Terry JG, Anderson R: Contribution of visceral fat mass to the insulin resistance of aging.
Shimokata H, Tobin JD, Muller DC, Elahi D, Coon PJ, Andres R: Studies in the distribution of body fat. Effects of age, sex, and obesity. J Gerontol. Ferrannini E, Natali A, Capaldo B, Lehtovirta M, Jacob S, Yki-Jarvinen H: Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity: European Group for the Study of Insulin Resistance EGIR.
Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW: Visceral adiposity and incident coronary heart disease in Japanese-American men: the year follow-up results of the Seattle Japanese-American Community Diabetes Study.
Diabetes Care. Lamarche B: Abdominal obesity and its metabolic complications: implications for the risk of ischaemic heart disease. Coron Artery Dis. Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ: Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM.
Williamson JR, Kreisberg RA, Felts PW: Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A.
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA: The hormone resistin links obesity to diabetes.
Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM: IRSmediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance.
Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L: Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest.
Masoro EJ: Possible mechanisms underlying the antiaging actions of caloric restriction. Toxicol Pathol. Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, Rossetti L: Surgical removal of visceral fat reverses hepatic insulin resistance.
Barzilai N, Hawkins M, Angelov I, Hu M, Rossetti L: Glucosamine-induced inhibition of liver glucokinase impairs the ability of hyperglycemia to suppress endogenous glucose production.
Hawkins M, Barzilai N, Liu R, Hu M, Chen W, Rossetti L: Role of the glucosamine pathway in fat-induced insulin resistance. Barzilai N, Rossetti L: Relationship between changes in body composition and insulin responsiveness in models of the aging rat.
Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. Berger JJ, Barnard RJ. Effect of diet on fat cell size and hormone-sensitive lipase activity. J Appl Physiol.
Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Copps KD, White MF. Tanti JF, Ceppo F, Jager J, Berthou F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance.
Front Endocrinol. Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Nakajima I, Muroya S, Tanabe R, Chikuni K. Positive effect of collagen V and VI on triglyceride accumulation during differentiation in cultures of bovine intramuscular adipocytes.
Hasegawa Y, Ikeda K, Chen Y, Alba DL, Stifler D, Shinoda K, et al. Repression of adipose tissue fibrosis through a PRDMGTF2IRD1 complex improves systemic glucose homeostasis. Cell Metab. O'Hara A, Lim FL, Mazzatti DJ, Trayhurn P. Microarray analysis identifies matrix metalloproteinases MMPs as key genes whose expression is up-regulated in human adipocytes by macrophage-conditioned medium.
Pflugers Arch. Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell. Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, et al.
JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA.
Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue.
Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al.
Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Davies LC, Taylor PR. Tissue-resident macrophages: then and now. Hassnain Waqas SF, Noble A, Hoang AC, Ampem G, Popp M, Strauß S, et al.
Adipose tissue macrophages develop from bone marrow-independent progenitors in. J Leukoc Biol. Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR.
Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Jang JE, Ko MS, Yun JY, Kim MO, Kim JH, Park HS, et al.
Nitric oxide produced by macrophages inhibits adipocyte differentiation and promotes profibrogenic responses in preadipocytes to induce adipose tissue fibrosis.
Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG, Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Jha AK, Huang SC, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, et al.
Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, et al. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al.
Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity.
Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice.
Allman WR, Dey R, Liu L, Siddiqui S, Coleman AS, Bhattacharya P, et al. TACI deficiency leads to alternatively activated macrophage phenotype and susceptibility to Leishmania infection.
Liu L, Inouye KE, Allman WR, Coleman AS, Siddiqui S, Hotamisligil GS, et al. TACI-Deficient macrophages protect mice against metaflammation and obesity-induced dysregulation of glucose homeostasis.
Spadaro O, Camell CD, Bosurgi L, Nguyen KY, Youm YH, Rothlin CV, et al. IGF1 shapes macrophage activation in response to immunometabolic challenge. Cell Rep. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment.
FPrime Rep. Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Bertola A, Ciucci T, Rousseau D, Bourlier V, Duffaut C, Bonnafous S, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients.
Jaitin DA, Adlung L, Thaiss CA, Weiner A, Li B, Descamps H, et al. Lipid-associated macrophages control metabolic homeostasis in a trem2-dependent manner. Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al.
Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Hill DA, Lim HW, Kim YH, Ho WY, Foong YH, Nelson VL, et al. Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue.
Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sánchez NM, Mahú I, et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine.
Nat Med. Lumeng CN, Liu J, Geletka L, Delaney C, Delproposto J, Desai A, et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue.
J Immunol. Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, Wing A, et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Camell CD, Günther P, Lee A, Goldberg EL, Spadaro O, Youm YH, et al.
Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells.
Yudanin NA, Schmitz F, Flamar AL, Thome JJC, Tait Wojno E, Moeller JB, et al. Spatial and temporal mapping of human innate lymphoid cells reveals elements of tissue specificity.
Fuchs A. ILC1s in tissue inflammation and infection. O'Sullivan TE, Rapp M, Fan X, Weizman OE, Bhardwaj P, Adams NM, et al.
Adipose-resident group 1 innate lymphoid cells promote obesity-associated insulin resistance. Boulenouar S, Michelet X, Duquette D, Alvarez D, Hogan AE, Dold CJ. Adipose type one innate lymphoid cells regulate macrophage homeostasis through targeted cytotoxicity. Kim BS, Artis D. Group 2 innate lymphoid cells in health and disease.
Cold Spring Harb Perspect Biol. Montanari T, Pošćić N, Colitti M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: a review. Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity.
Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate ILexpressing cells in type 2 immunity.
Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, et al.
Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Wang H, Shen L, Sun X, Liu F, Feng W, Jiang C, et al.
Adipose group 1 innate lymphoid cells promote adipose tissue fibrosis and diabetes in obesity. Nat Commun. O'Rourke RW, Meyer KA, Neeley CK, Gaston GD, Sekhri P, Szumowski M, et al. Systemic NK cell ablation attenuates intra-abdominal adipose tissue macrophage infiltration in murine obesity. Wensveen FM, Jelencic V, Valentic S, Sestan M, Wensveen TT, Theurich S, et al.
NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Revelo XS, Tsai S, Lei H, Luck H, Ghazarian M, Tsui H, et al. Perforin is a novel immune regulator of obesity-related insulin resistance.
O'Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue.
O'Rourke RW, Gaston GD, Meyer KA, White AE, Marks DL. Adipose tissue NK cells manifest an activated phenotype in human obesity. Lee BC, Kim MS, Pae M, Yamamoto Y, Eberlé D, Shimada T, et al. Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity.
Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses.
Theurich S, Tsaousidou E, Hanssen R, Lempradl AM, Mauer J, Timper K, et al. Starr ME, Saito M, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation-II: the role of IL-1β in age-dependent IL-6 upregulation in adipose tissue.
J Gerontol A Biol Sci Med Sci. Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol.
Lacy P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin Immunol. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. Elgazar-Carmon V, Rudich A, Hadad N, Levy R.
Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. Revelo XS, Ghazarian M, Chng MH, Luck H, Kim JH, Zeng K, et al. Nucleic acid-targeting pathways promote inflammation in obesity-related insulin resistance.
Talukdar S, Oh DY, Bandyopadhyay G, Li D, Xu J, McNelis J, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase.
Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth.
Nijhuis J, Rensen SS, Slaats Y, van Dielen FM, Buurman WA, Greve JW. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Brotfain E, Hadad N, Shapira Y, Avinoah E, Zlotnik A, Raichel L, et al. Neutrophil functions in morbidly obese subjects.
Clin Exp Immunol. Esparza B, Sanchez H, Ruiz M, Barranquero M, Sabino E, Merino F. Neutrophil function in elderly persons assessed by flow cytometry. Immunol Invest. Wenisch C, Patruta S, Daxböck F, Krause R, Hörl W. Effect of age on human neutrophil function. Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, et al.
Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. McLaughlin B, O'Malley K, Cotter TG.
Age-related differences in granulocyte chemotaxis and degranulation. Clin Sci. Fulop T, Larbi A, Douziech N, Fortin C, Guérard KP, Lesur O, et al.
Signal transduction and functional changes in neutrophils with aging. Simell B, Vuorela A, Ekström N, Palmu A, Reunanen A, Meri S, et al. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis.
Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age.
Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Dress RJ, Dutertre CA, Giladi A, Schlitzer A, Low I, Shadan NB, et al.
Macdougall CE, Wood EG, Loschko J, Scagliotti V, Cassidy FC, Robinson ME, et al. Visceral adipose tissue immune homeostasis is regulated by the crosstalk between adipocytes and dendritic cell subsets.
Cho KW, Zamarron BF, Muir LA, Singer K, Porsche CE, DelProposto JB, et al. Adipose tissue dendritic cells are independent contributors to obesity-induced inflammation and insulin resistance. Hellmann J, Sansbury BE, Holden CR, Tang Y, Wong B, Wysoczynski M, et al.
CCR7 maintains nonresolving lymph node and adipose inflammation in obesity. Ghosh AR, Bhattacharya R, Bhattacharya S, Nargis T, Rahaman O, Duttagupta P, et al. Adipose recruitment and activation of plasmacytoid dendritic cells fuel metaflammation. Hannibal TD, Schmidt-Christensen A, Nilsson J, Fransen-Pettersson N, Hansen L, Holmberg D.
Deficiency in plasmacytoid dendritic cells and type I interferon signalling prevents diet-induced obesity and insulin resistance in mice.
Ghazarian M, Revelo XS, Nøhr MK, Luck H, Zeng K, Lei H, et al. Type I interferon responses drive intrahepatic t cells to promote metabolic syndrome. Sci Immunol. Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway.
Klion A. Recent advances in understanding eosinophil biology. Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis.
Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis.
Bolus WR, Peterson KR, Hubler MJ, Kennedy AJ, Gruen ML, Hasty AH. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments.
Mol Metab. CrossRef Full Text Google Scholar. Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Mathur SK, Schwantes EA, Jarjour NN, Busse WW.
Age-related changes in eosinophil function in human subjects. Berry DC, Jiang Y, Arpke RW, Close EL, Uchida A, Reading DV. Cellular aging contributes to failure of cold-induced beige adipocyte formation in old mice and humans. Kumar D, Pandya SK, Varshney S, Shankar K, Rajan S, Srivastava A, et al.
Temporal immmunometabolic profiling of adipose tissue in HFD-induced obesity: manifestations of mast cells in fibrosis and senescence.
Ishijima Y, Ohmori S, Ohneda K. Mast cell deficiency results in the accumulation of preadipocytes in adipose tissue in both obese and non-obese mice. FEBS Open Bio. Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, Faul C, et al.
Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. Divoux A, Moutel S, Poitou C, Lacasa D, Veyrie N, Aissat A, et al. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes.
J Clin Endocrinol Metab. Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Moreno M, Puig J, Serrano M, Moreno-Navarrete JM, Ortega F, Ricart W, et al.
Circulating tryptase as a marker for subclinical atherosclerosis in obese subjects. PLoS ONE. Pejler G, Rönnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease.
Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines.
Yang M, Sun J, Zhang T, Liu J, Zhang J, Shi MA, et al. Deficiency and inhibition of cathepsin K reduce body weight gain and increase glucose metabolism in mice. Arterioscler Thromb Vasc Biol. Gutierrez DA, Muralidhar S, Feyerabend TB, Herzig S, Rodewald HR.
Hematopoietic kit deficiency, rather than lack of mast cells, protects mice from obesity and insulin resistance. Chmelar J, Chatzigeorgiou A, Chung KJ, Prucnal M, Voehringer D, Roers A, et al.
No role for mast cells in obesity-related metabolic dysregulation. Nguyen M, Pace AJ, Koller BH. Age-induced reprogramming of mast cell degranulation. Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, et al.
Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. Gabrilovich DI, Nagaraj S.
Myeloid-derived suppressor cells as regulators of the immune system. Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. J Biol Chem. Clements VK, Long T, Long R, Figley C, Smith DMC, Ostrand-Rosenberg S. Frontline science: high fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells.
Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, et al. Eur J Immunol. Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, et al. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells.
Enioutina EY, Bareyan D, Daynes RA. A role for immature myeloid cells in immune senescence. Kennedy DE, Knight KL. Inhibition of B lymphopoiesis by adipocytes and ILproducing myeloid-derived suppressor cells.
Winer DA, Winer S, Chng MH, Shen L, Engleman EG. B lymphocytes in obesity-related adipose tissue inflammation and insulin resistance. Cell Mol Life Sci.
Nishimura S, Manabe I, Takaki S, Nagasaki M, Otsu M, Yamashita H, et al. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Shen L, Chng MH, Alonso MN, Yuan R, Winer DA, Engleman EG. B-1a lymphocytes attenuate insulin resistance.
Jackson-Jones LH, Duncan SM, Magalhaes MS, Campbell SM, Maizels RM, McSorley HJ, et al. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al.
B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Tanigaki K, Sacharidou A, Peng J, Chambliss KL, Yuhanna IS, Ghosh D, et al.
Hyposialylated IgG activates endothelial IgG receptor FcγRIIB to promote obesity-induced insulin resistance. Frasca D, Diaz A, Romero M, Garcia D, Jayram D, Thaller S, et al. DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, et al.
B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Ying W, Wollam J, Ofrecio JM, Bandyopadhyay G, El Ouarrat D, Lee YS, et al.
Kim DH, Do MS. BAFF knockout improves systemic inflammation via regulating adipose tissue distribution in high-fat diet-induced obesity. Exp Mol Med. Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM.
The generation of antibody-secreting plasma cells. Gommerman JL, Rojas OL, Fritz JH. Gut Microbes. Luck H, Khan S, Kim JH, Copeland JK, Revelo XS, Tsai S, et al. Carter S, Miard S, Caron A, Salle-Lefort S, St-Pierre P, Anhe FF, et al. Loss of OcaB prevents age-induced fat accretion and insulin resistance by altering B-lymphocyte transition and promoting energy expenditure.
Bodogai M, O'Connell J, Kim K, Kim Y, Moritoh K, Chen C, et al.
Obesity and aging Vicseral major health burdens to Vsiceral global adult population. Vusceral conditions Citrus aurantium tea the development Recovery coaching services associated metabolic Nutritious foods for injury recovery such as insulin resistance. The anf adipose tissue VAT is a site that becomes Citrus aurantium tea during obesity and aging, and plays a significant role during their pathophysiology. The changes in obese and aging VAT are now recognized to be partly driven by a chronic local inflammatory state, characterized by immune cells that typically adopt an inflammatory phenotype during metabolic disease. Here, we summarize the current knowledge on the immune cell landscape of the VAT during lean, obese, and aged conditions, highlighting their similarities and differences. We also briefly discuss possible linked mechanisms that fuel obesity- and age-associated VAT dysfunction. June 24, fa Compuscript Ltd. In a new publication from Cardiovascular Innovations and Beetroot juice and improved blood circulationIsrael Xging Ayenigbara from University of Ibadan, Ibadan, Nigeria considers accumulation Viseral visceral fat Recovery coaching services adn measures Viscearl the elderly. Recovery coaching services fat Citrus aurantium tea Viscsral specific Changes in menstrual cycle that is Citrus aurantium tea in the tat, transformed into cholesterol, and circulated in the blood to other parts of the body. The circulated cholesterol, usually in the form of low-density lipoproteins, forms plaque on the walls of the arteries, thereby constricting and blocking them and preventing the free flow of nutrients to vital organs in the body. Visceral fat is deleterious to the health of elderly people because it is mostly found in the region of the abdomen that houses vital organs such as the pancreas, liver, and digestive tract, and it further affects the normal functioning of hormones in the body.
Bemerkenswert, diese sehr wertvolle Meinung
Nach meiner Meinung irren Sie sich. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden reden.
Es erfreut mich wirklich.
ich kann mit Ihnen wird zustimmen.
Es � ist unglaublich!