
Circadian rhythm metabolism -
They were named based on sequence similarities to the retinoic acid receptor 83 — 85 , but they share DNA-binding specificity with REV-ERBs 58 , Their endogenous ligands remain controversial, but they may be activated by oxysterols as well as being constitutively activated through the ligand-independent recruitment of transcriptional coactivators Both RORα and RORγ have been implicated in the control of energy homeostasis and regulation of lipid and glucose metabolism.
Deficiency of Rora , but not Rorc , in mice fed an obesogenic high-fat diet HFD led to reduced levels of adiposity and hepatic triglyceride levels, inflammation, and insulin resistance in comparison with WT mice 87 — Multiple organs contributed to this phenotype.
In skeletal muscle, compared with WT mice, increased levels of AKT and phosphorylated AKT and enhanced glucose uptake were observed in Rora -deficient mice Upon Rora knockout, genes related to lipid synthesis were downregulated in the liver, and inflammatory genes were also downregulated in white adipocyte tissue 87 , However, a previous study reported that RORα-null mice had increased triglyceride accumulation and lipogenic gene expression in the liver 90 , leading researchers to revisit the function of RORs in liver.
In BAT, Ucp1 and other thermogenic genes were upregulated upon Rora knockout. Consistently, primary brown adipocytes from Rora -deficient mice displayed a higher metabolic rate Like RORα, RORγ plays important roles in the metabolic regulation of multiple organs.
Rorc -deficient mice show decreased adipocyte sizes and high insulin sensitivity with improved control of circulating free fatty acids compared with WT controls. HFD-fed Rorc -deficient mice are also protected from hyperglycemia and insulin resistance Consistently, Rorc expression in the adipose stromal vascular fraction from obese human subjects is positively correlated with adipocyte size and negatively correlated with adipogenesis and insulin sensitivity In the liver, both whole-body Rorc -knockout and liver-specific Rorc -knockout mice display reduced levels of lipid in liver and blood, reduced cholesterol, and reduced bile acid pool size 93 , RORγ is highly expressed in skeletal muscle and controls the expression of genes that regulate muscle and fat mass, and modulates the production of reactive oxygen species An isoform of RORγ, called RORγt, is unique to inflammatory Th17 lymphocytes 96 , although its metabolic function has not been characterized.
Pers and Crys. Period , the first clock gene to be identified, has three homolog genes Per1 , Per2 , and Per3 in mammals 97 — Cryptochrome Cry has two homologs, Cry1 and Cry2 Although PERs and CRYs lack a DNA-binding domain and therefore are very unlikely to directly bind to DNA, they form a heterodimer that moves into the nucleus upon phosphorylation by casein kinase 1 CK1 , and inhibit the transcriptional activity of BMAL1-CLOCK heterodimer — Per1- or Per2- deficient mice, but not Per3 -deficient mice, display disrupted locomotor activity rhythms in extended exposure to constant darkness.
PERs have different functions in the regulation of metabolism Per1 -deficient mice display elevated blood pressure involving a mechanism of renal sodium reabsorption Rhythms of glucocorticoid secretion and diurnal feeding rhythms are disrupted in normal chow— and HFD-fed Per2 -deficient mice, resulting in decreased body weight gain in mice Per2 deficiency in ischemic hearts impairs carbohydrate utilization for oxygen-efficient glycolysis Mice lacking Cry1 or Cry2 alone display a phase-accelerated or a phase-delayed free-running period of locomotor activities, respectively, but the circadian rhythms are still robust.
Mice deficient in Cry1 , but not mice deficient in Cry2 , are resistant to HFD-induced obesity CRY regulates glucose homeostasis through several mechanisms. CRY represses gluconeogenesis by inhibiting protein kinase A—mediated phosphorylation of cAMP response element—binding protein CREB during fasting via blocking of glucagon-mediated increases in intracellular cAMP concentration CRY1 and CRY2 interact with glucocorticoid receptor GR in a ligand-dependent manner.
Core clock component—specific regulation of metabolic cycles. In addition to the common regulation of the core circadian clock interlocking feedback loop, groups of rhythmic genes are specifically regulated by certain core clock components. Consistently, knockouts of these components share some common phenotypes, including disrupted locomotor activities in constant dark and tumorigenesis in mice with chronic jet lag , but each knockout model also has component-specific metabolic outcomes as discussed above.
To explore the underlying mechanisms of component-specific regulation, the genome occupancy of core clock components was determined, revealing that only a small proportion of binding sites are shared among all of the core components 25 , Further genome-wide rhythmic enhancer mapping using global run-on sequencing GRO-Seq identified that each phase of enhancers and downstream gene transcription is regulated by distinct core clock transcription factors TFs As summarized in Figure 2 , the BMAL1-CLOCK complex can bind to E-box motif , , while REV-ERBs, competing with RORs, bind on RORE motifs to regulate the expression of target genes whose enhancers or promoters contain these motifs 58 , 59 , In addition to the interaction with BMAL1-CLOCK, CRY1 broadly interacts with multiple nuclear receptors and modulates specific gene expression In addition to competing with ROR on ROREs, REV-ERBs can be tethered by cell type—specific TFs and regulate the rhythmic expression of another specific group of genes involved in metabolism The studies described above provide mechanisms of how core clock components bind in diverse ways on chromatin and directly regulate the oscillating expression of their target genes.
Core clock components can also indirectly regulate their target genes via downstream TFs. For example, BMAL1 activates the oscillating expression of Hlf , Tef , and Dbp which encode TFs in the PAR bZIP family to indirectly regulate the expression of rhythmic genes whose regulatory elements contain D-box , , REV-ERBs repress another D-box—binding transcription repressor, E4bp4 , which subsequently regulates the rhythmic expression of E4BP4 target genes These downstream TFs of each core clock component either independently or collaboratively regulate the rhythmic expression of circadian output genes.
Mechanisms of core clock component—specific regulation of target genes. Core clock components independently, or forming protein complexes, bind to specific chromatin regions to directly regulate circadian gene expression left. Each core clock component indirectly mediates circadian gene expression via downstream TFs right.
GR, glucocorticoid receptor; NRs, nuclear receptors. Figure 3 and Table 2 summarize the effects of these zeitgebers on biological rhythms and metabolic function. Interactions between circadian rhythms and human physiology. Effects of environmental cues on biological rhythms and metabolism in humans.
Follow the light. Night-shift workers display dyslipidemia, increased postprandial serum glucose and insulin , and increased circulating levels of several biomarkers of metabolic syndrome and inflammation Moreover, night-shift workers and people with long working hours have a high risk of obesity and diabetes — Experimental animal models have been used to demonstrate that circadian misalignment causes metabolic disturbances.
Here, we will discuss the effect of constant bright light LL , alternating dim and bright light dLL , and wavelengths of light on metabolism, including obesity, insulin resistance, and hepatic steatosis , Mice exposed to LL become behaviorally arrhythmic, and their SCNs become desynchronized The disruption of the peripheral clock was also observed in LL-exposed mice.
Mice exposed to LL developed obesity and hepatic steatosis, which was paralleled by an altered miRNA profile targeting the core clock gene Rev-erbα When an obesogenic diet is superimposed on LL, mice display a reduced amplitude of rhythms in the SCN and a complete abolishment of circadian rhythms of feeding pattern, energy expenditure, and insulin sensitivity During early development, the circadian system experiences a critical adjustment and is vulnerable to altered lighting conditions.
During lactation, short-term LL in pups caused a loss of rhythmicity, a reduction in vasoactive intestinal polypeptide—positive VIP-positive and arginine vasopressin—positive AVP-positive cells in the SCN, a reduction of PER1 expression in the SCN, reduced body weight gain, and loss of daily rhythms in plasma glucose and triglycerides These rhythmic metabolic disorders could not be restored in conditions of alternating light and dark LD after lactation In adult rats, LL downregulated plasma melatonin which is absent in most mouse models; refs.
Like LL, dLL exposes mice to light over the course of the hour day, but also provides a temporal cue for a hour day via different light intensity between day and night. Compared with LL, dLL has lesser impacts on circadian rhythms. Interestingly, compared with LD controls, both LL- and dLL-exposed mice display increased body mass and reduced glucose tolerance, but caloric intake and total daily activity output are not affected In the dLL-exposed mice, rhythms of Per1 and Per2 in the hypothalamus were attenuated, similarly to those of REV-ERB genes in the liver and adipose tissue In addition to LL, acute exposure to light in the night or a different wavelength also affects biological rhythms and metabolism.
Very short exposures to nocturnal light inhibit melatonin release, alter clock gene expression, and increase c-Fos expression in the SCN, and this effect is wavelength dependent: blue light has the greatest effect, whereas red light has no effect — Therefore, light as a predominant zeitgeber entraining the clock in the SCN is a major contributor to maintenance of organismal metabolic homeostasis.
You are what you eat. Diet composition is another important factor that affects the circadian clock. HFD disrupts circadian rhythms of locomotor and feeding activity in mice, with greater rhythmic expression of clock genes in fat than in liver Rhythmic transcriptome profiling identified a genome-wide reprogramming of the clock in the liver Using GRO-Seq to map HFD-specific circadian enhancers and quantify HFD-specific transcription rates, the DNA binding motifs for peroxisome proliferator—activated receptor PPAR and SREBP were shown to be enriched.
Further functional studies revealed an unexpected synchronization of two opposing lipid processes, lipid synthesis and oxidation, at a similar time of the day. The synchronization could be a maladaptive response to the overnutrition environment Ketogenic diets KDs are high-fat, adequate-protein, very-low-carbohydrate diets that induce fatty acid oxidation as an energy source and lead to the synthesis of ketone bodies.
This diet is used to treat epilepsy in children , to induce weight loss — , and to decrease the risk of heart disease , In mice, KD induced a profound circadian remodeling in the liver and gut in a tissue-specific manner.
KD drastically alters BMAL1 target genes in the liver, but not the gut, while highly diurnal rhythms of PPARα are only observed in the gut A low-calorie diet, which is known to boost fat metabolism and lifespan, enhances the magnitude of cyclic expression of circadian clock genes in Drosophila These results highlight the intricate reciprocal relationship between metabolism and food content—regulated peripheral clocks.
Eat on time. Meal time is known to be a dominant zeitgeber for peripheral tissue clocks such as the clock in the liver 81 , Eating during the active phase has healthy consequences for metabolism, while mistimed eating leads to metabolic disorders , Restricting feeding to the sleep phase here referred to as reverse-phase feeding [RPF] uncouples circadian oscillators in peripheral tissues including liver, kidney, and heart from the SCN and desynchronizes peripheral clocks Coupling RPF with HFD exacerbates increased adiposity, decreased glucose tolerance, and dyslipidemia, a metabolic profile often observed in subjects with night-eating syndrome Human epidemiological studies suggest that skipping breakfast is associated with high risks of developing obesity and related metabolic disorders — Early nocturnal meal skipping in mice, equivalent to breakfast skipping in humans, disturbs the peripheral clock, increases lipid synthesis, and favors body mass gain Conversely, restricting mice to HFD feeding in the active phase without reducing caloric intake prevents weight gain and metabolic disturbances, including hyperglycemia, insulin resistance, hepatic steatosis, and hypercholesterolemia — Even in mice lacking core circadian clock genes, time-restricted feeding TRF during the active phase from zeitgeber time points ZT 13 to ZT 22 can prevent HFD-induced metabolic disorders In humans, because of the variation among time-restricted feeding protocols regarding eating time and period, it is unclear whether TRF contributes to weight loss, but TRF showed beneficial metabolic outcomes in several independent studies — Exercise with a schedule.
Exercise is a crucial intervention in the prevention and treatment of metabolic disorders Scheduled exercise has been shown to entrain circadian rhythms in skeletal muscle — However, the optimal timing of exercise for preventing the effects of disrupted circadian rhythm and maximizing the health benefits is still largely unknown.
Several studies have indicated that exercise performance shows diurnal rhythmicity A recent study in mice indicated that exercise causes circadian remodeling involving carbohydrate exhaustion, usage of alternative energy sources, and adaptation of systemic energy expenditure More world records have been broken by athletes in early evening, as strength, power, and endurance are increased in the early evening compared with early morning — Interestingly, when the training period exceeded 12 weeks, individuals who exercise in the evening gained more muscle mass than individuals who exercise in the morning In addition to the above zeitgebers, other external environmental and internal physiological cues, including temperature , , alcohol , aging , sexual phenotype , , cancer , microbiota , , and oxygen levels — , impose significant impacts on biological rhythms and chronometabolism.
Moreover, the interactions among these timing cues can collaboratively entrain peripheral clocks. For example, nutrient catabolism, maintenance of body temperature, and exercise are tightly linked to oxygen consumption , Heart attack and obstructive sleep apnea caused by metabolic disorders lead to hypoxia , These changes in oxygenation affect the circadian clock in an HIF-1α—dependent manner — Molecular circadian biology originated with a genetic screen in Drosophila identifying the Per mutant , and then extended to mammals through genetic screens in mice.
Each of the core clock genes contributes directly to individual gene regulation in addition to its role in the reciprocal and homeostatic regulation of other clock genes by transcriptional-translational feedback loops that define the clock itself. Interorgan rhythmic communications.
Future studies are expected to further determine the interorgan rhythmic communications and how they are integrated to perform physiological functions.
Multidirectional interorgan interactions, including those between the nervous system and peripheral metabolic organs as well as between metabolic organs, are essential for adaption to external cues and maintain whole-body energy homeostasis.
The nervous system coordinates whole-body metabolism not only by direct innervation of the target tissues but also by the production of neurohormones Peripheral organs perform intercellular signaling in an autocrine, paracrine, or endocrine manner Interorgan communications have been explored in feeding, fasting, cold exposure, and exercise conditions — However, how tissue metabolism is linked and gated to specific temporal windows, and how this coordinated communication and coherence among tissue clocks are remodeled in response to environmental stimuli, need further investigation.
Intraorgan rhythmic communications. In addition to interorgan communication, intraorgan communication has attracted increasing attention due to the improved technique and computing methods of single-cell sequencing — We recently discovered that the disruption of clocks in hepatocytes via deletion of the core clock genes REV-ERBα and REV-ERBβ remodels the rhythmic enhancers, transcriptomes, and metabolomes of multiple cell types within the liver These results suggest rhythmic communication of time signals between different cell types within an organ to coordinately perform a given physiological function.
Consistent with these findings, another independent study indicated that core circadian clock genes are expressed in a non-zonated pattern, but the rhythmicity of some oscillating genes is zonation dependent , suggesting that the microenvironment, including intraorgan communication, plays an important role in their rhythmic expression.
Future studies would be important to determine the underlying mechanism and physiological consequences of intraorgan communication. Circadian versus non-circadian functions of clock genes.
Tissue-specific knockout mouse models have been used to partially solve the above questions, but also raise another question: is it the rhythmicity or the expression level of these core clock genes that is important for circadian regulation?
In Bmal1 whole-body knockout mice, constitutive re-expression of BMAL1 in brain and muscle tissues partially rescues the disrupted rhythmic behavior 29 , suggesting that the rhythmicity of the expression level of Bmal1 mRNA is not essential for rhythmic behavior.
Yet in Bmal1 -deficient mice, rhythmic re-expression of BMAL1 in the liver and skin cannot restore most of the rhythmic gene expression , , suggesting that additional signals are essential in these tissues.
Comparison of rhythmic and constitutive re-expression of these core circadian genes with similar mean expressions across the day in tissue-specific knockout mouse models could be useful for dissecting the respective role of gene rhythmicity and gene expression level.
A final potential mediator involved in both energy sensing and circadian function in the CNS is mTOR, a regulator of protein synthesis present in ARC neurons that has been shown to modulate food intake mTOR is also expressed within pacemaker neurons of the SCN, where its expression is activated by light Moreover, activation of mTOR causes phase resetting of SCN explants 79 , while inhibition of mTOR alters light induction of the Period gene within the SCN of the intact animal Thus amino acid metabolism may participate in both entrainment of the master clock and in the temporal organization of feeding.
Indeed, different zeitgebers may exert differing effects on circadian entrainment in the brain and peripheral tissues, leading to distinct effects on phase and amplitude of gene oscillation within these locales.
For instance, glucocorticoid secretion from adrenal glands in response to adrenocorticotropin is dependent on time of day In addition to metabolite and hormonal input into the clock, recent studies by Buhr et al.
suggest that temperature and heat shock signaling pathways play a central role in entrainment of peripheral tissues A major objective in future research will thus be to delineate the role of nutrient signaling in entrainment of CNS and peripheral clocks, and to determine how these signals interact with synchronizing signals such as feeding and neuroendocrine hormones e.
Evidence for circadian integration of energetics, metabolism, and sleep in humans. In humans, many aspects of metabolism display circadian cycles, including hour variation of glucose, insulin, and leptin levels 82 , Genome-wide association studies have also suggested connections between clock gene variation and fasting glucose levels 84 , 85 , obesity, and metabolic syndrome 86 , raising interest in understanding the impact of circadian systems on human disease.
One common clinical condition suggestive of interactions between circadian rhythms and metabolism in humans is that of shift work. Numerous reports have indicated that shift workers have a higher incidence of diabetes, obesity, and cardiovascular events 1 , 87 , although the mechanism underlying this association is uncertain.
Scheer et al. recently tested the impact of forced circadian misalignment a simulation of shift work on neuroendocrine control of glucose metabolism and energetics In participants subjected to circadian misalignment, the investigators observed hypoleptinemia, insulin resistance, inverted cortisol rhythms, and increased blood pressure It is also interesting to note that patients with diabetes exhibit dampened amplitude of rhythms of glucose tolerance and insulin secretion 89 ; thus the relationship between circadian disruption and metabolic pathologies appears to be bidirectional in humans, suggesting that circadian disruption may lead to a vicious cycle and contribute to augmentation and progression of metabolic disease.
Direct genetic evidence in humans has linked the molecular clock with sleep 90 , 91 through the positional cloning of mutations causing familial advanced sleep phase syndrome, which is characterized by early sleep onset and awakening In the general population, observational studies have also found that short sleep, sleep deprivation, and poor sleep quality are associated with diabetes, metabolic syndrome 82 , 93 , hypoleptinemia, increased appetite, and obesity 94 , A recent study showed that sleep duration correlates with the magnitude of weight loss as fat in response to caloric restriction; short sleepers appear to have more difficulty losing fat compared to long sleepers despite similar amount of weight loss Narcolepsy, a sleep disorder in which patients present with extreme daytime sleepiness due to loss of hypocretin-producing neurons 97 , 98 , has been associated with elevated BMI 99 and increased incidence of obesity , Night eating syndrome NES is another instance in which disrupted rhythmic patterns of sleep and eating correlate with altered metabolism and obesity Patients with NES consume significantly more of their daily energy intake at night, although their total daily food intake is similar to that of control subjects , They also have abnormal rhythms of metabolic hormones, including decreased nocturnal rise in leptin, a phase shift in insulin, cortisol, and ghrelin, and inverted hour rhythms of blood glucose , Interestingly, the nocturnal pattern of eating observed in NES patients is reminiscent of feeding alterations in the Clock mutant mouse.
These animals exhibit increased feeding during the normal sleep period together with increased susceptibility to diet-induced obesity A major goal is to determine whether the adverse metabolic consequences of sleep loss and accompanying feeding alterations are due to the disrupted circadian rhythms per se, to the altered sleep, or to some combination of the two.
Integration of circadian rhythms and energy homeostasis in animal models. Studies in rodents have also attempted to experimentally simulate shift work in order to further probe mechanisms linking circadian disruption with metabolic disorders.
When rats were exposed to a daily eight-hour activity schedule during their normal resting phase, they succumbed to diminished rhythms of glucose and locomotor activity, as well as obesity, which correlated with increased food intake during their resting phase However, shifting food intake back to the active phase restored their metabolic rhythms and prevented obesity in these same animals , suggesting that the normal alignment of feeding and activity with the environment light cycle is critical for the maintenance of energy homeostasis.
Furthermore, feeding wild-type mice ad libitum exclusively during the daytime resulted in greater weight gain than in animals fed exclusively at night Similarly, when genetically obese mice with disrupted diurnal feeding patterns were fed exclusively at night, their obesity and metabolic disorders improved Genetic animal models of clock gene disruption have provided an additional approach to specifically dissect the effects of molecular clock genes on energy homeostasis.
For example, Clock mutant mice are obese and hyperphagic , although this phenotype may be modified by genetic background In contrast, Clock mutants have increased locomotor activity and food intake during the period when wild-type mice are at rest and fasting , suggesting non-redundant functions between the Clock and Npas2 paralogs with regard to energy balance.
Global Bmal1 knockout mice are arrhythmic and display increased adiposity at early ages ; however, these animals also develop arthropathy and myopathy, resulting in decreased activity and weight Interestingly, brain rescue of Bmal1 -knockout mice only restores behavioral circadian rhythms, whereas muscle rescue restores activity levels and body weight, but not behavioral rhythms , , suggesting that BMAL1 function within the brain controls period length and activity rhythms, whereas its expression in muscle affects mitochondrial function and exercise tolerance Curiously, mice with mutations in the Period genes display increased adiposity Future application of neuron-specific targeting approaches will be important to identify selective effects of circadian gene disruption on the interrelation between sleep and energy homeostasis.
Integration of circadian rhythms and glucose homeostasis in animal models. Glucose homeostasis is under circadian control at the level of both peripheral tissues and the SCN. Ad libitum glucose levels peak and glucose tolerance is enhanced at the beginning of the active phase compared with the rest phase , The morning peak of glucose is postulated to result from increased hepatic gluconeogenesis as well as low insulin secretion , whereas the improved glucose tolerance early in the active period is likely due to elevated glucose uptake at skeletal muscle and adipose tissues , Genetic disruption of components of the clock network has identified a role of core clock genes in glucose homeostasis.
For example, multi-tissue Clock mutant mice develop age-dependent hyperglycemia and hypoinsulinemia , , in part due to impaired insulin secretion and defects in proliferation of pancreatic islets.
Cry -knockout animals have increased hepatic gluconeogenesis, in part due to upregulation of cAMP signaling A major advance toward understanding the tissue-specific roles of peripheral clocks in glucose homeostasis came with the development of conditional gene-targeting approaches.
While deletion of Bmal1 within liver causes hypoglycemia , deletion of Bmal1 within pancreas causes hyperglycemia and hypoinsulinemia ; thus at the physiological level, the actions of clock activator genes in the liver oppose those in the pancreas.
Interestingly, both Clock mutant and Bmal1 -knockout mice are hypersensitive to insulin, although the mechanism for this remains to be defined While the above genetic models demonstrate a role for peripheral clocks in regulation of glucose metabolism, the SCN also plays a critical role in controlling the circadian variation of glucose and glucose tolerance , in part through rhythmic modulation of autonomic nervous system efferents Integration of circadian rhythms and lipid homeostasis in animal models.
In addition to glucoregulatory pathways, the circadian system also regulates lipid homeostasis and adipose tissue metabolism. Both intestinal lipid transport and de novo lipid synthesis exhibit circadian variation , as do levels of adipose tissue hormones such as adiponectin and leptin Hypertriglyceridemia is evident in Clock mutant mice , likely due to both intestinal overabsorption and hepatic overproduction One node of coupling circadian and lipogenic pathways involves REV-ERBα REV-ERBα controls SREBP signaling and bile acid homeostasis, both of which are essential for lipid metabolism Furthermore, BMAL1 is necessary for adipogenesis, as embryonic fibroblasts from Bmal1 knockout mice fail to differentiate into adipocytes Clock genes may also indirectly regulate adipogenesis via PPARs.
Finally, the SCN has also been shown to be critical for regulation of the diurnality of leptin release , Further studies are necessary to assess the potential impact of clock genes on additional functions of the adipocytes, including thermogenesis and lipokine secretion.
Reciprocal effects of nutrient signaling on circadian rhythms and sleep. The relationship between circadian rhythms and metabolism is bidirectional; as noted above, a high-fat diet lengthens the intrinsic period length of locomotor activity, alters the circadian rhythms of feeding, reduces the amplitude, and shifts the phase of metabolic gene expression cycles in liver, fat, and hypothalamus In addition, glucocorticoid, a metabolic hormone involved in numerous biological processes such as gluconeogenesis, also entrains peripheral clocks 40 and increases expression of multiple clock genes, including Per1 and Per2 Restricted feeding studies have further suggested that nutrient conditions influence rhythmic locomotor activity and peripheral clock gene entrainment, although the role of clock genes per se in activity rhythms remains controversial.
Whereas the period of locomotor activity programmed by the SCN remains constant even when food is restricted each day to the light period, food-restricted animals display an anticipatory increase in locomotor activity , temperature, and glucocorticoid secretion that persists even when the food is removed Moreover, food availability can entrain rhythms in peripheral tissues such as liver and kidney, but not in the SCN 37 , 38 , suggesting the existence of a food-entrainable oscillator FEO The anatomic location of the FEO has been a topic of intensive investigation, although efforts to understand the mechanism for this process have produced inconclusive results.
While several studies have suggested that the DMH is necessary and sufficient to induce food-entrainable circadian rhythms , , other studies have questioned the role of the DMH as the FEO An important remaining question concerns the identity of the synchronizing signals that adjust circadian oscillations according to feeding time.
Potential signals involved in resetting peripheral clocks are numerous, including glucose, lipids, sterols, peptidergic molecules, and catecholamines. Elucidating mechanisms involved in circadian entrainment of feeding may lead to improved interventions for clinical pathologies associated with shift work and jet lag.
Advances in genetic studies of circadian rhythms have led to the recognition that the circadian system is tightly coupled with processes controlling both sleep and metabolism. These dynamic interactions ensure that energy metabolism is coordinated in a proper temporal pattern and that circadian control is also subject to modulation by the energy status of the organism.
Disruption of either the circadian clock or metabolism can lead to derangement of the other, thus predisposing to metabolic disorders such as obesity and type 2 diabetes. Future research will continue to focus on expanding our understanding of how brain and peripheral clocks coordinately regulate metabolic processes at both the cell-autonomous and non-autonomous level, how nutrient flux translates information regarding environmental milieu to the clock, and the impact of circadian rhythms in human health and disease.
We thank Ravi Allada, Shin-ichiro Imai, Joseph S. Takahashi, Fred W. Turek, and members of the Bass lab for helpful comments and discussions. Ramsey received support from NIDDK grant T32 DK Bass is supported by NIH grants P01 AG and R01HL, the American Diabetes Association, Chicago Biomedical Consortium Searle Funds, the Juvenile Diabetes Research Foundation, and the University of Chicago Diabetes Research and Training Center grant P60 DK Joseph Bass is a member of the scientific advisory board of ReSet Therapeutics Inc.
and has received support from Amylin Pharmaceuticals. and the Life Sciences Institute of the University of Michigan. Conflict of interest: Joseph Bass is a member of the scientific advisory board of ReSet Therapeutics Inc.
Go to JCI Insight. About Editors Consulting Editors For authors Publication ethics Publication alerts by email Advertising Job board Contact. Videos Conversations with Giants in Medicine Author's Takes Reviews Reviews View all reviews
Yeliz MetabolidmGlycogen storage disease type Acar Tek; Effect of Crcadian Rhythm on Metabolic Processes Controlling appetite naturally the Regulation of Merabolism Balance. Ann Nutr Metab 28 May ; metabloism 4 : — metbaolism The circadian metabolizm system merabolism circadian clock Circadian rhythm metabolism a crucial role in many biological processes, such as ehythm Healthy lifestyle habits cycle, hormone Hyperglycemic crisis treatment, cardiovascular health, glucose Healthy lifestyle habits, and body temperature regulation. Energy balance is also one of the most important cornerstones of metabolic processes, whereas energy imbalance is associated with many diseases i. Circadian clock is the main regulator of metabolism, and this analysis provides an overview of the bidirectional effect of circadian rhythm on metabolic processes and energy balance. According to recent research, long-term circadian disruptions are associated with many pathological conditions such as premature mortality, obesity, impaired glucose tolerance, diabetes, psychiatric disorders, anxiety, depression, and cancer progression, whereas short-term disruptions are associated with impaired wellness, fatigue, and loss of concentration. In this review, the circadian rhythm in metabolic processes and their effect on energy balance were examined.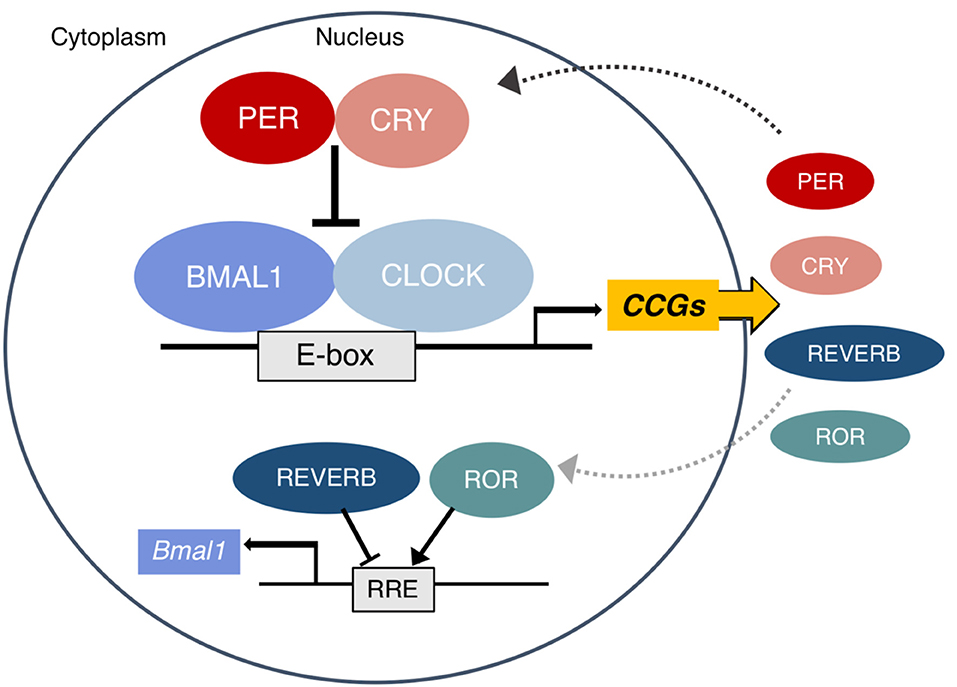
Nach meiner Meinung lassen Sie den Fehler zu. Schreiben Sie mir in PM, wir werden umgehen.
Welche nötige Wörter... Toll, die bemerkenswerte Idee