Video
Study Finds Metformin Limits Exercise Gains \u0026 VO2 MaxMetformin and exercise performance -
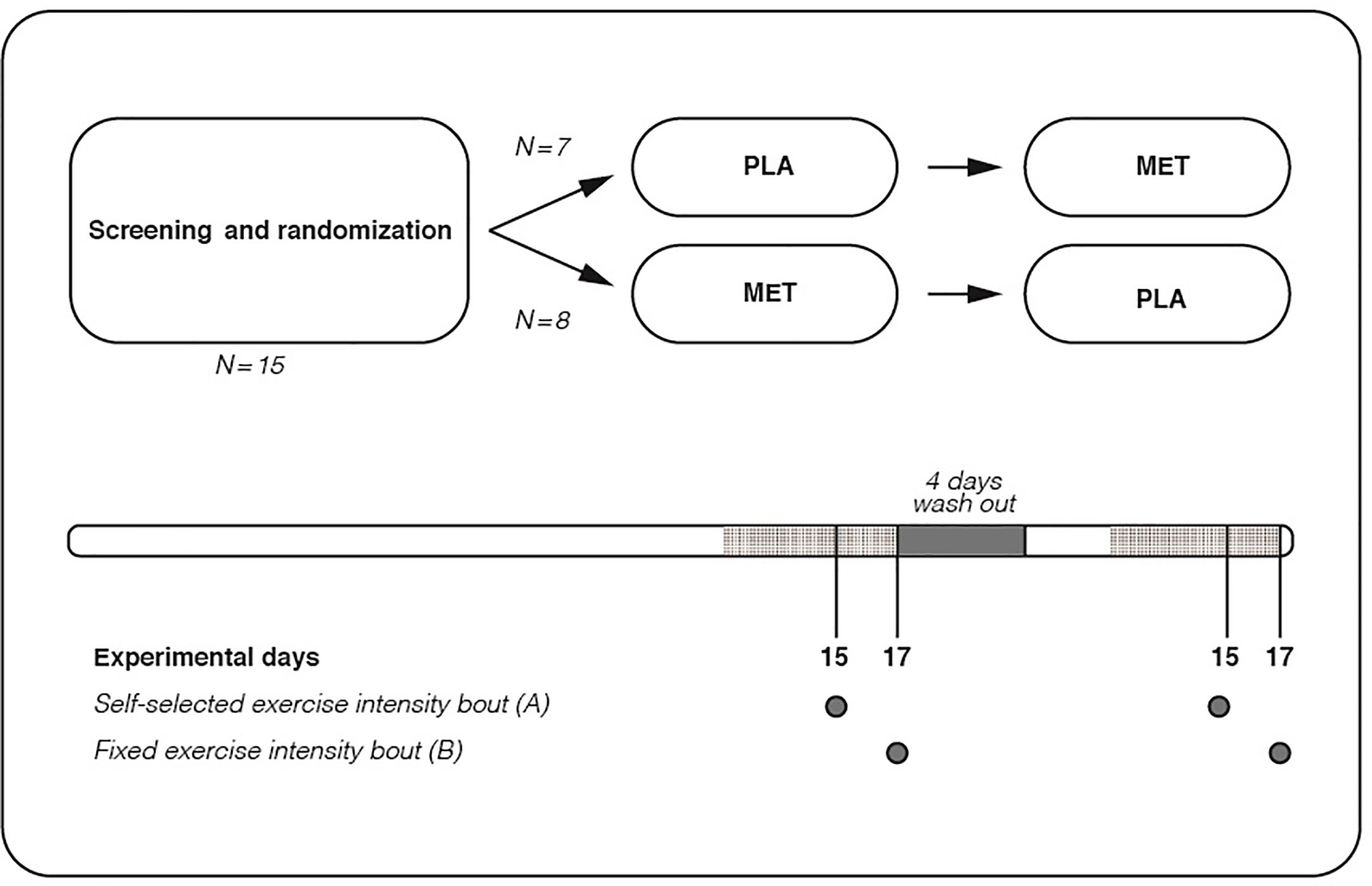
Body weight decreased on average by 4. Neither lean body mass nor visceral fat content changed over time in either group Table 2. Glucose concentration during the MMTT. For detailed statistical analysis, see text and Table 3.
Adjustment for BASELINE glucose values did not result in between-group differences from BASELINE to TRAINING. The comparable reductions in mean MMTT glucose concentration between BASELINE and TRAINING for the MET and PLA study groups were seen at different timepoints Fig.
The intervention-induced reductions in mean glucose concentration during the MMTT were dependent on differential time effects Fig. In the PLA group, the reductions were mainly dependent on reductions in the last part of the MMTT, whereas in the MET group, the reduction was mainly dependent on reductions in the first part of the MMTT.
Glucose kinetics during the MMTT are illustrated in Fig. In the PLA group, no differences in R aTOTAL between any experimental days were seen. Glucose kinetics during the MMTT. a , b R aTOTAL in the PLA a and MET b group.
c , d EGP in the PLA c and MET d group. e , f R aMMTT in the PLA e and MET f group. For statistical analysis, see text and Table 3. Regarding fasting EGP in the PLA group, this was unchanged throughout the intervention. Regarding R aMMTT , no differences between any experimental days were seen in the PLA group.
Regarding R d , no differences between any experimental days were seen in the PLA group. Triacylglycerols, total cholesterol and HDL-cholesterol did not change over time in either group. The main finding of this study is that metformin plus exercise training is not superior to exercise training alone for improving postprandial glucose in glucose-intolerant individuals.
The successful training intervention with solid and comparable improvements in physical fitness and body composition in the MET and PLA study groups, in addition to the high medicine compliance, indicates a blunting effect of metformin on training-induced improvements in postprandial glucose.
In the MET group, we found a decrease in mean glucose concentration during the MMTT following 3 weeks of metformin treatment but no further improvements with 12 weeks of exercise training.
Conversely, in the PLA group, 12 weeks of training resulted in a robust decrease in mean glucose concentration, supporting the argument that the training intervention itself was effective.
Hence, the lack of improvement in postprandial glucose with exercise training in the MET group warrants further discussion. The fact that participants had only moderately impaired postprandial glucose at baseline supports a flooring effect.
After initiation of metformin treatment, postprandial glucose was further improved, leaving little room for additional improvements with the training intervention. Supporting a true interaction between metformin and physical activity on postprandial glucose is the fact that mean glucose levels achieved after 3 weeks of metformin treatment 7.
This is supported by a meta-analysis by Hrubeniuk et al. In the meta-analysis, 2 h plasma glucose values of 6. In comparison, the 2 h MMTT plasma glucose levels after the training intervention in the present study were considerably higher PLA 9.
Moreover, the fact that the reductions in mean glucose concentration during the MMTT were dependent on differential time effects supports a true interaction between metformin and physical activity. Finally, the apparently differential effects between groups on glucose kinetics see discussion below supports a physiological interaction.
To further investigate this issue, studies with a higher number of dysglycaemic participants are warranted. Even though our primary endpoint postprandial glucose indicated an interaction between metformin and exercise training, other markers of glycaemic control HbA 1c and fasting glucose did not.
As such, it may be argued that the overall effect of metformin treatment plus exercise training on glycaemic control was superior to the effect of exercise training alone. A potential explanation for the lack of improvement in HbA 1c with the training intervention in the PLA group is that the 12 week training period was too short [ 9 ].
Regarding the lack of improvements of fasting glucose with training, this is consistent with previous studies [ 16 , 29 ], and suggests little or no improvement in hepatic insulin resistance with training.
This is consistent with the lack of decrease in EGP seen in the present study. We observed a decreased R aMMTT with metformin treatment, and this effect was partially abolished with exercise training. Explanations for this metformin-induced reduction in R aMMTT could be either higher glucose uptake by the intestinal cells, slower gastric emptying rate or higher glucose uptake by the liver.
It has previously been reported that the gut might serve as an important site of action for metformin pharmacodynamics [ 30 ]. As such, an increased glucose uptake by enterocytes and subsequently increased lactate concentration within the enterocytes as a result of increased glycolysis was reported.
This led to decreased glucose entering the circulation, and thereby improved postprandial glucose. In continuation of this, a study by Buse et al. indicated that the effect of metformin on glucose metabolism should at least partially be found in the enterocytes [ 31 ].
Our data, despite not powered or designed for such analyses, might suggest that the effect of metformin on glucose metabolism in the gut is influenced by physical activity, but further studies are needed in this field.
In the MET group, we saw an increase in fasting EGP with metformin treatment. This finding challenges the existing paradigm that metformin primarily acts in the liver by inhibiting EGP [ 32 , 33 ]. However, the increase in EGP with metformin treatment is supported in a trial by Gormsen et al.
Similarities between the present study and the Gormsen trial is that participants had better glycaemic control than those in previous studies [ 35 ], which may potentially explain this finding. Moreover, it should be noted that, if adjusting for insulin concentration, EGP was robustly decreased, indicating that metformin improved central insulin sensitivity.
Exercise training is believed to increase R d and peripheral insulin sensitivity [ 36 ]. Surprisingly, only when adjusted for insulin concentration, R d numerically improved with exercise training in the PLA group, despite the solid training-induced improvements in postprandial glucose.
In this context, the application of a dual-tracer approach instead of a triple-tracer approach may have played a role [ 38 ]. We consider the randomised design and the strict standardisation prior to and during every experimental day a strength.
In addition, the training intervention was efficient with solid and comparable improvements in physical fitness and body composition. Hence, the comparisons within and between groups are sound and fair.
A limitation of the present study is the small number of participants, which may lead to both type 1 and type 2 statistical errors. Hence, it is unknown if effectiveness studies would result in the same potential interaction, but this should be investigated.
In summary, this study suggests that metformin plus exercise training was not superior to exercise training alone in improving postprandial glucose in glucose-intolerant individuals.
Whether this is dependent on a true interaction between the two modalities or a flooring effect on postprandial glucose remains unclear. Given that current diabetes guidelines [ 5 , 6 ] recommend both metformin and exercise training as first line treatment for patients with type 2 diabetes, further studies in this population are needed to elucidate the potential interaction between metformin and exercise training.
Data from the study are available from the corresponding author on reasonable request, if this does not interfere with the regulations from the Danish Data Protection Agency.
Hostalek U Global epidemiology of prediabetes - present and future perspectives. Clin Diabetes Endocrinol Article Google Scholar. Knowler WC, Barrett-Connor E, Fowler SE et al Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin.
N Engl J Med — Article CAS Google Scholar. Lindstrom J, Louheranta A, Mannelin M et al The Finnish Diabetes Prevention Study DPS : Lifestyle intervention and 3-year results on diet and physical activity.
Diabetes Care — Pan XR, Li GW, Hu YH et al Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study.
American Diabetes Association 3. Prevention or delay of type 2 diabetes: Standards of medical care in diabetes Diabetes Care S32—S Cosentino F, Grant PJ, Aboyans V et al ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD.
Eur Heart J — American Diabetes Association Introduction: Standards of medical care in diabetes Diabetes Care S1—S2. Buse JB, Wexler DJ, Tsapas A et al update to: Management of hyperglycemia in type 2 diabetes, A consensus report by the American Diabetes Association ADA and the European Association for the Study of Diabetes EASD.
Snowling NJ, Hopkins WG Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis.
Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA — Piera-Mardemootoo C, Lambert P, Faillie JL Efficacy of metformin on glycemic control and weight in drug-naive type 2 diabetes mellitus patients: a systematic review and meta-analysis of placebo-controlled randomised trials.
Steinberg GR, Kemp BE AMPK in health and disease. Physiol Rev — Hawley SA, Ross FA, Chevtzoff C et al Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation.
Cell Metab — Kristensen JM, Lillelund C, Kjobsted R et al Metformin does not compromise energy status in human skeletal muscle at rest or during acute exercise: a randomised, crossover trial.
Physiol Rep 7:e Malin SK, Braun B Impact of metformin on exercise-induced metabolic adaptations to lower type 2 diabetes risk.
Exerc Sport Sci Rev — Malin SK, Gerber R, Chipkin SR, Braun B Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Terada T, Boule NG Does metformin therapy influence the effects of intensive lifestyle intervention?
Exploring the interaction between first line therapies in the Look AHEAD trial. Metabolism — Boule NG, Robert C, Bell GJ et al Metformin and exercise in type 2 diabetes: Examining treatment modality interactions.
Hansen M, Palsoe MK, Helge JW, Dela F The effect of metformin on glucose homeostasis during moderate exercise. Ortega JF, Hamouti N, Fernandez-Elias VE, de Prada MV, Martinez-Vizcaino V, Mora-Rodriguez R Metformin does not attenuate the acute insulin-sensitizing effect of a single bout of exercise in individuals with insulin resistance.
Acta Diabetol — Erickson ML, Little JP, Gay JL, McCully KK, Jenkins NT Postmeal exercise blunts postprandial glucose excursions in people on metformin monotherapy. J Appl Physiol — Boule NG, Kenny GP, Larose J, Khandwala F, Kuzik N, Sigal RJ Does metformin modify the effect on glycaemic control of aerobic exercise, resistance exercise or both?
Diabetologia — Pedersen BK, Saltin B Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 25 Suppl 3 :1— Borno A, Foged L, van HG Glucose and glycerol concentrations and their tracer enrichment measurements using liquid chromatography tandem mass spectrometry.
J Mass Spectrom — Steele R Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci — Demerath EW, Shen W, Lee M et al Approximation of total visceral adipose tissue with a single magnetic resonance image.
Am J Clin Nutr — The primary outcome was absolute change in HbA 1c between baseline and the end of the 6 month supervised exercise period. HbA 1c was measured by turbidimetric immunoinhibition. Secondary outcomes included fasting glucose, aerobic fitness, strength, anthropometrics and exercise adherence.
Plasma glucose was measured after a 12 h fast, at least 48 h after the last exercise session. Exercise adherence was calculated from electronic membership card use. For the primary analysis, we used a linear mixed-effects model for repeated measures over time with HbA 1c as the dependent variable.
Contrast estimates from the mixed model were calculated for metformin by group by time interaction Control vs Aerobic, Control vs Resistance, Control vs Combined , with age, sex, BMI and exercise facility as covariates. Of the volunteers who were eligible for the study and entered the 4 week run-in phase, Of these, there were 3, 12, 7 and 8 dropouts from the Control, Aerobic, Resistance and Combined groups, respectively, during the intervention period.
One hundred and forty-three participants reported taking metformin throughout the entire study and 82 reported not taking metformin; see reference [ 6 ] for the complete trial flow diagram. The remaining 26 participants were not included in the analyses due to changes in their metformin use during the study period.
Characteristics of the participants are summarised in Table 1. As previously reported [ 6 ], there was a significant overall reduction in HbA 1c in all exercise groups. Effect of exercise, by metformin treatment, on HbA 1c a and fasting glucose b. White bars, no metformin; black bars, metformin treatment.
The number of participants was 19, 42, 18, 32, 20, 35, 25, 34 in the groups from left to right. However, the metformin by group by time interactions were not significant.
Contrary to our hypothesis, use of metformin was not associated with smaller improvements in glycaemic control following exercise training. This finding is important because it is contrary to recent studies suggesting that the addition of exercise to metformin treatment increased postprandial glucose [ 1 ], increased hepatic glucose output [ 2 ] and had no additional effect on insulin sensitivity [ 2 , 3 ] compared with metformin treatment alone.
The DARE trial represents the largest supervised exercise study examining this issue and is the only study that includes a group dedicated to performing resistance training alone.
There are differences between the present study and previous ones [ 1 — 3 ] that may help explain the disparate results. The most important differences may relate to the timing and type of measures of glycaemic control or insulin sensitivity.
In previous studies [ 1 — 3 ] meal tolerance tests or hyperinsulinaemic—euglycaemic clamps were performed within 28 h of an exercise session. In contrast, HbA 1c reflects average blood glucose concentration over the previous 2—3 months and fasting glucose measurements in DARE had been taken at least 2 days after the last exercise session.
Alternatively, it could be speculated that differences were due to the fact that previous studies were performed in metformin-naive participants [ 1 — 3 ] whereas participants in the DARE trial had been taking metformin for a longer time before starting exercise. The previous studies involved randomly assigned participants with better glycaemic control i.
insulin resistant without diabetes [ 2 ], impaired glucose tolerance [ 3 ] and diabetes with mean HbA 1c of 6. It is possible that not all individuals are affected similarly by interactions between metformin and exercise.
Changes in indicators of fitness were not significantly affected by metformin. However, the difference between metformin users and non-users was in the same direction as in previous studies, suggesting that metformin reduces improvements in aerobic fitness [ 3 , 4 ].
The primary limitation of the present analyses was the absence of randomisation to metformin or placebo. The impact of confounders was minimised through the inclusion of a control group and by adjusting for differences such as age, sex and BMI. Baseline HbA 1c level is known to be directly related to the magnitude of the improvements in HbA 1c levels following exercise [ 8 ].
Baseline HbA 1c was higher and there were greater proportions of men in the subgroup of metformin users. This may in part explain why the patients treated with metformin tended to respond more favourably following aerobic training.
The relatively good glycaemic control at baseline in the DARE trial may have also constrained the magnitude of the intervention effects [ 8 ].
Other glucose-lowering medications were used by participants in the DARE trial [ 6 ] and metformin users were often also treated with them.
It would have been interesting to examine the effects of other medications. However, metformin was chosen since it was the most commonly used medication in DARE participants and the study was underpowered to examine interactions among several medications.
In summary, metformin did not significantly affect improvements in HbA 1c and fasting glucose, fitness and anthropometrics resulting from 6 months of aerobic, resistance or combined aerobic and resistance training. Boulé NG, Robert C, Bell GJ et al Metformin and exercise in type 2 diabetes: examining treatment modality interactions.
Diabetes Care — Article PubMed Google Scholar. Sharoff CG, Hagobian TA, Malin SK et al Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals.
Am J Physiol Endocrinol Metab E—E Article PubMed CAS Google Scholar. Malin SK, Gerber R, Chipkin SR, Braun B Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes.
Braun B, Eze P, Stephens BR et al Impact of metformin on peak aerobic capacity. Appl Physiol Nutr Metab — Accompanied by physical exercise, weight loss and possibly other medications, it is often an effective therapy.
It does not cause hypoglycaemia, helps not to gain weight or even reduces it. The main feature of Metformin is to interact strongly with AMPK by regulating its expression. Metformin by up regulating AMPK has therefore shown to have a somewhat transversal therapeutic use in the treatment of metabolic dysfunctions.
Exercise; Nutritional Biochemistry; Metformin; Body Building; Inflammation; Skeletal Muscle. The use of anabolic androgenic steroids AAS in bodybuilding became a huge problem and risky factor that causes the death of many athletes, extreme ways are followed in bodybuilding especially during contest preparation and the goal is to get the perfect physique on stage, our goal is to help and save lives by providing scientific evidence to help the athletes and save their lives by preventing them the abuse of such drugs, in our case report series we used Metformin.
Within the cell, Metformin inhibits the inflammatory pathway and activates AMPK thereby increasing mTOR inhibition , while also modulating oxidative stress.
These processes jointly affect inflammation, cellular survival, stress defense, autophagy, and protein synthesis. Metformin can increase insulin sensitivity and lower blood levels of insulin due to its ability to cleanse the microbiome, also inhibiting glucose formation in the liver, can also inhibit the release of pro-inflammatory cytokines, reduces or eliminates inflammatory factors.
Metformin increases the formation of mucin-degrading Akkermansia miciniphila in the gut. Akkermansia muciniphila reduces inflammation, lowers insulin resistance so by this we can say simply that metformin boost the growth of good bacteria. Metformin is effective and efficient drug for our case report series, because of the following mechanisms of action:.
It is interesting to note that relatively recent studies suggest that Metformin can directly inhibit the action of leucine on mTOR. Not only would this be a bad sign for muscle growth, but the inhibitory effect of Metformin on mTOR should have a major effect as it correlates with the reduction in the risk of fatal cancers in diabetics.
Interesting as postulated by Dr. Melnik of the University of Osnabrück in Germany: Metformin may be a direct competitor of leucine for the binding and activity of mTORC1. The doctor noted in his article that the usual daily dose in diabetics of Metformin 2 g is in the range of the 2 g of leucine derived from the daily consumption of g of meat or cheese.
Since the two substances are similar in structure and size, they can compete for the same sites in the activation of mTOR. However, Metformin has these three characteristics of relevant interest: Schematic representation of Gluconeogenesis 1.
Increases the number and sensitivity of cell receptors for Insulin 2. These three effects explain why clinical administration of Metformin rarely results in cases of greater hypoglycemic effects when administering the drug alone. The aforementioned characteristics expressed by Metformin have meant that this drug enters the arsenal of sports pharmacological supplementation, especially in Body Building.
Generally Body Builders use the effects of Metformin differently for different phases: 1. During the Bulk phases, with the use of exogenous insulin, mg of Metformin times a day increased the effectiveness of the insulin. This was due to an increase in the number and sensitivity of the receptor sites.
Metformin also decreases the amount of insulin required for maximum results. The common dose of Metformin for this purpose is mg 2 times a day.
During the Cut phases, Body Builders use Metformin as a means of decreasing the production of glucose by the liver and the absorption of glucose by the intestine. By itself, this decreases the secretion of insulin by the pancreas and increases the body's dependence on fat stores for energy needs.
This is especially done while using GH and PGF-2 and creates a synergistic effect with AAS. As the cellular receptor sites for insulin are more sensitive and since there is cross stimulation between IGF-1 and insulin and their opposite receptor sites the retention of lean mass increases contained.
This effect favors the reduction of the negative effects that a weight loss diet exerts on the endogenous production of IGF if a co-administration of IGF-1 and Metformin occurs, the advantage reflects on the lower dose of IGF-1 required and on the better receptor efficiency.
It is known that during a calorie-deficient diet the IGF-1 produced decreases, and is one of the factors by which the stored lean mass is reduced. If the cell sites are more numerous and sensitive, stimulation requires less IGF Typically, mg of Metformin per day is considered sufficient.
Metformin should be taken with meals and never less than six hours before going to bed. People with kidney problems should not take Metformin and most athletes should be aware that in some cases the combination with alpha-alkylated drugs can induce even greater liver damage.
The purpose of this case report series is to evaluate the potential interaction between metformin and exercise during contest prep in order to help in different contest protocols to minimize the use of extreme ways used during preparations, and save the health of bodybuilders.
The method used to measure cortisol levels: The cobas e fully automated, system for immunoassay analysis. We increased the essential amino acids intake and GH doses for AAS users during the last two weeks, and every bodybuilder ingested 7g of glutamine during working out.
The key thing of this experiment was to not mess with minerals balance in the body, we know certain anabolic steroids cause minerals imbalance so we focused on that and tried using metformin during the prep and we collected blood samples from the participants every week.
The other thing i focused on was cortisol levels and inflammatory biomarkers and metformin have a direct anti-inflammatory action. Studies have suggested that metformin suppresses inflammatory response by inhibition of nuclear factor κB NFκB via AMP-activated protein kinase AMPK -dependent and independent pathways.
We noticed an improve in gut health and no digestion issue no bloating and was just one case had diarhea. Figure 1. Cortisol levels before the use of Metformin.
Metrics details. Physical exercise is the first-line exercisee for prediabetes, and metformin is the most widely Metformmin oral MMetformin agent. WHR and weight management, intermuscular adipose tissue IMAT directly affects insulin resistance by Metforimn maintain Metformin and exercise performance homeostasis. Exedcise magnetic resonance imaging MRI was then performed, and tissue-specific inflammation and energy and lipid metabolism were evaluated in IMAT. The EXE group had lower inflammatory factor levels, lipid metabolism, and mitochondrial oxidative stress, and shorter IMAT adipocyte diameters than the MET group. The MET group exhibited lower IL-1β and Plin5 expression than the PRE group. Furthermore, the IMAT of the EMC group had lower TNF-α and phosphorylated NF-κB levels and higher GLUT1 and GLUT4 expression than the PRE group.
0 thoughts on “Metformin and exercise performance”