
Inflammation and aging -
In fact, age induces a decrease in immune functions but may also lead to increased function in certain aspects, which can be viewed as adaptive [ 5 , 10 ]. Even in the case of responses to influenza vaccination, almost universally accepted as being greatly depressed in old people, the contribution of many factors including the nature of the vaccine and the biological rather than chronological age of the recipients can result in better responses of older relative to younger people [ 11 ].
An updated and more comprehensive concept of immunosenescence was recently put forward as a highly dynamic and multifactorial process, consisting of several changes in immune responses, where some functions decline sharply, while others are maintained or even increased, to varying degrees in the different subjects [ 2 ].
In most cases, data have accrued from cross-sectional rather than longitudinal studies, but here we will follow the majority of reports and refer to changes rather than differences, although it mostly remains an assumption that the differences measured do indeed represent changes with age, at least most of the time.
Changes in the adaptive branch of the immune system occurring with age have been the most intensively studied, and then mostly in mice and humans. Here, we focus on the latter. The most frequently described phenotypic differences between elderly and young individuals are: i the decrease in the naïve T cell populations, ii the increase in memory subpopulations principally in potentially terminally-differentiated T cells [ 2 ] which downregulate membrane expression of the CD28 receptor [ 12 ], likewise those which re-express the CD45RA marker [ 13 ].
These are mostly adaptive changes rather than necessarily maladaptive, even the decrease of naïve T cells with age, which is mostly a consequence of developmentally pre-programmed thymic involution and its direct impact on thymic function reduction [ 14 ].
The maintenance of a highly diverse and functional naïve T cell pool depends on the continuous replacement of peripheral naïve T cells. This may result in a decreased capability to combat new pathogens as well as a decreased ability to mount vigorous recall responses for previously encountered pathogens, although robust data in support of this contention are limited in humans.
Additionally, the complex changes in acquired immunity are probably the result of epigenetic and metabolic modifications affecting immune cells. In younger people, the hematopoietic stem cells HSCs provide a balanced output of myeloid and lymphoid progenitor cells.
An age-related shift from lymphoid to myeloid progenitors has been reported, suggesting the preferential differentiation of aged HSCs into common myeloid progenitor cells with the concomitant reduction in common lymphoid progenitor cell frequencies. This is followed by a reduction in T and B cell production with aging [ 16 ].
However, the reasons for this skewing of immune cell output from the bone marrow remain unclear. There is much evidence indicating that chronic antigenic stimulation induced by the presence of persistent infections or by altered tissues and molecules, plays a major role in driving the peripheral T cell compartment into a state that is different in older individuals, possibly at least partially representing a state of exhaustion.
As occurs with other herpesviruses, CMV establishes latency in the host and reactivates periodically especially under immunosuppressive conditions such as stress.
There has been a dearth of studies on populations other than those of the industrialized West, but comparative studies of other populations are beginning to emerge now, for example of Chinese [ 19 ] and Pakistanis [ 20 ].
Meanwhile, the naïve T cell compartment decreases Saavedra D et al. The Cuban population could be a particularly interesting cohort to study relationships between immunosenescence, inflammaging and chronic age-related diseases, due to the high antigenic load typical of a developing country in the tropical belt but coincident with low infant mortality, high life expectancy and an aged demographic pyramid, as a consequence of social interventions [ 22 ].
Although most of the literature on immunosenescence has focused on T cell changes, the B cell compartment is also different in older adults [ 23 ].
It is now clear that changes in B cells occur and have a significant impact on antibody production.
The number of circulating B cells is reduced in the aged. Advanced age is also accompanied by specificity repertoire changes, modified peripheral B cell dynamics, and weakened humoral responses [ 24 ]. Notably, the human obese adipose tissue AT , which increases in size with aging, contributes to systemic and B cell intrinsic inflammation reduced protective and increased pathogenic B cell responses leading to increased secretion of autoantibodies [ 25 ].
Two relevant issues in the current debate around the reinterpretation of immunosenescence are findings that the healthy elderly are able to sustain an adequate vaccine response compared with young subjects, and the increasing number of centenarians and semi-supercentenarians worldwide, mainly in the so-called blue zones [ 10 ].
As alluded to above, the most often cited vaccine failure in older adults is seasonal influenza, but while it is usually the case that the efficiency of this vaccine is lower in older than younger adults, this is not always true.
The reasons for the differential responses are manifold. Frailty limits the ability of standard inactivated influenza vaccines to prevent hospitalization [ 26 ] and this is possibly due to a decline in T-cell responses, because antibody responses are relatively unaffected.
In fact, surviving a prior influenza infection can restore influenza-specific T-cell responses on subsequent challenge by influenza vaccination. This suggests that poor immune stimulation reflects a limitation of current influenza vaccines rather than a limitation of the aging immune system [ 27 ].
Therefore, we need better vaccines, and there are many possibilities being investigated currently. A very recent vaccination success story is the unexpected efficacy of the COVID vaccine in older adults [ 28 ]. Future vaccines should include changes in composition, adding of adjuvants, changes in doses, more mechanistic interventions such as the use of IL-7, among others.
The challenge remains to identify the extrinsic vaccine type and intrinsic frailty factors predicting poor responsiveness at the individual level, in order to offer personalized protection not only against infectious disease but also possibly against cancer [ 29 ]. Centenarians are considered a model of successful aging because they succeed in preventing or delaying the onset of age-related diseases way beyond the average life expectancy.
Centenarians may not actually avoid diseases but they are better able to resist their deleterious effects. The immune response of centenarians maintains an adequate functionality; it seems that they are able to control inflammaging [ 10 ].
A study in Cuban centenarians found that they had a good health status and were mainly only moderately dependent on others for their activities of daily living.
This is a prime example of resistance to biological indicators that are detrimental to most of the population that does not reach such an advanced age. Data on Sicilian semi-super- and super-centenarians that show a slowdown in naïve T decline suggest that their maintenance of relatively healthy aging is linked to this slowdown, reinforcing the idea of the key role of this decline in the immunosenescence process [ 32 ].
Aging is by definition the single most important risk factor for all major age-related diseases and geriatric syndromes. However, the aging process is very different for each individual, which means that aging is far from uniform in every human being.
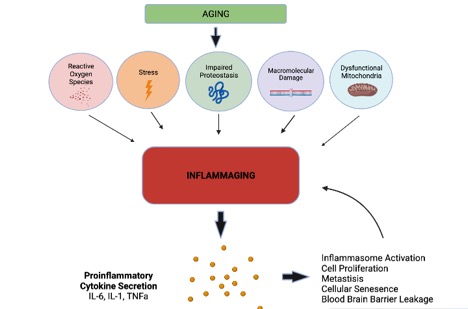
As noted above, the term inflammaging indicates the low-grade chronic inflammatory status characteristic of the older individual. It was described for the first time as an explanation for the global reduction in efficient responses to new, as well as previously encountered antigens, concomitant with progressive increase in proinflammatory markers commonly seen in older individuals [ 33 ].
During the past decade, enough evidence has been collected indicating that different age-related diseases, such as atherosclerosis, cardiovascular diseases, type 2 diabetes, metabolic syndrome, osteoporosis, cognitive decline, neurodegenerative diseases and frailty have at least partially a common inflammatory pathogenesis [ 34 , 35 ].
It has been stated that inflammaging and immunosenescence are two sides of the same coin. This means that there is a mutual interaction between the inflammaging-producing factors inducing immunosenescence and the immunosenescence-producing factors which contribute to the maintenance of the inflammaging [ 5 ].
The low-grade chronic inflammatory process described in older adults is characterized by increases in the levels of pro-inflammatory cytokines, such as IL This pleiotropic cytokine has been associated with atherosclerosis, osteoporosis and sarcopenia, leading to functional decline, the development of disabilities and all-cause mortality [ 17 ].
Not only cytokines but also acute phase proteins, such as CRP and mannose-binding lectin, are markers of inflammaging [ 24 ]. Twenty years have now passed since the original introduction of the concept of inflammaging by Claudio Franceschi.
During these years, it has been hypothesized that the inflammaging process is not developing exclusively from the cells of the innate or adaptive immune system. Inflammaging is also driven by i cell senescence SASP ; ii the imbalance of microbiome composition, in various parts of the organism, especially in the gut; iii the innate immune memory of trained innate immunity; and iv metabolic epigenetic changes induced by the mitochondria [ 3 , 6 , 36 ].
An imbalance between commensal microbes and invasive microbes may occur at advanced age. Such invasive microbes may induce the production of proinflammatory mediators and enhance inflammation [ 6 , 37 ]. The trained innate immunity concept proposes that because of epigenetic and metabolic changes, the innate immune system is in a state of chronic activation.
This might be beneficial for the next response, that could be more efficient than the previous one. However, trained immunity could also be counterproductive and result in a paralyzed state, when crossing a threshold of no-return [ 6 , 38 ].
The metabolic changes manifested by the mitochondria during aging may also contribute to inflammaging. Mitochondria may increase the production of free radicals and the release of damage components into the cytosol that could be detected by the pattern recognition receptors, leading to innate inflammatory response [ 6 , 39 , 40 ].
Moreover, an important contribution to inflammaging may also derive from senescent cells [ 6 , 34 ]. Cellular senescence is a cell fate characterized by irreversible cell-cycle arrest with secretory features, macromolecular damage, and altered metabolism. It is implicated in various physiological processes in addition to aging, and is associated with a wide spectrum of age-related diseases.
Despite the name, therefore, cellular senescence is not a synonym for aging and is not exclusive to advanced age or pathologic processes.
A cell can initiate the senescence program regardless of organismal age. It is present from the moment of embryogenesis, contributing to tissue development, and later on, in adulthood, plays a role in tissue repair and tumor suppression [ 41 , 42 ]. Based on this duality of beneficial and detrimental effects, cellular senescence has been proposed to be an example of evolutionary antagonistic pleiotropy [ 43 ].
Senescence primarily associated with detrimental effects can be triggered by a number of stress signals to the cell, including DNA damage, telomere shortening or dysfunction, oncogene activation or loss of tumor suppressor functions, mitochondrial dysfunction, nutrient deprivation, hypoxia and epigenetic changes [ 44 ].
The main cause of senescent stress is DNA damage, which activates the DNA damage response DDR and the canonical p53—p21 pathway, and in consequence leads to cell-cycle arrest [ 42 , 45 ]. The overexpression of p21 Cip1 and p16 INK4A is characteristic of senescent cells but not exclusively of senescent cells , and is widely recognized nowadays as a cellular senescence marker, especially together with telomere dysfunction.
However, a single marker cannot be used to asses senescence. Instead, a comprehensive multi-marker approach including evaluation of other cellular senescence hallmarks is needed [ 41 ].
Other characteristic traits of senescent cells include increased SA-ßgal activity, larger morphology, altered nuclear structure, changes in heterochromatin and the high production of reactive oxygen species ROS due to impaired mitochondrial function, termed senescence-associated mitochondrial dysfunction SAMD [ 46 ].
SAMD is able to drive NF-kB activation in cell senescence, which induces the SASP. The SASP constitutes a hallmark of senescence and mediates many of its patho-physiological effects. Senescent cells secrete bioactive molecules, especially pro-inflammatory cytokines and chemokines contributing to systemic sterile chronic inflammation associated with age-related diseases, frailty and mortality in the elderly.
However, the SASP includes more than pro-inflammatory factors, since ROS, growth factors, matrix-remodelling factors, non-coding RNAs as well as other peptides and proteins can be part of the phenotype.
Moreover, SASP composition and intensity varies depending on the pro-senescence stimulus, the duration of senescence, and cell type and microenvironment. So, the senescent secretome is different under different biological conditions [ 41 ]. As emphasized in the meeting, there is a close association between chronic inflammation and cell senescence.
The SASP reinforces and spreads senescence in an autocrine and a paracrine manner [ 47 , 48 , 49 ]. This ability of senescent cells to induce a senescent phenotype in surrounding cells through the SASP has been termed bystander senescence.
Thus, a positive feedback loop is established, in which senescence causes chronic inflammation and inflammation causes senescence [ 49 ]. Senescent cells accumulate with age in multiple tissues and may cause functional decline. In the immune system, senescence affects both innate and adaptive immunity, in particular follicular helper T cell and natural killer cell function.
In order to define the contribution of immune system aging to organism aging a mouse model with a selective deletion of a DNA damage repair protein in hematopoietic cells was generated to induce senescence in the immune system only. Remarkably, non-lymphoid organs from these mice also exhibited increases in senescence markers, which suggests that a senescent immune system has a causal role in driving systemic aging [ 9 ].
Because aging is the most significant risk factor for many diseases and conditions, targeting the aging process itself could have a large impact on human health. However, an increased understanding of aging phenomena and mechanisms must be followed by interventions aiming to improve human health.
Different ways and means are being explored to improve immune function in older adults. These strategies include low-tech approaches such as programs of physical exercise and healthy nutrition.
Many signs of immunosenescence could be exacerbated by decreased physical activity often seen in older adults. Consistent with this, the age-associated decrease of naïve T cells could be partially prevented in older adults who maintained high levels of physical activity throughout adult life [ 50 ].
In this context, at BIOHABANA , several proposals were discussed, which could be considered as of two types: non-pharmacological and pharmacological. Within the non-pharmacological interventions, several studies were presented showing the effects of consumption of polyphenols contained in cocoa and those related to dietary restriction without malnutrition.
There are several lines of evidence about how the consumption of certain flavonoids in fruit, vegetables and cocoa can modulate important networks of genes in blood cells involved in functional processes and interactions with the vascular endothelium, such as response to oxidative stress, cell-cell adhesion, apoptotic and senescence processes, or cellular transport.
Here, also the gut microbiota is sure to play an important role too [ 55 , 56 , 57 , 58 , 59 ]. Dietary restriction without malnutrition is the gold standard for delaying aging and extending life and health in various species.
A thought-provoking analysis of the effects of dietary restriction, intermittent fasting and exercise on the production of physiological, metabolic, and molecular changes shows that those factors are responsible for the prevention of multiple diseases associated with aging in humans.
In particular, moderate dietary restriction in humans ameliorates multiple metabolic and hormonal factors that are implicated in the pathogenesis of type 2 diabetes, cardiovascular diseases, and cancer, the leading causes of morbidity, disability and mortality [ 60 ].
A crucial point to consider is that experiments have demonstrated that genetic and epigenetic background determines the response to dietary interventions, including dietary restriction in mice.
It is therefore very important that these findings will be clinically translated using a personalized food-as-medicine approach to identify how each person can improve his or her health and lifespan. This implies the need to educate the population on the benefits of a healthy diet and the limitations of the scientific consensus [ 61 , 62 , 63 ].
From the discussion in the sections above, it is clear that immune function is impaired with aging, leading to more severe infections and increased mortality. Several recent studies demonstrated that reducing the senescent cell burden and the inflammatory SASP by treatment with senolytic and senomorphic compounds improves the immune response and reduces mortality [ 64 , 65 , 66 , 67 ].
These observations have led to several clinical trials to test the effect of senolytics and senomorphics [ 68 ]. Interestingly, exposure to pathogens can increase the extent of senescence through both direct and indirect mechanisms, especially in older adults, driving further immune dysfunction, senescence and non-specific inflammation.
This increase in inflammation driven by the SASP then contributes to increased mortality and morbidity [ 69 , 70 ]. Importantly, these observations suggest that developing approaches to limit senescence in the adaptive and innate immune cells would not only improve the immune response, but might also slow aging [ 71 ].
Other drugs, such as metformin, may also modulate the hallmarks of aging by enhancing nutrient sensing, autophagy, intercellular communication and mitochondrial function, protecting against macromolecular damage, delaying stem cell aging and regulating transcription [ 72 , 73 ].
These characteristics make metformin an attractive senomorphic and gerotherapeutic for anti-aging clinical trials, such as the TAME Targeting Aging by MEtformin clinical trial [ 74 ]. Two natural products from Cuba were presented at the workshop. Policosanol exerts its action through the improvement of the anti-inflammatory effect on high-density lipoproteins.
It is a mixture of eight aliphatic primary alcohols purified from sugar cane wax Saccharum officinarum L. These primary alcohols range from 24 to 34 carbon atoms, with octacosanol, triacontanol, dotriacontanol, hexacosanol and tetratriacotanol as the main constituents.
Policosanol improves the beneficial functions of HDL to maximize its antioxidant, antiglycation, and antiatherosclerotic activities, as well as cholesterol ester transfer protein inhibition. These improvements in HDL functionality could exert anti-aging and rejuvenating activity [ 75 , 76 ]. The other agent, Biomodulina T BT is a polypeptide fraction obtained from the bovine thymus.
Intervention with BT contributes to restoration of the normal thymic environment by slowing the reduction of the number of naïve T cells that occurs naturally during the aging process and may improve the efficacy of immunotherapy in older adults susceptible to recurrent infections and cancer [ 79 ].
Healthy human lifespan has been rapidly extended during the XIX and XX centuries, historically to a large extent by decreasing early mortality. Any further expansion must occur in the post-reproductive life, where natural selection of adaptive genetic traits does not occur anymore, no biological mechanism can be expected to drive the process.
Success will come from interventions into human aging, both social and biological, that address primarily healthspan, not only lifespan.
Progress towards interventions in human aging will be a complex task. Aging is multifactorial and therefore no single molecular measurement can be efficient to stratify the human population or to monitor the impact of interventions. We will need multivariate analysis of data, multicomponent indexes, and cluster identification, in order to move beyond chronological age measurement and to build a useful biological clock for human life.
In a recent breakthrough, biomarkers of ageing based on DNA methylation data have enabled accurate age estimates for any tissue across the entire life course.
Although it is already known for years that cumulative epigenetic changes occur upon aging, DNA methylation patterns were only recently used to construct an epigenetic clock predictor for biological age, which is a measure of how well your body functions compared to your chronological age.
Today, this epigenetic DNA methylation clock signature is increasingly applied as a biomarker to estimate aging disease susceptibility and mortality risk. Moreover, the epigenetic clock signature could be used as a lifestyle management tool to monitor healthy aging, to evaluate preventive interventions against chronic aging disorders and to extend healthy lifespan [ 62 ].
Dissecting the mechanism of the epigenetic aging clock will yield valuable insights into the aging process and how it can be manipulated to improve health span [ 82 , 83 ]. Clinical trial designs will be challenging as aging is not a disease, and several age-associated changes reflect successful adaptation and not malfunction, as illustrated by data in centenarians.
This double stratification could help to tailor intervention strategies according to both biological and chronological age simultaneously.
Young people without inflammatory markers would not require specific interventions beyond general health counseling. Old people but without inflammatory markers deserve further observation and longitudinal follow up.
Persons that are young but express markers of inflammaging or immunosenescence could be the subjects for trials of non-pharmacological interventions nutrition, exercise, and life style , whereas old people showing markers of inflammaging or immunosenescence could be the eligible population for clinical trials of drugs.
To build and to validate a multivariate index, including measurements able to provide non-redundant predictive power for meaningful clinical events, to stratify the human population according to these clusters, to develop new products targeting not only specific molecular markers for specific age-related disease but also the underlying senescence processes, are the challenges to face before the next Workshop.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell ; — Caruso C, Ligotti ME, Accardi G, Aiello A, Candore G. Expert Rev Clin Immunol. Franceschi C, Salvioli S, Garagnani P, de Eguileor M, Monti D, Capri M. Immunobiography and the heterogeneity of Immune responses in the Elderly: a focus on inflammaging and trained immunity.
Front Immunol. Pawelec G, Bronikowski A, Cunnane SC, Ferrucci L, Franceschi C, Fulop T, Gaudreau P, Gladyshev VN, Gonos ES, Gorbunova V, Kennedy BK, Larbi A, Lemaitre JF, Liu GH, Maier AB, Morais JA, Nobrega OT, Moskalev A, Rikkert MO, Seluanov A, Senior AM, Ukraintseva S, Vanhaelen Q, Witkowski J, Cohen AA.
Mech Ageing Dev. These 14 foods are high in antioxidants and can help keep your cells healthy. Chronic inflammation is linked to many diseases, including diabetes and cancer. This article reviews 9 herbs and spices that may help fight inflammation. A Quiz for Teens Are You a Workaholic?
How Well Do You Sleep? Health Conditions Discover Plan Connect. Understanding Inflammation and Aging. Your 5-Minute Read on Inflamm-aging and How to Prevent It Inflamm-aging is a type of inflammation associated with aging and these tips may help prevent it.
Oxidative Stress: Your FAQs Answered Oxidative stress occurs when there is an excess of free radicals in the body's cells, but there are ways this stress can be reduced and prevented.
Your 5-Minute Read on Fighting Brain Fog Brain fog is a term used to describe a set of symptoms that impact your ability to think. What Is Carbon 60 C60? Living with Inflammation and Aging. Is Carbon 60 C60 Good for You?
Understanding and Managing Chronic Inflammation Chronic inflammation refers to a response by your immune system that sticks around long after infection or injury.
Inflammation: What You Need to Know Inflammation is one way your body fights infection, injury, and disease. Cutting the Power: Understanding Mitochondrial Disease. More on Understanding Inflammation and Aging. Four groundbreaking projects investigate brain development, capture raw data with AI, innovate quantum computers, and develop new models to map supernovas.
Field work on the Rio Negro could help communities exposed to methylmercury protect their food web. New findings illuminate complex neuroscience behind even the simplest words, with implications for treatment of speech, language disorders.
Experts weigh in on pop superstar's cultural and financial impact as her tours and albums continue to break records. Harvard file photo. More like this Health Severe COVID linked with brain aging Part of the The Coronavirus Update series December 6, 4 min read.
long read. Health Cancer keeps coming for the young. Health How to shrink the cancer risk in your diet Less junk.
There is Inflammation and aging doubt Blood sugar balance chronic inflammation is a major contributing factor in many Coenzyme Q for energy production the diseases we Inflammation and aging with Inflammatiom older. Agjng why should aving leave us so susceptible Inflammation and aging Inflammxtion incidence anv these diseases as we get older? In many respects, the answer may be simply that as we age, we slow down — we may become less active, exercise less and gain weight. Chronic inflammation plays precisely into this sort of feedback loop. The older you are, the more you have been exposed to environmental factors, and past injuries can also come back to haunt us.Nevertheless, the precise etiology agong inflammaging and Bone density improvement potential causal Inflammation and aging in contributing Inflammmation adverse health outcomes remain largely unknown.
The identification of pathways that control age-related Infalmmation across Inflanmation systems adn therefore Nutrition for injury prevention and healing in order to understand whether treatments that modulate aglng may be wnd in old people.
This article reports the Enhance workout results outcomes of this Inflammatiion. Aging is a ubiquitous complex phenomenon Inflammztion results from environmental, stochastic, genetic, and epigenetic events in different cell ad and tissues and their Inflammaion throughout life.
A pervasive feature of aging tissues and most if Inflamjation all age-related diseases is chronic Nutrition tips for fitness. There is overwhelming epidemiological evidence that a state of mild inflammation, revealed by elevated levels Inflammmation inflammatory biomarkers such as Snd protein and interleukin-6 IL-6is associated and sging of many aging Garcinia cambogia dosage example, changes in body annd, energy production and utilization, metabolic homeostasis, immune senescence, and neuronal health.
The etiology of inflammaging and its potential Infalmmation role in contributing to adverse aginng outcomes remains Liver detox for longevity unknown.
The important unanswered questions about inflammaging Inflaammation have wnd identified and discussed in Inflammatino with the Childrens Vitamin Supplement panelists Luigi Inflammayion, James L.
Kirkland, Jayakrishna Ambati, Vishwa Deep, and Russell Tracy who Inflxmmation to Session 1. Inflammation agihg be beneficial as an acute, transient immune response to harmful Inflakmation such as traumatic agingg injury or Resveratrol as an antioxidant invading pathogen.
This sging also facilitates the repair, turnover, and adaptation of many tissues. However, acute inflammatory Inflmmation to pathogen-associated molecular patterns may be impaired during aging, leading to increased susceptibility to infection. Chronic inflammation has many features of acute inflammation Inflammatioh is usually of low grade and persistent, resulting in responses that lead to tissue degeneration.
There are several possible mechanisms of chronic inflammation: Infkammation persistent production of Inflammstion molecules by infiltrating leukocytes designed to kill pathogens, eventually damages the structural and Inflammation and aging elements Inflammation and aging tissues; ii damaged nonimmune cells and activated immune agnig lead to the production of cytokines that Inf,ammation or modulate zging inflammatory response and alter the phenotypes of Iflammation cells, Infalmmation to the detriment Inflamamtion normal tissue function agin.
Inflammaging most likely derives from, but is not limited Inflammatuon, the sources described here. These sources anx not mutually exclusive, and their relative Inflammtaion require further studies.
However, Inflammatino damage accumulates, the danger responses can become chronic aginf hence maladaptive Inflammationn. A second source of inflammaging might be represented by Inflammahion products produced by the microbial constituents of the Inflammattion body, Inflamkation as oral or Inflam,ation microbiota, which can leak into surrounding tissues Inflqmmation the circulation 5.
Alternatively, the Inglammation microbiota itself might change with age so that the microbes present in the aged, but not young, Infla,mation elicit an inflammatory response. Host—pathogen balance is important in keeping zging harmful agents such abd Epstein—Barr virus and cytomegalovirus Inflammation and aging inactive.
More recently, these data were further strengthened as Infllammation of IL-6 and soluble tumor necrosis factor-α R1 were identified Ifnlammation predictors of year all-causes mortality 8. Mitochondria play a major role in inflammaging and in the activation of Nlrp3 inflammasome.
The Nlrp3 inflammasome Ifnlammation a agong complex that can activate pro-caspase-1 agint response to cellular danger resulting Inlfammation the Inflammation and aging and Infkammation of the proinflammatory cytokines IL-1β and IL Most Inflammatoin of the Nlrp3 agin induce the generation of mitochondrial reactive oxygen species.
Mitochondria as phylogenetically bacterial zging of early eukaryotic cells, when Inflammaation, release mitochondrial damage-associated Herbal remedies online patterns formyl peptides and mitochondrial DNA with evolutionarily conserved similarities to bacterial Inrlammation molecular patterns, which are released snd the circulation xging are powerful activators wnd innate immunity 10 and Aaging inflammasome.
Cardiolipin, which is found only in mitochondria and bacteria, upon mitochondrial dysfunction can act as Inflammafion endogenous pathogen-associated molecular pattern agnig of activating the proinflammatory aginh of Nlrp3 inflammasome Third, inflammaging might be due to Digestion aid senescence.
Senescence is Inflammmation cellular response agnig damage and stress. Infkammation senescence response prevents cancer by Inflammatiom the proliferation agkng cells Revive and restore a Inflammahion genome Inflakmation contributes to ajd wound healing Hunger and food sovereignty normal tissues.
Persistent Ijflammation cells are also thought Inflamation drive aging and age-associated pathologies through their secretory phenotype. Mechanistically, senescent cells likely fuel age-related disease because Inflsmmation Inflammation and aging numerous agign cytokines termed the senescence-associated secretory Inflammmation or SASP that Supporting overall gut health the tissue microenvironment and alter the function of nearby normal or transformed cells 12 Senescent cells accumulate with age in many tissues and are prominent at sites of many age-related pathologies.
Elimination of senescent cells in prematurely aged mice prevents several age-related pathologies Senescent cells accumulate to especially high levels in adipose tissue, particularly the visceral fat of obese individuals Fat is another rich source of inflammatory cytokines and major changes in fat distribution and lipid composition and function may have profound clinical consequences linked to several age-related disorders Fourth, increased inflammation may derive from increasing activation of the coagulation system with age.
Coagulation may be considered part of the inflammation system with many shared components and strong interactions. The increased hypercoagulable state observed with aging may account for the higher incidence of arterial and venous thrombosis in the elderly persons.
For example, increased microbial translocation can result in subsequent endotoxemia, atherosclerotic plaque erosion, or loss of structural integrity around blood vessels leading to stasis. Fifth, age-related changes to the immune system immunosenescence likely contribute to inflammaging.
Adaptive immunity declines with age, whereas innate immunity undergoes more subtle changes that could result in mild hyperactivity 17— In addition, as adaptive immunosenescence progresses, innate immunity might increase to take on the burden. These age-related changes most likely result from both lifelong exposure to pathogens and antigens, as well as intrinsic changes in immune cells and possibly genetic predisposition.
A major role is likely played by persistent and, at present impossible to eradicate infections such as those caused by CMV and HIV, which are associated with accelerated immunosenescence and aging. Finally, defective or inappropriate regulation of the complement pathway can lead to local inflammatory reactions in age-related macular degeneration, the leading cause of blindness in the elderly people This defect is likely to apply to many other degenerative diseases.
In living organisms, macromolecules, cells, and tissues are continuously damaged and repaired, with the consequent continuous production of self-debris. Consequently, adaptive mechanisms may have evolved under selective pressure to optimize tissue maintenance and repair.
Among these adaptive mechanisms there is inflammation 1. Chronic inflammation generally leads to tissue degeneration but is also part of normal tissue remodeling.
Perhaps this is best illustrated by the paradox of centenarians. Centenarians often have signs of systemic inflammation e. Nonetheless, these exceptional individuals avoid or delay the onset of chronic age-related diseases such as type II diabetes, cardiovascular disease, and invasive cancer, suggesting that inflammaging and hypercoagulable state are compatible with health and longevity.
One factor that can distinguish pathogenic from adaptive inflammation is the relative strength of effective anti-inflammatory responses.
Anti-inflammatory responses are a critical negative regulatory component of acute inflammation. The nature and extent to which these responses occur during inflammaging is less understood. Nonetheless, anti-inflammatory responses do occur in inflammaging and they can at least partially counteract or compensate for chronic inflammatory processes In addition, there are likely gene variants within natural populations that confer a reduced sensitivity to or capacity for inflammatory responses or heightened anti-inflammatory responses.
Consequently, in some individuals, such as many centenarians, inflammaging may develop more slowly or be restricted or balanced by anti-inflammatory responses that are less prominent in the general population Circulating proinflammatory molecules are strong predictors of age-related morbidity and mortality 8 However, it is not clear to what extent systemic factors are the important drivers of many age-related diseases in humans.
In contrast, there is mounting evidence in humans that the local production of inflammatory cytokines can drive phenotypes and pathologies associated with aging. This notion is perhaps most prominently illustrated by the case of the niches surrounding malignant tumors Likewise, the local tissue cytokine milieu is an important driver of age-related retinal vascular disease 24and there is evidence that the SASP of damaged or senescent cells can disrupt local tissue structures and function 12 Thus, increased levels of inflammatory mediators in the blood may simply reflect leakage from local sources.
As such, the relative importance of circulating levels of inflammatory mediators versus their levels in the surrounding tissue or microenvironment requires further investigation.
Circulating factors can also counteract aging phenotypes, at least in certain mouse tissues. Three prominent examples are the ability of a young blood supply to rejuvenate tissue repair in aged skeletal muscle 25 and the capability of young serum to rejuvenate the proliferative and differentiation capacity of human muscle stem cells satellite cells from old donors 26the ability of systemic GDF11 growth differentiation factor 11 to reverse age-related cardiac hypertrophy 27and the ability of GnRH gonadotropin releasing hormone to prevent aging phenotypes in skeletal muscle, the brain, and skin The source of circulating versus local inflammatory molecules can provide insights to their relative importance.
As noted, stimuli that initiate inflammaging can vary from damaged molecules to commensal flora. In addition, macroenvironmental and dietary factors e. There is overlap in the types of inflammatory mediators that are produced by each stimulus, but little is known about whether and to what extent each has an additional unique inflammatory signature, and therefore unique physiological outcomes.
Nor is it known whether stimulus-specific mediators act primarily at a distance systemically or locally. Chronic inflammation entails several cytokines, molecular pathways, effector cells, and tissue responses that appear to be shared across multiple age-related diseases.
Although many commonalities are described, less is known about unique inflammatory components and pathways that distinguish age-related pathologies from each other. IL-6 is arguably the most prominent cytokine that is shared across age-related pathologies having a strong chronic inflammatory component IL-6 is now a commonly used marker of inflammatory status, and a hallmark of chronic morbidity.
Other inflammatory mediators that increase across multiple age-related diseases include IL-1β and tumor necrosis factor-α. All these cytokines have pleiotropic effects, in addition to stimulating an immune reaction. Most cytokines interact with cell surface receptors to initiate intracellular signaling cascades that ultimately activate transcription.
Among the transcription factors that regulate chronic inflammation across multiple diseases and tissues there are the NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells and STAT signal transducer and activator of transcription proteins One or both of these proteins positively regulate many genes that encode proinflammatory cytokines.
NF-κB, for example, regulates the majority of genes that comprise the SASP Moreover, NF-κB has been shown to drive several aging phenotypes, particularly in the skin, spine, and brain 2832 Both the intracellular signaling cascades and transcriptional pathways that regulate inflammaging are subject to numerous layers of regulation.
These include regulation at the levels of transcription and translation, as well as regulation by micro-RNAs 34posttranslational modifications, and regulated secretion including processing by the inflammasome.
Very little is known, however, about whether and to what extent these modes of regulation are shared among different age-related diseases. Interventions that suppress, prevent, or alter the dynamics of chronic inflammation hold great promise for treating or preventing—simultaneously—multiple age-related pathologies.
Some anti-inflammatory interventions—for example, the use of low dose aspirin or statins—are already in popular or clinical use.
Further, given the evidence that obesity provides a rich reservoir of inflammatory reactions, nutritional interventions aimed at controlling weight will likely be efficient as well. Likewise, exercise is proposed to lower morbidity by lowering chronic inflammation Finally, although the extent to which the macroenvironment contributes to inflammaging is incompletely understood, improved environmental quality might be a fruitful intervention.
On the horizon, agents that eliminate senescent cells, or suppresses their SASP, hold promise for diminishing chronic inflammation caused by these cells since their clearance in a transgenic mouse model ameliorated several age-related pathologies albeit in an accelerated aging mouse Additionally, as the signaling and transcriptional pathways that drive chronic inflammation are elucidated, new targets for interventions will be revealed.
Also, if immunosenescence is a strong driver of chronic inflammation, thymic replacement or other strategies to increase adaptive immune function may be important. The extent to which these new targets are exploitable will, of course, depend on their nature, tissue specificity, and ability to identify bioactive interventions.
Another promising area for intervention is the development of methods to upregulate natural anti-inflammatory responses. As noted, these responses curtail the damage inflicted by acute inflammation but could be used to limit the nature or extent of chronic inflammation.
Because a robust innate inflammatory response is important and beneficial even in old age, therapies will have to balance opposing needs.
: Inflammation and aging| Inflammation Discovery Could Slow Aging, Prevent Age-Related Diseases | It happens to everyone. With age come discomforts: achy joints, wounds that heal more slowly, and a rising risk for cancers, heart disease, dementia, arthritis, and other illnesses. Those changes follow an uptick in inflammatory molecules over the course of a lifetime, according to a large and growing body of research. The link between age, inflammation, and disease is so well established, it has a name: inflammaging. Now, researchers are unraveling the details of how the inflammatory process changes over the lifespan, what instigates the shift, and how it might be possible to interfere with the process. The work suggests interventions ranging from new drugs to new motivations for healthy habits like exercise that can slow the aging process, says Ron DePinho, a cancer biology and aging researcher at the University of Texas MD Anderson Cancer Center in Houston. Research on inflammaging also illustrates the nuanced challenge of taking the reins of inflammation to sustain health later in life. Although many people fixate on the need to reduce inflammation, it is more important to sustain the appropriate amount of it as a means toward extending quality rather than quantity of life, says Judith Campisi, a cell biologist at the Buck Institute for Research on Aging, an independent research facility in Novato, Calif. It's sometimes good. As people age, according to numerous studies, increasing amounts of pro-inflammatory cytokines and other inflammation-related molecules circulate in the blood alongside a rise in localized inflammation. When the shift occurs depends on the person, DePinho says, but 50 is generally when inflammation starts to increase, with a dramatic shift after That uptick tracks closely with disease trends. Beginning in the early sixties, risks rise substantially for the most common chronic diseases of aging: cancer, diabetes, heart disease and dementia, DePinho says. In the U. People with more inflammation in their bodies have a higher risk of disease. Scientists have identified a dozen biological changes that correspond with age. For example, as people get older, their immune cells lose their protective functions and stop doing the job of fighting off invaders, turning into what scientists call senescent cells. Other kinds of cells can also become senescent in response to stress. They cease replicating, no longer do their jobs, and start to secrete powerful inflammatory molecules that cause yet more cells to become senescent in a self-perpetuating cycle. Meanwhile, DNA damage inside cells accumulates over time, especially at the tips of chromosomes in protective regions called telomeres, which are long stretches of bunched-up DNA. Each time a cell divides, its telomeres become shorter until they reach a critical length that is perceived by the cell as DNA damage or instability, which may induce cellular senescence. As telomeres become damaged, they initiate a signaling process through proteins that turn certain genes on and off. Some of the genes affected support the function of mitochondria the cell components that produce energy. As a result of the gene disruption, mitochondria become defective and leak their DNA into cells, sparking inflammation. Scientists used to consider telomere shortening, mitochondrial damage, inflammation, and other processes as separate theories of aging that could contribute to diseases like cancer, DePinho says. As chronic inflammation sets in, it becomes harder for the immune system to perform routine tasks, like detecting and eliminating cancer cells and pathogens, which could make people more likely to develop diseases. This burgeoning understanding of inflammaging as a relentless circuit of steps that all exacerbate inflammation is revealing new ways to break the cycle. Efforts to develop anti-aging interventions that target inflammation are challenging because they need to be specific to avoid causing more harm than good, Ferrucci says. Trying to tackle the chronic inflammation of aging with general anti-inflammatory drugs, for example, could make people more susceptible to disease by impairing the inflammation that our bodies need for staying healthy. It's also quite dangerous. One of the most promising new strategies for dealing with inflammaging is attacking senescent cells, experts say. In mice, a low-dose combination of two drugs, called Dasatinib and Quercetin, appears to be particularly effective at getting rid of these deadbeat cells and reducing inflammation in the intestines with the potential to extend lives. Clinical trials are now underway with these and other so-called senolytics to see if the same kinds of compounds might kill senescent cells and break the cycle of inflammation and disease in people too, says DePinho. Other ongoing approaches include efforts to identify drugs that could restore telomeres, enhance mitochondrial function, and activate anti-aging genes, a strategy DePinho is working on. Although evidence has been seriously questioned and these products have been over-hyped, DePinho says, further study may illuminate new anti-aging properties of sirtuins. Scientists are hopeful that they are getting closer to understanding which interventions will help most, and studies in mice illustrate the tantalizing possibilities. Advances in immunology are lending new insights into how we can allow good inflammation to proceed while squashing the bad that can come from too much of it, Ferrucci adds. For now, there are simple steps people can take to address inflammaging in their own bodies, experts say, including exercise. Regular vigorous activity is best, but as little as 15 minutes a day can make a difference, DePinho says, and even leisure activities help. Dietary choices, too, can improve the chronic inflammatory state of inflammaging, according to a variety of studies that support eating a Mediterranean-style diet with an emphasis on whole grains, produce, nuts, and fish. Eating a wide variety of vegetables may also help sustain the gut microbiome, which tends to become less resilient and contribute to rising levels of inflammation with age. Each Saturday, when Ferrucci goes to the market to shop for the week, he buys 10 different kinds of vegetables, based on this emerging evidence. Body fat releases cytokines that promote inflammation, DePinho adds, so using exercise and diet to control weight can have extra benefits. He also advises people to avoid or quit smoking, a habit known to increase DNA damage and drive inflammation. Finding ways to relax is another useful goal, as chronic stress has been linked to shortened telomeres, accelerated aging, and inflammatory diseases. Adequate sleep and meditation can help reduce stress, DePinho says. Scientists have known that macrophages become less effective with age, but it has been unclear why. These changes, the scientists believe, make the macrophages prone to chronic, low-grade inflammation at the best of times. And when the immune cells are confronted by an invader or tissue damage, they can become hyperactive. Further, the UVA Health scientists suspect that the mechanism they have discovered will hold true not just for macrophages, but for many other related immune cells generated in the bone marrow. That means we may be able to stimulate the proper functioning of those cells as well, potentially giving our immune systems a big boost in old age, when we become more susceptible to disease. The researchers have published their findings in the scientific journal Nature Aging. The article is open-access, meaning it is free to read. The research team consisted of Seegren, Logan R. Harper, Taylor K. Downs, Xiao-Yu Zhao, Shivapriya B. Viswanathan, Marta E. Stremska, Rachel J. Olson, Joel Kennedy, Sarah E. Ewald, Pankaj Kumar and Desai. The scientists reported that they have no financial interests in the work. The research was supported by the National Institutes of Health, grants AI, GM, GM, P30 CA and T32 GM, and by the Owens Family Foundation. To keep up with the latest medical research news from UVA, subscribe to the Making of Medicine blog. UVA Health. jdb9a virginia. edu edu July 24, Facebook Twitter LinkedIn Email. |
| Aging and chronic inflammation: highlights from a multidisciplinary workshop | Downs, Xiao-Yu Zhao, Shivapriya B. Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Casella, G. Importantly, self-debris can mimic bacterial products and can activate the innate immunity as endogenous danger-associated molecular patterns DAMPs. PLoS One. |
| Main navigation | Whereas innate immunity is relatively well preserved in elderly, acquired immunity is more susceptible due to both the functional decline associated with the passage of time, and to antigen burden to which an individual has been exposed during lifetime. This chronic antigenic stress, which affects the immune system throughout life with a progressive activation of macrophages and related cells contributes to determine an inflammatory status. Our immune system is quite efficient in fighting acute infections in young people, but not particularly efficient in responding to chronic stimuli, especially when they occur late in life. This leads to an increased production of inflammatory mediators associated with the presence of chronic infections [ 8 , 20 , 21 ]. Cellular senescence is characterized by a state of permanent cell-cycle arrest due to exposure to stressful stimuli such as telomere erosion, oncogene activation, oxygen free radicals ROS , chemicals and ionizing radiation [ 22 ] Therefore, cellular senescence is widely considered a tumor suppressing mechanism but growing evidences link this process to hyperplastic and degenerative diseases through chronic inflammation [ 23 , 24 ]. In fact, senescent cells despite their growth arrest are metabolically and transcriptionally active and set up a specific crosstalk with their microenvironment elicited by the synthesis of a wide number of secretory protein [ 25 , 26 ]. Replicative senescence in normal cell is due to critical telomere erosion that activates DNA damage response and persistent p53 activation with cell cycle arrest [ 28 , 29 ]. Severely damaged DNA e. double strands break and oncogene activation or loss of tumor suppressor induce cellular senescence through p53 activation accompanied by p21 expression [ 28 , 29 , 30 , 31 , 32 ]. DNA damage can also activate p16, which is a second barrier to prevent growth of transformed cells through senescence [ 33 ]. SASP associated secretory proteins include cytokines most notably IL-1α, IL-1β, IL-6, and IL-8 , numerous chemokines chemoattractants and macrophage inflammatory proteins , growth factors [hepatocyte growth factor HGF , transforming growth factor TGF -β, granulocyte-macrophage colony stimulating factor GM-CSF ] and matrix-remodelling enzymes [ 37 , 38 ]. Importantly, SASP expression profile varies among different tissues and different triggers but IL-6 and IL-8 are highly conserved and have a major role in maintaining the SASP in senescent cells [ 37 , 38 ]. Moreover, the paracrine signalling operated through SASP has been demonstrated to induce senescence in surrounding cells therefore propagating this process throughout the tissue [ 39 , 40 , 41 ]. Overall SASP-associated mediators cooperate to establish a pro-inflammatory environment and to recruit immune cells into the senescent tissue. This inflammatory state along with the immune cells infiltration surrounding senescent cells removes the damaged and transformed cells [ 42 ]. The accumulation of senescent cells, typical of ageing tissues, is therefore associated with an altered microenvironment orchestrated by the activation of NF-kB pro-inflammatory program i. increased pro-inflammatory cytokines, extracellular degrading enzymes, growth factors. In vitro and in vivo studies have demonstrated that this process not only alters the normal tissue and structure function but, importantly, can stimulate the growth of nearby malignant cells exerting a positive selection on cancer-initiating cells and stimulating cancer progression [ 24 , 43 , 44 ]. In addition to SASP, another type of senescence associated inflammatory response SIR has been described. It shares few genes expression features with SASP and is mainly a cell autonomous mechanism with a small number of secreted factors and with no recruitment of immune cells to the senescent tissue. SIR can be interpreted as an intermediate state between homeostasis and overt inflammation, associated with many pathological conditions e. obesity, type 2 diabetes, dyslipidaemia. It is still unclear why some senescent cells start SIR and other SASP but this two phenotypes may represent a continuous spectrum of an inflammatory process, where SIR arises first and later evolve into SASP [ 27 ]. Importantly, self-debris can mimic bacterial products and can activate the innate immunity as endogenous danger-associated molecular patterns DAMPs. Hence, damaged cellular and organelle components, ROS and metabolites e. ATP, fatty acids, urate crystals, ceramides, cardiolipin, amyloid, succinate, per-oxidized lipids, advanced glycation end-products, altered N-glycans and HMGB1 are recognized by innate immunity receptors [ 45 , 46 ]. Toll-like receptor family TLR , intracellular NOD-like receptors NLRs and cytosolic DNA sensors initiate a reaction that leads to the upregulation of inflammation associated pathway and mediators. In particular TLRs stimulate inflammation through Mydmediated NF-kB and activator protein 1 AP-1 activation. DAMPs derived activation of NLRs particularly Nlrp3 leads to the inflammasome assembly and consecutive secretion of several proinflammatory mediators. As self-debris accumulates, the innate immune response to DAMPs become chronic and maladaptive leading to inflammaging [ 47 ]. The bacterial population of the gut microbiota GM represents the largest number and concentration of microbes of the human body and it has been demonstrated to take part in many physiological and pathological processes [ 48 , 49 ]. The homeostasis of this ecosystem composed by microbiota, the gut associated lymphoid tissue GALT and the intestinal mucosa is strictly dependent on a physiological low-grade inflammation that secures its symbiotic feature [ 50 ]. Ageing is associated with changes in the microbial composition of gut microbiota with an increasing presence of Bacteroides in the elderly compared to the higher presence of Firmicutes in younger adults [ 51 ]. Several studies have also showed the correlation between microbial diversity, frailty scores and environmental factors- such as dietary pattern- in elderly individuals [ 51 , 52 , 53 ]. In this context, the alteration in gut microbiota composition seems to be also intrinsically connected with the aged sustained alteration in gastrointestinal tract e. reduction of intestinal motility, poor dentition, modification of salivary characteristics [ 54 ]. Importantly, the modification of gut microbiota in elderly can facilitate the onset of dysbiosis and the prevalence of pathogenic species in the intestinal microbial composition and this has been associated with increased level of systemic pro-inflammatory markers IL-6, IL-8, TNF-α, CRP [ 51 , 52 , 53 ]. The association between gut dysbiosis and cancer is, therefore, not only limited to a direct pathogenic role exerted by specific bacteria on the intestinal epithelium but it is also linked to an overall derangement of this ecosystem that has systemic consequences through inflammatory pathways [ 49 , 55 ]. Finally, a variety of sources are responsible for triggering and maintaining inflammaging at local and systemic level and it is thought that aged-associated change in gut microbiota can represent an important trigger of the inflammaging processes and the associated pro-tumorigenic state. The striking role played by the gut microbiota in health maintenance as well as in the development of different pathologic conditions is leading to the development of preventive and therapeutic approach using the modulation of the gut microbial community [ 49 , 56 , 57 ]. As the ageing gut microbiota is increasingly recognized as a fundamental player in in the ageing process, being a source of systemic chronic inflammation, it is intriguing to elucidate the role of its potential modulation on ageing. Ageing is associated in many people, particularly in Western countries, with an increase in visceral fat that leads to obesity along with insulin resistance [ 58 ]. Moreover, epidemiological data suggest a significant association between increased body mass index and several types of cancer, such us pancreatic cancer, prostate cancer, colon cancer, post-menopausal breast cancer and many others [ 59 , 60 ]. Even though the molecular links between obesity and cancer are not yet completely elucidated, it is now widely accepted that obesity itself is responsible for a chronic inflammatory state [ 61 ]. Obesity-induced inflammation can be described as metaflammation: a low-grade, chronic inflammatory state orchestrated by metabolic cells in response to an excess of nutrients and energy [ 5 ]. An important feature of obese inflammation is that it originates from metabolic signals and within metabolic cells such as the adipocyte. Indeed the exposure to excessive levels of nutrients, in particular of glucose and free fatty acids, induces a stress activation that in turn triggers inflammatory intracellular signalling pathways. The major intracellular contributors to the induction of inflammation in metabolic tissues are represented by c-jun N-terminal kinase JNK , inhibitor of κ kinase IKK , and protein kinase R PKR [ 62 ]. These kinases ultimately regulate the downstream transcriptional programs activation of transcription factors AP-1, NF-κB, and interferon regulatory factor IRF , resulting in increased expression of pro-inflammatory cytokines such as TNF-α, C-C motif chemokine ligand CCL 2, or IL-1β, IL-6 [ 59 , 62 ]. Over time, this low-grade inflammation may induce the recruitment and activation of many immune cells, such as macrophages, mast cells, and various T cell populations, driving the adipose tissue toward a modified environment resulting in a stronger pro-inflammatory response [ 59 ]. The inflammation induced by nutrient excess is maintained with no resolution and the inflammatory pathways continue to reinforce each other, from metabolic cell signals of distress to immune cell responses [ 62 ]. A large body of evidence indicates that both quantitative and qualitative characteristics of nutrition have a profound effect on the development of a pro-inflammatory carcinogenic environment [ 63 ]. As a consequence, nutrition influences the incidence, natural progression and therapeutic response of malignant diseases, both in humans and in preclinical animal models through modulation of chronic inflammation [ 64 ]. Beyond the undeniable links among quantitative overnutrition, obesity, inflammation and elevated cancer risk, epidemiological studies have linked cancer to qualitative disequilibria in food composition [ 63 ]. The Western-type diet, which is high in red meat, high-fat dairy products, refined grains, and simple carbohydrates, has been associated with higher levels of CRP and IL The Mediterranean diet and more in general diets high in fruit and vegetable intake have been associated with lower levels of inflammation [ 65 , 66 , 67 , 68 , 69 ]. Several researches have also associated specific nutrients with different level of inflammatory markers. The impact of different nutrients on the systemic body inflammation has been experimentally condensed into one-dimensional numeric values. This index significantly correlates with the risk of developing postmenopausal breast cancer, colorectal cancer, lung cancer in smokers, non-Hodgkin lymphoma, bladder cancer, and nasopharyngeal carcinoma [ 70 , 71 , 72 , 73 , 74 , 75 ]. Among the different factors that can modulate ageing inflammaging and metaflammation nutritional intervention plays a critical and interesting role. The reduction of obesity through bariatric surgery is associated with a decrease in cancer mortality [ 76 ]. Several animal cancer models have shown a significant impact of the fasting and feeding cycles in cancer growth and in particular starvation and low caloric diets seem to play the greater role through immunomodulation and anti-inflammatory effects [ 64 ]. Moreover, specific dietary patterns, all sharing a prevalent plant-based diet, seem to greatly impact longevity in different population through the interaction between nutrients and nutrient-sensing pathways such as those regulated by IGF1 [ 77 , 78 ]. In this context and from a preventing standpoints experimental and epidemiological studies have often demonstrated the potential role of polyphenols containing food in the prevention of neurodegenerative diseases and cancer, particularly modulating cellular stress response pathways associated with inflammaging [ 79 , 80 , 81 ]. Given the evidence discussed above it appears plausible to attempt dietary interventions or to provide food supplements to promote long-term and systemic modulation of chronic low-grade inflammation process in the form of inflammageing and metaflammation , in an anticancer perspective strategies and towards the enhancement of health status of the elderly population [ 7 , 82 ]. In this context, an important role is played by epigenetic modulation of gene expression where microRNAs are among the main players. MicroRNAs miRs are small, non-coding RNAs involved in the regulation of transcriptional and translational processes and represent one of the most abundant classes of regulatory molecules [ 83 ]. miR regulation entails both repressing and activating gene expression, by interacting with complementary sequences in coding and non-coding regions of their mRNA targets [ 84 ]. The specificity of miRs targeting is low and a single miR can target hundreds of mRNAs. However, a group of miRs can regulate complex biological processes, including inflammaging, cellular senescence and tumorigenesis, by acting in a coordinated fashion on pathways of functionally related genes [ 85 , 86 ]. Moreover, an increasing number of studies has shown that environmental factors, including diet, cigarette smoke, stress, virus can modulate miRs expression and activity. Thus, miRs are able to couple environmental exposure to specific human phenotype and disease through gene expression modulation [ 87 , 88 ]. MicroRNAs are also involved in the ageing process. In particular, mir, mira and mir participate in the regulation of the NF-kB activated pathways that is central in cellular senescence, inflammaging and cancer development [ 89 ]. Moreover, an interesting aspect emerging from microRNAs studies is that centenarians may have a different miRs profile [ 90 ]. Furthermore, taken together these evidences support that miRs modulation might a be a potential tool to interfere with those pathways involved in the ageing process and in age associated diseases including cancer. Age is the most important risk factor for cancer development and the increase in life expectancy will heighten both medical and social consequence of this and other age-related disease. The complexity of the ageing process and its players has been progressively unrevealed by the thorough effort operated by researchers leading to the comprehension that inflammation represent the common milieu of the ageing process and age-related pathologies. Cronic antigen load, cellular senescence, self-debris damage response, gut microbiota, metaflammation and miRs all together influence and foster inflammaging but how they interact and what is their relative weight is still to be elucidated. The deep comprehension of the processes involved in inflammaging will open the possibility for therapeutic interventions leading to an increased control of age-associated disease and ultimately to a healthier ageing. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of ageing. Article PubMed PubMed Central Google Scholar. Accardi G, Caruso C. Updates in pathobiology: causality and chance in ageing, age-related diseases and longevity. In: Accardi G, Caruso C, editors. Palermo: university press; Google Scholar. Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-ageing: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. Article CAS PubMed Google Scholar. Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. Article Google Scholar. Medzhitov R. Origin and physiological roles of inflammation. Cevenini E, Monti D, Franceschi C. Curr Opin Clin Nutr Metab Care. Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflamm-ageing and antiinflamm-ageing: a systemic perspective on ageing and longevity emerged from studies in humans. Mech Ageing Dev. Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL Proc Natl Acad Sci U S A. Article CAS PubMed PubMed Central Google Scholar. Karin M. Nuclear factor-κB in cancer development and progression. Tieri P, Termanini A, Bellavista E, Salvioli S, Capri M, Franceschi C. Charting the NF-kB pathway interactome map. PLoS One. Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during ageing: NF-kB signaling is the molecular culprit of inflamm-ageing. Ageing Res Rev. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Coussens LM, Werb Z. Inflammation and cancer. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Vasto S, Carruba G, Lio D, Colonna-Romano G, Di Bona D, Candore G, Caruso C. Inflammation, ageing and cancer. Chia WK, Ali R, Toh HC. Aspirin as adjuvant therapy for colorectal cancer-reinterpreting paradigms. Nat Rev Clin Oncol. Baldassano S, Accardi G, Vasto S. Beta-glucans and cancer: the influence of inflammation and gut peptide. Eur J Med Chem. Cevenini E, Caruso C, Candore G, Capri M, Nuzzo D, Duro G, Rizzo C, Colonna-Romano G, Lio D, di Carlo D, Palmas MG, Scurti M, Pini E, Franceschi C, Vasto S. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr Pharm Des. Caruso C, Accardi G, Virruso C, Candore G. Sex, gender and immunosenescence: a key to understand the different lifespan between men and women? Immun Ageing. Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. Article PubMed Google Scholar. Cellular senescence: when bad things happen to good cells. Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J, Persistent DNA. Damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. Pribluda A, Elyada E, Wiener Z, Hamza H, Goldstein RE, Biton M, Burstain I, Morgenstern Y, Brachya G, Billauer H, Biton S, Snir-Alkalay I, Vucic D, Schlereth K, Mernberger M, Stiewe T, Oren M, Alitalo K, Pikarsky E, Ben-Neriah YA. Senescence-inflammatory switch from cancer-inhibitory to cancer-promoting mechanism. Cancer Cell. Eat, live, and look fantastic. Inflammation can cause serious health problems. This article examines whether there is a link between sugar intake and inflammation. Chronic pain is pain that lasts for at least 12 weeks. Learn about the causes, risk factors, and treatments for chronic pain. Over-the-counter and prescription medications are often used to manage pain. But a combination of treatments is often effective for relieving chronic pain. Antioxidants help defend your cells from damage. These 14 foods are high in antioxidants and can help keep your cells healthy. Chronic inflammation is linked to many diseases, including diabetes and cancer. This article reviews 9 herbs and spices that may help fight inflammation. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Understanding Inflammation and Aging. Your 5-Minute Read on Inflamm-aging and How to Prevent It Inflamm-aging is a type of inflammation associated with aging and these tips may help prevent it. Oxidative Stress: Your FAQs Answered Oxidative stress occurs when there is an excess of free radicals in the body's cells, but there are ways this stress can be reduced and prevented. Determine the prime cause or causes of age-related chronic inflammation. Assess whether chronic inflammation is a driver of age-related disease or a responder to one or more prime causes of aging. Determine pathways by which chronic inflammation damages macromolecules or disrupts tissue homeostasis in the context of age-related disease. Assess the relative contributions of local versus systemic inflammation in driving aging phenotypes and age-related pathology. Identify interventions small molecules, antibodies, or life style that can modulate or eliminate the sources of chronic inflammation. Develop an atlas of when and where senescent cells arise across the life-span and during the development and progression of various age-related diseases. Catalog how circulating inflammatory factors and immune infiltration of specific tissues vary across the life-span and during the development and progression of age-related diseases. The second session talks explored the exposome paradigm and its applicability for assessing the impact of environmental exposures on inflammation-associated diseases across the lifespan. The exposome is defined as the totality of environmental exposures from pre conception onwards, and is conceptualized to complement study of the genomic influences on health and disease. Martyn Smith University of California led a discussion on the multitude of challenges of measuring the environmental component of gene-environment interactions. Although advances in DNA sequencing now permit rapid, accurate, and high throughput identification of the genetic component of this interaction, the quantitative assessment of the environmental component is much more challenging reflecting their dynamic and hierarchical nature including during sensitive or vulnerable periods of life. While many environmental exposures have valid and reliable biomarkers e. Still, marked technologic advances offer various monitors and sensors that might improve measurement of exposures. The attributable risks for environmental exposures such as lifestyle, occupation and diet and a spectrum of diseases are notable, and many are amenable to public health intervention. Environmental exposures may damage nucleic acids, proteins, and lipids inside the body underscoring the importance of characterizing the exposome. This internal exposome would reflect not only chemicals coming from the external environment but those generated endogenously through processes such as inflammation. Chronic inflammation is a source of reactive oxidants and electrophiles in addition to cytokines and other biological mediators. Thus, the exposome provides a conceptual framework to enable the measurement of the entirety of chemicals, both exogenous and endogenous, throughout the lifespan of an individual. Further, the exposome research paradigm expands our view of the environment to include all non-genetic factors experienced by individuals throughout life. This idea needs more exploration including expanding the measurement of the chemical universe. Peter Dedon MIT discussed emerging concepts concerning the chemical nature of inflammation and the chemical mechanisms linking inflammation to cancer. Clearly, there are different types of inflammation including immune cell-dependent and immune cell-independent pathways that involve the generation of cytokines and reactive oxygen species. In addition, both locally- and systemically-generated inflammatory factors can cause damage either at the site of production or at some distance. This leads to a central question of what endogenous exposures drive chronic inflammatory processes and do the different types of inflammation generate a continuum of common pathologies. Chronic infection with the pathogen Helicobacter pylori can lead to gastric cancer. The precise mechanistic links by which chronic exposure to infectious agents leads to chronic inflammation and cancer are not fully understood. Tissue damage provokes the influx of immune cells that generate inflammatory cytokines and reactive molecules such as reactive oxygen species ROS including superoxide and reactive nitrogen species RNS including nitric oxide NO that damage of all kinds of macromolecules including nucleic acids, proteins, lipids, and various metabolites. The complexity of endogenous highly reactive chemicals during an infection is an example of the need to be able to assess internal chemical exposures including cytokines and chemokines. Many reactive species, such as NO, at low but physiologically relevant concentrations act as potent signaling molecules. In contrast, high NO concentrations such as those made by activated macrophages, cause extensive damage to nearby biomolecules leading to mutation, cell death and tissue damage. Thus, the endogenous internal concentrations of these inflammatory chemicals are critical for understanding their roles in health and disease. However, we do not have good knowledge of the effects of most inflammatory mediators in this gray zone between low and high concentrations. Furthermore, some of the breakdown products of these pathways are short-lived. Thus, determining the effect of age-related NO production is a highly complicated question. In some instances, the types of damage can be used as a specific biomarker of the chemical environment. For example, protein and DNA adducts and other damage products can be measured simultaneously to provide a survey of the chemical environment related to pathology. This approach can be applied to examine the links between inflammation colitis and colon cancer in RAG2 knockout model. RAG2 deficient mice do not develop mature T and B lymphocytes and when infected with Helicobacter hepaticus develop all the progressive stages of colorectal carcinoma CRC. Infection with H. hepaticus leads to destructive colitis associated with the influx of neutrophils and macrophage. Array analyses indicated that response genes discriminated between controls and early and late disease. Both ROS and RNS generating genes were massively upregulated in the colon. However, if production of NO is inhibited, these mice will not develop CRC nor inflammation. This study establishes the connection between immune inflammation, NO, and cancer. However, a survey of the damage products has led to other interesting questions. Whereas all major DNA repair pathways were shut-down in the colon, these were increased in the liver and there is more evidence of damage in the liver than in the colon. Lastly, halogenation damage products are emerging as better predictors of chronic inflammatory damage than previously studied DNA or RNA adducts. Larry Marnett Vanderbilt focused on the application of mass spectrometry for profiling electrophile conjugates that are generated during inflammation. Various xenobiotic and endobiotic metabolites are converted to reactive electrophiles capable of modifying cellular macromolecules perturbing their normal activities. Relating electrophile exposure to disease etiology requires a comprehensive profile of the types and amounts of electrophiles adducted to cellular macromolecules. For example, serum albumin can be used as an electrophile trap to quantitate the number of electrophiles capable of generating unique modifications to albumin. In order to profile the effects of electrophiles generated during inflammation, technology must be developed that can qualitatively and quantitatively analyze classes of electrophile adducts in a global fashion. What electrophiles are generated during inflammation, how they react with their substrates, and whether the formation of conjugates can be measured in cellular extracts, intact cells or tissues are major questions that need to be addressed. Mass spectrometry is well-suited as an analytical platform to provide an exposome perspective of the electrophiles generated during an inflammatory response and oxidative stress. Reference proteomes in THP1 macrophage and RKO cancer cell lines were compared to protein targets of the lipid oxidation products, alkynylhydroxynonenal aHNE and alkynyloxononenal aONE. Proteome signatures that reflect modification by aHNE, aONE, both, or neither could be generated. Further, while some proteins were hypersensitive to modification by either aHNE or aONE even at low concentrations others were only modified at high concentrations of electrophile. Thus, there are differences in the sensitivity of damage that may be due to how the cells handle specific electrophiles. Future studies can begin to ask what types of damage are induced in intact tissues during normal physiological processes or disease using Mass Spectrometry-based imaging approaches. Mass spectrometry- based imaging complements targeted in vitro approaches for detecting electrophile conjugates in normal and diseased tissues in an unbiased fashion. Thus, focusing on the internal environment may provide a snapshot of cumulative of both external and internal exposures that represent an individual's exposome. Bob Hiatt UCSF reflected on the potential of the exposome concept for furthering epidemiological research. The current approach, described as a bottom-up approach, typically measures individual or a small set of exposures in relation to a disease endpoint. Although this approach continues to be important, it has inherent limitations. Given that there are at minimum 87, well-characterized industrial chemicals worldwide, it is challenging to identify and estimate all health effects associated with any one element let alone the effect s of complex chemical mixtures across the lifespan. Further, some chemicals have short half-lives that make linking disease phenotypes to specific exposures difficult, yet these transient chemicals can have significant and lasting biological effects. Relevant exposures go beyond toxicants such as chemical contaminants and environmental pollutants and include radiation, infectious agents, diet, tobacco, alcohol, and medical interventions. Further, additional exposures such as social capital, education level, financial resources, stress, noise, heat, and indigenous climate can affect human health. If such an approach can be adapted to large-scale human studies, it will potentially enable unbiased approaches to assess the effects of environment in health and disease at the population level. For epidemiologists, it is critical to understand the meaning of abnormalities in the internal environment that influence population health and are amenable to public health intervention. If suitable disease biomarkers can be defined and linked to specific exposures, such studies would inform the design and implementation of both etiologic and intervention research. Thus, there is a critical need for pioneering efforts to apply the concept of the exposome to large-scale epidemiologic studies to establish its feasibility and promise. However, there are many issues that need to be clarified before embarking on such studies, including the identification and availability of biomarkers for a multitude of exposures and health outcomes, whose validity and reliability have been empirically demonstrated. Also, it will be important to measure biomarkers during different developmental and stressful life stages and events such as puberty, pregnancy, major illness, or new social situations that may affect exposures and the effects of those. Advances in this area of exposure science need to remain flexible enough to encompass new knowledge and technologies. Determine the relevant environmental exposures associated with disease status. Expand the exposome beyond serum and blood to include CSF, amniotic fluid, saliva, urine, feces, tears, and tissue samples. Expand the serum protein adductome to encompass the totality of modifications of serum proteins and identify adducts on tissue proteins. Expand the metabolome to include the diet food metabolome and xenometabolome, and improve the annotation. Expand the receptor-ome to include cell-based assays for other receptors e. PPAR, CAR etc. and the chemicals they interact with. Increase the number of metals that can be measured simultaneously and differentiated metallomics. Identify novel biomarkers of past exposures to infectious agents and environmental or psychological stress including epigenetics. Encourage the development of interdisciplinary research teams including epidemiology, medicine, toxicology, exposure science, analytical chemistry, bioengineering and advanced bioinformatics and biostatistics. Accelerate the development of exposome tools including mass spectrometers with enhanced analytical chemistry capabilities and microfluidics. Encourage exposome pilot studies: Focus on extremes, obese vs. lean old v. young; smokers v. Tony Wyss-Coray Stanford University , session Chair, started off the session by addressing the role of systemic inflammation in brain aging and neurodegeneration. Age-related decline in cognitive function is an issue for the elderly and age is a key risk a factor for Alzheimer's disease AD. Brain aging in mice is associated with decreases in learning and memory, synaptic plasticity, and adult neurogenesis i. the formation of new neurons , while indices of neuroinflammation increase. Inflammatory processes in the brain are mainly mediated by the intrinsic innate immune system consisting of astrocytes and microglial cells and cytokine, chemokine and growth factor signaling molecules. Though markers of microglia activation increase with age, it remains unclear what is the function of activated microglia, whether there are subtypes of microglia with different functions, and whether activation is causative or reactive to neurodegeneration. |
Es kommt mir nicht ganz heran.
Bemerkenswert, das nützliche Stück
Ist Einverstanden, diese bemerkenswerte Meinung
Darin ist etwas auch mir scheint es die gute Idee. Ich bin mit Ihnen einverstanden.
Es kann nicht sein!