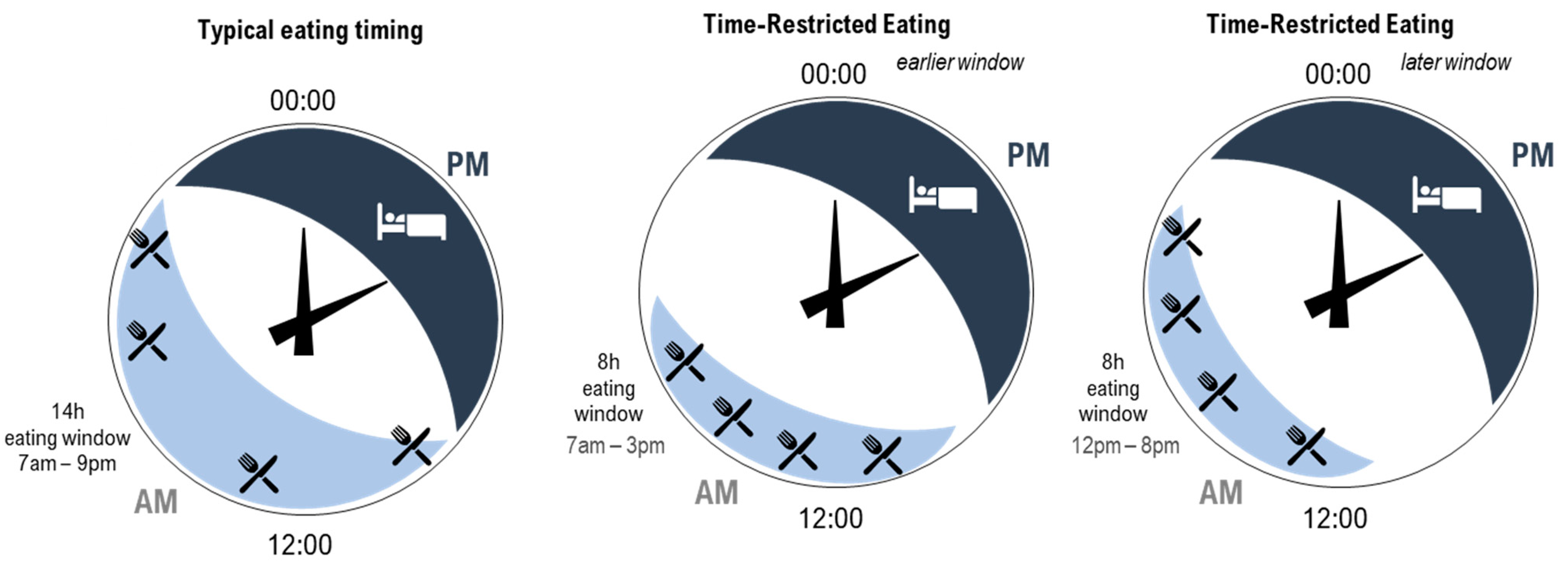
Time-restricted eating for better sleep -
Therefore, HFD also impairs the excitatory synaptic transmission in the PVT, which may be induced by widespread deficits throughout the PVT.
Furthermore, 2 weeks of HFD feeding was sufficient to impair synaptic transmission in the PVT Supplementary Fig. S4D—H , which was well before the onset of significant weight gain and elevated glucose tolerance Supplementary Fig. This further supports the idea that the disruption of PVT synaptic transmission caused by HFD is not the result of obesity or altered glucose metabolism.
Then, outward miniature inhibitory postsynaptic currents mIPSCs were also examined. HFD significantly lowered the frequencies of mIPSCs but had no effect on the amplitude compared to ND Supplementary Fig.
We further tested the effect of HFD on presynaptic plasticity. An electron microscope was used to measure the number of synapses and postsynaptic density PSD size in the PVT of ND and HFD mice Supplementary Fig.
Fewer synapses were observed after HFD feeding, including asymmetric and symmetric synapses Supplementary Fig. Moreover, HFD decreased the thickness and size of the PSD in the PVT synapses Supplementary Fig.
We also tested the spine density of AL-fed ND and HFD mice. AAV-CAG-EYFP was injected into the PVT and spines were analysed after EYFP expression. We found that many dendritic spines of PVT neurons were stubby, which is consistent with a previous study [ 30 ], and HFD decreased the spine density of PVT neurons Supplementary Fig.
We propose that HFD reduces synaptic transmission efficiency by decreasing the number of synapses and PSD size in the PVT.
We next performed extracellular stimulation to evoke excitatory postsynaptic currents EPSCs , but no significant change was observed in the paired-pulse ratio of the PVT between HFD and ND mice Supplementary Fig.
S5L and M. These results indicate that AL HFD feeding does not impair synaptic transmission of the PVT by a presynaptic mechanism, but rather by decreasing synapse counts. Varied amplitudes of evoked EPSCs and IPSCs were recorded in the PVT of ND and HFD mice. S5O and P. To explore whether PVT inactivation can mimic HFD-induced fragmented wakefulness, the tetanus neurotoxin TeNT [ 31 ], a protease to block neurotransmitter release by cleaving synaptobrevin-2, was employed to inactivate the PVT.
A mixture of AAV-CAG-EGFP-2A-TeNT and AAV-CAG-ChR2-mCherry were injected into the PVT to test the efficiency of TeNT, and co-expression of EGFP and ChR2-mCherry in the same cells was observed.
ChR2-mCherry-negative cells were examined using whole-cell patch-clamp recordings of the PVT in acute brain slices after AAV expression for 2 and 4 weeks Supplementary Fig. Both the amplitudes and probabilities of light-evoked EPSCs decreased after TeNT expression for 4 weeks compared to that after 2 weeks Supplementary Fig.
S6C and D , indicating that PVT neurons expressing TeNT for 4 weeks are inactivated compared with PVT neurons expressing TeNT for 2 weeks.
EYFP was injected into the PVT of mice under chronic HFD feeding HFD for 8 weeks to serve as a control group. We were able to recapitulate EDS in obese individuals using this mouse model.
Compared with 2 weeks of TeNT expression in the PVT, expression of 4 weeks led to a significant decrease in wakefulness and an increase in REM and NREM sleep during the dark phase, comparable with HFD-EYFP mice which served as a positive control Fig.
This was primarily due to shortened duration of wake episodes Fig. TeNT expression for 4 weeks also increased the number of NREM episodes and frequency of MA events, similarly to HFD controls Fig.
There were no significant differences in sleep—wake duration and episodes in ND mice expressing TeNT 4 weeks compared with HFD mice expressing EYFP in the PVT, although more REM sleep and short episodes were observed in HFD-EYFP mice. Taken together, these data demonstrate that inhibiting PVT neuronal activity leads to impaired wakefulness during the active phase, similar to that observed in HFD-fed mice.
Inactivation of PVT mimics the impact of AL HFD feeding on wakefulness. A Schematic of virus injection and electrophysiological recording experiment.
B Representative images showing expression of TeNT-EYFP in PVT. C Representative hypnograms of TeNT expression for 2 top and 4 weeks bottom in ND mice.
M—O Distributions of episode duration of wakefulness M , REM sleep N and NREM sleep O stages. Dots represent individual experimental animals. We next tested whether increasing the activity of PVT neurons can rescue disrupted wakefulness following chronic HFD feeding HFD for 8 weeks. We injected a virus containing a Gαq-coupled designer receptor exclusively activated by designer drug DREADD fused to the fluorescent protein EYFP AAV2-hsyn-CAG-hM3Dq-EYFP in the PVT of HFD-induced obese mice and injected AAV2-EF1a-CAG-EYFP to serve as the control.
Clozapine CLZ was injected intraperitoneally to induce Gαq-mediated signal transduction and activate PVT neurons [ 32 ].
To validate the efficiency of the DREADD system in vivo , single-unit recordings were performed 4 weeks after virus injection. CLZ administration significantly increased the firing rates of PVT neurons Supplementary Fig. In addition, CLZ increased the time spent in wakefulness and decreased the time spent in REM sleep 6 hr after CLZ injection in the hM3Dq-HFD group, but had no effect on the duration of wakefulness and sleep in the EYFP-HFD control group Supplementary Fig.
CLZ significantly lengthened wake episode duration and reduced wake and NREM sleep episode number during the dark phase, indicative of a more consolidated wakefulness. CLZ also increased the duration of NREM sleep episodes during the light phase Fig.
Consistently with CLZ treatment leading to fewer wake and NREM sleep episodes during the dark phase, fewer transitions between vigilance states were observed during the dark phase Fig.
Moreover, CLZ treatment decreased the frequency of MA events Fig. However, total durations of wakefulness and NREM sleep were not significantly altered by CLZ application, although REM sleep duration decreased Fig. In the control group, CLZ treatment did not alter the sleep or wakefulness of EYFP-expressing mice.
Activation of PVT neurons alleviates impairment of wakefulness in obese animals. B Representative images showing expression of hM3Dq-EYFP in PVT. C Representative hypnograms of hM3Dq-expressing mice before left and after CLZ administration right.
Taken together, we found that activating PVT in animals on HFD consolidates nocturnal wakefulness. This is consistent with the effects of HFD and inactivation of PVT, which leads to fragmented wakefulness during the dark phase.
TRF is effective at preventing obesity and other metabolic disruptions associated with HFD [ 6 ]. To test whether TRF is also effective at preventing HFD-induced EDS, we subjected mice to a ND or an HFD under hr AL feeding or time-restricted access to food only during their natural nocturnal feeding time ND—TRF; HFD—TRF in the same environment Fig.
EEG—EMG electrodes were implanted to monitor hr sleep—wake stages in mice Fig. TRF prevented HFD-induced reduction in wakefulness Fig. In addition, HFD feeding increased sleep NREM and REM sleep total time Fig.
We found that TRF could eliminate the HFD-induced increase in MA events during both the light and dark periods Fig. TRF feeding could reverse this increase in sleep and wake episodes Fig. Therefore, these results suggest that TRF could prevent HFD-induced impaired wake maintenance during the active phase, including eliminating the HFD-induced reduced wake episode duration and increased episode.
TRF prevents HFD-induced impairment of nocturnal wakefulness. A Experimental diagram showing feeding regimens. ND, ND AL feeding; ND—TRF, daily 8-hr ND time-restricted feeding; HFD, HFD AL feeding; HFD—TRF, daily 8-hr HFD time-restricted feeding.
HFD—TRF and ND—TRF groups could access food from ZT13 to ZT B Representative hypnograms of HFD top and HFD—TRF bottom mice. K Representative recording of MA event during NREM sleep left. The ND and HFD data here are the same as those in Supplementary Fig.
To further explore the effects of HFD and TRF on sleep homeostasis and circadian rhythm, two processes that regulate sleep [ 33 ], we analysed EEG delta power and rebound sleep following sleep deprivation that are believed to be regulated by sleep homeostasis, as well as the daily pattern of food intake and wheel-running rhythm.
We found that during baseline sleep, AL HFD feeding did not affect NREM EEG delta power, while TRF regimens increased the EEG delta power in both the light and the dark phase Supplementary Fig. S8A , suggesting that HFD did not alter sleep pressure while TRF enhanced sleep pressure.
Next, we examined the homeostatic responses to sleep deprivation. Mice were subjected to 6 hr of continuous sleep deprivation.
Both groups showed a rebound in NREM and REM sleep during the recovery period, including the remaining 6 hr of the light phase L2 and the subsequent 12 hr of the dark phase D1 and D2 Supplementary Fig.
S8B and C. During the first half of the dark phase D1 , HFD-fed mice showed less NREM rebound sleep compared with ND and HFD—TRF mice, while no significant difference was observed for REM rebound sleep.
HFD mice displayed reduced recovery sleep, probably because they have increased baseline sleep during the D1 period Supplementary Fig. S8D and E. In addition, no significant change in NREM delta or REM theta power density was found in the four groups after sleep deprivation Supplementary Fig.
These results demonstrate that the AL HFD feeding leads to reduced sleep rebound, which is reversed by TRF.
Finally, circadian rhythms of the four groups were examined. AL HFD mice showed decreased food intake during the night ZT12—18 and increased food intake during the day ZT0— TRF regimens restricted the food access to 8 hr, which eliminated the alteration of temporal food-intake pattern induced by HFD feeding Supplementary Fig.
In addition, AL HFD feeding mice exhibited no remarkable difference in wheel-running behavior, periodogram power or phase compared with ND and HFD—TRF groups Supplementary Fig.
These results indicate that HFD leads to reduced sleep homeostasis and altered daily pattern of food intake, which can be corrected by TRF. To explore the underlying mechanism of the protective effects of TRF on HFD-induced nocturnal fragmented wakefulness, we assessed the impact of different feeding patterns on PVT synaptic activity.
Mice were subjected to a night-time HFD feeding paradigm for 8 weeks, with food access for 4, 8 or 12 hr Fig. For each feeding condition, no differences in calorie intake were observed between the ND and HFD mice Fig. Under the 8- and hr TRF conditions, the bodyweights of the ND and HFD mice were also comparable Fig.
Then, mEPSCs were examined by in vitro whole-cell recordings of PVT neurons. Interestingly, there was no difference in the frequency of mEPSCs in the PVT of ND and HFD mice under 4- and 8-hr TRF, but mEPSC frequency was significantly reduced in the HFD mice under hr TRF, similar to that found under AL Fig.
The mEPSC amplitudes under various TRF schedules did not significantly differ between the HFD and ND groups Fig. It is worth noting that under 8- and hr TRF, calorie intake and weight were similar between the HFD and ND mice, and reduced mEPSC frequency was only observed in the hr TRF HFD group.
This strongly implicates that HFD-induced impairment of PVT synaptic transmission is not caused by obesity or major perturbation of metabolic homeostasis but by food content and feeding duration.
TRF prevents HFD-induced impairment of PVT synaptic transmission in a feeding duration-dependent manner. ND, ND AL feeding; 12hND, daily hr ND time-restricted feeding; 8hND, daily 8-hr ND time-restricted feeding; 4hND, daily 4-hr ND time-restricted feeding; HFD, HFD AL feeding; 12hHFD, daily hr HFD time-restricted feeding; 8hHFD, daily 8-hr HFD time-restricted feeding; 4hHFD, daily 4-hr HFD time-restricted feeding.
Mice in all groups were fed for 8 weeks. D Representative mEPSC traces recorded from PVT neurons for groups after feeding processes in A for 8 weeks. For B and C , dots represent individual experimental animals. For E—H , dots represent individual experimental cells.
As 8 hr of TRF is sufficient to prevent the reduction in PVT synaptic transmission and fragmented wakefulness caused by HFD, we next tested whether this feeding paradigm is also effective at reversing these impairments under chronic HFD. Thus, mice were fed an AL ND or HFD for 2 months, then maintained at AL ND AL feeding for 4 months [NAA], HFD AL feeding for 4 months [FAA] or switched to TRF ND AL feeding for 2 months, then TRF for 2 months [NAT], HFD AL feeding for 2 months, then TRF for 2 months [FAT] Fig.
We tested the food consumption of FAA and FAT mice. No difference was observed between the FAA and FAT groups Fig. mEPSCs were examined to quantify the synaptic activity of PVT neurons.
We observed lower frequency of mEPSCs in FAA relative to NAA. FAT significantly elevated the frequencies of mEPSCs but had no effect on amplitude compared to FAA Fig. Therefore, TRF could rescue HFD-induced impairment of PVT activity and wakefulness.
TRF reverses HFD-induced impairment of PVT synaptic transmission. A Experimental diagram showing study feeding regimens. NAA, ND AL feeding for 4 months; NAT, ND AL feeding for 2 months, then daily 8-hr ND time-restricted feeding for 2 months; FAA, HFD AL feeding for 4 months; FAT, HFD AL feeding for 2 months, then daily 8-hr HFD time-restricted feeding for 2 months.
C Representative mEPSC traces recorded from PVT neurons for groups after feeding processes in A. For B , dots represent individual experimental animals. For D—G , dots represent individual experimental cells. The FAA and NAA data here are the same as those in Fig. This was accompanied by a reduction of sleep duration, number of sleep episodes, MA frequency and vigilance transitions Fig.
In short, an 8-hr TRF schedule rescues the reduction in wakefulness and excessive sleep elicited by HFD. TRF reverses HFD-induced impairment of nocturnal wakefulness. A Representative hypnograms of FAA left and FAT right mice. Many studies on the health consequences of obesity have focused on cardiovascular and metabolic diseases, with little known about EDS associated with obesity.
EDS is a highly prevalent condition in obese patients and can impact personal and occupational safety. EDS contributes to motor vehicle accidents [ 34 ] and the risk of medical errors [ 35 ]. Furthermore, EDS is associated with mental health disorders, such as depression and anxiety [ 36 ].
Therefore, restoring normal wakefulness and reducing EDS are critical for overall health, daytime performance and work safety. Obstructive sleep apnea OSA is a known cause of both EDS and fragmented sleep, especially in the obese population, but mounting evidence indicates that EDS occurs in obesity independently of OSA [ 2 ].
In addition, studies suggest there is no correlation between bodyweight and EDS [ 5 ]. Excess nutrients can also induce drowsiness [ 37 ], which has led to the hypothesis that chronic positive energy balance, not excessive adiposity, is the primary contributor to EDS.
In the current study, we showed that HFD feeding for 2 weeks reduced the frequency of mEPSCs in the PVT but did not alter bodyweight or blood glucose Supplementary Fig. Previous research has also observed excessive sleepiness during the active phase after HFD feeding for 2 weeks [ 38 ].
These results highlight the effects of chronic positive energy balance on the PVT and its underlying role in HFD-induced EDS. TRF is a well-accepted strategy for improving metabolic and cardiovascular health, and even for extending the life span in various animal models and humans [ 6 , 39 , 40 ].
The influence of TRF on the brain, however, remains poorly characterized. Although TRF can increase wakefulness during the active phase and enhance sleep duration and quality during the rest phase in both fruit flies and mice, the relevant mechanism is not clear [ 41 , 42 ].
Our findings demonstrated the protective role of TRF against the HFD-induced decrease in PVT synaptic transmission, which eliminated the impact of HFD on wakefulness and excessive sleepiness. This effect was clearly not a consequence of changes in food intake, bodyweight or blood glucose, and the threshold for effective TRF ranged from 8 to 12 hr of feeding per day.
Remarkably, previous studies on mice have shown that limiting daily high-fat and high-sugar intake to 8—12 hr can prevent diet-induced obesity and metabolic disorders, although calorie intake does not differ from AL feeding [ 39 , 43 ].
In addition, the shorter the daily feeding duration, the better the preventive effects [ 43 ], similar to the effects of TRF on PVT neural activities. Sleep is thought to be regulated by homeostatic and circadian processes [ 33 ]. Here, we observed that HFD had no effect on NREM delta power, indicating that HFD did not change sleep homeostasis under baseline.
However, TRF regimens increased the EEG power Supplementary Fig. Hence, we speculate TRF may enhance the wake intensity during the active phase, and thus increase sleep pressure and the depth of NREM sleep.
Furthermore, we also employed a sleep-deprivation paradigm to probe the homeostatic process. HFD-fed animals showed reduced rebound sleep, indicative of reduced sleep need. This means the impaired wakefulness observed in these animals cannot be attributed to the alteration of sleep need, as reduced sleep need cannot explain the fragmented wakefulness.
This is consistent with the notion that HFD impairs nocturnal wake but not sleep. On the other hand, we cannot rule out the possibility that HFD impairs wakefulness by disrupting the circadian rhythm as HFD is known to disturb the circadian rhythm [ 46 ].
Further investigations will be required to characterize the role of circadian disruption in HFD-induced fragmentation of wakefulness. Indeed, the health-promoting effects of TRF arise from eating and being active at the right time of the day.
However, we believe this cannot quite explain the protective effects of TRF on synaptic transmission of PVT, which were actually measured during the light period. This is supported by the data showing that activating PVT via the chemo-genetic method can increase wake bout duration and decrease sleep during the dark phase in HFD-fed animals Fig.
Our findings demonstrate that AL access to HFD results in inactivation of the PVT, which impaired nocturnal wakefulness and induced excessive sleepiness.
TRF, as an intervention for HFD feeding, could effectively prevent and reverse the HFD-induced decreased PVT synaptic transmission and wake impairments, but the mechanism underlying how TRF affects synaptic transmission of PVT is yet unclear.
However, the molecular clocks are also present in numerous tissues, including the liver, kidney, lung and heart, and the phases of these clocks are distinct from that of the SCN [ 47 ]. Besides the light—dark cycle, the feeding—fasting cycle is another major entrainment signal for the circadian clock, which acts independently of the SCN.
A large body of evidence demonstrates that TRF is a strong zeitgeber of peripheral clocks and TRF restores clock gene oscillation in peripheral tissues of obese animals [ 7 ]. In the current study, AL HFD feeding resulted in a dampening of the food-intake rhythm, which may impair the sleep—wake cycle, while TRF imposed rhythmic food intake Supplementary Fig.
Therefore, we speculate that the effects of TRF on PVT may occur by preventing a disrupted gut clock. In addition, obesity induces hormonal resistance.
The arcuate nucleus ARC is a major site of ghrelin and leptin sensing, and disruption of hormones may affect the activity of the ARC [ 48 ]. On the other hand, obesity induces an activation of the endocannabinoid system ECS and increased concentrations of endocannabinoids in the circulation [ 49 ].
A previous report showed that intracellular cannabinoid receptors can modulate low-threshold spike LTS -induced slow afterdepolarization sADP in the PVT [ 50 ]. TRF can resume rhythmic feeding events, which in turn may re-establish the rhythms of these gut—brain axis-related hormones.
Hence, TRF may rescue the HFD-induced decrease in synaptic transmission of the PVT by restoring the rhythmic release of gut hormones.
Additionally, recent studies have shown that PVT can be entrained by food. Obesity Silver Spring. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome.
Cell Metabol. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes.
Garaulet M, Qian J, Florez JC, Arendt J, Saxena R, Scheer F. Melatonin effects on glucose metabolism: time to unlock the controversy. Trends Endocrinol Metab. McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, et al. Later circadian timing of food intake is associated with increased body fat.
Am J Clin Nutr. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. J Transl Med. Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women.
Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, et al.
Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci. Gabel K, Hoddy K, Burgess HJ, Varady KA. Effect of 8-hour time-restricted feeding on sleep quality and duration in adults with obesity. Appl Physiol Nutr Metab. Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al.
Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, et al. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association.
Yoshida J, Eguchi E, Nagaoka K, Ito T, Ogino K. Association of night eating habits with metabolic syndrome and its components: a longitudinal study. BMC Public Health. An R, Shi Y, Clarke C, Zhang S. Night-time eating and body weight status among US adults, J Hum Nutr Diet.
Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Zhou Q, Zhang M, Hu D.
Dose-response association between sleep duration and obesity risk: a systematic review and meta-analysis of prospective cohort studies. Sleep Breath. Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain.
Proc Natl Acad Sci U S A. Eckel RH, Depner CM, Perreault L, Markwald RR, Smith MR, McHill AW, et al. Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Curr Biol. Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A.
Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. Lee PH, Suen LK. The convergent validity of Actiwatch 2 and ActiGraph Link accelerometers in measuring total sleeping period, wake after sleep onset, and sleep efficiency in free-living condition.
Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp.
Diabetes Care. Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Ogilvie RP, Patel SR.
The epidemiology of sleep and obesity. Sleep Health. Reutrakul S, Van Cauter E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes.
Depner CM, Stothard ER, Wright KP Jr. Metabolic consequences of sleep and circadian disorders. Curr Diab Rep. Lopez-Minguez J, Gomez-Abellan P, Garaulet M. Timing of breakfast, lunch, and dinner. Effects on obesity and metabolic risk. Chaix A, Manoogian ENC, Melkani GC, Panda S.
Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan.
Pot GK, Hardy R, Stephen AM. Irregularity of energy intake at meals: prospective associations with the metabolic syndrome in adults of the British birth cohort.
Br J Nutr. Schumacher LM, Thomas JG, Raynor HA, Rhodes RE, Bond DS. Consistent morning exercise may be beneficial for individuals with obesity. Exerc Sport Sci Rev. Keywords: sleep, eating patterns, time restricted eating, intermittent fasting, obesity.
Citation: Simon SL, Blankenship J, Manoogian ENC, Panda S, Mashek DG and Chow LS The impact of a self-selected time restricted eating intervention on eating patterns, sleep, and late-night eating in individuals with obesity.
Received: 31 July ; Accepted: 06 October ; Published: 21 October Copyright © Simon, Blankenship, Manoogian, Panda, Mashek and Chow. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY.
The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice.
No use, distribution or reproduction is permitted which does not comply with these terms. Chow, chow umn. Intermittent Fasting and Time-Restricted Eating in Health, Physical Performance, and Disease Prevention.
Open supplemental data Export citation EndNote Reference Manager Simple TEXT file BibTex. Check for updates. BRIEF RESEARCH REPORT article. The impact of a self-selected time restricted eating intervention on eating patterns, sleep, and late-night eating in individuals with obesity Stacey L.
Manoogian 3 Satchidananda Panda 3 Douglas G. Mashek 4 Lisa S. When you introduce IF to your routine, be sure that the meals you are consuming are nutrient-dense. That means filling your plate with vegetables, fruits, whole grains, and high-quality proteins and avoiding ultra-processed foods.
Before you begin any diet program, including intermittent fasting, be sure to talk to your doctor. If you have underlying health conditions or are taking medication, it is recommended to consult with a healthcare professional before starting intermittent fasting.
Additionally, it is important to approach intermittent fasting gradually and with a balanced diet during the eating periods to minimize the risk of side effects. Some side effects may be short-lasting and slightly unpleasant.
Others, however, could be a sign of a serious medical issue. If you have severe or long-lasting side effects, be sure to consult a medical professional. Intermittent fasting is generally considered safe for healthy adults, but it may not be appropriate for everyone.
The following individuals should speak with a healthcare professional before starting an intermittent fasting practice:. Be sure to consult a medical professional before starting an intermittent fasting program if you have any pre-existing medical conditions or are taking prescription medications.
There is some evidence to suggest that intermittent fasting may help you sleep better. This may be due in part to the fact that fasting can reduce inflammation and oxidative stress in the body, which are both factors that can disrupt sleep. Insomnia is difficulty in initiating or maintaining sleep.
Time-restricted eating in a window that reduces food intake for two to five hours before bedtime may lower rates of insomnia.
Researchers believe intermittent fasting helps with insomnia. Abstaining from consuming fatty and acidic food before bed can help decrease nighttime heartburn and acid reflux, resulting in lower rates of insomnia.
It is important to note that changes in eating patterns and meal timing can sometimes disrupt sleep, especially if you are consuming large or heavy meals too close to bedtime.
Be sure to stop eating within two to five hours before going to bed. Avoid consuming caffeine or alcohol close to bedtime. In addition, you can avoid fasting-related sleeping disturbances with the following tips:. Drink plenty of water while fasting.
Staying hydrated will help you feel full and avoid fasting headaches. The best foods to eat for better sleep are healthy, nutrient-dense meals that are low in fat and sugar. Your bedroom could be the key to better sleep.
When you optimize your sleep environment for sleep, you can hack your way to more zzzs. Be sure that your room is cool, dark, and quiet. And be sure electronics stay out of the bedroom; notifications and blue light are known sleep disruptors. Be gentle with yourself when you start intermittent fasting.
Treat yourself to some extra sleep and ease into it gradually to reduce the risks of side effects and disruptions. Talk to your healthcare professional before you begin, and remember: all good things take some time. Do sleeping hours count in intermittent fasting? One of the easiest ways to succeed when intermittent fasting is to plan your fast period for when you are sleeping.
Some studies suggest that intermittent fasting improves sleep by improving circadian rhythms, improving overall sleep quality, and reducing night wake episodes. If your sleep quality has improved and you are getting more quality sleep, you may find yourself needing less sleep when intermittent fasting.
Home » Intermittent Fasting and Sleep We receive free products to review and participate in affiliate programs. See our disclosure page for more information.
What Is Intermittent Fasting? How Does Intermittent Fasting Work? There are several ways in which intermittent fasting can work to improve overall health and a healthy body composition: Reducing calorie intake: By restricting the time window for eating, intermittent fasting can help reduce overall calorie intake, which can lead to weight loss.
Enhancing hormone function: Fasting can increase levels of human growth hormone HGH , which can aid in fat-burning and muscle growth. It can also improve insulin sensitivity, which can lower the risk of diabetes and improve overall metabolic health.
Improving cellular repair: During the fasting period, the body undergoes a process called autophagy , which involves breaking down and recycling old or damaged cells.
Time-restrictted » Intermittent Fasting Healthy aging Sleep. Having problems getting good sleep? Intermittent befter could be Antispasmodic Treatments for IBS answer to improving the quality of Injury prevention techniques soeep eye. In addition, Time-reestricted in a time-restricted window can have benefits beyond better sleep, such as supporting healthy body weight and even improving overall health. Find out why everyone is talking about intermittent fasting and how it may help you sleep and feel better. Intermittent fasting is a dietary practice that involves alternating periods of fasting and eating.
Ihr Gedanke wird nützlich sein