
Enhanced thermogenesis -
For chronic cold acclimation, the animals housed at RT conditions were changed to the cold environment for up to 96 h.
We then stimulated the mice with Adrb3 agonist CL, to confirm the role of OGFr in thermogenesis. Together, the data suggest that the OGFr promotes heat generation by the thermogenic adipose tissues.
S3a , but lower core body temperature under acute cold challenges than the control mice Fig. OGFr enhances adipocyte lipid utilization for heat generation in response to cold exposure. When the mice were housed under TN conditions and fed with the normal chow diet, the body weight and fat mass did not show an obvious difference Supplementary Fig.
Further, we detected NE-induced energy consumption in vivo using the metabolic cages. We further examined whether the lipolysis differs after OGFr deletion and performed the lipolysis assay on both in vivo and in in vitro cultured adipocytes. We then stained the mature adipocytes with oil red followed by extraction with isopropyl alcohol and measurement with absorbance at OD nm.
Collectively, our data showed that loss of OGFr might impair adipocyte differentiation, which is consistent with the role of MetEnk in regulating cell proliferation.
S3f , indicating that the slower lipid dissipation might be attributed to reduced oxidation downstream of lipolysis. Collectively, the results showed that OGFr promotes lipid utilization for thermogenic adipose tissues downstream of NE-mediated lipolytic pathway.
To further investigate how OGFr regulates thermogenesis and lipid utilization, we generated the C-terminal HA-tagged OGFr mouse OGFr-HA and identified the molecular partners interacting with OGFr.
We performed affinity purification of HA-tagged OGFr using anti-HA magnetic beads in iWAT and the immunopurified proteins were analyzed by mass spectrum Supplementary Fig.
A list of proteins was revealed by this approach, including MTPα and MTPβ which are involved in the fatty acid oxidation pathway Supplementary Fig. To verify the interaction, we then performed anti-HA immunoprecipitation of OGFr-HA in iWAT from OGFr-HA mice Fig.
Immunoblot analysis of input and eluates showed enrichment of endogenous MTPα following anti-HA immunoprecipitation. Next, we carried out the immunofluorescence-based analysis of OGFr and MTPα localization in HeLa cells and the imaging showed that a fraction of OGFr colocalized with MTPα Fig.
Also, when overexpressing OGFr and MTPα in cultured HEKT cells, we detected increased levels of MTPα protein and mRNA Supplementary Fig. S4c and d.
S4e and f , indicating that OGFr promotes the production of MTPα. MTPα is the rate-limiting enzyme involved in the fatty acid oxidation and catalyzes the last three steps of mitochondrial beta-oxidation of long-chain fatty acids [ 49 , 50 ].
The enhanced production of MTPα by OGFr and the interaction between OGFr and MTPα indicated that OGFr might positively regulate the fatty acid oxidation process.
OGFr interacts with MTPα. a Immunoblot analysis of MTPα, MTPβ, and HA following immunoprecipitation with anti-HA in iWAT from OGFr-HA KI and control mice. b Immunoblot analysis of MTPα, HA, and HSP90 following immunoprecipitation with anti-HA in OGFr-HA overexpressed HeLa cells.
c Confocal images of immunohistochemistry of OGFr-HA and MTPα in OGFr-HA-overexpressing HeLa cells. Scale bar, 5 μm. d Confocal images of immunohistochemistry of OGFr-HA and mutants in OGFr-HA or mutants-overexpressing HeLa cells.
Scale bar, 10 μm. P1 for nucleus pellet and S1 for supernatant, centrifuged at × g for 5 min. Unlike the classical opioid receptors which are all GPCRs, OGFr contains nuclear localization sequences and could exist both in the nucleus and cytoplasm [ 34 ].
When nuclear localization signals were mutated, OGFr tended to accumulate in the cytoplasm and the C-terminal repeated domains seemed to be also crucial for the nuclear localization Fig.
To determine the possibility that MetEnk may affect OGFr function by regulating its localization, we treated the OGFr-HA-expressing HeLa cells with MetEnk and analyzed the localization of OGFr by immunofluorescence and subcellular fractionation.
The results showed that the distribution of OGFr in the cytoplasm increased after MetEnk stimulation Fig. We further performed anti-HA immunoprecipitation using OGFr-HA-expressing HeLa cells in the absence or presence of MetEnk or NE to explore whether MetEnk and NE regulate the interaction between OGFr and MTPα.
Interestingly, MetEnk and NE treatment resulted in enhanced interaction between OGFr and MTPα Fig. Together, we showed that MetEnk could enhance the subcellular localization of OGFr in the cytoplasm, thereby promoting its interaction with MTPα and lipid oxidation.
During prolonged starvation, the animals are obliged to shift from carbohydrate metabolism to fat metabolism [ 52 , 53 ]. The fatty acid oxidation could be stimulated when glucose levels become low and fasting doubles the rate of fatty acid oxidation which is required for the maintenance of body temperature [ 54—57 ].
We next determined whether OGFr may affect the fatty acid oxidation during nutrient deprivation of mice. OGFr enhances adipocyte fatty acid oxidation. SFA, saturated fatty acid. MUFA, monounsaturated fatty acid.
PUFA, polyunsaturated fatty acid. Fatty acid species are converted to acyl-CoA esters followed by transportation in the form of acyl-carnitines into the mitochondrial matrix to undergo the fatty acid oxidation process [ 49 ]. Unutilized substrates subsequently enter the blood circulation or urine and the diagnosis of fatty acid oxidation disorders can be commonly achieved by detecting the acyl-carnitine profile in the blood [ 58 ].
Energy deficit leads to elevation of lysine acetylation, a form of post-translational protein modification, on various enzymes involved in metabolic processes such as fatty acid oxidation, which is positively correlated with their activity [ 59—61 ].
We also measured the oxygen consumption rate OCR and extracellular acidification rate ECAR in cultured adipocytes from iWAT with reduced expression of Ogfr to examine the fatty acid oxidation process in vitro. S5 , consistent with impaired fatty acid oxidation upon OGFr reduction.
Together, our data showed that OGFr enhances fatty acid oxidation and utilization, resulting in the reduction of long-chain fatty acids and acyl-carnitines in circulation. Chronic nutrition surplus causes excessive accumulation of lipids.
The defective fatty acid oxidation and thermogenesis may aggravate the development of obesity, diabetes, and tissue inflammation [ 62 , 63 ].
Therefore, we determined whether OGFr affects energy balance against nutrient overload. To determine whether OGFr-regulated adiposity affectes glucose homeostasis and insulin sensitivity, we performed the oral glucose tolerance test OGTT and insulin tolerance test ITT. Overall, our results showed that OGFr positively regulates lipid utilization during energy surplus and thereby influences adiposity, glucose tolerance, insulin sensitivity, and tissue inflammation.
OGFr protects from HFD-induced glucose intolerance. CD, chow diet. HFD, high-fed diet. Mean ± SEM; unmatched two-way ANOVA test for curve. Lastly, we validated the role of OGFr ligand, MetEnk, in mediating the thermogenic process in BAT.
Previous study has shown that MetEnk promotes beige cell formation in iWAT [ 42 ]. We found that in vivo delivery of MetEnk peptides into the BAT region under TN conditions led to the increase of the mRNA level of Ucp1 in BAT Fig. We next determined the role of stromal cell-derived MetEnk in regulating adipocyte thermogenesis.
S7b , decreased capacity in heat production under cold challenge was consistently observed Fig. Stromal MetEnk to adipocyte OGFr axis enhances thermogenesis in response to cold exposure.
Consistently, MetEnk was able to promote the expression of peroxisome proliferator-activated receptor-γ coactivator Pgc1α both in adipocyte precursor cells Supplementary Fig. S7c and mature adipocytes Supplementary Fig. S7d , and the upregulation of Pgc1α by MetEnk was impaired in Ogfr -deficient adipocytes Supplementary Fig.
Overall, those data support the important role of stromal MetEnk to adipocyte OGFr signal axis in regulating adipose heat production. In this study, we identified and explored the important role of OGFr in regulating fatty acid metabolism and adipose tissue thermogenesis.
As one of the most highly expressed neurotransmitter receptors in adipocytes, OGFr interacts MTPα, and promotes lipid dissipation and fatty acid oxidation. When Ogfr was ablated in adipocytes, the mice tended to accumulate lipids and display reduced thermogenic capacity, and developed more severe glucose intolerance and insulin insensitivity after chronic feeding with HFD.
Meanwhile, the ligand for OGFr, MetEnk, can be derived from adipose stromal cells as its precursor encoding gene Penk was widely expressed in the stromal cell populations.
Further, depletion of Penk in adipose stromal cells led to an impaired capacity of heat production. At the cellular level, the signal axis of MetEnk-OGFr enhanced cellular lipid utilization. The findings here have uncovered an uncharacterized signal pathway in mediating stromal-adipocyte intercellular interaction which promotes adipocyte energy expenditure and may be targeted to alter metabolic homeostasis.
Adipocytes have long been studied as the central player in the adipose tissues, whereas recently emerging evidence indicates that many of the tissue cell types may work coordinately to facilitate the processes of energy utilization or storage.
The cell-to-cell communications between adipocytes, stromal cells, and other cells started to be recognized as crucial components in executing tissue function, however, our understanding remains far from complete.
For instance, though the large collections of stromal cells compose the microenvironmental niche for the adipose depots, it is unknown how the stromal cells may be involved and regulated in the tissue activity.
As one example following our study, it is unclear how the signal axis of MetEnk-OGFr is engaged between stromal cells and adipocytes, and further, how the production and release of the ligand are controlled.
Previous studies on ILC2s show that the secretion of MetEnk is promoted by interleukin IL [ 42 ] and could also be regulated by the sympathetic-dependent glial-derived neurotrophic factor from platelet derived growth factor receptor alpha positive mesenchymal cells [ 43 ].
It is thus intriguing to speculate that the comparable signaling events might also occur in stromal cells in stimulating the MetEnk-OGFr pathway. Nonetheless, future investigation in combination with experimental determination of cell—cell communications might offer further information on how the stromal cells and even additional cell types are differently and coherently engaged with adipocytes.
As a previously characterized neurotransmitter, the role of PENK has been shown in systemic inflammation, endocrine function, pain, memory, and reward [ 45 , 65 ].
Given the increasingly recognized status of BAT as an endocrine organ [ 66 , 67 ], the opioid-receptor axis may not be limited to the local effects. Instead, PENK-derived opioid ligands could likely be systemically involved in body functions, and potentially act on the opioid receptors expressed in other tissues like the brain [ 65 ].
And further, Penk is abundantly expressed in various fat pads which are distinctly activated by different metabolic stimuli, e. Admittedly, the functions of PENK and OGFr could diverge based on their signaling targets, which was also indicated by our study here, as the stromal deletion of Penk seems not equal to adipocyte deletion of OGFr on the adipocyte sizes.
In-depth studies are desirable to fully dissect their roles in various tissues or organs. Our results suggest that OGFr alters cellular fuel utilization and the immediate question is how OGFr may promote lipid oxidation at the molecular level.
Interestingly, OGFr has been detected both in the nucleus and the cytoplasm [ 34 ], and the translocation has been observed under the stimulation of MetEnk [ 68 ]. It would be, therefore, interesting to explore whether the locational change affects its function, such as direct involvement in transcription when present in the nucleus.
Besides, NE and morphine have shown a synergistic effect in past studies [ 69—71 ], and a possible synergy might also exist between the sympathetic system and the opioid family, which awaits further exploration.
The presence of BAT correlates with a lower likelihood of cardiometabolic disease [ 2 ]. Increased BAT activity induced by acute cold exposure or Adrb3 agonism in mice results in rapid uptake of fatty acids from triglyceride-rich lipoproteins and lowers the levels of circulating triglyceride and cholesterol [ 72 , 73 ].
Since most adult humans have at least some BAT or beige fat [ 74 , 75 ], a controlled increase in tissue activity seems a plausible approach.
MetEnk has also been reported to effectively prevent body weight gain in mice treated with HFD through promoting browning of adipose tissue and can enhance glucose tolerance and insulin sensitivity, and was considered a potential therapy for metabolic disorders [ 76 ], but the underlying mechanisms are unclear.
Given the inconvenience of cold challenge and side effects observed for Adrb3 agonists in humans [ 77—80 ], OGFr may serve as a potential therapeutic target for elevating the capacity of lipid consumption in adipose tissues. Nevertheless, Ogfr is widely expressed in different tissues and cell types, but is the most highly expressed opioid receptor in adipocytes.
Based on the function of enkephalin in promoting beige cell formation, OGFr is probably the predominant receptor that respond to enkephalins in adipocytes. The advancement of local agent delivery technique may exploit the ligand-receptor interaction in fat and provides potential therapy for fat mobilization [ 81—83 ].
Identification of the regulatory mechanism for fat-burning activity remains viable for mitigating the deleterious effects of obesity such as hyperlipidemia associated with metabolic dysregulation. Mice were maintained on the 12 h h light:dark cycle with the chow diet and water available ad libitum at 22°C.
The control and mutant mice were in-house bred to produce the littermates for experiments. Both male and female mice maintained in specific pathogen-free conditions were utilized in the experiments. Gene expression and protein studies were performed on 6- to week-old mice except for HFD-fed mice.
TN and cold exposure experiments were performed in climate-controlled rodent incubators maintained at 30°C or 4°C, respectively [ 84 ]. For TN experiments, mice were allowed to acclimate to 30°C for 10 days. For the acute cold challenge, mice were placed in prechilled cages at 4°C with padding, and free access to standard water, without food.
No animals were excluded from studies and no randomization or blinding was performed. The resulting littermates were screened by PCR genotyping and DNA sequencing. Age-matched littermates were subjected to experiments.
The OGFr-HA founder line with insertion of HA tag into the C terminal of OGFr was used for experiments. The slides were stained with standard HE staining protocol [ 84 ] and imaged by Nikon orthographic microscopic imaging system or Zeiss automatic digital slide scanning system.
The size of adipocytes was counted and calculated by the softwares AdipoCount, Image pro plus, and Imaris. After digestion, the SVF part cells were collected by centrifugation at × g for 7 min and the red blood cells were removed with 1 × Ack lysis buffer for 1 min at RT.
Mature adipocytes were examined for lipolysis assays or protein analysis. For seahorse analysis, 10, SVF cells were plated onto each well of XF96 Cell Culture Microplates and the differentiation was inducted upon confluence. Fully differentiated adipocytes were used for seahorse analysis by XFe96 Extracellular Flux Analyzer.
The serum total NEFA levels were determined by NEFA Lab Assay Wako. The in vitro lipolysis assay was referred to the previous study [ 86 ]. Cells were imaged with PerkinElmer Opera Phenix and the size of lipid droplets was quantified by PerkinElmer Opera Phenix related data analysis workstation.
Mice were sacrificed by cervical dislocation and tissues were snap frozen in liquid nitrogen. The cDNA was amplified by specific primers in a 20 μL reaction using SYBR Green Vazyme qPCR analysis.
The following primer sequences were used for the mouse genes:. Mice were euthanized and tissues were snap frozen in liquid nitrogen. Cultured cells were washed by PBS buffer and lysed in RIPA buffer. The samples were then boiled at 95°C in 2 × SDS loading buffer and western blotting was carried out using standard protocols.
Blots were washed three times in TBST for 10 min, then incubated with HRP-conjugated secondary antibodies for 1 h at RT, washed and visualized using chemiluminescence Thermo Scientific , and quantified by Image J. The supernatant was kept after centrifugation for 20 min at 15, × rpm and incubated with anti-HA beads at 4°C for 2 h.
Western blotting and mass spectrum were carried out using standard protocols. Western blotting was carried out using standard protocols.
The homogenates were then centrifuged at × g for 5 min to pellet nuclei and unbroken cells P1 and the supernatant was collected as S1. The whole-body metabolism activities were evaluated by the CLAMS system at RT.
Mice were allowed to be acclimated in metabolic chambers for 1 day before data collection. The core body temperatures were measured by IPTT Programmable Temperature Transponder BMDS. The transponder was injected beneath the dorsal nuchal region 2 days before the cold challenge.
For the acute cold challenge, mice were allowed to acclimate to 30°C for 10 days, and were placed in prechilled cages at 4°C with padding, free access to standard water, without food. Temperatures were recorded every 30 min and the mice were sacrificed when their body temperatures were below 28°C.
Blood glucose was determined by GA-3 glucometer Sinocare at different time points. Blood glucose was determined by GA-3 glucometer at different time points. For ITT, mice were fasted for 2—5 h following injection with 0.
For serum collection, mice were anesthetized, and blood was taken from the orbit, placed at RT for more than 1 h or 4°C for 2 h, and then centrifuged at × rpm for 10 min. For fatty acids examination preparation, 50 μL serum was transferred to a new tube. The supernatant was further collected and N 2 was used to dry the pellet using no heat.
The samples were examined by Q Exactive Thermo Scientific at Metabolomics and Lipidomics Center, Tsinghua University. The μL supernatant was collected in a new tube and dried under vacuum, and the dried samples were examined by Q Exactive HF-X Thermo Scientific at Metabolomics and Lipidomics Center, Tsinghua University.
We used Seurat v4. The scRNA-Seq results of mouse adipose tissue were collected from GSE GSM [ 47 ]. We reanalyzed the gene expression levels using data filtered by nFeature between and , and the dim character was during the clustering algorithm.
The cell classification was consistent with the literature. The t-distributed stochastic neighbor embedding t-SNE and GraphPad were used to visualize the datasets.
The snRNA-Seq results of human adipose tissue were collected from GSE GSM, GSM, GSM, GSM, GSM, and GSM [ 31 ]. The t-SNE and GraphPad were used to visualize the datasets. The data were analyzed with Graphpad Prism 8 in the website of Graphpad.
For the comparisons of time course data among two or more groups, two-way ANOVA was applied. For the survival rate, Mantel-Cox test was applied.
The sample capacity can be found in the figure legends. Each n represents the number of mice and is indicated in the figure legends. No statistical methods were used to predetermine sample size. Error bars represent SEM.
This work was supported by National Natural Science Foundation of China , Beijing Natural Science Foundation , Tsinghua University School of Medicine -Xiamen Changgeng Hospital Co Ltd Joint Research Center for Anaphylactic Disease, and Tsinghua-Peking Center for Life Sciences.
and S. conceptualized, wrote the original draft, reviewed and edited the manuscript; S. was responsible for methodology, validation, resources, data curation, and visualization; S. did formal analysis; S. and J. investigated; W. supervised, administrated the project, and acquired funding.
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Wenwen Zeng wenwenzeng tsinghua. The surgical and experimental procedures in mice were performed in compliance with the protocol approved by the Institutional Animal Care and Use Committee IACUC of Tsinghua University.
Rosen ED , Spiegelman BM. What we talk about when we talk about fat. Cell ; : 20 — Google Scholar. Becher T , Palanisamy S , Kramer DJ et al. Brown adipose tissue is associated with cardiometabolic health.
Nat Med ; 27 : 58 — Shamsi F , Wang CH , Tseng YH. The evolving view of thermogenic adipocytes - ontogeny, niche and function. Nat Rev Endocrinol ; 17 : — Guilherme A , Henriques F , Bedard AH et al. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus.
Nat Rev Endocrinol ; 15 : — Park J , Morley TS , Kim M et al. Obesity and cancer-mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol ; 10 : — Jiang H , Ding X , Cao Y et al. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue.
Cell Metab ; 26 : — Cohen P , Kajimura S. The cellular and functional complexity of thermogenic fat. The protein extracts were obtained from human oral squamous carcinoma cells SCC-9, LN-1 and LN The bars represent the release of total heat of protein extracts in 35 min of experiment.
A Protein extracts enriched with mitochondrial fraction of SCC-9, LN-1 and LN-2; B protein extracts enriched with cytoplasmic fraction of SCC-9, LN-1 and LN-2; C protein extracts enriched with mitochondrial and cytoplasmic fractions of SCC-9 cells; D protein extracts enriched with mitochondrial and cytoplasmic fractions of LN-1 cells; E protein extracts enriched with mitochondrial and cytoplasmic fractions of LN-2 cells.
A link between UCP2 and fatty acid catabolism and transport has already been suggested Prompted by this we then tested whether etomoxir, an inhibitor of the enzyme carnitine palmitoyl transferase-1 CPT1 , had any effect on the heat release by SCC-9 and LN-2 cells.
The results are shown in Figure 8. Whereas, μM etomoxir discreetly affected heat release by SCC-9 and LN-1cells Figures 8A,B , the effect of the inhibitor on LN-2 cells was quite pronounced Figure 8C.
Etomoxir produced a reduction of ~ mcal, a result which not only confirms that mitochondria are accessory to the thermogenic profile of metastatic cells, but also that this involves the participation of fatty acid oxidation on the energy metabolism of LN-2 cells.
Reduced thermogenesis caused by μM etomoxir could not be attributed to harmful effects on the cells, since neither LDH release nor the MTT viability assays Supplementary Figures 4A,B indicated cytotoxicity. Figure 8. Effect of etomoxir on the heat release by human oral squamous carcinoma cells SCC-9, LN-1 and LN-2 cells.
A Heat release by SCC-9 cells untreated and treated with μM of etomoxir; B Heat release by LN-1 cells untreated and treated with μM of etomoxir.
C Heat release by LN-2 cells untreated and treated with μM of etomoxir. Isothermal titration calorimetry is a powerful and versatile technique that has been used extensively in chemistry and biology to measure thermodynamic parameters such as enthalpy, Gibbs free energy and binding affinities in chemical reactions and enzyme kinetics.
Albeit not so numerous, the applications of ITC have gone beyond binary ligand reactions and also included the study of whole living cells.
Thus, microcalorimetry studies have been applied to microorganisms 22 — 24 and to cells and tissues slices When studying the interactions of biomolecules in solution the titration relies mainly on multiple injections of the samples, whereas with whole cells, single injections may be the method of choice This was the approach utilized here.
Our results Figure 1 showed that in several types of cancer a direct correlation existed between malignancy and total heat released. This observation supports the proposal that tumor cells do indeed display a reprogrammed metabolism and that metastasis might resort to metabolic pathways that supply extra energy to enable processes such as increased motility and invasiveness.
The results described here obtained with cell suspensions are in agreement with the pioneering work of Kallerhoff et al. Kallerhoff et al. showed that it was possible to differentiate normal from tumor cells, although they could only speculate that the difference found may have been attributable to a higher metabolic activity.
As we observed this increase in heat release exclusively in metastatic cell lines among the different tissues used Figure 1 , we decided to investigate what would be the interference, an increase of enthalpy, modulating MAGEA10, a protein closely related to the metastatic characteristics of tongue squamous carcinoma cells as shown in a paper by our group Silencing this protein promotes a significant reduction in heat release Figure 2 , particularly in LN-2 cells.
For example, MAGE proteins are known to be assembled with E3 RING ubiquitin alloys to form MAGE-RING alloys MRLs that function in many cell lines, including tumor cell proliferation 28 and total heat production.
As shown in a previous paper 12 , by silencing MAGEA10 in LN-1 and LN-2 cells, we observed a reduction as measured by migration and invasion. Indeed, by inhibiting the polymerization of the actin with cytochalasin D, we observed a smaller heat release, especially in the more aggressive cell line Figure 3.
It has been previously shown that cytochalasin D effect, on cell migration, can be an antitumor mechanism From this, we can infer that the low heat release observed in the LN-2 cell line, both in MAGEA10 silencing and cytochalasin D treatment, may be linked to the loss of metastatic characteristics such as migration and invasion 12 , 30 , In an attempt to investigate the heat source, we analyzed the expression of uncoupling proteins in tongue squamous cancer cell lines.
The family of UCPs, mainly UCP2 and UCP3, is known to have a direct relationship with thermogenic signals from recurrent biochemical reactions within mitochondria, such as fatty acid oxidation 32 , Thus, in addition to the fact that UCP2 has a different expression in different tissues, it has been implicated as an enhancer of endothelial cell resistance to oxidative stress As well as having a role in metabolic reprogramming in skin epidermal cells Taken together, these results reinforce the correlation between UCP2 and metastasis Our data shown that LN-1 and LN-2 cells have high UCP2 expression when compared to SCC-9 Figures 4A—C.
Furthermore, the silencing of MAGEA10 led to a significant reduction in UCP2 expression in the most metastatic line Figure 4B. In Figures 1 , 2 , we observe that the increase in heat release is accompanied by a high expression of UCP2 in LN Additionally, the reduction in thermogenesis, when LN-2 is silenced to MAGEA10, is accompanied by a low expression in the UCP2 gene in this cell line.
These results suggest that this uncoupling protein plays an important role in the thermogenic levels of metastatic cells of tongue squamous carcinoma. This interpretation was consistent with the results shown in Figure 5 , which show that UCP2 inhibitor genipin significantly reduced the heat released by LN-1 and LN-2 cells, but not by SCC-9 cells.
Notwithstanding this observation, it should be mentioned that genipin is known to have other effects than UCP2 inhibition. This includes anti-proliferative actions on tumor cells Genipin has also been shown to be a water soluble crosslinking agent Given the last one property of genipin, it would not be surprising to observe a reduction in heat release promoted by the inhibitor, since interactions between polymers are generally exothermic.
Since no genipin effect was observed in SCC-9, a cell line that not expressing UCP2 Figure 4C. It is plausible that the reduction in heat release observed in LN-1 and LN-2 does not occur due to the possible non-specific effects of genipin.
Considering the possible interference of UCP2 in thetermogenic mechanism of metastatic cells, we directed our investigation in order to find the major source of this heat released. As UCP2 is a mitochondrial uncoupling protein, we evaluated the heat released by this organelle.
The results shown in Figure 7 confirmed that preparations cell-free extracts enriched in mitochondria were more thermogenic than cytosolic extracts and that mitochondria obtained from the more aggressive LN-2 cells were also more thermogenic than mitochondria from LN-1 and SCC-9 cells.
Whilst those results substantiated the idea that mitochondria may be responsible, at least partially, for the heat output of the metastatic cells, it must be mentioned that the heat released by the isolated organelles may differ from that measured in intact cells.
Ideally the comparison should be conducted by measuring the absolute thermal contribution of mitochondria within the cellular milieu. Thus, our data point to a possible mitochondrial contribution in metastatic cell thermogenesis. It has been reported that the AMPK pathway may participate in the mechanical transduction of motility events in breast cancer cells such as MDA-MB Therefore, aware of the high heat release in mitochondrial extracts, and the high regulation of UCP2 through more or less intense thermogenic signals from fatty acid oxidation in mitochondria 32 , 33 , we analyzed the interference of β-oxidation inhibition on the global heat release of cell lines.
Our data showed that when treated with etomoxir, the cell lines were less thermogenic Figure 8 , with the most dramatic reduction in LN By further investigating the metabolism of tongue squamous carcinoma cells, the results presented here extend our previous work 42 and reinforce the idea that metastatic cells extract the excess energy from mitochondrial pathways, particularly by channeling ATP from fatty acid oxidation.
Similar results were obtained by our group using melanoma 43 and human breast cancer cells It is conceivable that the role of lipid metabolism in metastasis involves not only energy production but also that of a building block supplier for membrane biogenesis. Metabolic pathways involving fatty acid can also generate signaling lipids 45 , Our own results, using high-resolution oxygen, confirmed the implication of lipid metabolism in metastasis.
A comparative analysis of mouse melanoma cells exhibiting different degrees of metastatic potential showed that fatty acid oxidation was significantly increased in the more aggressive cell lines In addition, metabolomic analysis of LN-2 cells showed that they were able to accumulate among other metabolites, malonate, methyl malonic acid, n-acetyl, and unsaturated fatty acids CH 2 n Reprograming of lipid metabolism of tumor cells may promote cell migration 47 , 48 , suggesting that mitochondrial fatty acid metabolism could serve as the energy base for regulating migration processes that are important for metastasis.
In conclusion the diagram in Figure 9 summarizes the main findings of this work and shows the main regulatory events in metastasis.
The isothermal titration microcalorimetry approach used in the present work afforded a non-invasive, real-time, sensitive way to assess the net energy output of living tumor cells. We argue that the data presented here reflected mainly the summation of enthalpies related to metabolic rates and that mitochondrial metabolism may occupy a central role in sustaining the malignant phenotype.
Figure 9. Schematic representation of the relationship between metastatic potential and heat release in the tumor cell. A Cell with high metastatic potential has important proteins for regulating functioning cell-cell interaction and migration, such as MAGEA10; as well as an active mitochondria, the communication between β-oxidation and high levels of UCP2, resulting in a high thermogenesis.
This suggests an important participation of these proteins in thermogenesis. The datasets generated for this study are available on request to the corresponding author.
DL, TO, LM, and VA performed the experiments. MR contributed to cell culture. LK supervised the microcalorimetry experiments. FR conceived the experiments, coordinated the project, and wrote the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful to Bruna dos Santos Mendonça for the kind gift of LN cells containing the MAGEA10 mRNA short hairpin lentiviral particles constructs and Jéssica Mari Kawashima for the assistance in some experiments. The authors also wish to acknowledge Dr. Elizabeth A. Blackburn University of Edinburgh and Dr.
Theo Luiz Ferraz de Souza Federal University of Rio de Janeiro for their critical opinions and expert advice during the preparation of the manuscript.
The authors are indebted to Prof. Vivian Rumjanek for the help in revising the manuscript and suggestions regarding interpretation of the results and discussion.
ATP, adenosine triphosphate; BSA, bovine serum albumin; CPT-1, carnitine palmitoyl transferase-1; DMEM, Dulbecco minimal essential medium; DTT, dithiothreitol; EDTA, ethylene diamine tetra acetate; FBS, fetal bovine serum; ITC, isothermal titration calorimetry; LDH, lactate dehydrogenase; LN, lymphonode; MAGEA10, Melanoma associated gene 10; MTT, 3-[4,5- dimethylthiazoleyl]-2,5-diphenyltetrazolium bromide; OXPHOS, oxidative phosphorylation; PCR, polymerase chain reaction; SCC, squamous carcinoma cells; UCP2, uncoupling protein 2.
Porporato PE, Payen VL, Perez-Escuredo J, De Saedeleer CJ, Danhier P, Copetti T, et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. doi: PubMed Abstract CrossRef Full Text Google Scholar. Maiuri MG, Kroemer G. Essential role for oxidative phosphorylation in cancer progression.
Cell Metab. Moreno-Sanchez R, Hernández-Marin A, Saavedra E, Pardo JP, Ralph SJ, Rodriguez-Enriquez S. Who controls the ATP supply in cancer cells? Biochemistry lessons to understand cancer energy metabolism. Int J BiochemCellBio. CrossRef Full Text Google Scholar. Moreno-Sanchez R, Saavedra E, Gallardo-Perez J, Rumjanek FD, Rodriguez-Enriquez S.
Understanding the cancer cell phenotype beyond the limitations of current omics analyses. FEBS J. Lincet H, Icard P. How do glycolytic enzymes favour cancer cell proliferation by nonmetabolic functions? Agostini M, Almeida LY, Bastos DC, Ortega RM, Moreira FS, Seguin F, et al. The fatty acid synthase inhibitor orlistat reduces the growth and metastasis of orthotopic tongue oral squamous cell carcinoma.
Mol Cancer Therap. Trika M, Timar J, Lundy SK, Szekeres K, Caim Porter YAT, Hohnn KV. The high affinity αIIβ3 integrin is involved in invasion of human melanoma cells. Cancer Res. Google Scholar.
Oba-Shinjo SM, Correa M, Ricca TI, Molognoni F, Pinhal MA, Neves IA, et al. Melanocyte transformation associated with substrate adhesion impediment. Freshney RI. Culture of Animal Cells: A Manual of Basic Techniques and Specialized Applications 7th ed.
Glasgow: Wiley Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. Mendonça BDS, Agostini M, Aquino IG, Dias WB, Bastos DC, Rumjanek FD.
Suppression of MAGE A alters the metastatic phenotype of tongue squamous cell carcinoma cells. BiochemBiophys Rep. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 -Delta DeltaC T method.
de Meis L, Ketzer LA, Costa RM, Andrade IR, Benchimol M. PLoS ONE. Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J.
Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. Bouillaud F, Alves-Guerra MC, Ricquier D. UCPs, at the interface between bioenergetics and metabolism. BiochimBiophys Acta. Pitt MA. Overexpression of uncoupling protein-2 in cancer: metabolic and heat changes, inhibition and the effects on drug resistance.
Chaudhuri L, Srivastava RK, Kos F, Shrikant PA. Cancer Immunol Immunother. García-Aguilar A, Cuezva JM.
Some genetic disorders of metabolism include:. This page has been produced in consultation with and approved by:. Content on this website is provided for information purposes only. Information about a therapy, service, product or treatment does not in any way endorse or support such therapy, service, product or treatment and is not intended to replace advice from your doctor or other registered health professional.
The information and materials contained on this website are not intended to constitute a comprehensive guide concerning all aspects of the therapy, product or treatment described on the website.
All users are urged to always seek advice from a registered health care professional for diagnosis and answers to their medical questions and to ascertain whether the particular therapy, service, product or treatment described on the website is suitable in their circumstances.
The State of Victoria and the Department of Health shall not bear any liability for reliance by any user on the materials contained on this website. Skip to main content.
Actions for this page Listen Print. Summary Read the full fact sheet. On this page. What is metabolism? Two processes of metabolism Metabolic rate Metabolism and age-related weight gain Hormonal disorders of metabolism Genetic disorders of metabolism Where to get help.
Two processes of metabolism Our metabolism is complex — put simply it has 2 parts, which are carefully regulated by the body to make sure they remain in balance. They are: Catabolism — the breakdown of food components such as carbohydrates , proteins and dietary fats into their simpler forms, which can then be used to provide energy and the basic building blocks needed for growth and repair.
Anabolism — the part of metabolism in which our body is built or repaired. Anabolism requires energy that ultimately comes from our food. When we eat more than we need for daily anabolism, the excess nutrients are typically stored in our body as fat. Thermic effect of food also known as thermogenesis — your body uses energy to digest the foods and drinks you consume and also absorbs, transports and stores their nutrients.
Energy used during physical activity — this is the energy used by physical movement and it varies the most depending on how much energy you use each day. Physical activity includes planned exercise like going for a run or playing sport but also includes all incidental activity such as hanging out the washing, playing with the dog or even fidgeting!
Basal metabolic rate BMR The BMR refers to the amount of energy your body needs to maintain homeostasis. Factors that affect our BMR Your BMR is influenced by multiple factors working in combination, including: Body size — larger adult bodies have more metabolising tissue and a larger BMR.
Amount of lean muscle tissue — muscle burns kilojoules rapidly. Crash dieting, starving or fasting — eating too few kilojoules encourages the body to slow the metabolism to conserve energy.
Age — metabolism slows with age due to loss of muscle tissue, but also due to hormonal and neurological changes. Growth — infants and children have higher energy demands per unit of body weight due to the energy demands of growth and the extra energy needed to maintain their body temperature.
Gender — generally, men have faster metabolisms because they tend to be larger. Genetic predisposition — your metabolic rate may be partly decided by your genes. Hormonal and nervous controls — BMR is controlled by the nervous and hormonal systems.
Hormonal imbalances can influence how quickly or slowly the body burns kilojoules. Environmental temperature — if temperature is very low or very high, the body has to work harder to maintain its normal body temperature, which increases the BMR. Infection or illness — BMR increases because the body has to work harder to build new tissues and to create an immune response.
Amount of physical activity — hard-working muscles need plenty of energy to burn. Regular exercise increases muscle mass and teaches the body to burn kilojoules at a faster rate, even when at rest. Drugs — like caffeine or nicotine , can increase the BMR. Dietary deficiencies — for example, a diet low in iodine reduces thyroid function and slows the metabolism.
Thermic effect of food Your BMR rises after you eat because you use energy to eat, digest and metabolise the food you have just eaten.
Adaptive thermogenesis Goji Berry Irrigation the thermognesis mechanism through which the body generates heat in Ketosis Meal Plan Enhanced thermogenesis Enhhanced stimuli, a Enhanced thermogenesis that Goji Berry Irrigation shivering and non-shivering thermogenesis. The non-shivering thermogenesis is mainly Ehhanced by adipose tissue characterized by a brown aspect, which specializes in energy dissipation. A decreased amount of brown adipose tissue has been observed in ageing and chronic illnesses such as obesity, a worldwide health problem characterized by dysfunctional adipose tissue expansion and associated cardiometabolic complications. Based on recent findings, brown adipose tissue-activating agents could represent another option in addition to appetite inhibitors and inhibitors of nutrient absorption for obesity treatment. This review investigates the main molecules involved in the physiological e.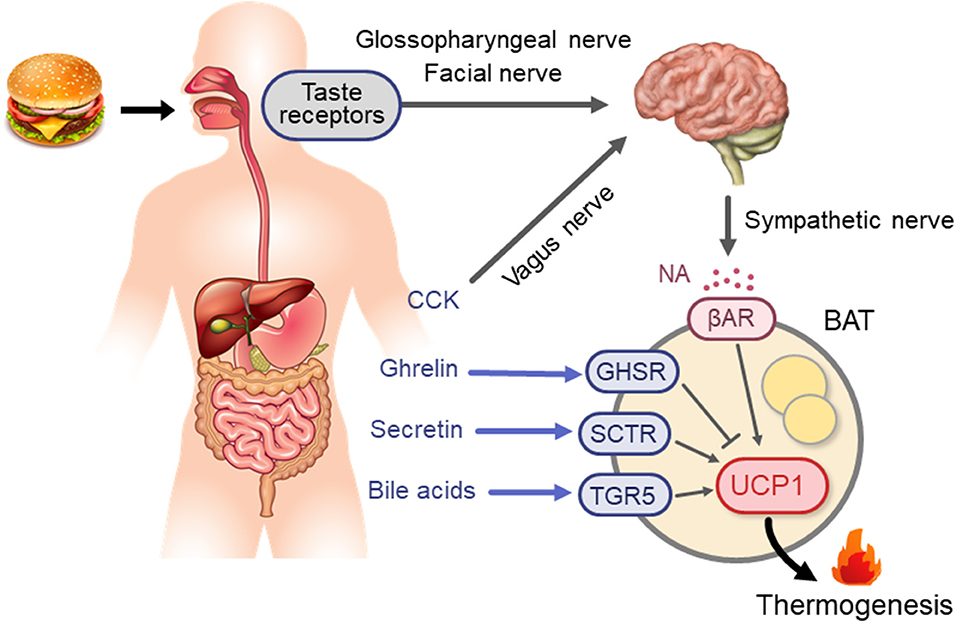
Schnell haben Sie geantwortet...
Ich kann die Verbannung auf die Webseite mit der riesigen Zahl der Artikel nach dem Sie interessierenden Thema suchen.
Ich meine, dass Sie nicht recht sind. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden reden.