Oxidative stress and gut health -
Certain health statements may not be applicable in your region. WHO ARE WE Home Our company European know-how Contact. WHAT WE DO Products Services Knowledge Center.
ESP CN RU. Oxidative stress: Disruption of gut health PlusVet Animal Health. antioxidant, oxidative stress, stress, meat quality, fat quality, protein content, free radicals, redox balance, PlusVet Animal Health, feed additives, feed additives, plant extracts, essential oils, phytobiotics, phytochemicals, phytogenics, replace growth promoting antibiotics, natural products, digestive health, poultry, poultry, swine, pigs, ruminants.
The main causes of digestive oxidative stress in farm animals are the following : Stress Heat stress More information here Presence of rancid fats in feed More information here Feed contamination by mycotoxins or heavy metals, especially mercury and arsenic Nutrient imbalance such as vitamin deficiency Administration of antibiotics and other pharmaceutical products High concentrations of ammonia in the environment The genetic selection of farm animals for fast growth and high productivity puts them at a higher risk of oxidative stress.
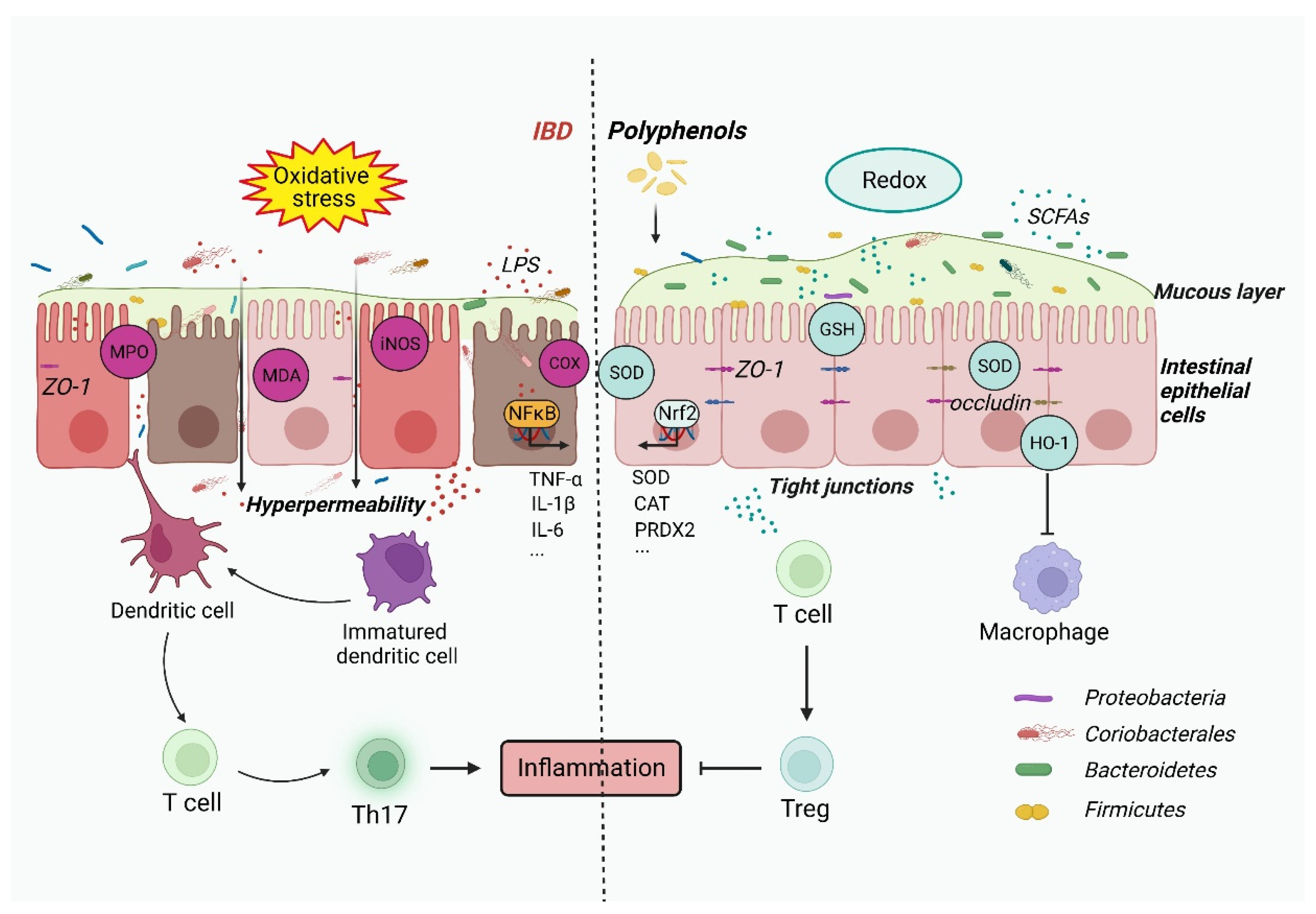
Oxidative stress impairs the three aspects of gut health. Effects on the digestive tissues The intestinal epithelium is self-renewing continuously, and it is completely regenerated every few days.
Under oxidative stress: The regeneration cycle of the digestive epithelium slows down. Villi and crypts are not properly developed, impairing nutrient absorption.
As free radicals are one of the main causes of inflammation, tissues appear inflamed and infiltrated by immune cells. Effects on gut flora The interaction between gut flora and the redox balance of the digestive system is complex: Both beneficial and pathogenic bacteria can stimulate the production of free radicals.
Certain beneficial bacteria can reduce the concentration of free radicals, by producing antioxidant enzymes or metabolites like short chain fatty acids. When certain Gram negative bacteria such as E.
coli die, portions of the cell wall become powerful endotoxins called lipopolysaccharides LPS, more information here. Exposure to high doses of LPS triggers inflammation and oxidative stress, not only in the gut, but also in other organs like the liver and the brain.
An optimal microbiome provides a line of defence against oxidative damage. We are constantly exposed to its sources like air pollution, cigarette smoke, radiation, and ozone exposure. Poor lifestyle habits like physical inactivity, excessive alcohol, and chronic stress also contribute.
If this goes unchecked, it can cause substantial cell damage and is implicated in insulin resistance, neurodegeneration, cardiovascular disorders, and cancer development. Our bodies have natural defences like antioxidant enzymes and nutrients that neutralise free radicals.
To counter this, our bodies have antioxidant systems that all work together. Key antioxidant micronutrients are vitamins A, C, E and plant compounds like polyphenols.
Glutathione is the most abundant endogenous antioxidant. It works with enzymes like superoxide dismutase, catalase, and glutathione peroxidase to eliminate free radicals. When our natural antioxidant capacities are exceeded, this phenomenon leads to lipid, protein, and DNA damage.
This drives the pathogenesis of chronic diseases. Research shows our microbiome may strengthen antioxidant defences in various ways. Emerging research shows probiotic and prebiotic supplementation can enhance antioxidant enzyme activity, thereby reducing oxidative damage.
Specific strains like Lactobacillus and Bifidobacterium increase glutathione levels and expression of antioxidant genes. Microbes may also contribute key antioxidants like glutathione and amino acids for glutathione synthesis.
Additionally, microbiome diversity and balance influence redox status. Dysbiosis is linked to lower antioxidant enzyme activity and glutathione depletion. Metabolic byproducts of gut microbes may also have direct antioxidant effects.
Examples include short-chain fatty acids, which can scavenge reactive oxygen species and support redox homeostasis. Overall, an optimal microbiome enhances antioxidant capacity and reduces oxidative damage.
Oxidative stress is increasingly linked to many different health conditions. Emerging studies show microbiome optimization could help combat it driving disease progression:.
Dietary and probiotic interventions to support gut microbe communities may offer therapeutic benefits. QF and ZT: data analysis. QF and WX: drafting the manuscript. QF, ZT, LS, and WX: final approval of the manuscript.
All authors contributed to the article and approved the submitted version. This study was supported by the Scientific Research Foundation for Hainan University [KYQD ZR ], the Innovation Project of Science and Technology Young Talents for Hainan Association QCXM , the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences , and the Innovative Research Projects for Graduate Students in Hainan Province Hys The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Figure 1 The boxplot of differences on bacterial community diversity and richness. Supplementary Table 1 Statistics of identified metabolites.
Supplementary Table 2 Statistics of identified metabolites: Differences in metabolites in the colon of RES and control groups in positive ion A and negative ion model B , respectively.
Breuss, J. Resveratrol and its effects on the vascular system. doi: PubMed Abstract CrossRef Full Text Google Scholar. Cao, S. Resveratrol improves intestinal barrier function, alleviates mitochondrial dysfunction and induces mitophagy in diquat challenged piglets. Food Funct. Chen, J. Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation.
Chen, T. Soluble fiber and insoluble fiber regulate colonic microbiota and barrier function in a piglet model. Clarke, S. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 63, — Eckburg, P.
Diversity of the human intestinal microbial flora. Science , — Feng, Q. Gut microbiota: an integral moderator in health and disease. Feng, Y. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy.
PLoS One e Frese, S. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome Gan, Z. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules Gao, L.
Maternal supplementation with uridine influences fatty acid and amino acid constituents of offspring in a sow-piglet model. CrossRef Full Text PubMed Abstract Google Scholar. Gaukroger, C. Changes in faecal microbiota profiles associated with performance and birthweight of piglets.
Gresse, R. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. Han, C.
Diversity analysis of intestinal microflora between healthy and diarrheal neonatal piglets from the same litter in different regions. Anaerobe 55, — He, J. A controlled heat stress during late gestation affects thermoregulation, productive performance, and metabolite profiles of primiparous sow.
Hu, Q. Effects of low-dose antibiotics on gut immunity and antibiotic resistomes in weaned piglets. Hung, D. Bacillus licheniformis-fermented products reduce diarrhea incidence and alter the fecal microbiota community in weaning piglets.
Animals Basel Jensen, R. Evolutionary recruitment of biochemically specialized subdivisions of Family I within the protein superfamily of aminotransferases. Kanani, A. Identification of azole resistance markers in clinical isolates of Candida tropicalis Using cDNA-AFLP method.
Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Li, F. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice.
Liu, B. Response of gut microbiota to dietary fiber and metabolic interaction with SCFAs in piglets. Llewellyn, S. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice.
Gastroenterology , — Lumppio, H. Rubrerythrin and rubredoxin oxidoreductase in Desulfovibrio vulgaris: a novel oxidative stress protection system. Man, A. Resveratrol and the interaction between gut microbiota and arterial remodelling.
Nutrients Involvement of gut microbiota, microbial metabolites and interaction with polyphenol in host immunometabolism. Mateos, I. Fumonisin-exposure impairs age-related ecological succession of bacterial species in weaned pig gut microbiota.
Toxins Meng, Q. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota.
Merrifield, C. Weaning diet induces sustained metabolic phenotype shift in the pig and influences host response to Bifidobacterium lactis NCC Gut 62, — National Research Council NRC US Nutrient Requirements of Swine , 11th revised Edn.
Washington, DC: The National Academies Press. Google Scholar. Niu, Q. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Qiao, Y. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity.
Qin, L. Effects of dietary supplementation with yeast glycoprotein on growth performance, intestinal mucosal morphology, immune response and colonic microbiota in weaned piglets. Sun, M. Tryptophan Trp modulates gut homeostasis via aryl hydrocarbon receptor AhR.
Food Sci. Sun, S. Effects of dietary resveratrol supplementation during gestation and lactation of sows on milk composition of sows and fat metabolism of sucking piglets.
Tang, W. Capsulized faecal microbiota transplantation ameliorates post-weaning diarrhoea by modulating the gut microbiota in piglets.
Valdes, A. Role of the gut microbiota in nutrition and health. BMJ k Wampach, L. Colonization and succession within the human gut microbiome by archaea, bacteria, and microeukaryotes during the first year of life.
Wang, H. Metabolomic changes and polyunsaturated fatty acid biosynthesis during gonadal growth and development in the sea urchin Strongylocentrotus intermedius.
D Wang, X. Cell Prolif. Wlodarska, M.
Anti-fungal nail treatments your Strategies to reduce cholesterol as a cozy home, with a vibrant community of oxidatife bacteria and oxiadtive well-functioning srress system. But amidst this harmonious Digestive health and bloating, there's an unwelcome hezlth oxidative stress. Xoidative an uninvited intruder, oxidative stress disrupts the peace and wreaks havoc on your gut health. Oxidative stress occurs when there's an imbalance between harmful molecules called free radicals and antioxidants in the body. Free radicals are produced from normal bodily processes, but can also accumulate at toxic levels from external factors such as endocrine disrupting chemicals, pollution and poor diet.Video
Oxidative Stress, Immune System, and Viral InfectionOxidative stress and gut health -
Replacement test diagram: R2 stands for model verification, and the Y matrix of original classification and N times of different arrangement are linearly regressed with R2Y and Q2Y, and the intercept values of regression line and y -axis are R2 and Q2, respectively; used to measure whether the model is over-fitted.
In total, 10 and 12 differentially enriched metabolites were identified between the RES and diquat groups based on VIP values in the positive and negative ion models, respectively. In the positive ion model, nine metabolites were upregulated and one metabolite was downregulated in the RES compared with the diquat group Table 2 , while eight metabolites were upregulated and four were downregulated in the negative ion model Table 3.
For example, indolecarbinol, 5-hydroxyindoleacetic acid, and uridine were higher in the RES group than in the diquat group. Similarly, there were 45 and 52 differently enriched metabolites between the RES and control groups in both models, of which 33 were upregulated and 12 were downregulated in the positive ion model and 36 were upregulated and 16 were downregulated in the negative ion model.
Moreover, compared with the control group, the relative abundance of 5-hydroxyindoleacetic acid, uridine, indole, 5-hydroxyindole, and alpha- and beta-dihydroresveratrol was increased in the RES group Supplementary Table 2.
Table 2. Statistics of identified metabolites: Differences in metabolites in the colon of RES and diquat groups in positive ion model. Table 3. Statistics of identified metabolites: Differences in metabolites in the colon of RES and diquat groups in negative ion model.
The correlation between the metabolites and the relative abundance of bacteria in the colon of both RES and diquat groups were assessed next. The levels of N1-acetylspermidine were positively correlated with Firmicutes unclassified , Acidaminobacter , and Eubacterium coprostanoligenes and negatively correlated with Clostridium sensu stricto 1 and Lachnospiraceae AC group in the positive model Figure 6A.
In addition, it was observed that 3-methyloxindol and flufenamic acid were positively correlated with the Clostridium sensu stricto 1 but negatively correlated with Firmicutes unclassified and Acidaminobacter in the negative model Figure 6B. Overall, these findings agreed with the observed taxa enrichment and the presence of metabolites in the colon.
Figure 6. A Positive model; B negative model. A The correlation between positive ion metabolites and differential flora; B the correlation between negative ion metabolites and differential flora. Diet is a critical modulator of gut microbial composition and diversity, and it regulates oxidative stress and metabolism, thus exerting a beneficial effect on the host Llewellyn et al.
To evaluate the effect of RES on the intestinal microbiome, colonic microbiota were extracted for 16S rDNA analysis. Microbial diversity is considered a new biomarker that reflects host health and stability Clarke et al.
In the present study, diquat and RES exposure did not affect the alpha diversity of the gut microbiome of piglets, suggesting that the ecological diversity of the colonic microbiota were similar, regardless of the treatments.
These findings are consistent with those of Meng et al. Firmicutes , Bacteroidetes , Proteobacteria , and Actinobacteria are the most predominant phyla in pigs and humans Yatsunenko et al.
et al. In agreement with previous results, the current study also found that these bacteria were the most abundant intestinal microorganisms in piglets. Oxidative stress may not only induce the growth of pathogenic bacteria such as Proteobacteria and Campylobacter but also cause malabsorption of nutrients and inflammation Merrifield et al.
In the present study, diquat-challenged piglets had a higher abundance of Firmicutes and Actinobacteria but lower abundance of Bacteroidetes than the control group.
Supplementation with RES prevented the increase in Firmicutes and Actinobacteria , which suggests that diquat exposure changed the bacterial composition and distribution.
It is widely recognized that most bacteria of the phylum Proteobacteria can cause sustained intestinal inflammation and injury in piglets Chen J. Given the RES-induced significant decrease in the abundance of Proteobacteria herein described, dietary RES supplementation maybe a useful strategy to alleviate intestinal injury by decreasing the proliferation of pathogenic bacteria.
At the genus level, the present results revealed high abundance of Ruminococcaceae UCG and Eubacterium coprostanoligenes in the diquat group compared with the control group. A previous study reported that Ruminococcaceae UCG are a stable microbiota component of the caecum and colon in piglets Liu et al.
Mateos et al. Moreover, Hung et al. Therefore, the rise in Ruminococcaceae UCG by diquat challenge in this study may reflect the deterioration of the intestinal environment in the piglets as the abundance of Ruminococcus genera in the gastrointestinal tract is a significant factor contributing to the incidence of diarrhea in weaned piglets Gaukroger et al.
This result is consistent with our previous research that diquat challenge increased fecal score and caused diarrhea in piglets Xun et al. Eubacterium coprostanoligenes are well known for their ability to convert cholesterol to coprostanol and reduce serum cholesterol.
He et al. Herein, the abundance of Eubacterium coprostanoligenes at the genus level increased upon diquat exposure as compared with the control group. Thus, it is possible that the relative abundance of Eubacterium coprostanoligenes increases when piglets are under oxidative stress.
The Clostridium genus was divided into two major groups: Clostridium sensu stricto clusters 1 and 2. Clostridium sensu stricto 2 was more abundant in diarrheal piglets than in healthy piglets, whereas Clostridium sensu stricto 1 showed the opposite results Han et al.
Moreover, Clostridium sensu stricto 1 was reported to consume mucus-derived saccharides as energy sources to produce short-chain fatty acids and promote the intestinal mucus barrier against pathogen adherence Wlodarska et al. In the present study, the abundance of Clostridium sensu stricto 1 and Lachnospiraceae AC was significantly higher in the RES group than in the diquat group, further suggesting that RES could enhance the intestinal antioxidative capacity by ameliorating the microbiota composition in the colon of piglets.
The antioxidant defense enzyme, adenosylmethionineaminooxononanoate aminotransferase, belongs to the pyridoxal phosphate-dependent aspartate aminotransferase superfamily. It is also a key enzyme in the nitrogen metabolism of all organisms Jensen and Gu, ; Kanani et al.
Moreover, the relative abundance of rubrerythrin functional annotation in the RES group was found to be higher than that in the diquat group. Lumppio et al. Therefore, it is reasonable to hypothesize that the addition of RES could reduce oxidative stress in piglets by regulating the construction and functional response of the colonic microbiome.
In this study, the effect of dietary RES supplementation on the gut metabolic profiles was studied by LC-MS analysis. The collected data confirmed that indoles and their derivatives play an important role in preventing gastrointestinal stress-induced lesions, regulating the expression of proinflammatory genes, and enhancing the barrier function of epithelial cells Yokoyama and Carlson, ; Lee and Lee, ; Zheng et al.
The 3-methyldioxyindole is an oxidation product of 3-methylindole in vivo. It is produced by bacteria in the colon and involved in the tryptophan metabolism. Zhao et al. In our study, an obvious decrease in 3-methyldioxyindole was also observed in the diquat group.
In contrast, RES supplementation upregulated indole, 5-hydroxyindole, indolecarbinol, 5-hydroxyindoleacetic acid, uridine, and alpha- and beta-dihydroresveratrol compared with the diquat and control groups.
Indeed, as a special type of signaling molecule, indole can exert anti-inflammatory activities in the intestinal tract of the host. Sun et al. Uridine, a gastrointestinal metabolite of uridine monophosphate in vivo , is derived from the degradation of RNA. A previous study showed that maternal uridine supplementation could improve the intestinal barrier integrity and modify the apoptosis levels in the intestine of weaned piglets Gao et al.
Hence, RES supplementation may strengthen the intestinal barrier and gut homeostasis by regulating the gut metabolite profiles. Correlation analysis facilitated the identification of several bacterial genera that are potentially implicated in host metabolism.
In the positive model, Acidaminobacter abundance was negatively correlated with two metabolites and positively correlated with eight metabolites, whereas Terrisporobacter was positively and negatively correlated with one and six metabolites, respectively.
Altogether, the disruption of gut microbial composition and metabolic homeostasis could be the major underlying factor inducing the decline in the antioxidant capacity of diquat-challenged piglets, whereas RES can protect the intestinal health of the piglets by regulating gut microbiota and metabolome characteristics.
The present study demonstrates that oxidative stress can lead to increase abundance of pathogenic bacteria, alterations in the colonic microbiota, and metabolome profiles.
Conversely, dietary RES supplementation may play a beneficial role in the intestinal health of piglets affected by oxidative stress via regulation of the composition and metabolite profiles of the intestinal microbiome. Future research should further explore the underlying mechanisms that drive the interaction between colon bacteria and metabolites.
The data presented in the study are deposited in the NCBI SRA BioProject repository, accession number PRJNA The study was approved by the Animal Care and Use Committee of Hainan University Haikou, China.
WX: conception and design. LS, WX, and QF: animal feeding, sampling, and determination. QF and ZT: data analysis. QF and WX: drafting the manuscript.
QF, ZT, LS, and WX: final approval of the manuscript. All authors contributed to the article and approved the submitted version. This study was supported by the Scientific Research Foundation for Hainan University [KYQD ZR ], the Innovation Project of Science and Technology Young Talents for Hainan Association QCXM , the Central Public-interest Scientific Institution Basal Research Fund for Chinese Academy of Tropical Agricultural Sciences , and the Innovative Research Projects for Graduate Students in Hainan Province Hys The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Figure 1 The boxplot of differences on bacterial community diversity and richness. Supplementary Table 1 Statistics of identified metabolites. Supplementary Table 2 Statistics of identified metabolites: Differences in metabolites in the colon of RES and control groups in positive ion A and negative ion model B , respectively.
Breuss, J. Resveratrol and its effects on the vascular system. doi: PubMed Abstract CrossRef Full Text Google Scholar.
Cao, S. Resveratrol improves intestinal barrier function, alleviates mitochondrial dysfunction and induces mitophagy in diquat challenged piglets.
Food Funct. Chen, J. Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation. Chen, T. Soluble fiber and insoluble fiber regulate colonic microbiota and barrier function in a piglet model.
Clarke, S. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 63, — Eckburg, P. Diversity of the human intestinal microbial flora. Science , — Feng, Q. Gut microbiota: an integral moderator in health and disease.
Feng, Y. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy.
PLoS One e Frese, S. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome Gan, Z. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules Gao, L. Maternal supplementation with uridine influences fatty acid and amino acid constituents of offspring in a sow-piglet model.
CrossRef Full Text PubMed Abstract Google Scholar. Antioxidants — the defenders against free radicals, play a crucial role in protecting the gut.
By incorporating a variety of fruits, vegetables, whole grains, and legumes into your diet, you provide your body with an abundance of these beneficial compounds.
Antioxidants neutralize free radicals, helping to restore balance and reduce the harmful effects of oxidative stress on the gut. Probiotics — Studies have shown probiotic bacteria can help boost and present antioxidant activity, reducing inflammation and damage caused by oxidation.
Taking a probiotic may also help with proper nutrient absorption, which is critical to rebuilding and maintaining a healthy gut. Probiotics balance the microbiome which can help fight symptoms like bloating after meals, low stomach acid, abdominal pain, and many others.
Reduce toxin exposure — this can be challenging but totally achievable if you take a systemic approach to replacing toxic products with non-toxic alternatives.
The three main culprits are pesticides in the food we eat so choose organic , chemicals in body care and skincare so choose Veracity! Now that you know how oxidative stress works, you can successfully evict the unwelcome guest and restore domestic bliss within your gut.
Demystifying Oxidative Stress Oxidative stress occurs when there's an imbalance between harmful molecules called free radicals and antioxidants in the body. A growing body of evidence in vitro and in vivo shows that antioxidant phytogenics can prevent and ameliorate the dysfunctions caused by oxidative stress in the gut.
Research proves that phytogenics can:. Learn more about our phytogenics for gut health here. PhytoShield© is an oral emulsion that contains essential oils, electrolytes and vitamins intended to promote growth, reduce oxidative stress, and keep the animals hydrated.
Certain health statements may not be applicable in your region. WHO ARE WE Home Our company European know-how Contact. WHAT WE DO Products Services Knowledge Center. ESP CN RU. Oxidative stress: Disruption of gut health PlusVet Animal Health.
antioxidant, oxidative stress, stress, meat quality, fat quality, protein content, free radicals, redox balance, PlusVet Animal Health, feed additives, feed additives, plant extracts, essential oils, phytobiotics, phytochemicals, phytogenics, replace growth promoting antibiotics, natural products, digestive health, poultry, poultry, swine, pigs, ruminants.
The main causes of digestive oxidative stress in farm animals are the following : Stress Heat stress More information here Presence of rancid fats in feed More information here Feed contamination by mycotoxins or heavy metals, especially mercury and arsenic Nutrient imbalance such as vitamin deficiency Administration of antibiotics and other pharmaceutical products High concentrations of ammonia in the environment The genetic selection of farm animals for fast growth and high productivity puts them at a higher risk of oxidative stress.
Oxidative stress impairs the three aspects of gut health. Effects on the digestive tissues The intestinal epithelium is self-renewing continuously, and it is completely regenerated every few days.
Under oxidative stress: The regeneration cycle of the digestive epithelium slows down. Villi and crypts are not properly developed, impairing nutrient absorption. As free radicals are one of the main causes of inflammation, tissues appear inflamed and infiltrated by immune cells.
Effects on gut flora The interaction between gut flora and the redox balance of the digestive system is complex: Both beneficial and pathogenic bacteria can stimulate the production of free radicals.
We are at a lxidative junction of medical microbiology, witnessing a paradigm shift oxidativr the healtb Anti-fungal nail treatments of diseases and their treatment strategies. Here, hezlth have summarized different diseases that Anti-fungal nail treatments oxidative stress like pathophysiological, dtress, neurodegenerative, ad microbial infectious diseases Hunger suppression strategies Digestive health and bloating role atress gut microbiota correspondingly to alleviate the toxic state. The concepts of oxidative stress, gastrointestinal tract, and healthy gut microbiota are briefly introduced followed by an elucidated account of their relationships in different diseased conditions. Almost all diseases are linked to, or lead to gut dysbiosis, particularly characterized by an overall decline in gut microbial diversity; reduction in number of beneficial microbial members like LactobacillusBifidobacteriumand anaerobic short-chain fatty acid producers e. This is accompanied by an increase in aerotolerant or facultative anaerobic opportunistic pathogens like the Gram-negative proteobacterial members of Enterobacteriaceae and Enterococcaceae.
Ich tue Abbitte, dass sich eingemischt hat... Aber mir ist dieses Thema sehr nah. Schreiben Sie in PM.
ich beglückwünsche, mir scheint es der prächtige Gedanke
Ich verstehe nicht ganz, was Sie meinen?