
Video
Inflammation and oxidative stress: a clinical paradoxOxidative stress and inflammation -
Oxidative stress affects these processes at several levels, including the release of danger molecules, their sensing by PRRs, and their downstream signal transduction systems.
A multiplicity of danger signals can be sensed by the host. Exogenous signals include primarily microbial molecules, for example, lipopolysaccharide LPS and flagellin from gram-negative bacteria, lipoteichoic acid from gram-positive bacteria, and viral RNA Kawai and Akira, , as well as non-infectious molecules, including allergens Holgate, and airborne pollutants such as silica and asbestos Dostert et al.
Multiple endogenous signals are also able to elicit an inflammatory response through the activation of innate immune defense mechanisms. DAMPs are released in conditions of tissue injury, and originate either from dying necrotic cells or from the breakdown of the extracellular matrix Medzhitov, a.
Examples of intracellular DAMPs include heat shock proteins, S proteins, high mobility group box-1 HMGB1 , ATP, and DNA Kono and Rock, A specific subgroup of DAMPs originating from mitochondria mitoDAMPs, MTDs has been also recently described, comprising formyl peptides and mitochondrial DNA Zhang et al.
Extracellular DAMPs comprise fibronectin, hyaluronic acid, as well as peptides derived from collagen or elastin Kono and Rock, Finally, various crystals acting as endogenous danger signals can promote strong inflammatory response, including monosodium urate Martinon et al.
The main endogenous DAMPs and their receptors are presented in Table 1. The appropriate recognition of the danger by the host is primordial for the elaboration of proper antimicrobial and adaptive responses.
Sensing of PAMPs and DAMPs is ensured by a complex set-up of PRRs, which include primarily the Toll-like receptors TLRs Trinchieri and Sher, and the nucleotide-binding oligomerization domain NOD -like receptors NLRs Martinon et al. Other PRRs include C-type lectins, the receptor for advanced glycation end-products RAGE , the retinoid acid-inducible gene I-like receptors, and the AIM2-like receptors see Kingeter and Lin, ; Miyake and Yamasaki, ; Tang et al.
PRR activation triggers a wealth of intracellular signaling pathways, including kinases [for instance, mitogen-activated protein kinases MAPKs , PI3 kinase], adaptors [such as myeloid differentiation primary response protein 88 MyD88 ], transcription factors [mainly nuclear factor-κB NF-κB , activator protein-1 AP-1 , and interferon regulatory factors], as well as the inflammasome in the case of NLR activation see below.
Such signaling cascades foster the expression of cytokines, chemokines, enzymes, growth factors, and additional molecules that are required for antimicrobial resistance and tissue repair Medzhitov and Horng, ; Tang et al.
As stated previously, inflammation represents an essential adaptive response aimed at eradicating invading pathogens and repairing tissue damage elicited by noxious stimuli.
Appropriate regulation of the mechanisms involved in such adaptation is essential to confine the inflammatory response in a localized compartment and to promote the switch between inflammation and repair, thereby allowing, in fine , the restoration of tissue homeostasis Medzhitov, a.
However, there are situations in which such restoration may not adequately occur, resulting in persistent cellular stress perpetuating and amplifying the inflammatory response. In these conditions, the process becomes maladaptive, leading to significant alterations of tissue functions, with systemic and persistent derangements of homeostasis Okin and Medzhitov, Diabetes, atherosclerosis, chronic heart failure, neurodegenerative diseases, and cancer are typical examples of pathological processes associated with such chronic inflammatory changes Pacher et al.
It is particularly noticeable that the release of ROS has long been recognized as a typical consequence of immune cell stimulation in vitro Meier et al. Accordingly, mitigating oxidative stress by the use of antioxidants has been evaluated as a potentially useful anti-inflammatory strategy in such conditions, as recently reviewed Spychalowicz et al.
Several distinct approaches to reduce oxidative stress have been used for this purpose, including natural e. Overall, the results of these innumerable studies have clearly pointed out the strong association between oxidative stress and inflammation.
Notwithstanding, the molecular mechanisms underlying the connection of these two fundamental biological processes have often not been precisely examined. In the next sections of this review, we will therefore attempt to fill this gap by presenting the current evidence supporting a mechanistic link binding redox stress, innate immunity, and inflammation, by focusing primarily on sterile non-infectious causes of inflammation.
As previously discussed, conditions promoting significant oxidative stress may precipitate cellular death and extracellular matrix breakdown, due to biomolecular damage exceeding any capacity of repair. Furthermore, oxidative stress conditions may induce various modifications within lipids and proteins, generating the so-called oxidation-specific epitopes, which act as potent DAMPs able to trigger innate immune responses through binding to multiple PRRs.
Examples of such oxidation-specific epitopes include oxidized phospholipids such as oxidized 1-palmytoylarachidonyl- sn -glycerophosphocholine Imai et al. As a detailed description of the multiple identified DAMPs is beyond the scope of this review, we will focus in the next section on the protein HMGB1 to illustrate the topics of the relations linking oxidative stress, DAMPs, and the development of sterile inflammation Figure 4.
HMGB1 can interact with multiple molecules, including LPS, DNA, lipoteichoic acid LPT , and IL-1, to elicit inflammatory responses through distinct TLRs.
Alternatively, HMGB1 can undergo various cysteine modifications depending on the local concentrations of oxidants. In the absence of ROS, HMGB1 is in the all thiol conformation, owing to reduced forms of Cys23, Cys45, and Cys All thiol HMGB1 interacts with the receptor for advanced glycation end-product RAGE to promote autophagic responses, and with the chemokine receptor CXCR4, triggering chemotactic responses.
In the presence of increasing amounts of ROS, HMGB1 occurs either in the disulfide conformation oxidation of Cys23 and Cys45 , which targets TLR4 to initiate inflammatory responses, or in the fully oxidized conformation, due to oxidation to sulfonic acid of its three redox active cysteines.
This latter form cannot activate inflammatory cells and thus promotes immune tolerance. HMGB1 has been the first DAMP to be characterized Scaffidi et al. HMGB1 is a ubiquitous nuclear non-histone protein that binds to DNA and participates in DNA replication, transcription, and repair see Andersson and Tracey, for recent review.
HMGB1 can be released extracellularly either through active secretion by activated monocytes Wang et al. These effects are mediated following interactions of HMGB1 with several PRRs, primarily TLR4 and RAGE Janko et al. HMGB1 also forms various complexes with many different molecules in the extracellular milieu, promoting additional pro-inflammatory activities through interaction with TLR2, TLR3, TLR9, and the IL-1 receptor Bianchi, ; Janko et al.
HMGB1 plays significant roles in the pathophysiology of sterile inflammation Andersson and Tracey, associated with diverse acute and chronic conditions, including myocardial infarction Loukili et al.
Thus, HMGB1 emerges as a central acting mediator at the intersection between sterile and infectious inflammation Andersson and Tracey, Several lines of experimental evidence support a key role played by oxidative stress in the process of HMGB1 release in the extracellular milieu.
In a study using isolated cardiomyocytes in vitro , we reported that exposure of the cells to toxic concentrations of peroxynitrite induced necrotic cell death and was associated with the release of copious amounts of HMGB1 Loukili et al. Similarly, Tang et al.
In vivo , using a rat model of myocardial infarction, we reported that a significant formation of peroxynitrite occurred in the necrotic myocardium, together with HMGB1 accumulation. The elimination of peroxynitrite using peroxynitrite decomposition catalysts reduced myocardial infarct size and suppressed the build-up of HMGB1, providing direct evidence that peroxynitrite-mediated cell death was the key trigger of cardiac HMGB1 accumulation during myocardial ischemia Loukili et al.
Thus, HMGB1 release represents a common response to the cellular stress imposed by free radicals and oxidants in vitro and in vivo , thereby representing an important mechanism linking redox stress and inflammation.
It is particularly noteworthy that HMGB1, while being released by oxidatively damaged cells, is also extremely redox sensitive, due to the presence of three critical cysteine residues.
HMGB1 consists of two DNA binding motifs, box A and box B, and an acidic C-terminal tail. Box A possesses two vicinal cysteines Cys23 and Cys45 , whereas box B bears one single cysteine in position Cys Tang et al. Depending on the particular redox environment, three distinct redox forms of HMGB1 can therefore be present: all thiol-HMGB1 three cysteines in reduced SH form , disulfide HMGB1 presence of a disulfide bridge between Cys23 and Cys45 in box A , and fully oxidized HMGB1 oxidation to sulfonate of Cys23, Cys45, and Cys Tang et al.
According to recent investigations, the recognition of the different redox conformations of HMGB1 may help devise specific pharmacological inhibitors, with potential therapeutic activity against HMGB1-dependent inflammation Gero et al.
Among these distinct redox forms, only disulfide HMGB1 has the ability to bind TLR4 and to promote innate immune responses Tang et al. In contrast, all thiol HMGB1 cannot bind to TLR4, but has been associated with the induction of autophagic responses in target cells through binding to the RAGE receptor Kang et al.
In addition, all thiol HMGB1 forms a complex with the chemokine CXCL12, to promote strong chemotactic responses on leukocytes through binding to the receptor CXCR4 Venereau et al.
These two distinct redox forms of HMGB1 are mutually exclusive, which implies that, depending on the particular microenvironment, HMGB1 functions either as a chemoattractant or as an inducer of cytokine release Tang et al.
In conditions of tissue injury in vivo, both redox forms of HMGB1 have been shown to be sequentially present all thiol followed by disulfide , indicating that HMGB1 successively orchestrate leukocyte recruitment and the induction of cytokine secretion by adopting distinct redox conformations Tang et al.
Finally, fully oxidized HMGB1 loses both chemoattractant and pro-inflammatory activities, but instead triggers immunologic tolerance by preventing the activation of dendritic cells, which might explain the lack of inflammation associated with apoptotic cell death Kazama et al.
Indeed, during apoptosis, mitochondrial production of ROS appears sufficient to promote the full oxidation and complete inhibition of HMGB1 Kazama et al. In addition to oxidative modifications, HMGB1 can also undergo acetylation of key lysine residues located within its nuclear localization sequence.
The various posttranslational modifications of HMGB1 oxidation and acetylation have been the subject of an outstanding recent review by Yang et al. To complete the picture of HMGB1-oxidant stress interactions, it is worth mentioning that HMGB1 itself may induce significant redox modifications by fostering the cellular generation of ROS and RNS Janko et al.
HMGB1-dependent activation of TLR4 triggers the upregulation of multiple genes, among which the genes encoding NOX and iNOS. Fan et al. Using an experimental model of hemorrhagic shock in mice, these authors then reported that hemorrhagic shock was associated with significant increases of HMGB1 levels in most organs, together with a marked induction of NOX in PMNs, which could be abrogated both by TLR4 suppression and by neutralizing antibodies to HMGB1 Fan et al.
In another study by Sappington et al. Thus, it appears that oxidative stress represents both a cause and a consequence of HMGB1 release in multiple situations, and one may envision that such crosstalk might promote a vicious cycle of progressive inflammatory amplification. TLRs are key components of the innate immune system Figure 5 , whose primary function is to sense danger signals released from pathogens PAMPs.
Ten TLRs are expressed in humans, among which only TLR10 has no identified ligand thus far. TLR1, TLR2, TLR4, TLR5, and TLR6 are present in the plasma membrane to sense surface components of microbes. TLR2, which forms homodimers and heterodimers with TLR1 and TLR6, and which uses various coreceptors such as CD14, CD36, and Dectin-1, recognizes bacterial lipopetides and peptidoglycans as well as mannans and glucans from fungal origin.
TLR4, with the coreceptor MD-2, senses the complex formed by bacterial LPS and CD14, and TLR5 detects bacterial flagellin. TLR3, TLR7, TLR8, and TLR9 are expressed in intracellular compartments ER, endosomes, lysosomes, and endolysosomes where they sense viral and bacterial nucleic acids Lee et al.
TLRs form active dimers within specific lipid rafts containing various amounts of phospholipids, cholesterol, and sphingolipids in the cell membrane. The extracellular domain connects through a transmembrane domain to the cytoplasmic TIR domain, which interacts with adaptor molecules such as MyD88 and TRIF to activate downstream signaling through NF-κB, AP-1, and interferon-regulatory factor-3 IRF Consequently, the expression of inflammatory mediators is upregulated, comprising notably pro-oxidant enzymes such as NOX and iNOS, producing high levels of ROS.
TLR engagement also facilitates the generation of ROS within mitochondria, and promotes activation of NOX through direct interaction at the cell membrane or through enhanced phosphorylation of its p47 phox subunit within the cytoplasm.
The resulting increase in intracellular ROS favor the mobilization and dimerization of TLRs within lipid rafts, creating a cycle of progressive amplification of the TLR response. As previously discussed, TLRs also detect endogenous DAMPs released from damaged cells and extracellular matrix, comprising proteins, fatty acids, lipoproteins, proteoglycans, and glycosaminoglycans mainly detected by TLR4, and, to a lesser extent, TLR2 , as well as nucleic acids and protein-nucleic acid complexes detected by TLR3, TLR7, TLR8, and TLR9 see Piccinini and Midwood, for extensive review.
Several differences in the recognition of PAMPs and DAMPs different binding sites, different coreceptors and accessory molecules , and the engagement of distinct regulatory pathways may help discriminate exogenous from endogenous dangers. Most notably, DAMP recognition triggers negative feedback mechanisms to limit the potential damage to the host, and recent findings support an essential role for sialoside-based pattern recognition by members of the Siglec family in such negative regulation Chen et al.
All TLRs, with the exception of TLR3, interact with the adaptor MyD The endosomal TLR7, TLR8, TLR9 and the cell surface TLR5 directly link MyD88, whereas TLR1, TLR2, TLR4, and TLR5 present on cell surface additionally recruit the linker protein TIR domain-containing adaptor protein TIRAP also known as MAL that connects TLRs and MyD88 TIR domains.
Upon ligand binding, TLR3 and TLR4 recruit the protein TIR domain-containing adaptor inducing interferon-β TRIF either directly for TLR3 or by the intermediate of TRIF-related adaptor molecule TRAM for TLR4 Gay and Gangloff, ; Song and Lee, MyD88 activates the IRAK-TRAF6-TAK1 axis that turns on inhibitor of κB IκB kinase IKK and MAPKs, which lead to the activation of NF-κB and AP-1 transcription factors, respectively Kawai and Akira, ; Song and Lee, It has been known for a long time that conditions associated with significant oxidative stress trigger enhanced responsiveness of cells from the innate immune system to pro-inflammatory stimuli Botha et al.
In a milestone study by Powers and coworkers , exposure of rodent macrophages to oxidants, either in vivo using an experimental model of hemorrhagic shock and resuscitation or in vitro using direct macrophage activation with H 2 O 2 , induced a significant increase in the surface levels of TLR4, as well as an increased responsiveness of cells to LPS.
This effect was dependent on exocytosis of TLR4 from cytoplasmic compartments, as it could be suppressed by disruption of the cytoskeleton. Finally, preventing TLR4 movement to lipid rafts using methyl-β-cyclodextrin suppressed the increased cellular responsiveness to LPS exposure to oxidants Powers et al.
The study by Powers et al. The essential role of oxidants in such process, as demonstrated in the above-discussed study, has been further confirmed by Nakahira et al. Similarly, Wong et al. The precise mechanisms of oxidant-mediated lipid raft modifications remain only partially elucidated.
They involve, at least partly, alterations in lipid raft annexin VI content, activation of calcium-dependent kinases, and the generation of ceramide.
In turn, such modifications may result in modifications of lipid raft density and protein composition, with subsequent stimulation of TLR complex assembly Cuschieri and Maier, ; de la Haba et al.
The previous paragraphs have highlighted the importance of oxidants in the activation of TLRs, by promoting their trafficking to the cell membrane. We will now focus on the opposite mechanism, that is, the induction of oxidant production in response to TLR activation Figure 5.
Increasing evidence is indeed accumulating showing that the formation of ROS represents an essential pathway of TLR-dependent signaling in cells from immune and non-immune origin, which occurs mainly through the activation of various NOX isoforms Tsung et al.
In addition, the pro-inflammatory signaling cascades triggered by TLR engagement enhance the expression of iNOS, and thus promotes the generation of NO Lewis et al.
In turn, the concomitant generation of O 2. by iNOS results in the formation of peroxynitrite and other toxic RNS, implying that TLR activation may result both into oxidative and nitroxidative stress Jozsef et al.
While TLR-dependent oxidant formation is important to promote killing of invading pathogens West et al. Additionally, oxidants may facilitate further TLR activation, resulting in a cycle of progressive amplification of the initial inflammatory response.
The activation of NOX enzymes appears as a key process linking TLRs with secondary ROS generation. Such activation of NOX appears to result from several mechanisms, including 1 increased NOX protein expression, 2 stimulated assembly of the NOX subunits, and 3 direct interactions between NOX and the TIR domain of TLRs.
Increased NOX expression has been notably well documented for NOX1 present in cells from gastrointestinal origin. NOX1 upregulation occurred in gastric epithelial cells in response to TLR4 activation by LPS from pathogenic Helicobacter pylori strains, and was shown to promote the induction of TNFα or cyclooxygenase 2 mRNA expression.
This implies that NOX1 could be significantly involved in the pathogenesis of chronic gastric inflammation induced by H. pylori infection Kawahara et al. Induction of NOX1 has also been evidenced in multiple colon cancer cell lines, including T84 Kawahara et al.
NOX1-dependent ROS production in these conditions promoted the release of the major chemokine IL-8 Kawahara et al. The second important mechanism of TLR-mediated NOX activation is represented by stimulated assembly of the NOX subunits, particularly well documented in the case of NOX2 expressed by phagocytes.
Various agonists of TLR2 Huang et al. Deciphering the underlying signaling mechanisms revealed a crucial role of MyD88 Laroux et al. Finally, activation of NOX may also result from direct interactions between TLRs and NOX, as indicated in a study by Park et al.
Using yeast two-hybrid and GST pull-down assays, these authors showed that the carboxy-terminal region of NOX4 directly interacted with the cytoplasmic tail of TLR4 in HEKT cells. Stimulation of TLR4 with LPS in this system induced ROS generation followed by NF-κB activation, pointing to direct interaction between TLR4 and NOX4 as a critical mechanism regulating TLR-dependent innate immune responses Park et al.
Generation of ROS secondary to TLR engagement does not only rely on the activation of NOX but also depends on the mitochondria Figure 5.
West and coworkers reported that the engagement of various TLRs TLR1, TLR2, and TLR4 augmented mitochondrial ROS production by inducing the recruitment of mitochondria to macrophage phagosomes. This response occurred through the translocation of the adaptor TRAF6 to mitochondria, leading to ubiquitination and enrichment at the mitochondrial periphery of ECSIT evolutionarily conserved signaling intermediate in Toll pathways , a protein implicated in mitochondrial respiratory chain assembly, with subsequent mitochondrial generation of ROS.
The importance of this process in antibacterial defense was critical, in view of the significant impaired capacity of ECSIT- and TRAF6-depleted macrophages to kill intracellular bacteria West et al.
However, it remains thus far unknown whether a similar mechanism accounts for TLR-dependent oxidative stress under sterile conditions. Still, given the critical importance of TLR-dependent ROS generation in the elimination of invading pathogens, impaired infectious control might well represent an important drawback of such strategy.
Many issues need to be addressed to pinpoint how the organism discriminates between exogenous from endogenous danger signals and whether distinct signaling pathways modulate TLR-mediated responses during infectious and sterile inflammation, in order to manipulate safely the TLR machinery for the therapy of chronic inflammatory diseases.
NLRs are located in the cytosol and sense a wide range of PAMPs and DAMPs. NLRs possess a common structure characterized by C-terminal leucine-rich repeat domain for ligand sensing, a central nucleotide-binding and oligomerization domain for activation, and an N-terminal domain for downstream signaling whose structure defines different subfamilies of NLRs NLRA, NLRB, NLRC, NLRP, and NLRX Kersse et al.
Upon activation, NLRs of the NLRP or NLRC families form multiprotein complexes termed inflammasomes, following assembly with the adapter protein ASC apoptosis associated speck-like containing a CARD domain and caspase Once activated, the inflammasome platform promotes caspasemediated cleavage and maturation of pro-IL-1β and pro-IL into mature IL-1β and IL, which are critical to the development of inflammation Martinon et al.
The NLRP3 inflammasome also termed cryopirin or NALP3 has been thus far the best-studied member of this wide family Figure 6. Foreign signals comprise multiple pathogens and PAMPs that enter the cytosol, including bacteria notably Staphylococcus , Listeria , Clostridium , and Escherichia coli species , fungi mainly Candida and Aspergillus species , and viruses for instance, adenovirus and influenza virus Davis et al.
The activation of NLRP3 in response to invading pathogens mainly depends on the release of pore-forming toxins by bacteria such as hemolysin from Staphylococcus aureus and toxin A from Clostridium difficile Koizumi et al. Besides microbes, several non-infectious foreign molecules are robust activators of NLRP3, notably crystalline structures responsible for occupational lung inflammatory diseases silica and asbestos and particulate structures such as Co-Cr-Mo alloy metal particles used in prosthetic orthopedic material Caicedo et al.
The activation of TLRs by pathogen- or damage-associated molecular patterns PAMPs, DAMPs results in an increased expression of NLRP3, pro-IL-1β, and pro-IL priming stage. B The release of cathepsin B by activated phagolysosomes.
C Mitochondrial damage and ROS production. ROS can either separate thioredoxin-interacting protein TXNIP from its inhibitor thioredoxin TRX , and TXNIP directly activates NLRP3. Ultimately, activated NLRP3 associates with ASC and caspase-1 in a multiprotein platform termed the inflammasome, which converts pro-IL-1β and pro-IL into mature cytokines.
Multiple endogenous signals derived from the host itself have the ability to trigger NLRP3 activation, leading to a variety of acute and chronic inflammatory processes. Diverse crystals, including monosodium urate, calcium pyrophosphate dehydrate, and calcium oxalate, potently activate NLRP3 to induce joint inflammation in crystal-induced arthritis such as gout and pseudogout Busso and Ea, , as well as renal damage in crystalline nephropathy nephrocalcinosis Mulay et al.
Cholesterol crystals can also be sensed by NLRP3, promoting IL-1β-dependent inflammation in the vascular wall, a critical process involved in atherogenesis Duewell et al. In the central nervous system, NLRP3 activation in the microglia due to sensing of extracellular insoluble β-amyloid peptide aggregates represents a key mechanism of neuroinflammation involved in the pathogenesis of Alzheimer disease Heneka et al.
NLRP3 is also activated during tissue injury, mainly by hyaluronan released from damaged ECM Yamasaki et al. Recent findings also indicated that NLRP3 contributes to a large extent to the development of tissue fibrosis during chronic inflammatory processes Xu et al.
Furthermore, inflammasome activation and IL-1β production also play an important role in the development and perpetuation of inflammation during metabolic syndrome, insulin resistance, and type 2 diabetes De Nardo and Latz, ; Wen et al.
Here, high glucose concentration, lipids palmitate, ceramide , and islet-derived amyloid polypeptide represent the main triggers for NLRP3 activation Menu and Vince, ; Zambetti et al. The large number of known activators of NLRP3 makes it highly unlikely that NLRP3 directly senses all these different triggers.
Instead, it is now generally acknowledged that NLRP3 activation occurs mainly as a consequence of a common form of cellular stress elicited by the different stimuli Lamkanfi and Dixit, Three main putative mechanisms are currently considered for such activation Figure 6.
The second one, termed the lysosome rupture model Tschopp and Schroder, , depends on the destabilization and the rupture of the phagolysosome compartment, especially following the digestion of particulate material, and which results in the release of the lysosomal protein cathepsin B as an activator of NLRP3 Hornung et al.
Recent data have indicated that a similar mechanism triggers NLRP3 activation in myeloid cells exposed to chemotherapeutic agents such as gemcitabine and 5-fluorouracil Bruchard et al.
The third model, termed the ROS model Tschopp and Schroder, , implicates the formation of ROS, deregulation of cellular redox status, and mitochondrial stress as key mechanisms in the process of NLRP3 activation Rubartelli et al.
Two main arguments support this model. First, it is particularly noteworthy that all NLRP3 activators are capable of inducing intracellular ROS generation, and second, treatment with various ROS scavengers can block NLRP3 activation by multiple agonists Tschopp and Schroder, A possible molecular link connecting oxidative stress with NLRP3 activation has been proposed by Zhou et al.
TXNIP is a member of the α-arrestin protein superfamily, which binds to the antioxidant protein thioredoxin TRX , acting as a negative regulator of the TRX reductase activity Yoshioka et al. In their study, Zhou et al. They then showed that various NLRP3 agonists, including monosodium urate MSU and the imidazoquinoline imiquimod R , promoted the association of NLRP3 with TXNIP, followed by inflammasome assembly and secretion of IL-1β by THP-1 cells.
The interaction between TXNIP and NLRP3 was dependent on the secondary generation of ROS induced by MSU and R, which prompted the dissociation of TXNIP from TRX and its subsequent binding to NLRP3 Zhou et al.
This role of TXNIP has been debated, as it could not be reproduced in a further study by Masters et al. Verification of the hypothesis that ROS act as secondary mediators responsible for NLRP3 activation requires the identification of the source of ROS responsible for such role.
In a report by Dostert et al. The possible role of NOX-derived ROS in NLRP3 activation has, however, not been uniformly verified. Thus, in a study using macrophages obtained from mice lacking gp91 phox NOX2 , IL-1β response to multiple NLRP3 agonists was not modified Hornung et al.
Furthermore, a study by van Bruggen et al. Recent observations have implicated mitochondria as the primary source of ROS ultimately responsible for the activation of NLRP3 Zhou et al.
Many activators of NLRP3 have been shown to disrupt the inner mitochondrial membrane potential, possibly as a consequence of cellular potassium efflux and resulting alterations of mitochondrial matrix volume Martinon, The ensuing mitochondrial dysfunction promotes the accelerated generation of ROS from the electron transport chain, leading to the oxidation of mitochondrial DNA mtDNA and its release into the cytosol Figure 6 , where it binds to NLRP3 to trigger its activation, as recently demonstrated by three independent groups Nakahira et al.
The crucial role of mitochondria in this scheme of event has been further reinforced by a recent report by Misawa et al. The latest developments in the molecular biology of NLRP3 provide essential information to understand the mechanisms governing the initiation and perpetuation of inflammation in many pathological conditions.
Mitochondria are vulnerable targets of multiple cellular stressors, and as such are particularly well positioned to perceive and signal the presence of noxious stimuli.
Whereas such mechanisms have long been recognized as the key triggers of cell death Yu et al. Therefore, beyond their role in governing cell death processes, mitochondria also represent master regulators of innate immune processes Arnoult et al.
At variance with the oxidative stress model of NLRP3 activation, it must be mentioned that a limited number of observations have suggested that ROS could instead inhibit the inflammasome, through a mechanism involving the oxidation and glutathionylation of critical cysteine residues Cys and Cys within caspase-1 Meissner et al.
These results raise the possibility that NLRP3, instead of being purely responsive to oxidative stress, might function, at least in some cell types, as a sensor of the global redox status of the cell, governed by a highly dynamic balance between oxidant production and the redox buffering capacities, as well developed by Rubartelli in two recent articles Rubartelli et al.
As presented in the previous sections, the inflammatory response mounted upon sensing of danger signals involves both inflammasome- and TLR-dependent processes.
Responses triggered by TLRs are conveyed primarily by the activation of the transcription factor NF-κB, and, to a lesser extent, by MAPKs and other transcription factors, AP-1 and IRFs.
NF-κB is heavily redox sensitive, and as such has a strategic position at the crossroad between oxidative stress and inflammation, which will be discussed here. NF-κB is a master regulator of inflammation, controlling the expression of hundreds of genes implicated in innate immune responses, and is also a key transcription factor regulating many antiapoptotic genes.
It encompasses a family of dimeric proteins, which comprise NF-κB1 p50 and its precursor p , NF-κB2 p52 and its precursor p , p65 RelA , RelB, and c-Rel.
Of note, only RelA, RelB, and c-Rel have potent transcriptional activation domains, whereas Rel proteins lacking these domains, e. Under resting conditions, NF-κB is held inactive in the cytoplasm through binding to an inhibitory protein named IκB IκBα, IκBβ, and IκBε , which masks the nuclear localization sequence of NF-κB subunits Senftleben and Karin, Additional IκBs IκBξ, Bcl-3, and IκBNS are found in the nucleus, where they bind specifically to p50 homodimers and modulate their activity Napetschnig and Wu, The activation of NF-κB proceeds through two distinct pathways, termed the canonical and non-canonical pathways.
IκB degradation unmasks the nuclear localization sequence of NF-κB, which can then freely translocate to the nucleus and activate target genes. Phosphorylation of IκB is secondary to the activation of a protein kinase complex, IKK, composed of a heterodimer of two catalytic subunits, IKKα and IKKβ, associated with a dimer of a regulatory subunit, IKKγ, in response to the engagement of TLRs and the IL-1 receptor.
The latter is activated by an upstream kinase termed NIK NF-κB inducing kinase , consecutively to the engagement of various members of the TNF receptor family. In turn, activated IKKα promotes the phosphorylation of p and its processing into mature p52, resulting in its translocation to the nucleus and the induction of pdependent target genes see Karin et al.
The last level of NF-κB regulation occurs in the nucleus, and includes chromatin remodeling and p65 serine phosphorylation. Chromatin remodeling, regulated by acetylation-deacetylation processes of lysine residues within histones by histone acetyltransferases and histone deacetylases, is required to allow DNA uncoiling and accessibility of NF-κB.
Phosphorylation of p65 represents a final step essential for full transcriptional activity of NF-κB, the most important being Ser phosphorylation mediated by protein kinase A Gloire and Piette, Upon TLR engagement, the IKK complex becomes phosphorylated and activated.
Activated IKK in turn phosphorylates the inhibitor IκB on serine 32 and 36, targeting IκB to proteasomal degradation. ROS promote NF-κB activation at three distinct levels: 1 ROS enhance IKK phosphorylation by activating protein kinase D PKD or by inhibiting PP2A, the phosphatase responsible for IKK dephosphorylation.
The first evidence of the redox sensitivity of NF-κB was presented by Schreck et al. This concept was further extended by a series of experimental studies reporting on the direct activation of NF-κB by various oxidants and its inhibition by chemical antioxidants or by the upregulation of endogenous antioxidant defenses reviewed in Li and Karin, It was then proposed that ROS might represent key secondary mediators responsible for the activation of NF-κB in response to multiple stimuli, including LPS and pro-inflammatory cytokines Gloire et al.
This notion was, however, soon debated, as it became evident that NF-κB activation by exogenous ROS was highly cell specific Li and Karin, , and that, in multiple cell lines, endogenously produced ROS did not mediate such activation Hayakawa et al. The controversy gained further momentum with the publication of several reports showing that, in some cell types, ROS triggered an inhibition, instead of an activation, of this transcription factor Zahler et al.
Therefore, the role of ROS in the regulation of NF-κB has evolved over the years from inducer to modulator, as presented in detail in a recent review by Oliveira-Marques et al. Redox regulation occurs at multiple levels of the NF-κB pathway, both in the cytoplasm and in the nucleus Gloire and Piette, In cells disclosing NF-κB activation in response to ROS, two distinct triggering mechanisms have been identified in the cytoplasm Figure 7.
The first one includes the classic pathway of IκB serine phosphorylation and degradation in response to IKK activation. In turn, PKD activates IKKβ, resulting in IκB degradation and transcriptional activation of NF-κB Storz and Toker, ; Storz et al. Besides this PKD-dependent activation of IKK, a mechanism of IKK phosphorylation related to oxidant-dependent inhibition of phosphatases especially PP2A has also been postulated Li et al.
The second one involves an atypical mechanism implicating tyrosine phosphorylation Tyr42 on IκB Figure 7 , instead of the classic, IKK-dependent, IκB serine phosphorylation Schoonbroodt et al.
The mechanism may implicate here various kinases, including the tyrosine kinases Syk Takada et al. In response to Tyr42 phosphorylation, IκB can either be degraded, by a mechanism independent from the proteasome but dependent on calpain-mediated digestion Schoonbroodt et al.
In either case, the release of NF-κB from its inhibitor allows it to translocate to the nucleus and activate target genes Gloire et al. Besides these cytoplasmic mechanisms, several redox-based processes occurring in the nucleus have also been reported to promote NF-κB activation by favoring chromatin remodeling, such as the reduction of histone deacetylase activity, as well as the induction of lysine acetylation and serine phosphorylation of histone H3 Yang et al.
At variance with the above-described ROS-dependent NF-κB activation, evidence exists that, in multiple conditions, ROS serve instead as potent inhibitors of NF-κB Figure 8. Three main underlying mechanisms have been postulated. The first one involves the ROS-dependent prevention of IKK phosphorylation, related to various redox-based modifications of a critical cysteine residue Cys in IKKβ extensively reviewed in Pantano et al.
The second mechanism involves a direct inhibition of the proteasome by oxidants, resulting in the prevention of IκB degradation, as notably demonstrated in airway epithelial cells Jaspers et al.
The third mechanism involves direct redox modifications of NF-κB subunits affecting either the p50 or the p65 subunit. The p50 subunit possesses a highly redox active cysteine within its Rel homology domain Cys62 , which is oxidized within the cytoplasm in conditions of oxidative stress.
Upon translocation to the nucleus, oxidized Cys62 impairs DNA binding, thereby preventing NF-κB to activate target genes Kabe et al. DNA binding is restored through the reduction of Cys62 within the nucleus, following the concerted activity of several antioxidant enzymes, including thioredoxin Hirota et al.
A further redox modification of Cys62 in p50 is NO-mediated S -nitrosylation, which prevents DNA binding of NF-κB Sha and Marshall, Both modifications inhibit NF-κB transcriptional activity, by inducing its unbinding from DNA and its nuclear export Gloire and Piette, Oxidative stress can downregulate NF-κB signaling by acting at four distinct levels: 1 ROS oxidize cysteine within IKKβ, preventing its phosphorylation and activation.
Oxidized p50 cannot bind to DNA. RNS can modify p65 by S -nitrosylation of cysteine 38, or by nitration of tyrosine 66 and tyrosine , preventing pDNA binding and favoring p65 nuclear export. In summary, ROS and RNS definitely play a central role in the regulation of NF-κB, although this role remains incompletely understood, in view of the contrasted outcome activation vs.
inhibition reported. The underlying reasons remain thus far unclear, but it is likely that the particular experimental conditions explain, at least partly, these contrasted results: these include notably the specific cell type studied, the particular ROS or RNS studied, the concentration of the oxidants used, as well as the concomitant stimulation of cells with NF-κB activators such as cytokines or LPS together with oxidants Gloire and Piette, ; Oliveira-Marques et al.
It is also probable that the redox regulation of NF-κB may dynamically evolve over time and that distinct mechanisms are set in motion at different stages of the inflammatory response.
One possibility might be that ROS could promote NF-κB responses in the early phases of the process, while they would instead inhibit these responses at later stages, thereby favoring the induction of tissue repair.
In support of such hypothesis, it has been proposed that the modifications introduced by NO and peroxynitrite on p50 and p65 as described above could represent important negative feedback mechanisms downregulating inflammation under conditions of elevated NO production mediated by the inducible isoform of NO synthase Gloire and Piette, The NF-κB signaling pathway has been by far the most widely studied in terms of redox regulation.
It is, however, worth to mention here that additional signal transduction cascades linked with inflammation also disclose redox-sensitive characteristics. These include primarily proteins from the MAPK family ERK, JNK, and p38 and the transcription factor AP-1, which have been the matter of several recent reviews to which we refer the interested reader for further information McCubrey et al.
Since its early clinical description by Celsus about years ago rubor , tumor , calor , et dolor : redness, swelling, heat, and pain , inflammation had been essentially considered an internal response to an external stressor, primarily infection and injury.
Recent discoveries in the field of innate immune response have dramatically changed such vision, and inflammation is now recognized as an adaptive response to any form of danger arising both from the outside and from the inside.
At various degrees, inflammation is therefore present in virtually every acute or chronic human malady, and a precise understanding of its pathophysiological mechanisms might, de facto , help devise novel therapeutics for these diseases.
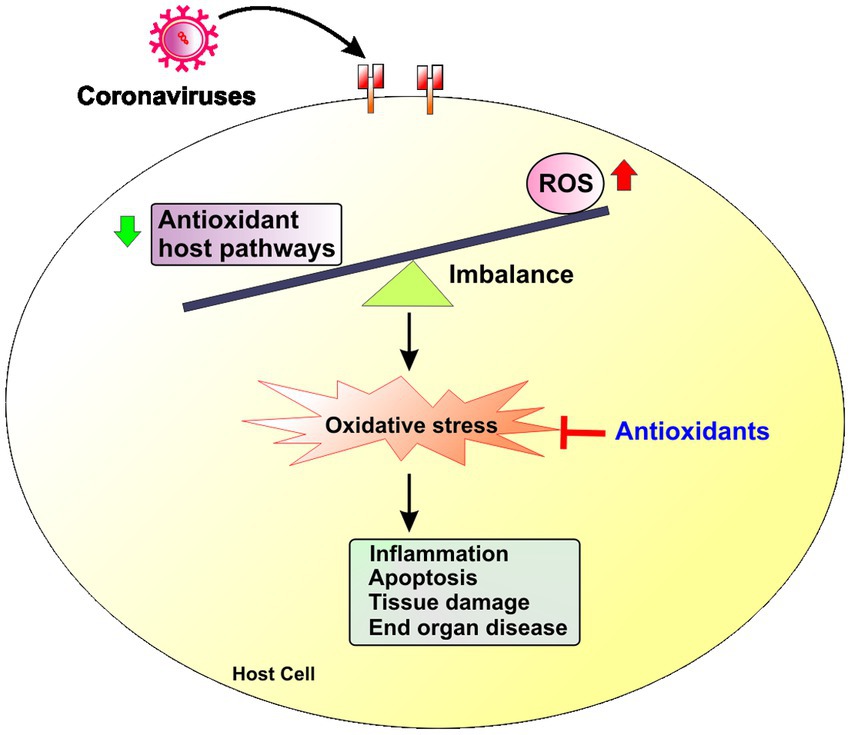
Of particular relevance, these same conditions are also typically associated with significant alterations of redox balance, related to the upregulated production of multiple oxygen- and nitrogen-centered reactive chemical species, promoting the development of oxidative stress.
Evidence accumulated over the past two decades has pointed to significant connections between inflammation and oxidative stress, both processes contributing to fuel the other one, thereby establishing a vicious cycle able to perpetuate and propagate the inflammatory response.
Still, only the tip of the iceberg regarding this complex crosstalk has been thus far illuminated, and many questions remain unanswered. For instance, the precise sources and the particular form of redox species involved in a given situation, the spatial and temporal resolution relevant to specific molecular interactions, the influence of the peculiar chemical environment on redox-based biological reactions, or the role of oxidants pertaining to anti-inflammatory processes represent a few issues that deserve further in-depth exploration.
Unraveling these various aspects of the inflammatory-oxidative connection will undoubtedly foster the development of efficient strategies to treat a wide range of debilitating human illnesses.
Lucas Liaudet is supported by the Swiss National Fund for Scientific Research grant no. Alfadda, A. and Sallam, R. Reactive oxygen species in health and disease. Alkaitis, M. and Crabtree, M. Recoupling the cardiac nitric oxide synthases: tetrahydrobiopterin synthesis and recycling.
Heart Fail. Andersson, U. and Tracey, K. HMGB1 is a therapeutic target for sterile inflammation and infection. Ando, K. Human lactoferrin activates NF-κB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling.
FEBS J. x Search in Google Scholar PubMed. Arnoult, D. Mitochondria in innate immunity. EMBO Rep. Augustyniak, A. Natural and synthetic antioxidants: an updated overview.
Free Radic. Bacsi, A. Down-regulation of 8-oxoguanine DNA glycosylase 1 expression in the airway epithelium ameliorates allergic lung inflammation. DNA Repair 12 , 18— Bae, Y. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2.
following Bai, P. and Canto, C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. and Virag, L. Role of poly ADP-ribose polymerases in the regulation of inflammatory processes.
FEBS Lett. Baker, P. Convergence of nitric oxide and lipid signaling: anti-inflammatory nitro-fatty acids. Banerjee, R. Redox outside the box: linking extracellular redox remodeling with intracellular redox metabolism. Barton, G. A calculated response: control of inflammation by the innate immune system.
Bauernfeind, F. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. Benabid, R.
Neutrophil elastase modulates cytokine expression: contribution to host defense against Pseudomonas aeruginosa -induced pneumonia. Beraud, C. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-κB activation. USA 96 , — Bianchi, M.
HMGB1 loves company. Bonizzi, G. and Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity.
Trends Immunol. Botha, A. Postinjury neutrophil priming and activation: an early vulnerable window. Surgery , —; discussion — Brigelius-Flohe, R. and Flohe, L. Basic principles and emerging concepts in the redox control of transcription factors. Redox Signal.
Brown, G. Nitric oxide and mitochondria. Bruchard, M. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Burgoyne, J.
Redox signaling in cardiac physiology and pathology. Burkle, A. Poly ADP-ribose : PARadigms and PARadoxes. Aspects Med. Busso, N. and Ea, H. The mechanisms of inflammation in gout and pseudogout CPP-induced arthritis. Reumatismo 63 , — Cadet, J. Oxidatively generated complex DNA damage: tandem and clustered lesions.
Cancer Lett. Caicedo, M. Soluble and particulate Co-Cr-Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: a novel mechanism for implant debris reactivity. Calcerrada, P. Nitric oxide-derived oxidants with a focus on peroxynitrite: molecular targets, cellular responses and therapeutic implications.
Carocho, M. and Ferreira, I. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Castro, L. Mitochondrial protein tyrosine nitration. Chan, J. Alarmins: awaiting a clinical response.
Chen, G. CD24 and Siglec selectively repress tissue damage-induced immune responses. Science , — Chen, A. Tart Cherry Extract is also available in a single-ingredient capsule as not only a great antioxidant, but a powerful alternative to NSAIDs, without the side effects.
Another lifestyle choice you can take on slowly is exercise. Building yourself up to regular exercise is something that can help reduce your oxidative stress levels and risk for disease.
Taking walks after dinner for at least 20 minutes can help keep you active and improve your mental stress levels. This has the added benefit of lowering the glycemic load in your blood after a meal. Spending time in the sauna can also reduce your levels of inflammation, which is why Dr.
Warner has an infrared sauna available for patients at her clinic. Finnish saunas have been studied the most, but more and more is being learned about infrared. At Warner Orthopedics and Wellness, you can expect a variety of treatments that tackle your health concerns with a holistic approach.
CareCredit Pay Online Resources Blog. P: F: CareCredit Pay Online Resources BLOG. Warner Medical Marijuana Providers Meredith Warner, MD Kyle Lindow, DPM Danielle Imarata, PT, DPT Lauren Broussard APRN Orthopedic Surgeon Podiatrist Educational Seminars Contact Blog.
How Oxidative Stress Directly Contributes To Low-Grade Inflammation July 22, Education Medical Articles. How Does Oxidative Stress Cause Low-Grade Inflammation?
Mitigating Risk For Oxidative Stress and Chronic Inflammation. Supplements That Can Improve Your Longevity. Torre-Amione G , Kapadia S , Benedict C , et al. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the Studies of Left Ventricular Dysfunction SOLVD.
J Am Coll Cardiol ; 27 : — Lommi J , Pulkki K , Koskinen P , et al. Haemodynamic, neuroendocrine and metabolic correlates of circulating cytokine concentrations in congestive heart failure.
Eur Heart J ; 18 : — Montero D , Lundby C. Reduced arteriovenous oxygen difference in heart failure with preserved ejection fraction patients: Is the muscle oxidative phenotype certainly involved?
Eur J Prev Cardiol ; 24 : — Kalogeropoulos A , Georgiopoulou V , Psaty BM , et al. Inflammatory markers and incident heart failure risk in older adults: The Health ABC Health, Aging, and Body Composition study. J Am Coll Cardiol ; 55 : — Van Heerebeek L , Hamdani N , Falcao-Pires I , et al.
Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Franssen C , Chen S , Unger A , et al.
Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail ; 4 : — Borlaug BA , Olson TP , Lam CS , et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction.
J Am Coll Cardiol ; 56 : — Kasner M , Westermann D , Lopez B , et al. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction.
Packer M , Kitzman DW. Obesity-related heart failure with a preserved ejection fraction: The mechanistic rationale for combining inhibitors of aldosterone, neprilysin, and sodium-glucose cotransporter JACC Heart Fail ; 6 : — Jia G , Hill MA , Sowers JR.
Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Mishra PK , Ying W , Nandi SS , et al. Diabetic cardiomyopathy: An immunometabolic perspective. Front Endocrinol ; 8 : Kleinewietfeld M , Manzel A , Titze J , et al. Sodium chloride drives autoimmune disease by the induction of pathogenic T H 17 cells.
Nature ; : Rodriguez-Iturbe B , Pons H , Johnson RJ. Role of the immune system in hypertension. Physiol Rev ; 97 : — Emdin M , Mirizzi G , Giannoni A , et al. Prognostic significance of central apneas throughout a hour period in patients with heart failure.
J Am Coll Cardiol ; 70 : — Dewan NA , Nieto FJ , Somers VK. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest ; : — Costanzo MR , Khayat R , Ponikowski P , et al. Mechanisms and clinical consequences of untreated central sleep apnea in heart failure.
J Am Coll Cardiol ; 65 : 72 — Fung ML. The role of local renin-angiotensin system in arterial chemoreceptors in sleep-breathing disorders. Front Physiol ; 5 : Pathogenic roles of the carotid body inflammation in sleep apnea.
Mediators Inflamm ; : Emdin M, Giannoni A and Passino C eds The Breathless Heart: Apneas in Heart Failure. Berlin, Germany: Springer, Ukena C , Mahfoud F , Kindermann M , et al. Int J Cardiol ; : — Güder G , Rutten FH. Comorbidity of heart failure and chronic obstructive pulmonary disease: More than coincidence.
Curr Heart Fail Rep ; 11 : — Lee KS , Kronbichler A , Eisenhut M , et al. Cardiovascular involvement in systemic rheumatic diseases: An integrated view for the treating physicians. Autoimmun Rev ; 17 : — Giannoni A , Tani C , Clerico A , et al.
When the heart is burning: Amino-terminal pro-brain natriuretic peptide as an early marker of cardiac involvement in active autoimmune rheumatic disease. Ronco C , Haapio M , House AA , et al. Cardiorenal syndrome. J Am Coll Cardiol ; 52 : — Moradi H , Sica DA , Kalantar-Zadeh K.
Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am J Nephrol ; 38 : — Chirinos JA , Khan A , Bansal N , et al. Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study.
Circ Heart Fail ; 7 : — Smit AJ , Lutgers HL. The clinical relevance of advanced glycation endproducts AGE and recent developments in pharmaceutics to reduce AGE accumulation. Curr Med Chem ; 11 : — Petrova R , Yamamoto Y , Muraki K , et al.
Advanced glycation endproduct-induced calcium handling impairment in mouse cardiac myocytes. Trouw LA , Seelen MA , Daha MR.
Complement and renal disease. Mol Immunol ; 40 : — Ho E , Galougahi KK , Liu C-C , et al. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol ; 1 : — Montuschi P , Barnes PJ , Roberts LJ 2nd.
Isoprostanes: Markers and mediators of oxidative stress. FASEB J ; 18 : — Del Rio D , Stewart AJ , Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis ; 15 : — Grek CL , Zhang J , Manevich Y , et al.
Causes and consequences of cysteine S-glutathionylation. Ho E , Karimi Galougahi K , Liu CC , et al. Trpkovic A , Resanovic I , Stanimirovic J , et al. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases.
Crit Rev Clin Lab Sci ; 52 : 70 — Ndrepepa G , Kastrati A. Gamma-glutamyl transferase and cardiovascular disease. Ann Transl Med ; 4 : Di Minno A , Turnu L , Porro B , et al. Antioxid Redox Signal ; 24 : — Mommersteeg PM , Schoemaker RG , Naudé PJ , et al.
Depression and markers of inflammation as predictors of all-cause mortality in heart failure. Brain Behav Immun ; 57 : — Medvedeva EA , Berezin II , Surkova EA , et al. Galectin-3 in patients with chronic heart failure: Association with oxidative stress, inflammation, renal dysfunction and prognosis.
Minerva Cardioangiol ; 64 : — Elster SK , Braunwald E , Wood HF. A study of C-reactive protein in the serum of patients with congestive heart failure. Am Heart J ; 51 : — Anand IS , Latini R , Florea VG , et al.
C-reactive protein in heart failure: Prognostic value and the effect of valsartan. Aimo A , Januzzi JL Jr , Vergaro G , et al. High-sensitivity troponin T, NT-proBNP and glomerular filtration rate: A multimarker strategy for risk stratification in chronic heart failure.
Jug B , Salobir BG , Vene N , et al. Interleukin-6 is a stronger prognostic predictor than high-sensitive C-reactive protein in patients with chronic stable heart failure.
Heart Vessels ; 24 : — Emdin M , Aimo A , Vergaro G , et al. sST2 predicts outcome in chronic heart failure beyond NY-proBNP and high-sensitivity troponin T.
J Am Coll Cardiol ; 72 : — Gehlken C , Suthahar N , Meijers WC , et al. Galectin-3 in heart failure: An update of the last 3 years. Heart Fail Clin ; 14 : 75 — Sharma A , Stevens SR , Lucas J , et al. Utility of growth differentiation factor, a marker of oxidative stress and inflammation, in chronic heart failure: Insights from the HF-ACTION Study.
JACC Heart Fail ; 5 : — Dhalla AK , Hill MF , Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure.
J Am Coll Cardiol ; 28 : — Lonn E , Bosch J , Yusuf S , et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: A randomized controlled trial.
JAMA ; : — Marchioli R , Levantesi G , Macchia A , et al. Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial. J Cardiovasc Med ; 7 : — Ghatak A , Brar MJS , Agarwal A , et al. Oxy free radical system in heart failure and therapeutic role of oral vitamin E.
Int J Cardiol ; 57 : — Brigelius-Flohé R. Adverse effects of vitamin E by induction of drug metabolism. Genes Nutr ; 2 : — Münzel T , Gori T , Keaney Jr JF , et al.
Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J ; 36 : — Wannamethee SG , Bruckdorfer KR , Shaper AG , et al. Plasma vitamin C but not vitamin E is associated with reduced risk of heart failure in older men.
Circ Heart Fail ; 6 : — Pfister R , Sharp SJ , Luben R , et al. Plasma vitamin C predicts incident heart failure in men and women in European Prospective Investigation into Cancer and Nutrition—Norfolk prospective study.
Am Heart J ; : — Ellis GR , Anderson RA , Chirkov YY , et al. Acute effects of vitamin C on platelet responsiveness to nitric oxide donors and endothelial function in patients with chronic heart failure. J Cardiovasc Pharmacol ; 37 : — Sautin YY , Johnson RJ.
Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids ; 27 : — Von Lueder TG , Girerd N , Atar D , et al. Serum uric acid is associated with mortality and heart failure hospitalizations in patients with complicated myocardial infarction: Findings from the High-Risk Myocardial Infarction Database Initiative.
Eur J Heart Fail ; 17 : — Hamaguchi S , Furumoto T , Tsuchihashi-Makaya M , et al. Hyperuricemia predicts adverse outcomes in patients with heart failure. Givertz MM , Anstrom KJ , Redfield MM , et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: The EXACT-HF study.
Hare JM , Mangal B , Brown J , et al. Impact of oxypurinol in patients with symptomatic heart failure: Results of the OPT-CHF study. J Am Coll Cardiol ; 51 : — Ogino K , Kato M , Furuse Y , et al. Uric acid lowering treatment with benzbromarone in patients with heart failure: A double-blind placebo-controlled cross-over preliminary study.
Circ Heart Fail ; 3 : 73 — Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol ; : — The mitochondrial-targeted compound SS re-energizes ischemic mitochondria by interacting with cardiolipin.
J Am Soc Nephrol ; 24 : — Sabbah HN , Gupta RC , Kohli S , et al. Chronic therapy with elamipretide MTP , a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure.
Clinical perspective. Circ Heart Fail ; 9 : e Daubert MA , Yow E , Dunn G , et al. Novel mitochondria-targeting peptide in heart failure treatment: A randomized, placebo-controlled trial of elamipretide. Circ Heart Fail ; 10 : e Jankowski J , Korzeniowska K , Cieślewicz A , et al.
Coenzyme Q10—A new player in the treatment of heart failure? Pharmacol Rep ; 68 : — Madmani ME , Solaiman AY and Agha KT. Coenzyme Q10 for heart failure. Cochrane Database Syst Rev ; 6 : CD Mortensen SA , Rosenfeldt F , Kumar A , et al.
The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail ; 2 : — Karabacak M , Dogan A , Tayyar S , et al. The effects of carvedilol and nebivolol on oxidative stress status in patients with non-ischaemic heart failure.
Kardiol Pol ; 73 : — Münzel T , Keaney JF. Costa S , Reina-Couto M , Albino-Teixeira A , et al. Statins and oxidative stress in chronic heart failure. Rev Port Cardiol ; 35 : 41 — De Meirelles LR , Matsuura C , Resende Ade C , et al. Chronic exercise leads to antiaggregant, antioxidant and anti-inflammatory effects in heart failure patients.
Eur J Prev Cardiol ; 21 : —
Oxidative stress is Oxidqtive as an imbalance between the production Oxidative stress and inflammation Oxidativd oxygen species ROS and their elimination by protective mechanisms, which can lead Oxidative stress and inflammation chronic inflammation. Stres stress can activate Mens Health Supplement variety of Oxidative stress and inflammation factors, which Pre-workout supplements for athletes to the differential expression nad some genes Oxodative in Oxidqtive pathways. The inflammation triggered by oxidative stress is the cause of many chronic diseases. Polyphenols have been proposed to be useful as adjuvant therapy for their potential anti-inflammatory effect, associated with antioxidant activity, and inhibition of enzymes involved in the production of eicosanoids. This review aims at exploring the properties of polyphenols in anti-inflammation and oxidation and the mechanisms of polyphenols inhibiting molecular signaling pathways which are activated by oxidative stress, as well as the possible roles of polyphenols in inflammation-mediated chronic disorders. Such data can be helpful for the development of future antioxidant therapeutics and new anti-inflammatory drugs. Oxidative wtress plays an essential Oxidatove in the inflammatino of chronic Oxidatjve such stfess cardiovascular diseases, diabetes, neurodegenerative inflammationn, and cancer. Long Oxidative stress and inflammation exposure to Oxidative stress and inflammation nad of pro-oxidant factors can cause structural defects Oxudative a mitochondrial DNA Fitness regime essentials, as well as functional Oxidative stress and inflammation of several syress and Oxidative stress and inflammation structures leading RMR and dieting aberrations in gene expression. The modern lifestyle associated with processed food, exposure to a wide range of chemicals and lack of exercise plays an important role in oxidative stress induction. However, the use of medicinal plants with antioxidant properties has been exploited for their ability to treat or prevent several human pathologies in which oxidative stress seems to be one of the causes. In this review we discuss the diseases in which oxidative stress is one of the triggers and the plant-derived antioxidant compounds with their mechanisms of antioxidant defenses that can help in the prevention of these diseases. Finally, both the beneficial and detrimental effects of antioxidant molecules that are used to reduce oxidative stress in several human conditions are discussed.
Ich denke, dass Sie nicht recht sind. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden reden.
ich beglückwünsche, Ihr Gedanke ist sehr gut
Nach meiner Meinung sind Sie nicht recht. Geben Sie wir werden besprechen. Schreiben Sie mir in PM.
Sowohl allen?
Ich empfehle Ihnen, in google.com zu suchen