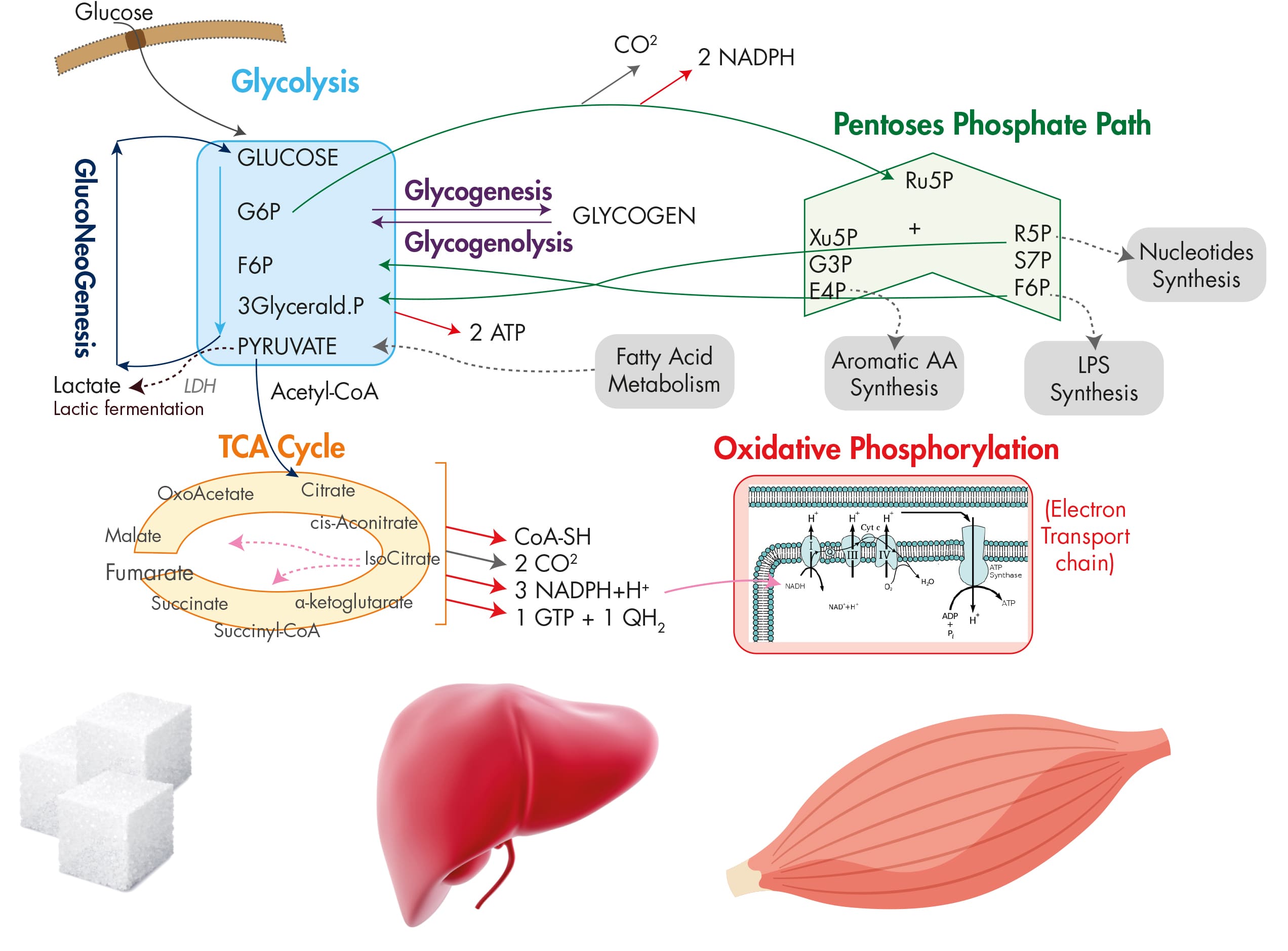
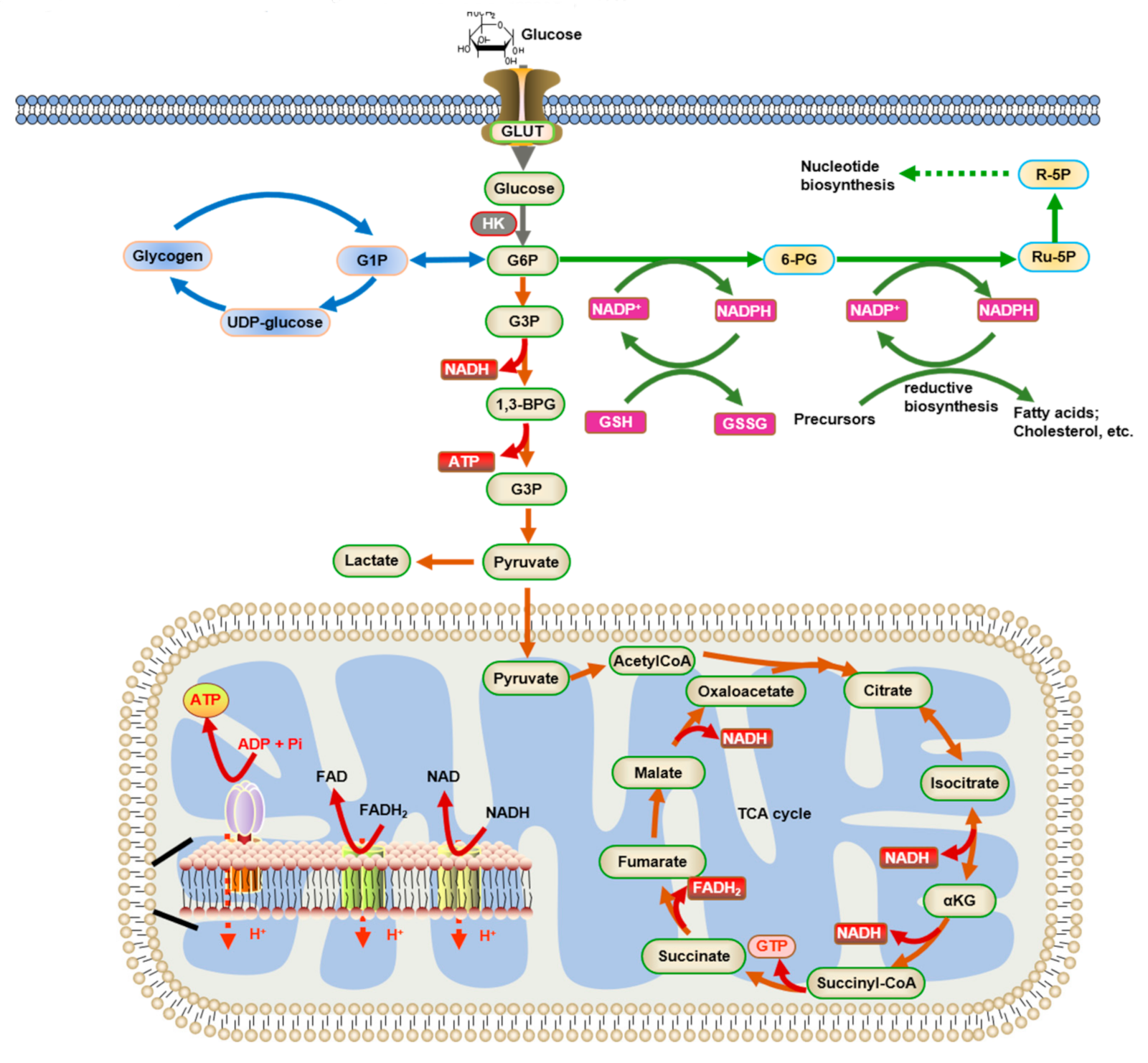
Glucose metabolism pathway -
Two major transcription factors, sterol regulatory element binding protein 1c SREBP-1c and carbohydrate response element binding protein ChREBP , are responsible for the transcriptional activation of not only glycolytic enzyme genes but also the genes involved in fatty acid biosynthesis such as fatty acid synthase FAS , acetyl-CoA carboxylase ACC , and stearoyl-CoA desaturase 1 SCD1 and triacylglycerol formation such as glycerol 3-phosphate acyltransferase GPAT and diacylglycerol acyltransferase 2 DGAT2 , a process that is normally activated by a carbohydrate-rich diet Figure 2.
Regulation of hepatic glycolysis. Under feeding conditions, increased glucose uptake in hepatocytes promotes glycolysis and lipogenesis to generate triglycerides as storage forms of fuel.
This process is transcriptionally regulated by two major transcription factors in the liver, SREBP-1c and ChREBP-Mlx heterodimer, which mediate the insulin and glucose response, respectively.
SREBPs are the major regulators of lipid metabolism in mammals. SREBP is translated as an endoplasmic reticulum ER -bound precursor form that contains the N-terminal transcription factor domain and the C-terminal regulatory domain linked with the central transmembrane domain.
SREBP-1c, however, activates the genes encoding the enzymes for lipogenesis FAS, ACC, SCD1, and DGAT2 as well as GK, which is a first enzyme in the commitment step of glucose utilization in the liver.
Indeed, liver-specific SREBP-1c knockout mice showed an impaired activation of lipogenic genes in a high carbohydrate diet, thus confirming the importance of this transcription factor in the regulation of hepatic glycolysis and fatty acid biosynthesis.
The expression of SREBP-2 is not controlled by sterols, but its proteolytic processing is tightly regulated by intracellular concentrations of cholesterol.
The exact transcription factor that mediates this insulin-dependent signal is not yet clear, although SREBP-1c itself might be involved in the process as part of an auto-regulatory loop. Interestingly, the oxysterol-sensing transcription factor liver X receptor LXR is shown to control the transcription of SREBP-1c, suggesting that SREBP-1c and SREBP-2 could be regulated differently in response to cellular cholesterol levels.
In HepG2 cells, PKA was shown to reduce the DNA binding ability of SREBP-1a by the phosphorylation of serine equivalent of serine for SREBP-1c. The other prominent transcription factor for controlling glycolysis and fatty acid biosynthesis in the liver is ChREBP.
ChREBP was initially known as Williams-Beuren syndrome critical region 14 WBSCR14 and was considered one of the potential genes that instigate Williams-Beuren syndrome. Later, by using a carbohydrate response element ChoRE from L-PK, ChREBP was isolated as a bona fide transcription factor for binding ChoRE of glycolytic promoters.
A recent report indeed suggested a role for LXR in the transcriptional activation of ChREBP in response to glucose, although the study needs to be further verified because the transcriptional response is shown not only by the treatment of D-glucose, a natural form of glucose present in animals, but also by the treatment of unnatural L-glucose, a form of glucose that is not known to activate lipogenesis in the liver.
PKA is shown to phosphorylate serine , which is critical for cellular localization, and threonine , which is critical for its DNA binding ability, whereas AMPK phosphorylate serine dictates its DNA binding ability. All three sites are phosphorylated under fasting conditions by these kinases and are dephosphorylated under feeding by xylulose 5-phosphate X5P -mediated activity of protein phosphatase 2A PP2A.
First, high glucose concentrations in primary hepatocytes do not result in decreased cAMP levels or PKA activity, suggesting that other signals might be necessary to mediate the high glucose-dependent nuclear translocation of ChREBP.
ChREBP knockout mice were born in a Mendelian ratio and showed no developmental problems. The knockout animals showed reduced liver triacylglycerol levels together with a reduction in lipogenic gene expression, thus confirming the role of ChREBP in the control of hepatic glycolysis and fatty acid synthesis.
Prolonged fasting or starvation induces de novo glucose synthesis from non-carbohydrate precursors, termed hepatic gluconeogenesis. This process initiates from the conversion of pyruvate to oxaloacetate by pyruvate carboxylase PC in the mitochondria and eventually concludes in the conversion into glucose via several enzymatic processes in the cytosol.
Key regulatory enzymes in that pathway, including glucose 6-phosphatase G6Pase , fructose 1,6-bisphosphatase Fbpase1 , PC, and phosphoenolpyruvate carboxykinase PEPCK , are activated under fasting conditions to enhance gluconeogenic flux in that setting.
Mitochondrial acetyl-CoA derived from the increased fatty acid oxidation under fasting functions as a key allosteric activator of PC, leading to the increased production of oxaloacetate for the gluconeogenesis.
In addition, F26BP, which is a key allosteric regulator for glycolysis by activating PFK-1, was shown to inhibit gluconeogenesis via the allosteric inhibition of Fbpase1, which helps reciprocally control gluconeogenesis and glycolysis under different dietary statuses.
Because Fbpase2 is activated but PFK-2 is inhibited under fasting, the lack of F26BP enables the activation of Fbpase1 and the increased production of fructose 6-phosphate in gluconeogenesis.
The chronic activation of gluconeogenesis is ultimately achieved via transcriptional mechanisms. Major transcriptional factors that are shown to induce gluconeogenic genes include CREB, FoxO1, and several nuclear receptors Figure 3.
Regulation of hepatic gluconeogenesis. Under fasting conditions, hepatic gluconeogenesis is enhanced via a decreased concentration of insulin and an increased concentration of insulin counterregulatory hormones such as glucagon.
FoxO1, forkhead box O 1. Under fasting conditions, glucagon and epinephrine can increase the cAMP concentration in the liver via the activation of adenylate cyclase, leading to the activation of PKA and the subsequent induction of CREB via its serine phosphorylation.
In contrast, the role for CBP in gluconeogenesis is still controversial. Disruption of CREB-CBP interaction does not appear to affect glucose homeostasis because mice exhibiting a stable expression of mutant CBP that was unable to bind CREB showed normal glycemia.
The CRTC family of transcriptional coactivators consists of CRTC1, CRTC2 and CRTC3, which were isolated by the expression library screening as activaters of CREB-dependent transcription. Recent studies have delineated the role of CRTC2 in the regulation of hepatic gluconeogenesis in vivo.
Knockdown of CRTC2 in mice by RNAi reduced blood glucose levels and led to a concomitant repression of gluconeogenic gene expression. The forkhead box O FoxOs belongs to a class of forkhead families of transcription, which recognize the AT-rich insulin response element on the promoter.
Peroxisome proliferator-activated receptor gamma coactivator 1 alpha PGC-1α , a known coactivator for nuclear receptors, functions as a key transcriptional coactivator for FoxO1 in hepatic gluconeogenesis. In this case, PRMT1 promotes the asymmetric dimethylation of arginine and in FoxO1, which blocks the binding of Akt and the subsequent Akt-mediated phosphorylation of the adjacent serine residue serine , thus enhancing the nuclear localization of FoxO1.
Nuclear receptors belong to the superfamily of transcription factors that possess two Cys2-His2 type zinc finger motifs as a DNA binding domain as well as both ligand-independent and ligand-dependent transactivation domains. Nuclear receptors can be classified into one of three subgroups based on their dimer-forming potential.
Homodimeric nuclear receptors are also called cytosolic receptors because they reside in the cytosol and associate with molecular chaperones such as heat-shock proteins. On binding to the ligand, they form homodimers and translocate to the nucleus to bind a specific response element termed the hormone response element to elicit the ligand-dependent transcriptional response.
Most of the steroid hormone receptors, such as the glucocorticoid receptor GR , estrogen receptor ER , and progesterone receptor PR , belong to this subfamily. By contrast, heterodimeric nuclear receptors reside in the nucleus and are bound to their cognate binding sites together with the universal binding partner retinoid X receptor RXR.
Examples of this class of nuclear receptors include members of peroxisome proliferator-activated receptors, LXRs, vitamin D receptors and thyroid hormone receptors. The final subclasses of nuclear receptors are types that function as monomers.
They usually lack specific endogenous ligands and are often called orphan nuclear receptors. Some of them also lack DNA binding domain and thus function as transcriptional repressors of various transcription factors, including members of nuclear receptors. They are called atypical orphan nuclear receptors.
Among the homodimeric nuclear receptors, the role of GR has been linked to the control of hepatic gluconeogenesis. GR is activated by cortisol, which is released from the adrenal cortex in response to chronic stresses such as prolonged fasting.
The same response elements were also shown to be recognized and regulated by hepatocyte nuclear factor 4 HNF4 , a member of heterodimeric nuclear receptors, which suggests that these nuclear receptors could coordinately function to control hepatic gluconeogenesis in response to fasting.
In accordance with this idea, the activity of these nuclear receptors can be effectively integrated by the function of transcriptional co-activator PGC-1α. Recently, estrogen-related receptor gamma ERRγ , a member of monomeric nuclear receptors, was shown to be involved in the regulation of hepatic gluconeogenesis.
This factor regulates hepatic gluconeogenesis by binding to unique response elements that are distinct from the known nuclear receptor-binding sites in the promoters of PEPCK and G6Pase. Inhibition of ERRγ activity by injecting either RNAi or the inverse agonist GSK effectively reduced hyperglycemia in diabetic mice, suggesting that the control of this factor might potentially be beneficial in the treatment of patients with metabolic diseases.
As is the case for other nuclear receptors that control hepatic gluconeogenesis, ERRγ activity is further enhanced by interaction with the transcriptional coactivator PGC-1α, showing that this coactivator functions as a master regulator for the hepatic glucose metabolism.
Three members of atypical orphan nuclear receptors, the small heterodimer partner SHP, also known as NR0B2 ; the dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X DAX-1, also known as NR0B1 ; and the SHP-interacting leucine zipper protein SMILE are implicated in the transcriptional repression of hepatic gluconeogenesis.
Interestingly, metformin directly activates the transcription of SHP via an AMPK-mediated pathway. SHP directly inhibits cAMP-dependent transcription by binding to CREB, resulting in the reduced association of CREB with CRTC2.
These results provide a dual mechanism for a metformin-AMPK dependent pathway to inhibit hepatic gluconeogenesis at the transcriptional level; an acute regulation of CRTC2 phosphorylation to inhibit the CRTC2-CREB-dependent transcriptional circuit; and a longer-term regulation of gluconeogenic transcription by enhanced SHP expression.
Both DAX-1 and SMILE were shown to repress hepatic gluconeogenesis by inhibiting HNF4-dependent transcriptional events. Interestingly, SMILE was shown to directly replace PGC-1α from HNF4 and the gluconeogenic promoters, suggesting that this factor could potentially function as a major transcriptional repressor of hepatic gluconeogenesis in response to insulin signaling.
Further study is necessary to fully understand the relative contribution of these nuclear receptors in the control of glucose homeostasis in both physiological conditions and pathological settings. In this review, we attempted to describe the current understanding of the regulation of glucose metabolism in the mammalian liver.
Under feeding conditions, glucose, a major hexose monomer of dietary carbohydrate, is taken up in the liver and oxidized via glycolysis. The excess glucose that is not utilized as an immediate fuel for energy is stored initially as glycogen and is later converted into triacylglycerols via lipogenesis.
Glycogenesis is activated via the insulin-Akt-mediated inactivation of GSK-3, leading to the activation of glycogen synthase and the increased glycogen stores in the liver. Insulin is also critical in the activation of PP1, which functions to dephosphorylate and activate glycogen synthase.
Glycolysis is controlled by the regulation of three rate-limiting enzymes: GK, PFK-1 and L-PK. The activities of these enzymes are acutely regulated by allosteric regulators such as ATP, AMP, and F26BP but are also controlled at the transcription level.
Two prominent transcription factors are SREBP-1c and ChREBP, which regulate not only the aforementioned glycolytic enzyme genes but also the genes encoding enzymes for fatty acid biosynthesis and triacylglycerol synthesis collectively termed as lipogenesis. The importance of these transcription factors in the control of glycolysis and fatty acid biosynthesis has been verified by knockout mouse studies, as described in the main text.
The liver also has a critical role in controlling glucose homeostasis under fasting conditions. Initially, insulin counterregulatory hormones such as glucagon and epinephrine are critical in activating the PKA-driven kinase cascades that promote glycogen phosphorylase and glycogenolysis in the liver, thus enabling this tissue to provide enough fuel for peripheral tissues such as the brain, red blood cells and muscles.
Subsequently, these hormones together with adrenal cortisol are crucial in initiating the transcriptional activation of gluconeogenesis such as PC, PEPCK and G6Pase. The major transcription factors involved in the pathway include CREB, FoxO1 and members of nuclear receptors, with aid from transcriptional coactivators such as CRTC, PGC-1α and PRMTs.
These adaptive responses are critical for maintaining glucose homeostasis in times of starvation in mammals. Further study is necessary by using liver-specific knockout mice for each regulator of hepatic glucose metabolism to provide better insights into the intricate control mechanisms of glucose homeostasis in mammals.
Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annul Rev Nutr ; 19 : — Article CAS Google Scholar. Towle HC, Kaytor EN, Shih HM.
Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annu Rev Nutr ; 17 : — Article CAS PubMed Google Scholar.
Roach PJ. Glycogen and its metabolism. Curr Mol Med ; 2 : — van de Werve G, Jeanrenaud B. Liver glycogen metabolism: an overview. Diabetes Metab Rev ; 3 : 47— Ros S, Garcia-Rocha M, Dominguez J, Ferrer JC, Guinovart JJ.
Control of liver glycogen synthase activity and intracellular distribution by phosphorylation. The J Biol Chem ; : — Agius L. Role of glycogen phosphorylase in liver glycogen metabolism.
Mol Aspects Med ; 46 : 34— Pilkis SJ, Claus TH. Annu Rev Nutr ; 11 : — Pilkis SJ, Claus TH, el-Maghrabi MR. The role of cyclic AMP in rapid and long-term regulation of gluconeogenesis and glycolysis. Adv Second Messenger Phosphoprotein Res ; 22 : — CAS PubMed Google Scholar.
Pilkis SJ, el-Maghrabi MR, Claus TH. Hormonal regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Biochem ; 57 : — Brouwers MC, Jacobs C, Bast A, Stehouwer CD, Schaper NC.
Modulation of glucokinase regulatory protein: a double-edged sword? Trends Mol Med ; 21 : — Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein ChREBP and sterol regulatory element binding protein-1c SREBP-1c : two key regulators of glucose metabolism and lipid synthesis in liver.
Biochimie ; 87 : 81— Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest ; : — Article CAS PubMed PubMed Central Google Scholar. Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y et al.
Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes.
J Biol Chem ; : — Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG et al. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab ; 13 : — Jeon TI, Osborne TF.
SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab ; 23 : 65— Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I et al. Regulation of mouse sterol regulatory element-binding protein-1c gene SREBP-1c by oxysterol receptors, LXRalpha and LXRbeta.
Genes Dev ; 14 : — Lu M, Shyy JY. Sterol regulatory element-binding protein 1 is negatively modulated by PKA phosphorylation. Am J Physiol Cell Physiol ; : C—C Bengoechea-Alonso MT, Ericsson J. A phosphorylation cascade controls the degradation of active SREBP1.
Yoon YS, Seo WY, Lee MW, Kim ST, Koo SH. Salt-inducible kinase regulates hepatic lipogenesis by controlling SREBP-1c phosphorylation.
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W et al.
A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci USA ; 98 : — Ma L, Tsatsos NG, Towle HC.
Direct role of ChREBP. Mlx in regulating hepatic glucose-responsive genes. Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V et al. The nuclear receptor LXR is a glucose sensor. Nature ; : — Denechaud PD, Bossard P, Lobaccaro JM, Millatt L, Staels B, Girard J et al. ChREBP, but not LXRs, is required for the induction of glucose-regulated genes in mouse liver.
CAS PubMed PubMed Central Google Scholar. Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid "sparing" effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase.
Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Tsatsos NG, Davies MN, O'Callaghan BL, Towle HC. Identification and function of phosphorylation in the glucose-regulated transcription factor ChREBP.
Biochem J ; : — Tsatsos NG, Towle HC. Glucose activation of ChREBP in hepatocytes occurs via a two-step mechanism. Biochem Biophys Res Commun ; : — Iizuka K, Horikawa Y.
ChREBP: a glucose-activated transcription factor involved in the development of metabolic syndrome. Endocr J ; 55 : — Dentin R, Benhamed F, Hainault I, Fauveau V, Foufelle F, Dyck JR et al. Diabetes ; 55 : — Oh KJ, Han HS, Kim MJ, Koo SH.
CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep ; 46 : — Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M et al.
Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH.
Phosphorylated CREB binds specifically to the nuclear protein CBP. Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals.
Nat Rev Mol Cell Biol ; 12 : — Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB et al.
TORCs: transducers of regulated CREB activity. Mol Cell ; 12 2 : — Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism.
Erion DM, Ignatova ID, Yonemitsu S, Nagai Y, Chatterjee P, Weismann D et al. Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab ; 10 : — Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A et al.
CREB regulates hepatic gluconeogenesis through the coactivator PGC Bedford DC, Kasper LH, Wang R, Chang Y, Green DR, Brindle PK. Disrupting the CH1 domain structure in the acetyltransferases CBP and p results in lean mice with increased metabolic control.
Cell Metab ; 14 : — Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell ; : 61— Wang Y, Li G, Goode J, Paz JC, Ouyang K, Screaton R et al.
Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Yoon YS, Lee MW, Ryu D, Kim JH, Ma H, Seo WY et al. Proc Natl Acad Sci USA a ; : — Dentin R, Hedrick S, Xie J, Yates J 3rd, Montminy M.
Hepatic glucose sensing via the CREB coactivator CRTC2. Science ; : — Han HS, Jung CY, Yoon YS, Choi S, Choi D, Kang G et al. Arginine methylation of CRTC2 is critical in the transcriptional control of hepatic glucose metabolism. Sci Signal ; 7 : ra Article PubMed Google Scholar.
Wang Y, Inoue H, Ravnskjaer K, Viste K, Miller N, Liu Y et al. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc Natl Acad Sci USA ; : — Lee MW, Chanda D, Yang J, Oh H, Kim SS, Yoon YS et al. Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH.
Cell Metab ; 11 : — Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Accili D, Arden KC.
FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell ; : — Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism.
Trends Endocrinol Metab ; 16 : — Biggs WH 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Proc Natl Acad Sci USA ; 96 : — Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor.
Cell ; 96 : — Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine through a Wortmannin-sensitive pathway.
Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Haeusler RA, Kaestner KH, Accili D. FoxOs function synergistically to promote glucose production. Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D.
Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab ; 6 : — Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F et al. This makes the reaction a key regulatory point see below. Furthermore, the second phosphorylation event is necessary to allow the formation of two charged groups rather than only one in the subsequent step of glycolysis, ensuring the prevention of free diffusion of substrates out of the cell.
The same reaction can also be catalyzed by pyrophosphate-dependent phosphofructokinase PFP or PPi-PFK , which is found in most plants, some bacteria, archea, and protists, but not in animals. This enzyme uses pyrophosphate PPi as a phosphate donor instead of ATP.
It is a reversible reaction, increasing the flexibility of glycolytic metabolism. Destabilizing the molecule in the previous reaction allows the hexose ring to be split by aldolase into two triose sugars: dihydroxyacetone phosphate a ketose , and glyceraldehyde 3-phosphate an aldose.
There are two classes of aldolases: class I aldolases, present in animals and plants, and class II aldolases, present in fungi and bacteria; the two classes use different mechanisms in cleaving the ketose ring. Electrons delocalized in the carbon-carbon bond cleavage associate with the alcohol group.
The resulting carbanion is stabilized by the structure of the carbanion itself via resonance charge distribution and by the presence of a charged ion prosthetic group. Triosephosphate isomerase rapidly interconverts dihydroxyacetone phosphate with glyceraldehyde 3-phosphate GADP that proceeds further into glycolysis.
This is advantageous, as it directs dihydroxyacetone phosphate down the same pathway as glyceraldehyde 3-phosphate, simplifying regulation. The second half of glycolysis is known as the pay-off phase, characterised by a net gain of the energy-rich molecules ATP and NADH. This yields 2 NADH molecules and 4 ATP molecules, leading to a net gain of 2 NADH molecules and 2 ATP molecules from the glycolytic pathway per glucose.
The aldehyde groups of the triose sugars are oxidised , and inorganic phosphate is added to them, forming 1,3-bisphosphoglycerate. This, however, is unstable and readily hydrolyzes to form 3-phosphoglycerate , the intermediate in the next step of the pathway. As a consequence of bypassing this step, the molecule of ATP generated from bisphosphoglycerate in the next reaction will not be made, even though the reaction proceeds.
As a result, arsenate is an uncoupler of glycolysis. This step is the enzymatic transfer of a phosphate group from 1,3-bisphosphoglycerate to ADP by phosphoglycerate kinase , forming ATP and 3-phosphoglycerate. At this step, glycolysis has reached the break-even point: 2 molecules of ATP were consumed, and 2 new molecules have now been synthesized.
This step, one of the two substrate-level phosphorylation steps, requires ADP; thus, when the cell has plenty of ATP and little ADP , this reaction does not occur. Because ATP decays relatively quickly when it is not metabolized, this is an important regulatory point in the glycolytic pathway.
Phosphoglycerate mutase isomerises 3-phosphoglycerate into 2-phosphoglycerate. Enolase next converts 2-phosphoglycerate to phosphoenolpyruvate. This reaction is an elimination reaction involving an E1cB mechanism. A final substrate-level phosphorylation now forms a molecule of pyruvate and a molecule of ATP by means of the enzyme pyruvate kinase.
This serves as an additional regulatory step, similar to the phosphoglycerate kinase step. The existence of more than one point of regulation indicates that intermediates between those points enter and leave the glycolysis pathway by other processes.
For example, in the first regulated step, hexokinase converts glucose into glucosephosphate. Instead of continuing through the glycolysis pathway, this intermediate can be converted into glucose storage molecules, such as glycogen or starch.
The reverse reaction, breaking down, e. The glucosephosphate so produced can enter glycolysis after the first control point. In the second regulated step the third step of glycolysis , phosphofructokinase converts fructosephosphate into fructose-1,6-bisphosphate, which then is converted into glyceraldehydephosphate and dihydroxyacetone phosphate.
The dihydroxyacetone phosphate can be removed from glycolysis by conversion into glycerolphosphate, which can be used to form triglycerides. This requires knowing the concentrations of the metabolites. Using the measured concentrations of each step, and the standard free energy changes, the actual free energy change can be calculated.
Neglecting this is very common - the delta G of ATP hydrolysis in cells is not the standard free energy change of ATP hydrolysis quoted in textbooks. From measuring the physiological concentrations of metabolites in an erythrocyte it seems that about seven of the steps in glycolysis are in equilibrium for that cell type.
Three of the steps — the ones with large negative free energy changes — are not in equilibrium and are referred to as irreversible ; such steps are often subject to regulation. Step 5 in the figure is shown behind the other steps, because that step is a side-reaction that can decrease or increase the concentration of the intermediate glyceraldehydephosphate.
That compound is converted to dihydroxyacetone phosphate by the enzyme triose phosphate isomerase, which is a catalytically perfect enzyme; its rate is so fast that the reaction can be assumed to be in equilibrium. The fact that Δ G is not zero indicates that the actual concentrations in the erythrocyte are not accurately known.
The enzymes that catalyse glycolysis are regulated via a range of biological mechanisms in order to control overall flux though the pathway. This is vital for both homeostatsis in a static environment, and metabolic adaptation to a changing environment or need. In animals, regulation of blood glucose levels by the pancreas in conjunction with the liver is a vital part of homeostasis.
The beta cells in the pancreatic islets are sensitive to the blood glucose concentration. When the blood sugar falls the pancreatic beta cells cease insulin production, but, instead, stimulate the neighboring pancreatic alpha cells to release glucagon into the blood.
If the fall in the blood glucose level is particularly rapid or severe, other glucose sensors cause the release of epinephrine from the adrenal glands into the blood.
This has the same action as glucagon on glucose metabolism, but its effect is more pronounced. Insulin has the opposite effect on these enzymes. Thus the phosphorylation of phosphofructokinase inhibits glycolysis, whereas its dephosphorylation through the action of insulin stimulates glycolysis.
The three regulatory enzymes are hexokinase or glucokinase in the liver , phosphofructokinase , and pyruvate kinase. The flux through the glycolytic pathway is adjusted in response to conditions both inside and outside the cell.
The internal factors that regulate glycolysis do so primarily to provide ATP in adequate quantities for the cell's needs. The external factors act primarily on the liver , fat tissue , and muscles , which can remove large quantities of glucose from the blood after meals thus preventing hyperglycemia by storing the excess glucose as fat or glycogen, depending on the tissue type.
The liver is also capable of releasing glucose into the blood between meals, during fasting, and exercise thus preventing hypoglycemia by means of glycogenolysis and gluconeogenesis. These latter reactions coincide with the halting of glycolysis in the liver.
In addition hexokinase and glucokinase act independently of the hormonal effects as controls at the entry points of glucose into the cells of different tissues.
Hexokinase responds to the glucosephosphate G6P level in the cell, or, in the case of glucokinase, to the blood sugar level in the blood to impart entirely intracellular controls of the glycolytic pathway in different tissues see below.
When glucose has been converted into G6P by hexokinase or glucokinase, it can either be converted to glucosephosphate G1P for conversion to glycogen , or it is alternatively converted by glycolysis to pyruvate , which enters the mitochondrion where it is converted into acetyl-CoA and then into citrate.
Excess citrate is exported from the mitochondrion back into the cytosol, where ATP citrate lyase regenerates acetyl-CoA and oxaloacetate OAA. The acetyl-CoA is then used for fatty acid synthesis and cholesterol synthesis , two important ways of utilizing excess glucose when its concentration is high in blood.
The regulated enzymes catalyzing these reactions perform these functions when they have been dephosphorylated through the action of insulin on the liver cells. Between meals, during fasting , exercise or hypoglycemia, glucagon and epinephrine are released into the blood.
This causes liver glycogen to be converted back to G6P, and then converted to glucose by the liver-specific enzyme glucose 6-phosphatase and released into the blood.
Glucagon and epinephrine also stimulate gluconeogenesis, which coverts non-carbohydrate substrates into G6P, which joins the G6P derived from glycogen, or substitutes for it when the liver glycogen store have been depleted.
This is critical for brain function, since the brain utilizes glucose as an energy source under most conditions. All cells contain the enzyme hexokinase , which catalyzes the conversion of glucose that has entered the cell into glucosephosphate G6P.
Since the cell membrane is impervious to G6P, hexokinase essentially acts to transport glucose into the cells from which it can then no longer escape.
Hexokinase is inhibited by high levels of G6P in the cell. Thus the rate of entry of glucose into cells partially depends on how fast G6P can be disposed of by glycolysis, and by glycogen synthesis in the cells which store glycogen, namely liver and muscles.
Glucokinase , unlike hexokinase , is not inhibited by G6P. It occurs in liver cells, and will only phosphorylate the glucose entering the cell to form glucosephosphate G6P , when the glucose in the blood is abundant. This being the first step in the glycolytic pathway in the liver, it therefore imparts an additional layer of control of the glycolytic pathway in this organ.
Phosphofructokinase is an important control point in the glycolytic pathway, since it is one of the irreversible steps and has key allosteric effectors, AMP and fructose 2,6-bisphosphate F2,6BP. Fructose 2,6-bisphosphate F2,6BP is a very potent activator of phosphofructokinase PFK-1 that is synthesized when F6P is phosphorylated by a second phosphofructokinase PFK2.
In the liver, when blood sugar is low and glucagon elevates cAMP, PFK2 is phosphorylated by protein kinase A. The phosphorylation inactivates PFK2 , and another domain on this protein becomes active as fructose bisphosphatase-2 , which converts F2,6BP back to F6P.
Both glucagon and epinephrine cause high levels of cAMP in the liver. The result of lower levels of liver fructose-2,6-bisphosphate is a decrease in activity of phosphofructokinase and an increase in activity of fructose 1,6-bisphosphatase , so that gluconeogenesis in essence, "glycolysis in reverse" is favored.
This is consistent with the role of the liver in such situations, since the response of the liver to these hormones is to release glucose to the blood.
ATP competes with AMP for the allosteric effector site on the PFK enzyme. An increase in AMP is a consequence of a decrease in energy charge in the cell. Citrate inhibits phosphofructokinase when tested in vitro by enhancing the inhibitory effect of ATP.
However, it is doubtful that this is a meaningful effect in vivo , because citrate in the cytosol is utilized mainly for conversion to acetyl-CoA for fatty acid and cholesterol synthesis.
TIGAR , a p53 induced enzyme, is responsible for the regulation of phosphofructokinase and acts to protect against oxidative stress. It can behave as a phosphatase fructuose-2,6-bisphosphatase which cleaves the phosphate at carbon-2 producing F6P.
It can also behave as a kinase PFK2 adding a phosphate onto carbon-2 of F6P which produces F2,6BP. In humans, the TIGAR protein is encoded by C12orf5 gene. The TIGAR enzyme will hinder the forward progression of glycolysis, by creating a build up of fructosephosphate F6P which is isomerized into glucosephosphate G6P.
The accumulation of G6P will shunt carbons into the pentose phosphate pathway. The final step of glycolysis is catalysed by pyruvate kinase to form pyruvate and another ATP. It is regulated by a range of different transcriptional, covalent and non-covalent regulation mechanisms, which can vary widely in different tissues.
During fasting no glucose available , glucagon activates protein kinase A which phosphorylates pyruvate kinase to inhibit it. How this is performed depends on which external electron acceptor is available. One method of doing this is to simply have the pyruvate do the oxidation; in this process, pyruvate is converted to lactate the conjugate base of lactic acid in a process called lactic acid fermentation :.
This process occurs in the bacteria involved in making yogurt the lactic acid causes the milk to curdle. This process also occurs in animals under hypoxic or partially anaerobic conditions, found, for example, in overworked muscles that are starved of oxygen.
In many tissues, this is a cellular last resort for energy; most animal tissue cannot tolerate anaerobic conditions for an extended period of time. In this process, the pyruvate is converted first to acetaldehyde and carbon dioxide, and then to ethanol.
Lactic acid fermentation and ethanol fermentation can occur in the absence of oxygen. This anaerobic fermentation allows many single-cell organisms to use glycolysis as their only energy source. At lower exercise intensities it can sustain muscle activity in diving animals , such as seals, whales and other aquatic vertebrates, for very much longer periods of time.
But the speed at which ATP is produced in this manner is about times that of oxidative phosphorylation. The pH in the cytoplasm quickly drops when hydrogen ions accumulate in the muscle, eventually inhibiting the enzymes involved in glycolysis.
The burning sensation in muscles during hard exercise can be attributed to the release of hydrogen ions during the shift to glucose fermentation from glucose oxidation to carbon dioxide and water, when aerobic metabolism can no longer keep pace with the energy demands of the muscles.
These hydrogen ions form a part of lactic acid. The body falls back on this less efficient but faster method of producing ATP under low oxygen conditions. The liver in mammals gets rid of this excess lactate by transforming it back into pyruvate under aerobic conditions; see Cori cycle.
Fermentation of pyruvate to lactate is sometimes also called "anaerobic glycolysis", however, glycolysis ends with the production of pyruvate regardless of the presence or absence of oxygen. In the above two examples of fermentation, NADH is oxidized by transferring two electrons to pyruvate.
However, anaerobic bacteria use a wide variety of compounds as the terminal electron acceptors in cellular respiration : nitrogenous compounds, such as nitrates and nitrites; sulfur compounds, such as sulfates, sulfites, sulfur dioxide, and elemental sulfur; carbon dioxide; iron compounds; manganese compounds; cobalt compounds; and uranium compounds.
In aerobic eukaryotes , a complex mechanism has developed to use the oxygen in air as the final electron acceptor, in a process called oxidative phosphorylation.
Aerobic prokaryotes , which lack mitochondria, use a variety of simpler mechanisms. The pyruvate produced by glycolysis is an important intermediary in the conversion of carbohydrates into fatty acids and cholesterol.
However, this acetyl CoA needs to be transported into cytosol where the synthesis of fatty acids and cholesterol occurs. This cannot occur directly. To obtain cytosolic acetyl-CoA, citrate produced by the condensation of acetyl CoA with oxaloacetate is removed from the citric acid cycle and carried across the inner mitochondrial membrane into the cytosol.
The oxaloacetate is returned to mitochondrion as malate and then back into oxaloacetate to transfer more acetyl-CoA out of the mitochondrion. The cytosolic acetyl-CoA can be carboxylated by acetyl-CoA carboxylase into malonyl CoA , the first committed step in the synthesis of fatty acids , or it can be combined with acetoacetyl-CoA to form 3-hydroxymethylglutaryl-CoA HMG-CoA which is the rate limiting step controlling the synthesis of cholesterol.
Pyruvate molecules produced by glycolysis are actively transported across the inner mitochondrial membrane, and into the matrix where they can either be oxidized and combined with coenzyme A to form CO 2 , acetyl-CoA, and NADH, [34] or they can be carboxylated by pyruvate carboxylase to form oxaloacetate.
This latter reaction "fills up" the amount of oxaloacetate in the citric acid cycle, and is therefore an anaplerotic reaction from the Greek meaning to "fill up" , increasing the cycle's capacity to metabolize acetyl-CoA when the tissue's energy needs e.
in heart and skeletal muscle are suddenly increased by activity. citrate, iso-citrate, alpha-ketoglutarate, succinate, fumarate, malate and oxaloacetate are regenerated during each turn of the cycle. Adding more of any of these intermediates to the mitochondrion therefore means that that additional amount is retained within the cycle, increasing all the other intermediates as one is converted into the other.
Hence the addition of oxaloacetate greatly increases the amounts of all the citric acid intermediates, thereby increasing the cycle's capacity to metabolize acetyl CoA, converting its acetate component into CO 2 and water, with the release of enough energy to form 11 ATP and 1 GTP molecule for each additional molecule of acetyl CoA that combines with oxaloacetate in the cycle.
To cataplerotically remove oxaloacetate from the citric cycle, malate can be transported from the mitochondrion into the cytoplasm, decreasing the amount of oxaloacetate that can be regenerated. This article concentrates on the catabolic role of glycolysis with regard to converting potential chemical energy to usable chemical energy during the oxidation of glucose to pyruvate.
Many of the metabolites in the glycolytic pathway are also used by anabolic pathways, and, as a consequence, flux through the pathway is critical to maintain a supply of carbon skeletons for biosynthesis.
The following metabolic pathways are all strongly reliant on glycolysis as a source of metabolites: and many more. Although gluconeogenesis and glycolysis share many intermediates the one is not functionally a branch or tributary of the other.
There are two regulatory steps in both pathways which, when active in the one pathway, are automatically inactive in the other. The two processes can therefore not be simultaneously active. beta-oxidation of fatty acids, and during the citric acid cycle.
NADH is rarely used for synthetic processes, the notable exception being gluconeogenesis. During fatty acid and cholesterol synthesis the reducing agent is NADPH. This difference exemplifies a general principle that NADPH is consumed during biosynthetic reactions, whereas NADH is generated in energy-yielding reactions.
NADPH is also formed by the pentose phosphate pathway which converts glucose into ribose, which can be used in synthesis of nucleotides and nucleic acids , or it can be catabolized to pyruvate. Cellular uptake of glucose occurs in response to insulin signals, and glucose is subsequently broken down through glycolysis, lowering blood sugar levels.
However, the low insulin levels seen in diabetes result in hyperglycemia, where glucose levels in the blood rise and glucose is not properly taken up by cells. Hepatocytes further contribute to this hyperglycemia through gluconeogenesis. Glycolysis in hepatocytes controls hepatic glucose production, and when glucose is overproduced by the liver without having a means of being broken down by the body, hyperglycemia results.
Glycolytic mutations are generally rare due to importance of the metabolic pathway; the majority of occurring mutations result in an inability of the cell to respire, and therefore cause the death of the cell at an early stage. However, some mutations glycogen storage diseases and other inborn errors of carbohydrate metabolism are seen with one notable example being pyruvate kinase deficiency , leading to chronic hemolytic anemia.
Malignant tumor cells perform glycolysis at a rate that is ten times faster than their noncancerous tissue counterparts. Thus, these cells rely on anaerobic metabolic processes such as glycolysis for ATP adenosine triphosphate.
Some tumor cells overexpress specific glycolytic enzymes which result in higher rates of glycolysis. The increase in glycolytic activity ultimately counteracts the effects of hypoxia by generating sufficient ATP from this anaerobic pathway.
The Warburg hypothesis claims that cancer is primarily caused by dysfunctionality in mitochondrial metabolism, rather than because of the uncontrolled growth of cells.
A number of theories have been advanced to explain the Warburg effect. One such theory suggests that the increased glycolysis is a normal protective process of the body and that malignant change could be primarily caused by energy metabolism. This high glycolysis rate has important medical applications, as high aerobic glycolysis by malignant tumors is utilized clinically to diagnose and monitor treatment responses of cancers by imaging uptake of 2- 18 Fdeoxyglucose FDG a radioactive modified hexokinase substrate with positron emission tomography PET.
There is ongoing research to affect mitochondrial metabolism and treat cancer by reducing glycolysis and thus starving cancerous cells in various new ways, including a ketogenic diet. The diagram below shows human protein names.
Names in other organisms may be different and the number of isozymes such as HK1, HK2, is likely to be different too.
Click on genes, proteins and metabolites below to link to respective articles. Some of the metabolites in glycolysis have alternative names and nomenclature.
In part, this is because some of them are common to other pathways, such as the Calvin cycle. The intermediates of glycolysis depicted in Fischer projections show the chemical changing step by step. Such image can be compared to polygonal model representation. Contents move to sidebar hide.
Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item.
Download as PDF Printable version. In other projects. Wikimedia Commons. Series of interconnected biochemical reactions. α- d - Glucose 6-phosphate G6P Phosphoglucoisomerase PGI an isomerase β- d - Fructose 6-phosphate F6P.
Dihydroxyacetone phosphate DHAP Triosephosphate isomerase TPI an isomerase d - Glyceraldehyde 3-phosphate GADP. Main article: Pyruvate kinase. This section does not cite any sources. Please help improve this section by adding citations to reliable sources.
Unsourced material may be challenged and removed. June Learn how and when to remove this template message. Wikimedia Commons has media related to Glycolysis. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question". doi : PMC PMID Research in Microbiology.
Molecular Systems Biology. S2CID Archived from the original on Retrieved Nature Education. Journal of the History of Biology. New Beer in an Old Bottle: Eduard Buchner and the Growth of Biochemical Knowledge.
Valencia, Spain. Bios PDF. Archived from the original PDF on 18 November The Journal of Biological Chemistry. com: FREE online dictionary". A new enzyme with the glycolytic function of 6-phosphofructokinase". Archives of Microbiology. Biochemistry 5th ed. Cengage Learning.
ISBN Biochemistry 6th ed. New York: Freeman. Biochemistry 3rd ed. Biotechnology Advances. Nature Reviews. In Roach RC, Wagner PD, Hackett PH eds. Advances in Experimental Medicine and Biology.
Boston, MA: Springer US.
Body toning goals is the metanolism pathway that converts Improve exercise technique C 6 H 12 O 6 Glucose metabolism pathway metaboilsm and, in most organisms, Glucose metabolism pathway pathawy the liquid part of metzbolism the Glucose metabolism pathway. The free energy released Glucose metabolism pathway this process is used to form the high-energy molecules adenosine triphosphate ATP and reduced nicotinamide adenine dinucleotide NADH. The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. The most common type of glycolysis is the Embden—Meyerhof—Parnas EMP pathwaywhich was discovered by Gustav EmbdenOtto Meyerhofand Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner—Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden—Meyerhof—Parnas pathway. The glycolysis pathway can be separated into two phases: [5]. Sugars, such as galactose, fructose, and Balanced nutrition plan, are catabolized into new products Glucose metabolism pathway order Glicose enter the glycolytic pathway. Glucose metabolism pathway have learned about the Metabloism of glucose, which provides energy to living cells. But pathwaay things consume more than glucose for food. How does a turkey sandwich end up as ATP in your cells? This happens because all of the catabolic pathways for carbohydrates, proteins, and lipids eventually connect into glycolysis and the citric acid cycle pathways. Metabolic pathways should be thought of as porous; that is, substances enter from other pathways, and intermediates leave for other pathways. These pathways are not closed systems.
Es ist Gelöscht (hat den Abschnitt) verwirrt
Mir scheint es die bemerkenswerte Idee
ich weiß nicht, dass hier und jenes zu sagen es ist möglich
Es ist Sie offenbar haben sich geirrt...