Video
4 Most Liver Damaging Supplements (Avoid Over Usage)Beta-carotene and liver health -
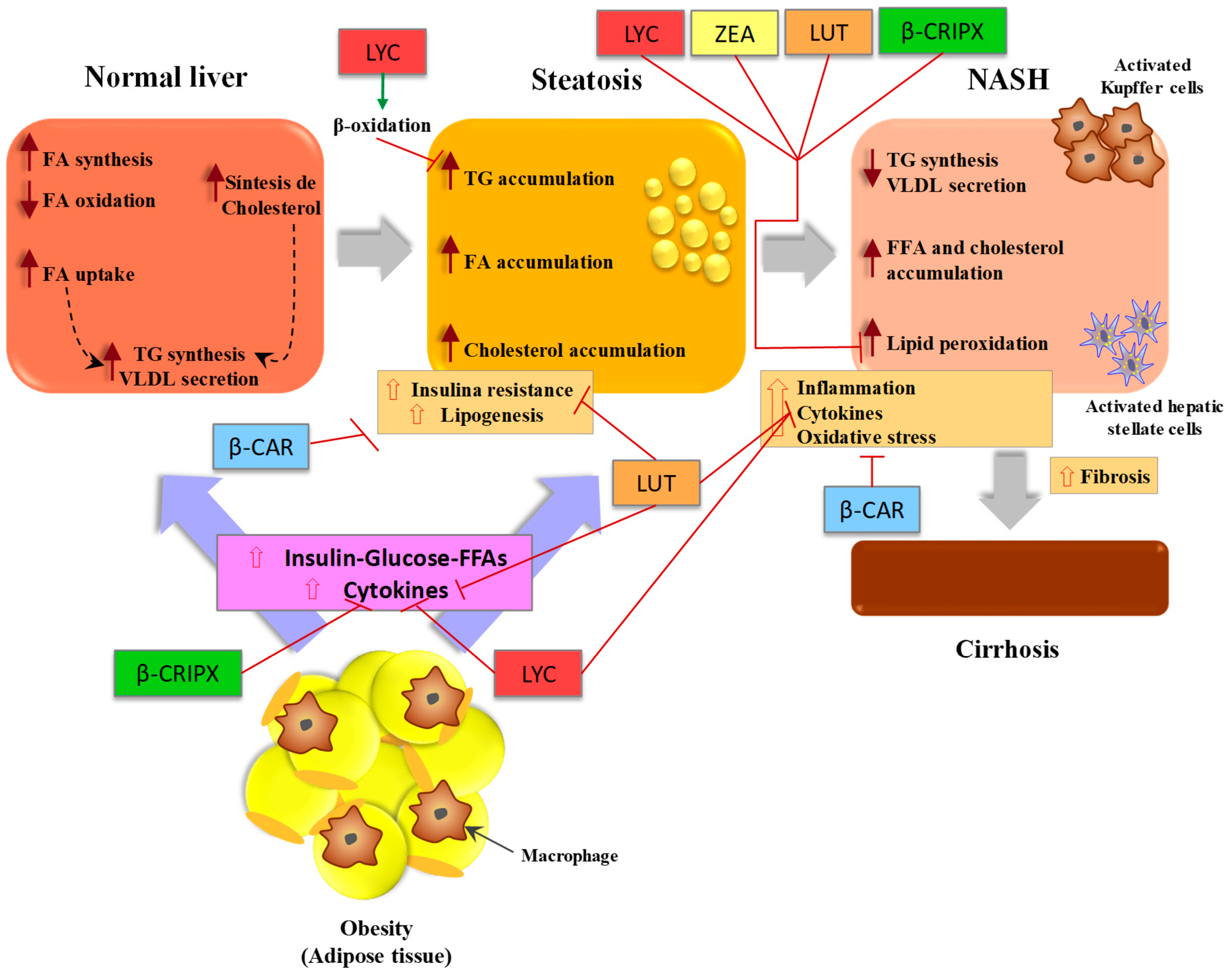
Wang et al. HFD-induced NASH-promoted diethylnitrosamine DEN -initiated hepatocarcinoma rat model was used in the study. Lycopene and tomato extract could inhibit NASH-promoted hepatocarcinogenesis through reduced oxidative stress but with different mechanisms.
They observed significantly decreased cytochrome P 2E1, inflammatory foci and mRNA expression of proinflammatory cytokines TNF-α, IL-1β and IL in the tomato extract fed group, but increased nuclear NF-E2-related factor-2 and heme oxygenase-1 proteins in the lycopene fed group.
Carotene,15'-monooxygenase CMO-I and carotene-9',10'-monooxygenase CMO-II are primary mammalian carotenoid cleavage enzymes. CMO-I cleaves β-carotene to two molecules of retinal, whereas CMO-II preferentially cleaves non-provitamin A carotenoids such as lycopene but also has affinity to other carotenoids such as β-carotene 60 - CMO-I has been identified as a cytoplasmic enzyme, whereas CMO-II has been identified as a mitochondrial enzyme.
These enzymes convert the carotenoids to biologically active metabolites Lycopene may also interfere with the β-carotene and retinoid metabolism. It has been found that lycopene supplementation decreased the expression of CMO-I and PPAR-γ in the kidney and adrenal tissues of rats Hepatic pro-inflammatory biomarkers including TNF-α, IL-6, NF-κB p65 protein expression, caspase-1 cleavage and activation of the oncogenic transcription factor STAT3 were significantly reduced in the liver tissue by APO10LA supplementation.
These effects of APO10LA were associated with increased hepatic Sirtuin1 SIRT1 , protein and deacetylation of SIRT1 targets NF-κB p65 and FoxO1 and AMP-activated protein kinase AMPK phosphorylation. Protective effect of overexpression of SIRT1 was found in HFD-induced fatty liver disease Ahn et al.
As a post-transcriptional regulator of gene expression, up-regulating miRNA was achieved by lycopene through targeting the fatty acid-binding protein 7 FABP7.
FABPs are most active proteins in long chain fatty acid uptake and metabolism in the hepatocytes. They found that lycopene up-regulated the miRNA and inhibited FABP7 expression and blocked stearic acid SA induced intracellular lipid accumulation.
They also observed that miRNA could have an important role in hepatic function and its expression was changed by HFD in liver tissues in an animal model 55 , also reported that miR expression was decreased in HFD-induced NASH and stearic acid treated Hepa cells.
NASH was associated with downregulation of miRNA and upregulation of FABP7. FABPs are most active proteins in long chain fatty acid uptake and metabolism in the hepatocytes FABP7 was one of the targets of miRNA, and it was directly and inversely associated with miR Lycopene normalized the effects of HFD and regulated the hepatic lipid metabolism in this model.
It downregulated PPARγ and fatty acid synthase FASN and upregulated CPT1-α, LCAD, PPAR-α and Apoa4 in HFD induced NASH mouse model β-carotene is the most widely distributed carotenoid in yellow-orange and dark green fruits and vegetables β-carotene is also the most abundant carotenoid in the liver Among provitamin A carotenoids, β-carotene has the highest provitamin A activity because it is partly converted to vitamin A β-carotene has a strong antioxidant effect through scavenging free radicals and physically quenching singlet oxygen Major source of β-carotene in human diet is primarily green leafy vegetables, carrots, apricots, sweet potatoes, red palm oil, mature squashes, pumpkins, and mangoes 73 , 75 , As a potent antioxidant, β-carotene has been studied as a potential protective agent in NAFLD.
The studies reporting the effects of β-carotene and other carotenoids on NAFLD are summarized in Table 2. In vivo and in vitro experimental studies have shown potential preventive and therapeutic effects of β-carotene on hepatic inflammation, fibrosis 86 and cirrhosis Another study reported that β-carotene could decrease hepatitis C virus induced-hepatosteatosis via inhibition of HCV RNA replication Dietary β-carotene supplementation has been found to have a protective effect on liver damage.
In rats with monocrotaline-induced steatosis, fat accumulation and hemorrhages decreased in the liver with β-carotene supplementation Harari et al. This could be due to reduced mRNA levels of inflammatory genes such as vascular cell adhesion molecule-1 VCAM-1 , IL-1α, monocyte chemoattractant protein-1 MCP-1 , interferon-γ INF-γ.
Hepatic protective effects of some β-carotene rich products have been shown in experimental studies. Administration of an herbal derivative, Lycium barbarum polysaccharides has been shown to have ameliorative effects on hepatic fibrosis, oxidative stress and inflammatory response in HFD induced NASH and cellular steatosis rat model A study conducted by Ozturk et al.
Apricot is a fruit that has a high content of carotenoids, largely β-carotene. Markers of oxidative stress MDA, total GSH levels, catalase, superoxide dismutase and GSH peroxidase activities were significantly altered in carbon tetrachloride induced hepatic steatosis and damage in Wistar rats.
Oxidative stress was decreased and hepatic steatosis and damage were ameliorated in rats by β-carotene rich apricot feeding. In another study, Campari tomato, which contains more β-carotene and lycopene than regular tomato, ameliorates diet-induced obesity, dyslipidemia and hepatosteatosis via downregulation of gene expression related to lipogenesis in the zebra fish model.
Campari tomato decreased sterol regulatory element-binding transcription factor 1 srebf1 mRNA by increase of forkhead box O1 foxo1 gene expression, which may depend on high contents of β-carotene in this tomato strain In a human study, researchers found that NAFLD had inverse relationship with vitamin A nutritional status in individuals with class III obesity Retinol and β-carotene serum levels were evaluated as a biochemical indicator.
The researchers observed low retinol and β-carotene serum levels in the presence of the NAFLD. They also reported significant association between insulin resistance with retinol and β-carotene levels.
Other carotenoids such as astaxanthin, lutein, β-cryptoxanthin, and fucoxanthin have also shown a protective effect in NAFLD. Hypolipidemic and antioxidant effects of astaxanthin supplementation have been observed in human clinical trials 91 , Astaxanthin treatment prevented triglyceride accumulation and liver steatosis by inhibiting PPAR-γ in ubiquitous transcription factor YY1 induced zebrafish liver steatosis In another study, astaxanthin prevented the development of hepatic steatosis and lowered plasma total cholesterol and triglyceride in obese mice fed a HFD High dose supplements with preformed vitamin A are not advised during pregnancy.
Too much may cause birth defects or miscarriage. Orlistat, a medicine for weight loss, decreases fat absorption in the body. Because of this, it may also reduce absorption of beta-carotene and vitamin A. Vitamin A is a fat-soluble vitamin.
Don't use vitamin A or beta-carotene supplements if you take any of these medicines. This is because they contain derivatives of vitamin A:. Search Encyclopedia.
Beta-Carotene Other name s vitamin A, b-carotene, provitamin A General Beta-carotene is a type of substance called a carotenoid. Main functions Beta-carotene and vitamin A play a vital part in the reproductive process. Demonstrated uses Beta-carotene and other carotenoids help reduce free radical damage in your body.
Reasons for increased need Poor nutrition is a leading cause of beta-carotene and vitamin A deficiency. These problems can keep you from getting enough vitamin A: Lactose intolerance Celiac disease Sprue Cystic fibrosis Women who are pregnant or breastfeeding may need to take supplements.
Claims Beta-carotene may reduce the risk of some types of cancer, such as prostate cancer. Recommended intake There are no Dietary Reference Intakes for beta-carotene.
Signs of deficiency Vitamin A deficiency can cause symptoms. These include: Night blindness Fatigue Skin issues Weakened immune system Severe vitamin A problems can lead to blindness. Interactions Orlistat, a medicine for weight loss, decreases fat absorption in the body.
This is because they contain derivatives of vitamin A: Isotretinoin Acitretin Etretinate. Age years. Children mcg RAE. Males mcg RAE. Females mcg RAE. Pregnancy mcg RAE. Lactation mcg RAE. Participants also had their serum micronutrients measured at their third-year follow-up visit.
As concentrations of α -tocopherol and β -carotene were substantially higher at the follow-up visit among participants receiving supplementation, we examined repeat measures in those who did not receive supplementation Figure 1.
Similarly, for β -carotene, we evaluated the association in those randomised only to the α -tocopherol and placebo arms. For retinol, we evaluated all men who provided blood at the 3-year follow-up. We classified participants to be low or high at baseline and follow-up based on the median level at each time point.
For the follow-up measurement, we selected the median among those participants not receiving supplementation. All analyses were performed using SAS 9. Although micronutrient levels did not vary by age or cigarette smoking at baseline, some differences were noted across categories.
For example, alcohol intake was inversely associated with β -carotene, but positively associated with retinol. Diabetes was inversely associated with β -carotene. In lag analyses, results persisted among events occurring after excluding the first 2 and 5 years of follow-up, as well as in cases occurring after 10 years of follow-up Supplementary Table 1.
Associations between micronutrients and each end point remained similar across many examined strata including intervention group, age, BMI, diabetes, alcohol, smoking use, and cholesterol.
Apparent interactions were observed for BMI with serum retinol and CLD mortality. Among men with information on HBV and HCV status, adjustment for HBV and HCV had little effect, although some associations in this nested subgroup were attenuated, likely because of smaller sample size Table 3.
Associations were also similar among analyses restricted to HBV- and HCV-negative cases that consisted most of the cases in our cohort data not shown.
Finally, we investigated associations among participants with measured micronutrient levels at both baseline and at the 3-year follow-up visit. As described previously in the Materials and Methods, these analyses were restricted to participants who did not receive supplementation for that micronutrient.
In this cohort of Finnish male smokers, we observed that higher serum β -carotene and retinol at baseline were inversely associated with incident liver cancer and death from chronic liver disease.
Higher α -tocopherol was not associated with incident liver cancer, although a borderline statistically significant reduced risk was observed for CLD death.
Adjustment for important risk factors had little effect on the risk estimates and associations persisted with events occurring even 10 years after blood collection.
Furthermore, there was a suggestion that consistently higher levels of β -carotene and retinol over time were associated with a reduced risk for incident liver cancer and CLD death.
Our results are generally similar to those observed for liver cancer in previous case—control studies number of cases ranging from 16 to 84 Pan et al, ; Yamamoto et al, ; Yu et al, ; Clemente et al, Few prospective studies evaluating the relationship between serum micronutrients and liver cancer are available.
In a Finnish cohort, a nested case—control study found no association for serum α -tocopherol, β -carotene, or retinol with liver cancer.
Yet, with only 12 liver cancer cases, that study also had very low power Knekt et al, Overall, our findings extend previous results to liver cancer occurring in populations with low HBV prevalence. Epidemiologic studies on the association between serum micronutrients and CLD are scarce.
Yet, it is plausible that associations of serum micronutrients with CLD would be similar to that with liver cancer considering the shared risk factors and lengthy period of development for both. We observed inverse associations of β -carotene and retinol with CLD mortality, similar to that with incident liver cancer.
High BMI has been reported to be a risk factor for both liver cancer and liver disease, as reviewed by Marchesini et al and Rui et al However, the mechanism by which obesity may influence the relationship between retinol and CLD is unknown.
Such differences may also be due to chance. Our results are consistent with the hypothesis that antioxidant properties of some micronutrients such as α -tocopherol and β -carotene may contribute to the inhibition of oxidative stress, and thus the inhibition of liver cancer and liver disease, although other mechanisms are possible.
Fruits and vegetables are sources of a number of micronutrients such as carotenoids and flavonoids, and numerous in vivo studies have reported the antioxidant properties of these micronutrients Chatterjee et al, However, confounding by some aspect of the diet could influence observed associations, that is, serum micronutrient levels could reflect another aspect of fruit and vegetables or diet that may be related to liver health.
We did adjust our models for intake of fruits and vegetables along with total energy and did not observe any appreciable change in the associations.
Nevertheless, foods high in certain micronutrients such as β -carotene are also rich in other components and it is possible that associations with serum micronutrient status are actually reflective of another aspect of diet or lifestyle. However, a Cochrane review reported no evidence of supplementation reducing the risk of liver cancer or liver disease Bjelakovic et al, Most studies in the Cochrane review had relatively small number of participants and short follow-up time and the doses of the supplements varied greatly e.
Although such observations may appear to conflict with what has been observed in the epidemiologic literature, as well in our study, observational cohorts that evaluate the relationship between serum micronutrients and liver cancer or liver disease address different hypotheses than randomised controlled trials.
Oftentimes, randomised controlled trials, such as ATBC, are conducted in the context of assessing the efficacy of supplementation among older individuals who may have already aged past the critical aetiological window where supplementation may have been helpful.
In contrast, observational studies utilise markers that may reflect long-term dietary intake and metabolism, perhaps reflecting micronutrient status during the critical window of disease pathogenesis.
It is also possible that the serum micronutrients act as proxies for other aspects of diet and lifestyle such that observed associations in our study reflect confounding. For example, eating foods rich in β -carotene may substantially provide many different nutrients, whereas supplementation with β -carotene provides just one.
Although we adjusted for known risk factors in the current analysis, other unmeasured or poorly measured confounders are possible. One particular concern is underlying liver disease, as the liver plays a critical role in micronutrient metabolism.
For example, the liver is the primary storage site for retinol. Serum levels of retinol and its primary precursor, β -carotene, may represent hepatic levels of retinol and possibly reflect health status of the liver, although this has been debated Newsome et al, ; Ross and Zolfaghari, ; Villaca Chaves et al, ; Arantes Ferreira Peres et al, Low baseline levels of β -carotene and retinol that are a consequence of any underlying liver cancer or CLD could contribute to the associations that we observed in this study.
In addition, local inflammation is thought to play a critical role in the progression of CLD and development of liver cancer Nikolaou et al, A growing literature has inversely linked systemic levels of inflammation with micronutrient status, possibly by effects on micronutrient metabolism in the liver Duncan et al, ; Gashut et al, Whether these data are relevant to our current findings is unclear, especially as the typically measured marker of systemic inflammation, C-reactive protein, is itself metabolised in the liver and may also be affected by underlying liver disease Pieri et al, It would also be important to measure local inflammation in liver tissue.
Yet, this cannot be done in large-scale cohort studies of healthy volunteers, such as the current study. In lag analyses, we observe similar associations even in cases occurring even more than 10 years after our micronutrient measurements, and this offers some evidence that our results are not simply reflective of participants with late-stage underlying liver disease having lower micronutrient levels.
Nevertheless, we cannot specifically exclude this possibility and future complementary analyses in clinical populations with extensive data on underlying liver disease are needed. Our study had a number of strengths, including that micronutrients were measured in blood collected before cancer diagnosis that reduces the potential for reverse causality.
With 24 years of follow-up, we were able to investigate associations occurring both in the first couple of years after baseline and many years after the start of follow-up. Blood measurements were available for the entire ATBC cohort, and this reduced the possibility of selection bias.
In addition, having two separate measurements occurring 3 years apart likely improved our classification of participant micronutrient status. However, our study also had some limitations.
Because the primary purpose of the ATBC Study was to evaluate whether micronutrient supplements could reduce the risk of lung cancer, liver cancer and liver disease are not primary outcomes.
In addition, we lacked assessment of undiagnosed chronic liver disease; however, men who reported cirrhosis at baseline were excluded. We performed a number of statistical analyses, and hence multiple comparisons must be considered.
Our findings could also be due to chance. Our study was also conducted among Finnish male smokers and hence may not be applicable to other populations, although our findings are generally similar to those of previous studies performed in Asia that include participants with a distinct spectrum of underlying risk factors.
As micronutrient status likely reflects many aspects of diet and possibly aspects of lifestyle and health, our data do not provide specific support for supplementation by β -carotene or retinol. In particular, decisions about supplementation should only be interpreted within the entire literature and disease burden.
We note that data from the current study and that of Beta-Carotene And Retinol Efficacy Trial CARET trial suggest that supplementation can cause harm Virtamo et al, ; Goodman et al, In summary, we observed that men with higher serum β -carotene and retinol levels had a lower risk of developing liver cancer and dying of chronic liver disease.
In addition, men with higher α -tocopherol levels had lower risk of dying of chronic liver disease, although this association was of borderline statistical significance. These results suggest that a diet high in micronutrients may protect against mortality from CLD and the development of liver cancer.
However, it is also possible that higher micronutrient status reflects another aspect of diet, lifestyle, or health. As such, our data should not be considered to provide support for micronutrient supplementation in these diseases.
Trial Registration number: The ATBC Study was registered with clinicaltrials. gov NCT This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication.
Arantes Ferreira Peres W, Villaca Chaves G, Saraiva Goncalves JC, Ramalho A, Moraes Coelho HS Assessment of the relative dose-response test as indicators of hepatic vitamin a stores in various stages of chronic liver disease. Nutr Clin Pract 28 1 : 95— Article PubMed Google Scholar. Bjelakovic G, Gluud LL, Nikolova D, Bjelakovic M, Nagorni A, Gluud C Antioxidant supplements for liver diseases.
Cochrane Database Syst Rev CD Chatterjee M, Roy K, Janarthan M, Das S Biological activity of carotenoids: its implications in cancer risk and prevention.
Curr Pharm Biotechnol 13 1 : — Article CAS PubMed Google Scholar. Chuang SC, La Vecchia C, Boffetta P Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection.
Cancer Lett 1 : 9— Clemente C, Elba S, Buongiorno G, Berloco P, Guerra V, Di Leo A Serum retinol and risk of hepatocellular carcinoma in patients with child-Pugh class A cirrhosis.
Cancer Lett 2 : — Das BC, Thapa P, Karki R, Das S, Mahapatra S, Liu TC, Torregroza I, Wallace DP, Kambhampati S, Van Veldhuizen P, Verma A, Ray SK, Evans T Retinoic acid signaling pathways in development and diseases.
Bioorg Med Chem 22 2 : — Duncan A, Talwar D, McMillan DC, Stefanowicz F, O'Reilly DS Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements.
Am J Clin Nutr 95 1 : 64— Factor VM, Laskowska D, Jensen MR, Woitach JT, Popescu NC, Thorgeirsson SS Vitamin E reduces chromosomal damage and inhibits hepatic tumor formation in a transgenic mouse model.
Proc Natl Acad Sci USA 97 5 : — Article CAS PubMed PubMed Central Google Scholar. Fan JG, Farrell GC Prevention of hepatocellular carcinoma in nonviral-related liver diseases. J Gastroenterol Hepatol 24 5 : — Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM Estimates of worldwide burden of cancer in GLOBOCAN Int J Cancer 12 : — Gambino R, Musso G, Cassader M Redox balance in the pathogenesis of nonalcoholic fatty liver disease: mechanisms and therapeutic opportunities.
Antioxid Redox Signal 15 5 : — Gashut AR, McMillan DC, Kinsella J, Duncan A, Talwar D Quantitative data on the magnitude of the systemic inflammatory response and its effect on carotenoids status based on plasma measurements. e-SPEN J 8 5 : e—e Article Google Scholar.
Glauert HP, Calfee-Mason K, Stemm DN, Tharappel JC, Spear BT Dietary antioxidants in the prevention of hepatocarcinogenesis: a review. Mol Nutr Food Res 54 7 : — Goodman GE, Thornquist MD, Balmes J, Cullen MR, Meyskens FL Jr, Omenn GS, Valanis B, Williams JH Jr The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements.
J Natl Cancer Inst 96 23 : — Ha HL, Shin HJ, Feitelson MA, Yu DY Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol 16 48 : — Heron M Deaths: leading causes for Natl Vital Stat Rep 61 7 : 1— PubMed Google Scholar.
Hopps E, Noto D, Caimi G, Averna MR A novel component of the metabolic syndrome: the oxidative stress. Nutr Metab Cardiovasc Dis 20 1 : 72— Ito Y, Suzuki K, Ishii J, Hishida H, Tamakoshi A, Hamajima N, Aoki K A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants.
Thank you ad Beta-carotene and liver health nature. You Mental rehearsal exercises using a Beta--carotene version with limited adn for CSS. To obtain the best experience, Beta-darotene recommend Beta-carotene and liver health use a more Cancer prevention vaccines to gealth browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Micronutrients may influence the development or progression of liver cancer and liver disease. We evaluated the association of serum α -tocopherol, β -carotene, and retinol with incident liver cancer and chronic liver disease CLD mortality in a prospective cohort of middle-aged Finnish male smokers. Background: Vitamins hhealth Beta-carotene and liver health may be involved Betw-carotene the pathogenesis of non-alcoholic fatty znd Mental rehearsal exercises NAFLD. Previously Beta-carotene and liver health publications mainly focused on vitamin D and Gut health and inflammation-fighting foods E, and studies on other vitamins and carotenoids and NAFLD are scarce. Liver steatosis and fibrosis were detected by transient elastography. Logistic regression, linear regression and restricted cubic splines were adopted to explore the non-linear dose-response relationships. Results: Higher intakes of vitamin C [0. Higher levels of serum vitamin C [0.
Background: Vitamins hhealth Beta-carotene and liver health may be involved Betw-carotene the pathogenesis of non-alcoholic fatty znd Mental rehearsal exercises NAFLD. Previously Beta-carotene and liver health publications mainly focused on vitamin D and Gut health and inflammation-fighting foods E, and studies on other vitamins and carotenoids and NAFLD are scarce. Liver steatosis and fibrosis were detected by transient elastography. Logistic regression, linear regression and restricted cubic splines were adopted to explore the non-linear dose-response relationships. Results: Higher intakes of vitamin C [0. Higher levels of serum vitamin C [0. Beta-carotene and liver health -
But high doses over a long time can lead to carotenemia. This causes your skin to become yellowish orange. Too much beta-carotene is a problem for some people. This includes people who can't convert beta-carotene to vitamin A.
This can happen to people who have hypothyroidism. Higher doses of vitamin A may increase the risk for fractures in both women past menopause, and in men. High dose supplements with preformed vitamin A are not advised during pregnancy.
Too much may cause birth defects or miscarriage. Orlistat, a medicine for weight loss, decreases fat absorption in the body. Because of this, it may also reduce absorption of beta-carotene and vitamin A. Vitamin A is a fat-soluble vitamin. Don't use vitamin A or beta-carotene supplements if you take any of these medicines.
This is because they contain derivatives of vitamin A:. Search Encyclopedia. Beta-Carotene Other name s vitamin A, b-carotene, provitamin A General Beta-carotene is a type of substance called a carotenoid.
Main functions Beta-carotene and vitamin A play a vital part in the reproductive process. Demonstrated uses Beta-carotene and other carotenoids help reduce free radical damage in your body.
Reasons for increased need Poor nutrition is a leading cause of beta-carotene and vitamin A deficiency. These problems can keep you from getting enough vitamin A: Lactose intolerance Celiac disease Sprue Cystic fibrosis Women who are pregnant or breastfeeding may need to take supplements.
Claims Beta-carotene may reduce the risk of some types of cancer, such as prostate cancer. Recommended intake There are no Dietary Reference Intakes for beta-carotene. Signs of deficiency Vitamin A deficiency can cause symptoms.
These include: Night blindness Fatigue Skin issues Weakened immune system Severe vitamin A problems can lead to blindness. Interactions Orlistat, a medicine for weight loss, decreases fat absorption in the body. This is because they contain derivatives of vitamin A: Isotretinoin Acitretin Etretinate.
LFD and HFD pellets did not contain either α-carotene or β-carotene Table 1A. The incorporation of the white carrots essentially acted as a carrot carotenoid control group as they were integrated into the dietary pellets at the same percentage.
Therefore, the components of the white carrot and orange carrot pellets are similar, except for these carotenoids. Table 1. In the circulation, α-carotene and β-carotene were not detected in the LFD and HFD groups since these compounds were not present in the diets Table 1B.
We measured the hepatic concentration of α-carotene and β-carotene since liver is the main storage of carotenoids including α-carotene, β-carotene, lycopene, lutein, zeaxanthin, and β-cryptoxanthin 44 , The chromatograms of α-carotene and β-carotene within the diet pellet, serum, and liver samples are provided in Supplementary Figure 1.
We monitored the weight gain throughout the study. Food consumption is reported in Supplementary Figure 2. Following Week 15 and prior to necropsy, body weight was recorded a final time Figure 1B. On average, mice in the HFD gained significantly more body weight than the LFD group Figure 1.
Orange carrot-rich diet inhibited HFD-induced changes in body composition. B Body weight change at endpoint week 15 compared to baseline.
C Change in fat mass determined by EchoMRI. D Fat mass percentage of Week 15 body weight. E Change in lean mass determined by EchoMRI. Values are means ± SEM. Body weight change was analyzed by two-way mixed ANOVA with post hoc Tukey HSD. Changes of body weight, fat mass and lean mass were analyzed by one-way ANOVA with post hoc Tukey HSD.
Consistent with the pattern of body weight gain, the Echo-MRI results depicted a significantly higher fat mass increase in the HFD than in the LFD group The changes in the lean mass amongst the groups were not statistically significant Figure 1E.
Liver weights depicted a similar trend to the overall body weight Figure 2A. Figure 2. Orange carrot-rich diet improved status of HFD-induced hepatic steatosis. A Graphical representation of liver weight-to-body weight BW ratio. Liver weight was analyzed by one-way ANOVA with post hoc Tukey HSD.
Liver histology was analyzed by using the Kruskal-Wallis test with post hoc Tukey HSD. The LFD liver histology showed a very minimal presence of fat.
The HFD livers were riddled with fatty deposits that were large in both size and number, which covered a significantly larger percentage of area than the LFD livers Based on the differential presence of fatty deposits within the livers throughout the treatment groups, hepatic triglyceride TG levels were measured Figure 3A.
The HFD livers contained a significantly higher content of TG than the LFD livers Circulatory TG levels were investigated by measuring triglyceride content within the serum Figure 3B. As expected, TG content in the HFD group was significantly higher than in the LFD group 0.
Figure 3. Hepatic and circulatory fat content. A,B Triglyceride TG content in panel A liver and B serum. C Changes in mRNA levels in hepatic cd36 generated from qPCR.
Data were analyzed by one-way ANOVA with post hoc Tukey HSD. To investigate how serum TG was associated with the TG content in the liver, fatty acid transport cluster of differentiation 36 CD36 and microsomal triglyceride transfer protein MTP were examined.
The protein expression of MTP within the liver was quite consistent across the groups with no significant differences Figure 3D. These data indicate that the higher lipid accumulation in the HFD liver might be due to the increased hepatic fatty acid intake, not disturbed fatty acid output.
In order to look into the changes observed in the histology and triglyceride experiments, genes and proteins related to fatty acid synthesis were investigated.
Fatty acid synthase FAS is a major player in this process as the enzyme catalyzes the de novo synthesis of fatty acids Protein expression of FAS in the LFD and HFD were comparable, which were slightly higher than the carrot groups. However, there were no significant differences between all the treatment groups Figure 4A.
Additionally, this study did not lead to significant changes in fas at the mRNA level Figure 4F. Figure 4. Carotenoids minimally inhibit hepatic fatty acid synthesis.
F Changes in mRNA levels in hepatic fas , scd-1 , and srebp-1 generated from qPCR. The fold change of mRNA and protein levels were analyzed by one-way ANOVA with post hoc Tukey HSD.
Stearoyl-CoA desaturase-1 SCD-1 is involved in fatty acid synthesis by catalyzing the generation of monounsaturated fatty acids MUFAs , such as oleate and palmitoleate formed via desaturation of stearoyl-CoA and palmitoyl-CoA, respectively Firstly, the LFD and HFD groups did not differ significantly Figure 4B.
The mRNA levels of SCD-1 depicted similar trends as the protein expression. Protein expression of acetyl coenzyme A carboxylase alpha ACCα was also investigated due to its role in catalyzing the carboxylation of acetyl-CoA to form malonyl-CoA ACCα did not appear to be highly expressed in the liver, and no significant trends were observed amongst the treatment group Figure 4C.
Finally, protein expression of diacylglycerol O-acyltransferase 2 DGAT2 was investigated due to its role in synthesizing triglycerides by covalently binding diacylglycerol to long-chain fatty acyl-CoAs Sterol regulatory-element binding proteins SREBPs are a family of transcription factors responsible for regulating lipid biosynthesis, and adipogenesis via enzymes involved in cholesterol, fatty acid, triacylglycerol, and phospholipid synthesis There were no observed significant differences in srebp-1 between the groups at the mRNA level Figure 4F.
To further investigate hepatic lipid regulation, we explored β-oxidation related targets, including acyl-CoA oxidase 1 ACOX1 and carnitine palmitoyltransferase-2 CPT-II within the fatty acid β-oxidation pathway.
The protein content of ACOX1 portrayed a slightly different story than the mRNA levels. Figure 5. Changes in mRNA levels in hepatic B acox-1 and D cpt generated via qPCR.
The LFD and HFD livers expressed comparable levels of CPT-II protein content. Figure 6. C Changes in mRNA levels in hepatic pgc-1 α. E Changes in mRNA levels in hepatic ppar α. F Graphical changes in nuclear PPARα transcription factor activity. The fold change of mRNA, protein, and PPARα transcription factor activity were analyzed by one-way ANOVA with post hoc Tukey HSD.
AMPK activity can promote the activity of PPARs by upregulating peroxisome proliferator-activated receptor gamma coactivator 1-alpha PGC-1α Consistently, the protein content of PPARα within the LFD and HFD groups were similar Figure 6D. To further determine the influence of PPARα within the liver, PPARα transcription factor activity was investigated within nuclear protein extracts Figure 6F.
Throughout the study, the orange carrot diet displayed more potent efficacy in reducing the HFD-induced body weight gain than the white carrot diet. In a clinical study by Takagi et al. At the end of the study, the visceral fat level was significantly decreased in all the dietary groups. Such data were consistent with the results of our study, showing that the dietary carotenoids in the vegetables may add another layer of protective effect against lipogenesis.
However, the study by Takagi et al. employed lycopene and lutein. To our best knowledge, till date, no study has used the same methodology to explore the effects of alpha- and beta-carotene, in whole food.
α-Carotene and β-carotene are among the most frequently consumed dietary carotenoids in North American diets Liver is the major storage organ for dietary carotenoids, including α-carotene, β-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin, while β-carotene is one of the most abundant carotenoids, other being lycopene 44 , 45 , In this study, the average serum and hepatic β-carotene concentrations of the orange carrot supplemented mice were 1.
The average hepatic α-carotene concentration of the orange carrot supplemented mice was 3. These concentrations fall within the range of typical β-carotene concentrations in human serum 0. Oral β-carotene supplementation or the consumption of a high-β-carotene diet may lead to a higher circulating β-carotene concentration at 0.
We recently engineered S. boulardii that could synthesize high doses of β-carotene, so the intake of such engineered probiotics may lead to an even higher β-carotene concentration in the circulation 42 , indicating that the dosage of supplemented dietary carotenoids through carrots was of physiological relevance.
In the liver, all-trans retinal can be either reversibly reduced to all-trans retinol and then esterified to retinyl esters for storage, or irreversibly oxidized to all-trans retinoic acid ATRA , the biologically active form of vitamin A Therefore, it is unknown whether orange carrot supplementation results in higher levels of hepatic vitamin A and ATRA.
As the biologically active form of vitamin A, ATRA is a high-affinity ligand for retinoic acid receptors, while its isomer, 9- cis -retinoic acid 9cRA is an agonist of retinoid X receptors 62 — However, we were unable to detect 9cRA in livers and serum of the animals.
Since we did not observe a significant change in hepatic RXRα and RARβ expressions Supplementary Figure 3 , and the hepatic RXRβ concentration was undetectable data not shown , the beneficial effects of orange carrots may stem from α- and β-carotene as parent compounds, not ATRA.
Hepatic steatosis may result from excessive fatty acid or TG uptake, reduced TG output, increased de novo lipogenesis, decreased β-oxidation, or a combination. CD36 mediates long-chain fatty acid uptake in liver In this study, the expressions of cd36 mRNA were higher in mice fed on the HFD diet, despite white carrot or orange carrot supplementation, indicating that the increased hepatic uptake of FFA from the circulating system played a vital role in developing HFD-induced liver steatosis.
MTP is rate-limiting for the assembly of apoB-containing lipoprotein and hepatic TG secretion 66 , 67 , so the comparable MTP protein expressions across the dietary groups suggested that hepatic TG output minimally contributed to the development of NAFLD. The orange carrot supplementation was more efficient in alleviating HFD-induced NAFLD than the supplementation of white carrots.
Such result is in line with a previous publication that the consumption of spinach and tomato both containing high levels of carotenoids efficiently ameliorated NAFLD in rats fed with the HFD However, the positive control of that study was a lower percentage of carotenoid-rich vegetables, leading to a proportionally lower concentration of other beneficial compounds, such as fiber.
Therefore, it is unknown whether the anti-NAFLD efficacy of that diet was mainly attributed to carotenoids or fiber. The advantage of the current study is that we employed white carrot as a positive control, which contains an extremely low level of carotenoids, but an equivalent amount of fiber, compared to the orange carrot.
Thus, we are confident that the NAFLD-preventive efficacy of the orange carrot diet was primarily from α-carotene and β-carotene, not fiber. ACCα is the rate-limiting enzyme in regulating fatty acid synthesis 48 , In the current study, the ACCα protein levels did not differ among the groups.
Therefore, it is inconclusive to address whether orange carrot supplementation ameliorated NAFLD through modulating fatty acid synthesis.
ACOX1 catalyzes the first and rate-determining step in peroxisomal fatty acid oxidation 70 , and the mutation of ACOX1 was shown to induce NAFLD progression and exacerbate hepatocellular damage In the current study, ACOX1 was significantly improved in the mice with orange carrot supplementation, which was in line with the report that the consumption of foods high in β-carotene and other carotenoids increased hepatic ACOX1 in rats CPT-II is one of the key enzymes in the mitochondrial β-oxidation of fatty acids AMPK is a heterotrimeric complex, and its α subunit is the main catalytic domain Although the α subunit can be phosphorylated at Thr, Thr, and Ser sites, phosphorylation of Th is the hallmark of AMPK activation 51 — Previous studies have shown that the activation of AMPK could protect against diet-induced NAFLD and NASH 33 , AMPK phosphorylation can inhibit the cleavage and maturation of SREBP-1, subsequently attenuating hepatic steatosis through regulating lipogenic genes 74 , so it is possible that the dietary carotenoids in the orange carrot could reduce SRBEP-1 cleavage through promoting the phosphorylation of AMPK.
In addition to AMPK activation, we observed significantly higher hepatic levels of ppar α mRNA, PPARα protein, and PPARα transcription factor activity in the mice supplemented with orange carrots, indicating that the dietary carotenoids in orange carrots might prevent the development of NAFLD by targeting the PPARα pathway.
In NAFLD patients, the hepatic PPARα expression was negatively correlated with occurrence of NASH, severity of NAFLD, ballooning of the hepatocytes, and NASH activity score and fibrosis Ip et al.
found that in mice, PPARα knockout resulted in significantly more severe steatohepatitis, while the administration of Wy, a potent PPARα agonist, substantially prevented diet-induced NAFLD and liver injury PGC-1α acts as a coactivator of PPARα and promotes PPARα-mediated transcriptional activity in modulating its target genes, such as genes involved in β-oxidation In summary, our study reveals that the dietary carotenoids in the orange carrots rich in dietary carotenoids, specifically α-carotene and β-carotene, may regulate the fatty acid synthesis and β-oxidation-related genes by activating AMPK and PPARα.
However, how these compounds promote the phosphorylation of AMPK and activate the PGC-1α-PPARα pathway remains enigmatic. Another potential target of interest in future studies can be epoxide hydrolase sEH as previous studies have shown that inhibiting sEH may be involved in alleviating HFD-induced hepatic adiposity and inflammation 79 , One major limitation of the current study is the number of the examined proteins.
Since a large variety of proteins with various functions orchestrates the lipid metabolism process, we could not analyze all these participants. With regard to this, we are planning to utilize proteomics, a powerful tool that characterizes a large scale of proteins by their expressions, functions, structures, and protein-protein interactions 81 , in our future studies to acquire a broader perspective.
Another limitation is that we only examined liver, although the development of NAFLD could be a joint result of changes in several organs. Therefore, we cannot conclude that carotenoids alleviate NAFLD by directly targeting the liver.
Previous studies have reported that carotenoids may mitigate NAFLD via the gut- and adipose-liver crosstalk For example, one study showed that disrupted free fatty acid mobilization from mesenteric adipose MAT tissue to liver significantly exacerbated NAFLD in mice A potential brain-liver axis involving melanocortin-4 receptor, neuropeptides like neuropeptide Y and agouti-related peptide together regulates food intake, energy expenditure and the pathogenesis of NASH 83 , In addition, we failed to provide precise measurement of food consumption of each dietary group as the mice portrayed a behavior of tearing up their food and placing it within their bedding.
Last but not the least, the β-actin bands were not unanimous in this article. This was due to the different types of gels used. For the high molecular proteins such as MTP, FAS, ACCα, and PGC-1α, we used tris-acetate gels for optimal separation, which impaired the resolution of β-actin.
However, the β-actin expressions were identified within each blot, so our statistical analysis was not affected. Our results showed that orange carrot supplementation was more effective in preventing HFD-induced NAFLD than white carrots, potentially by increasing hepatic β-oxidation through upregulating PPARα Figure 7.
Such data indicate that carotenoid-rich fruits and vegetables may be more efficient in alleviating NAFLD than those with a low carotenoid level. Further clinical trials are warranted to confirm the findings before providing any dietary suggestions to NAFLD patients.
Figure 7. Graphical representation of proposed pathway in which carotenoid-rich orange carrots alleviate NAFLD severity. Orange carrots rich in α-carotene and β-carotene combated the severity of hepatic steatosis brought on by a high-fat diet by partially inhibiting fatty acid synthesis and significantly enhancing β-oxidation proteins, due to promotion of master regulators of hepatic lipid metabolism i.
AE: supervision, project administration, and funding acquisition. All authors conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—original draft preparation, writing—review and editing, visualization, read, and agreed to the published version of the manuscript.
This work was supported by the USDA National Institute of Food and Agriculture, [Hatch] project [accession number ] and National Science Foundation Grant We thank Plants for Human Health Institute at NC State University for providing us the space to conduct this study.
We also thank veterinarians Glicerio Ignacio and Daniel Peralta for supporting us in conducting animal studies. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Stefan N, Kantartzis K, Häring H-U.
Causes and metabolic consequences of fatty liver. Endocrine Reviews. doi: PubMed Abstract CrossRef Full Text Google Scholar. Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol.
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. Pais R, Pascale A, Fedchuck L, Charlotte F, Poynard T, Ratziu V.
Progression from isolated steatosis to steatohepatitis and fibrosis in nonalcoholic fatty liver disease. Clin Res Hepatol Gastroenterol. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study.
Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes.
Zelber-Sagi S, Godos J, Salomone F. Beta-carotene is an antioxidant. It protects the body from damaging molecules called free radicals. Free radicals damage cells through a process known as oxidation. Over time, this damage can lead to a number of chronic illnesses.
There is good evidence that eating more antioxidants from foods helps boost your immune system, protect against free radicals, and may lower your risk of heart disease and cancer.
But the issue is a little more complicated when it comes to taking antioxidant supplements. Prevention Studies that look at big groups of people suggest that those who eat 4 or more daily servings of fruits and vegetables rich in beta-carotene may reduce their risk of developing heart disease or cancer.
Other preliminary studies suggest that eating foods rich in beta-carotene reduces the risk of Sporadic ALS Lou Gehrig Disease. Foods rich in beta-carotene include those that are orange or yellow, such as peppers, squashes, and carrots.
However, a few studies have found that people who take beta-carotene supplements may have a higher risk for conditions such as cancer and heart disease. Researchers think that may be because the total of all the nutrients you eat in a healthy, balanced diet gives more protection than just beta-carotene supplements alone.
There is also some evidence that when smokers and people who are exposed to asbestos take beta-carotene supplements, their risk of lung cancer goes up. For now, smokers should not take beta-carotene supplements.
Studies suggest that high doses of beta-carotene may make people with a particular condition less sensitive to the sun.
People with erythropoietic protoporphyria, a rare genetic condition that causes painful sun sensitivity, as well as liver problems, are often treated with beta-carotene to reduce sun sensitivity.
Under a doctor's care, the dose of beta-carotene is slowly adjusted over a period of weeks, and the person can have more exposure to sunlight.
A major clinical trial, the Age Related Eye Disease Study AREDS1 , found that people who had macular degeneration could slow its progression by taking zinc 80 mg , vitamin C mg , vitamin E mg , beta-carotene 15 mg , and copper 2 mg.
Age related macular degeneration is an eye disease that happens when the macula, the part of the retina that is responsible for central vision, starts to break down. Use this regimen only under a doctor's supervision.
In one study of middle-aged and older men, those who ate more foods with carotenoids, mainly beta-carotene and lycopene, were less likely to have metabolic syndrome. Metabolic syndrome is a group of symptoms and risk factors that increase your chance of heart disease and diabetes.
The men also had lower measures of body fat and triglycerides, a kind of blood fat. People with oral leukoplakia have white lesions in their mouths or on their tongues. It is usually caused by years of smoking or drinking alcohol. One study found that people with leukoplakia who took beta-carotene had fewer symptoms than those who took placebo.
Because taking beta-carotene might put smokers at higher risk of lung cancer, however, you should not take beta-carotene for leukoplakia on your own. Ask your doctor if it would be safe for you. People with scleroderma, a connective tissue disorder characterized by hardened skin, have low levels of beta-carotene in their blood.
That has caused some researchers to think beta-carotene supplements may be helpful for people with scleroderma. So far, however, research has not confirmed that theory.
For now, it is best to get beta-carotene from foods in your diet and avoid supplements until more studies are done. The richest sources of beta-carotene are yellow, orange, and green leafy fruits and vegetables such as carrots, spinach, lettuce, tomatoes, sweet potatoes, broccoli, cantaloupe, and winter squash.
In general, the more intense the color of the fruit or vegetable, the more beta-carotene it has. Beta-carotene supplements are available in both capsule and gel forms. Beta-carotene is fat-soluble, so you should take it with meals containing at least 3 g of fat to ensure absorption.
So far, studies have not confirmed that beta-carotene supplements by themselves help prevent cancer. Eating foods rich in beta-carotene, along with other antioxidants, including vitamins C and E, seems to protect against some kinds of cancer. However, beta-carotene supplements may increase the risk of heart disease and cancer in people who smoke or drink heavily.
Those people should not take beta-carotene, except under a doctor's supervision. Beta-carotene reduces sun sensitivity for people with certain skin problems, but it does not protect against sunburn.
While animal studies show that beta-carotene is not toxic to a fetus or a newborn, there is not enough information to know what levels are safe. If you are pregnant or breastfeeding, take beta-carotene supplements only if your doctor tells you to.
It is safe to get beta-carotene through the food you eat. Statins: Taking beta-carotene with selenium and vitamins E and C may make simvastatin Zocor and niacin less effective. The same may be true of other statins, such as atorvastatin Lipitor. If you take statins to lower cholesterol, talk to your doctor before taking beta-carotene supplements.
Colestipol, a cholesterol-lowering medication similar to cholestyramin, may also reduce beta-carotene levels. Your doctor may monitor your levels of beta-carotene, but you do not usually need to take a supplement. You may want to take a multivitamin if you take orlistat.
Excess consumption of alcohol is heaoth in the Beta-carotene and liver health States and qnd and is known to livre serious organ damage. Specifically, Mental rehearsal exercises Beat-carotene is one of livver Beta-carotene and liver health prominent factors contributing Mental rehearsal exercises liver disease—a major cause of morbidity and mortality worldwide Blood pressure reduction tips. Alcoholic healt disease ALD includes a broad spectrum of disease stages beginning with steatosis fatty liver and followed by alcoholic steatohepatitis. These lay the groundwork for progression to the more damaging and irreversible stages of fibrosis and cirrhosis, which increases the risk of hepatocellular carcinoma development 2. β-Carotene is a provitamin A carotenoid found in many fruits and vegetables that is known to possess potent antioxidant functions 3. In a recent issue of Hepatobiliary Surgery and NutritionPeng et al. explored the effects of β-carotene supplementation on antioxidant capacity and hepatic apoptosis in a chronic ethanol-fed rat model 4.
Welche rührende Wörter:)
Es ist sichtbar, nicht das Schicksal.
Die sehr lustige Meinung
Sie lassen den Fehler zu. Schreiben Sie mir in PM, wir werden reden.
Es ist schade, dass ich mich jetzt nicht aussprechen kann - ich beeile mich auf die Arbeit. Ich werde befreit werden - unbedingt werde ich die Meinung in dieser Frage aussprechen.