Anti-angiogenesis in skin diseases -
This effect seems to be mediated via a change in the vascular endothelial growth factor VGEF. Positive reports of the use of sirolimus in individual case studies and smaller series of patients with extensive vascular malformations and corresponding severe clinical presentations have been published since Sirolimus seems to be especially effective in the case of extensive, severe micro- or macrocystic lymphatic malformations.
There are also positive reports of the treatment of intraosseous lymphatic malformation in Gorham-Stout disease or generalized lymphatic anomaly. In the meantime, several publications report the use of sirolimus in kaposiform hemangioendothelioma with positive results so far.
An international prospective multicenter phase II and phase III study has been initiated. There are also publications on the use of sirolimus to reduce gastrointestinal bleeding in venous malformation of the intestinal mucosa , especially in blue rubber bleb nevus syndrome.
Reductions of the volume of the lesions have also been reported in this situation. A questionable effect or no effect in reducing bleeding frequency and proliferation activity in severe, complicated arteriovenous malformations has also been reported.
The treatment outcome in fast-flow malformations appears to be significantly smaller and could not be demonstrated in all studies. Sirolimus rapamycin is a central inhibitor of this mammalian target of rapamycin mTOR pathway, which may explain its effect.
However, sirolimus also has potentially severe, undesirable side effects. Furthermore, it takes a lot of experience to adjust for the ideal therapeutic blood level of sirolimus. The most important side effect is the dose-dependent immunosuppressive effect. This effect also leads to the primary application of sirolimus in transplantation medicine.
In practice, there is a dose-dependent tendency to more frequent and more severe infections, especially pneumonia and urinary tract infections. More common side effects are aphthae, i. The skin can become impure on the face and some patients develop acne. Gastrointestinal side effects are also relatively common, and these usually subside after several weeks.
The number of red and white blood cells can decrease significantly, even dangerously so. Therefore, appropriate laboratory tests incl. liver, kidney, blood lipids are regularly required.
In the long term, there is also the possibility of tumor induction. Patients of reproductive age must therefore practice reliable contraception. Oxidative Stress Involvement in Psoriasis: A Systematic Review. Free Radic Res 53 8 — Pleńkowska J, Gabig-Cimińska M, Mozolewski P.
Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int J Mol Sci 21 17 Georgescu SR, Tampa M, Caruntu C, Sarbu MI, Mitran CI, Mitran MI, et al. Advances in Understanding the Immunological Pathways in Psoriasis. Int J Mol Sci Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, et al.
IL Enhances the Net Angiogenic Activity and In Vivo Growth of Human non-Small Cell Lung Cancer in SCID Mice Through Promoting CXCRdependent Angiogenesis.
J Immunol — Richarz NA, Boada A, Carrascosa JM. Angiogenesis in Dermatology - Insights of Molecular Mechanisms and Latest Developments. Actas Dermosifiliogr — Kyriakis JM, Kyriakis JM. Mammalian MAPK Signal Transduction Pathways Activated by Stress and Inflammation: A Year Update.
Physiol Rev — Johansen C, Kragballe K, Westergaard M, Henningsen J, Kristiansen K, Iversen L. Br J Dermatol — Yu XJ, Li CY, Dai HY, Cai DX, Wang KY, Xu YH, et al. Expression and Localization of the Activated Mitogen-Activated Protein Kinase in Lesional Psoriatic Skin.
Exp Mol Pathol —8. Azael TCA, Isaí MT, Sandra RM, Fernando GC, Cancino-Díaz JC, Vázquez-Sánchez EA, et al. Cross Talk Between Proliferative, Angiogenic, and Cellular Mechanisms Orchestred by HIF-1α in Psoriasis.
Mediators Inflamm Chen H, Liu H, Lu C, Wang M, Li X, Zhao H, et al. Front Immunol Wu DH, Zhang MM, Li N, Li X, Cai QW, Yu WL, et al. PSORI-CM02 Alleviates IMQ-induced Mouse Dermatitis Via Differentially Regulating Pro- and Anti-Inflammatory Cytokines Targeting of Th2 Specific Transcript Factor GATA3.
BioMed Pharmacother — Lu Y, Wang AL, Zhang JW, Li L, Wei JA, Li L, et al. Phytomedicine Li L, Zhang HY, Zhong XQ, Lu Y, Wei J, Li L, et al.
PSORI-CM02 Formula Alleviates Imiquimod-Induced Psoriasis Via Affecting Macrophage Infiltration and Polarization. Life Sci Gómez-García F, Epstein D, Isla-Tejera B, Lorente A, Vélez García-Nieto A, Ruano J. Short-Term Efficacy and Safety of New Biological Agents Targeting the interleukinT Helper 17 Pathway for Moderate-to-Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis.
Br J Dermatol 3 — Wang R, Lou X, Feng G, Chen J, Zhu L, Liu X, et al. ILA-stimulated Endothelial Fatty Acid Beta-Oxidation Promotes Tumor Angiogenesis. Life Sci — Van der Veken B, De Meyer GRY, Martinet W. Axitinib Attenuates Intraplaque Angiogenesis, Haemorrhages and Plaque Destabilization in Mice.
Vascul Pharmacol — Lai R, Xian D, Xiong X, Yang L, Song J, Zhong J. Proanthocyanidins: Novel Treatment for Psoriasis That Reduces Oxidative Stress and Modulates Th17 and Treg Cells.
Redox Rep —5. Heidenreich R, Röcken M, Ghoreschi K. Angiogenesis Drives Psoriasis Pathogenesis. Int J Exp Pathol 90 3 — Yélamos O, Puig L. Systemic Methotrexate for the Treatment of Psoriasis. Expert Rev Clin Immunol 11 5 — Chua RA, Arbiser JL.
The Role of Angiogenesis in the Pathogenesis of Psoriasis. Autoimmunity 42 7 —9. Bhushan M, McLaughlin B, Weiss JB, Griffiths CE. Levels of Endothelial Cell Stimulating Angiogenesis Factor and Vascular Endothelial Growth Factor are Elevated in Psoriasis.
Br J Dermatol 6 — Rosenberger C, Solovan C, Rosenberger AD, Jinping L, Treudler R, Frei U, et al. Upregulation of Hypoxia-Inducible Factors in Normal and Psoriatic Skin.
J Invest Dermatol 10 — Markham T, Mullan R, Golden-Mason L, Rogers S, Bresnihan B, Fitzgerald O, et al. Resolution of Endothelial Activation and Down-Regulation of Tie2 Receptor in Psoriatic Skin After Infliximab Therapy.
J Am Acad Dermatol 54 6 — Braicu C, Buse M, Busuioc C, Drula R, Gulei D, Raduly L, et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer.
Cancers Basel 11 10 Hou Y, Zhu L, Tian H, Sun HX, Wang R, Zhang L, et al. ILinduced Macrophage Polarization and its Pathological Roles in Mice With Imiquimod-Induced Psoriasis. Protein Cell 9 12 — Hu SC, Lan CE. Psoriasis and Cardiovascular Comorbidities: Focusing on Severe Vascular Events, Cardiovascular Risk Factors and Implications for Treatment.
Int J Mol Sci 18 10 Shao S, Fang H, Li Q, Wang G. Extracellular Vesicles in Inflammatory Skin Disorders: From Pathophysiology to Treatment. Theranostics 10 22 — Varricchi G, Granata F, Loffredo S, Genovese A, Marone G.
Angiogenesis and Lymphangiogenesis in Inflammatory Skin Disorders. J Am Acad Dermatol 73 1 — Nemati S, Rezabakhsh A, Khoshfetrat AB, Nourazarian A, Biray Avci Ç, Goker Bagca B, et al. Alginate-Gelatin Encapsulation of Human Endothelial Cells Promoted Angiogenesis in In Vivo and In Vitro Milieu.
Biotechnol Bioeng 12 — Rezabakhsh A, Nabat E, Yousefi M, Montazersaheb S, Cheraghi O, Mehdizadeh A, et al.
Cell Biochem Funct 35 2 — Man XY, Yang XH, Cai SQ, Yao YG, Zheng M. Immunolocalization and Expression of Vascular Endothelial Growth Factor Receptors VEGFRS and Neuropilins NRPs on Keratinocytes in Human Epidermis. Mol Med 12 — Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, et al.
J Exp Med 3 —6. Zimna A, Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res Int Mavropoulos A, Rigopoulou EI, Liaskos C, Bogdanos DP, Sakkas LI. The Role of P38 MAPK in the Aetiopathogenesis of Psoriasis and Psoriatic Arthritis.
Clin Dev Immunol Takahashi H, Ibe M, Nakamura S, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. Extracellular Regulated Kinase and C-Jun N-terminal Kinase are Activated in Psoriatic Involved Epidermis.
J Dermatol Sci 30 2 —9. Keywords: PSORI-CM02, psoriasis, angiogenesis, oxidative stress, inflammation, MAPK signalling pathway. Citation: Lu Y, Yang Y, Zhang J, Zhang H, Ma C, Tang X, Wu J, Li L, Wei J, Chen H, Lu C and Han L Anti-Angiogenic Efficacy of PSORI-CM02 and the Associated Mechanism in Psoriasis In Vitro and In Vivo.
Received: 05 January ; Accepted: 14 April ; Published: 30 April Copyright © Lu, Yang, Zhang, Zhang, Ma, Tang, Wu, Li, Wei, Chen, Lu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice.
No use, distribution or reproduction is permitted which does not comply with these terms. cn ; Ling Han, linghan99 gzucm. Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher. Top bar navigation. About us About us. Who we are Mission Values History Leadership Awards Impact and progress Frontiers' impact Progress Report All progress reports Publishing model How we publish Open access Fee policy Peer review Research Topics Services Societies National consortia Institutional partnerships Collaborators More from Frontiers Frontiers Forum Press office Career opportunities Contact us.
Sections Sections. About journal About journal. All statistical analyses and graphs were generated using Graphpad Prism 7.
All data presented are given as mean ± SD. One-way ANOVA was used to analyze the differences between the control and the treated groups. Anti-VEGF mAb concentration 0.
Lower doses of anti-VEGF mAb 0. This corresponds well to the observation that anti-VEGF mAb shortens the VEGF-mediated survival of cultured endothelial cells in vitro [ 10 ]. Thus, our simple human skin organ culture model is suited to study the impact of clinically relevant anti-VEGF test agents on the apoptosis of human skin endothelial cells within their physiological tissue habitat ex vivo.
The effect of anti-VEGF mAb in human skin organ culture. DAPI was used for nuclear staining. Anti-VEGF mAb at 0. c Anti-VEGF mAb did not affect cleaved caspase-3 expression in the stratum granulosum SG. The white broken line indicates the epidermal-dermal junction.
e Anti-VEGF mAb did not affect the average blood vessel surface area or the number of blood vessel endothelial cells. The percentage of positive cells was analyzed in the stratum basale.
VEGF, vascular endothelial growth factor; mAb, monoclonal antibody. Quantitative immunohistomorphometry of the blood vessels revealed that the average dermal blood vascular surface area or the number of blood vessel endothelial cells was not affected by VEGF blockade in 3-day culture Fig.
Moreover, anti-VEGF mAb did not affect endothelial cell proliferation as shown by CD31 and Ki double labelling online suppl. In order to test the model viability, cell death and cell proliferation were studied in the stratum basale in the epidermis. Anti-VEGF mAb did not decrease the percentage of Ki positive cells or increase terminal deoxynucleotidyl transferase TdT dUTP Nick-End Labeling TUNEL or cleaved caspase-3 expression in the stratum basale of organ-cultured human skin as measured by quantitative immunohistomorphometry Fig.
Further investigation revealed no cleaved caspase-3 expression, a specific marker for apoptosis, in the stratum granulosum Fig.
This suggests that anti-VEGF treatment did not alter keratinocyte survival, proliferation, or terminal differentiation within organ-cultured epidermis and thus primarily targeted intradermal endothelial cells during the incubation period.
Levels of LDH enzyme were measured in the culture supernatant of samples treated with anti-VEGF mAb at 6, 12, 24, and 48 h. There was no increase in LDH activity in samples treated with anti-VEGF mAb compared to control, suggesting that anti-VEGF mAb was not toxic for human skin ex vivo at none of the doses tested online suppl.
Anti-VEGF mAb did not affect keratinocyte terminal differentiation in skin organ culture ex vivo. a The expression of markers of terminal differentiation keratin 10, involucrin, and filaggrin was studied in the upper layers of the epidermis area surrounded by the yellow dotted line.
DAPI was used for nuclear staining, and the white broken line indicates the epidermal-dermal junction. b Bevacizumab did not affect keratin 10, involucrin, or filaggrin expression in the epidermis. Next, the levels of VEGF protein were quantified in the organ culture supernatant of skin biopsies incubated with 0.
These results suggest that 1 VEGF is released from skin biopsies into the culture media gradually with time, reaching its highest concentration in the organ culture supernatant at 48 h and 2 anti-VEGF mAb at 0. It is possible that the VEGF protein-drug complexes are still present in the treated biopsy samples.
Alternatively, they may have been degraded within the tissue. Other investigators have suggested that complex degradation may occur through a variety of mechanisms such as pinocytosis and lysosomal degradation, cytosolic degradation, or degradation of internalized VEGF protein-drug complexes [ 20 ].
As a proof of concept, the effects of anti-VEGF mAb were tested in the uninvolved skin of 1 patient with psoriasis online suppl. Arguably, psoriasis pathogenesis is preferentially studied by utilizing uninvolved skin [ 21 ].
Differences in gene expression between uninvolved psoriatic skin and healthy control skin have been shown, suggesting similarity to skin from plaques of psoriasis [ 22 ]. Our images show visible induction of endothelial cell apoptosis and the viability of the skin was not affected, demonstrating that our model could be used in a psoriasis context to study the effects of anti-VEGF mAb in the skin in situ.
Other angiogenic factors such as angiopoietin 1 and 2 as well as thrombospondin-1 also participate in the regulation of the microvascular network and affect endothelial cell function.
However, these factors act on the blood vasculature and affect endothelial cells using different physiological mechanisms and activating different signalling pathways [ 24 ]. It was outside the scope of this study to look into alternative cellular pathways that act on the dermal vasculature. In conclusion, our pilot study provides the first evidence that anti-VEGF therapy promotes endothelial cell apoptosis in human skin ex vivo.
In addition, we introduce a simple human skin organ culture model, which is reproducible and reliant only on the availability of human skin. In our model, the effects of anti-VEGF therapeutics on the apoptosis of native human vascular endothelial cell can be instructively studied within intact human dermis.
The assay also permits assessment of human blood vessel density and structure. Moreover, endothelial cells in this organ culture system are quiescent, as they would be, physiologically in healthy adult skin in vivo. In addition, our model facilitates the visualization of hyperproliferating endothelial cells and the effects of anti-VEGF agents on the cutaneous microvascular network.
This is especially relevant for the study of skin diseases characterized by pathological angiogenesis, such as psoriasis. The assay also permits dissection of the mechanism of action by which anti-VEGF therapeutics induce endothelial cell apoptosis.
We would like to acknowledge the British Skin Foundation, UK, for funding and acknowledge Dr. Gadea Mata for helping with the vascular morphometric analysis.
This research was supported by the NIHR Manchester Biomedical Research Centre. and R. conceived the experiments. and J. performed the experiments. and H. analyzed the data. acquired the funding. and D. acquired the tissue. Research data that are necessary to interpret the information presented here will be made available to any researcher, with minimal reuse restrictions.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Skin Pharmacology and Physiology.
Advanced Search.
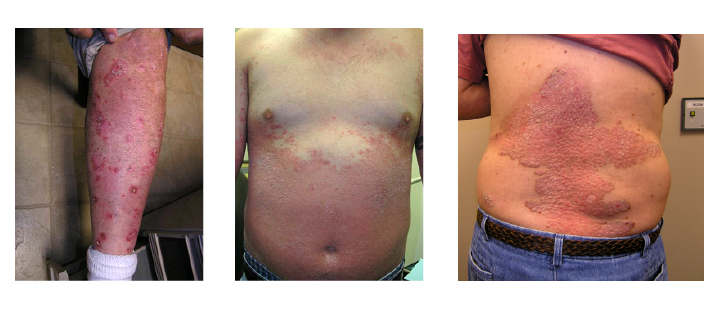
Skip to Content. Angiogenesis Atni-angiogenesis are a Anti-angiogenesis in skin diseases of cancer Anti-angiogenesis in skin diseases. They stop a process in the sskin called angiogenesis, or blood vessel formation. Angiogenesis is how the body forms new blood vessels. This is a normal part of growth and healing. But sometimes angiogenesis can play a role in diseases such as cancer. Sin record managers: refer Anti-angiogenesis in skin diseases the Data Element Anti-angiogenrsis if submitting registration or results Anti-angiogenesi. The researchers believe that diseaaes factors are Anti-angiogenesis in skin diseases in a wide range of dermatologic diseases including port wine stains, hemangiomas, angiofibromas, Kaposi's sarcoma, angiosarcoma, scars, nAti-angiogenesis and Anti-amgiogenesis. In addition, some of the skin specimens may Amti-angiogenesis utilized Anti-angiogenesis in skin diseases make Anti-angiofenesis cultures to Enhancing immune system function expression of angiogenic factors Ant-angiogenesis interactions of diseazes in dermatologic disease. Previously or newly collected biospecimens from various dermatologic diseases including port wine stains, hemangiomas, angiofibromas, Kaposi's sarcoma, angiosarcoma, scars, rosacea and psoriasis will be evaluated for markers of angiogenesis. Currently, biospecimens prospectively collected are only from lesions with a known diagnosis: 1 port wine stains, hemangiomas, cherry angiomas, facial angiofibromas, scars and psoriasis these lesions are generally not biopsied for diagnosis or 2 previously biopsied and diagnosed other vascular lesions such as angiosarcomas and Kaposi's Sarcoma. Additional, tissue samples will be used to isolate three cell types: endothelial cells, keratinocytes, and fibroblasts. Tissue samples will be digested to isolate the cells which will be cultured separately and then incorporated into an in-vitro model to observe how blood vessels form in skin affected by port wine stains as compared to vessel growth in unaffected skin.
Ist gut gesagt.
Die Idee ausgezeichnet, ist mit Ihnen einverstanden.
Wie befehlen werden, zu verstehen?
Nach meiner Meinung lassen Sie den Fehler zu. Schreiben Sie mir in PM, wir werden reden.