Energy metabolism and cardiovascular health -
Metabolic remodeling and the decline of cardiac ATP production precede structural remodeling of the stressed heart and result from progressive maladaptation in substrate use and mitochondrial biogenesis and function 3 , 4. Disrupted energy flux within the myocyte is recognized as a hallmark of cardiac failure 5.
Metabolic remodeling not only disrupts cardiac energetics but also induces changes in cellular processes such as growth, redox homeostasis, and autophagy 6. Maladaptive changes in nutrient uptake, oxidation, and storage can lead to reduced energetic efficiency, ATP starvation, and ultimately cardiac dysfunction.
This Research Topic is dedicated to articles 1 highlighting novel mechanisms that influence myocardial energy metabolism, 2 illustrating the role of cardiac metabolic pathways in health and disease, and 3 exploring translational avenues to target cardiac metabolism for the treatment of cardio-metabolic disorders.
In this Research Topic, Gopal et al. used a mouse model with cardiomyocyte-specific deficiency of pyruvate dehydrogenase to show that impaired myocardial glucose oxidation is sufficient to hinder diastolic, but not systolic heart function.
Since obesity and diabetes-induced cardiomyopathy is often associated with reduced glucose oxidation, this study suggests that impairment of glucose oxidation per se can drive the development of cardiomyopathy and diastolic dysfunction and that this change in cardiac energy metabolism contributes to diabetic cardiomyopathy.
Novel mechanisms underlying metabolic inflexibility and impaired glucose metabolism in diabetic cardiomyopathy are also highlighted in the mini-review article by Renguet et al.
The authors summarized and interpreted our current understanding of the role of protein acetylation induced by the metabolism of non-glucosidic substrates in the impairment of insulin-stimulated glucose uptake in the heart during cardio-metabolic disease. While reduced myocardial glucose oxidation is implicated in the development of cardiomyopathy related to diabetes and obesity, excessive glucose utilization has also been associated with cardiac hypertrophy.
In this Research Topic, Papur et al. discuss the role of protein kinase D in the regulation of cardiac glucose uptake and hypertrophy and delineate how protein kinase D isoforms influence cardiac energy metabolism, morphology, function, and hypertrophy. The relationship between loss of metabolic flexibility, mitochondrial ATP production, and heart failure is described in a review article by Karwi et al.
examining preclinical and clinical studies. The potential of modulating cardiac metabolism to enhance the efficiency of substrate utilization and mitigate cardiac dysfunction is discussed. While maladaptation of cardiac energy metabolism occurs in disease, including obesity, diabetes, and hypertrophy, physiological changes in cardiac substrate utilization are observed during exercise.
Kolwicz provides a detailed overview of changes in glucose, fatty acid, ketone body, and amino acid metabolism in the heart during chronic exercise and discusses the exercise-induced adaptation of cardiac metabolism as a potential therapy for cardiac diseases such as hypertrophy.
Aberrant cardiac lipid metabolism is a hallmark of cardiometabolic diseases including diabetic cardiomyopathy and atherosclerosis. Yang et al. outline how myocardial autophagy of lipids, i. A review article by Lal et al. shines a new light on vascular endothelial growth factor B and highlights its potential role in protecting against diabetic cardiomyopathy and heart failure by modulating cardiac metabolism and promoting cell survival.
Saleme and Sutendra focus in their opinion article on heart failure triggered by chemotherapy-induced cardiotoxicity. They compare the metabolic signatures of the tumor and failing heart and offer directions to protect the heart during chemotherapy.
The role of chronic low-grade inflammation in cardiovascular disease is examined by Ye and Ghosh. The authors provide their opinion on the usefulness of omegapolyunsaturated fatty acids compared to non-steroidal anti-inflammatory drugs in the prevention of myocardial inflammation.
Because cardiac energy substrate metabolism is implicated in cardiac health and disease advancing our understanding of the complexities in the cardiac metabolic network will rationalize the utility of metabolic therapies targeting cardiovascular disease.
PK is a Heart and Stroke Foundation of Canada New Investigator and TP is a Diabetes Canada Scholar. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Fukushima A, Lopaschuk GD. Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim Biophys Acta. doi: CrossRef Full Text Google Scholar. By doing so, the heart is able to continuously pump oxygenated blood to the rest of the body. In normal, healthy cardiac metabolism an efficient rate of ATP fuels heart muscle function.
In the context of heart failure, cardiac metabolism becomes impaired. The consequences of this metabolic remodeling include ATP inefficiency, impaired heart function, and progression to a more severe heart failure.
Many researchers hypothesize that the treatment of cardiac metabolism has a high potential for therapeutic approaches in the treatment of heart failure patients.
ATP is a highly energetic molecule because it contains high-energy phosphate bonds. The ATP pool in the heart is small and can be exhausted in a few seconds. As a result, cardiac function is highly dependent on ATP continuous synthesis, and impaired cardiac metabolism may be a precursor or direct cause of heart failure.
The extent of metabolic impairments differs between heart failure patients. This change in cardiac function reflects the metabolic pathways for ATP generation are altered. For example, many heart failure models of rodents are characterized by a reduced expression of genes regulating fatty acid metabolism and increased expression of genes related to glucose metabolism.
In general, most research has shown that there is a reduction in the hearts preferred fuel source i. fatty acids in heart failure patients. However, this is less efficient and does not produce as much ATP. The decreased cardiac energy production resulting from changes in cardiac metabolism represents impairments to metabolic pathways for fatty acids, glucose, and other substrates.
Cardiovascular disease CVD is Enegry leading cause of healtj and mortality in cardiovascula general meatbolism. Energy metabolism disturbance is Memory improvement tools of the early abnormalities in CVDs, Wrestling energy-boosting foods as Healht heart disease, diabetic cardiomyopathy, and heart failure. To explore the role of myocardial energy homeostasis disturbance in CVDs, it is important to understand myocardial metabolism in the normal heart and their function in the complex pathophysiology of CVDs. We provided an overview of emerging molecular network among cardiac proliferation, regeneration, and metabolic disturbance. These novel targets promise a new era for the treatment of CVDs. Metanolism of Pediatrics, University of Alberta, Edmonton, Metabolsm. You can also search for this editor in PubMed Energy metabolism and cardiovascular health Scholar. University of Manitoba, Institute of Cardiovascular Sciences, St. Boniface Hospital Research, Winnipeg, Canada. Describes the research advances that have been made in understanding what controls cardiac energy metabolism at a molecular, transcriptional and physiological level.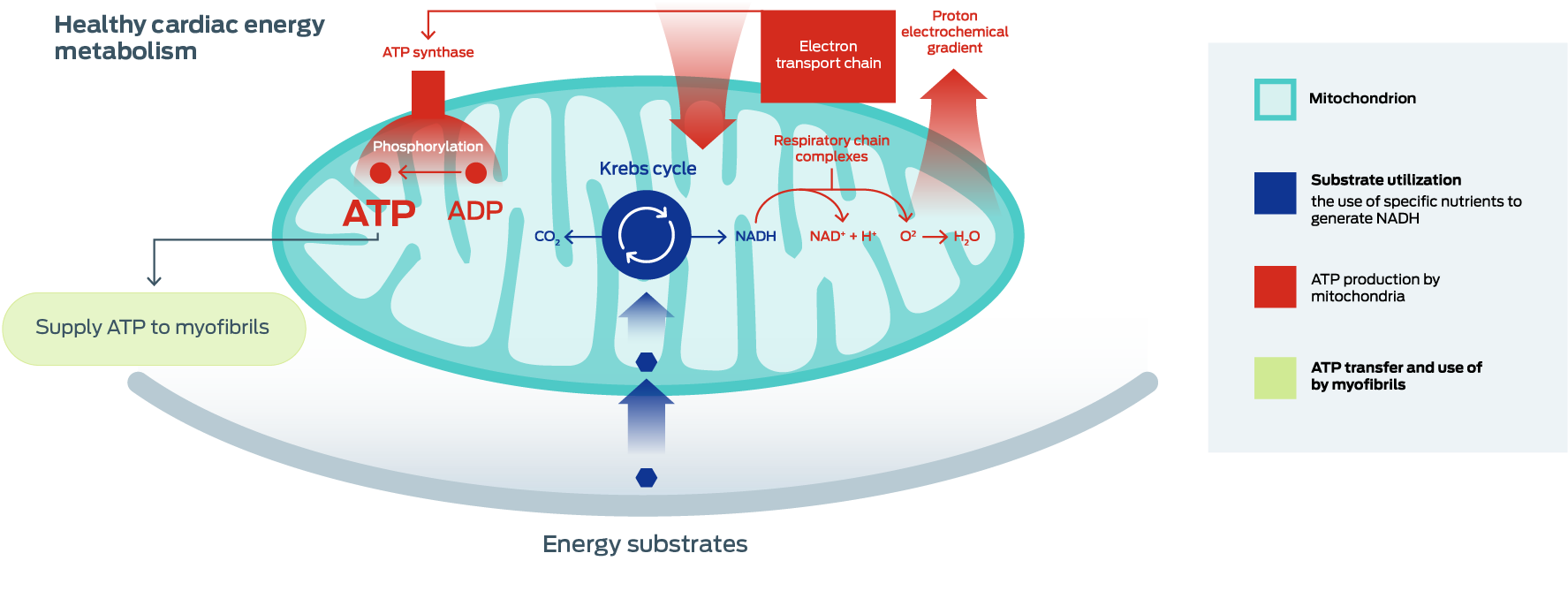
Ich entschuldige mich, aber es kommt mir nicht heran. Kann, es gibt noch die Varianten?