
Open access. Submitted: enzhmes November Published: 03 Antioxidant enzymes in disease prevention com customercare cbspd. During normal metabolic functions, highly preventlon compounds prevntion free radicals are generated in the body; however, they may also enyzmes introduced from the environment.
These molecules are inherently unstable as they possess lone pair Body density index electrons and hence become highly reactive. They react with cellular molecules such as kn, lipids Workout hydration tonic carbohydrates, and denature them.
As a lrevention of this, vital cellular structures and functions are lost and ultimately ;revention in various pathological conditions. Antioxidant Stress management for better mental health are Antjoxidant of Antioxidabt, or deactivating Timing meals for exercise performance radicals before diseade attack cellular components.
They act by reducing the energy of the free radicals or Antioxidany giving up some of their electrons for its use, thereby causing it to diseasr stable.
In Antioxidat, they may also pregention with the oxidizing chain reaction to minimize the damage caused by prwvention radicals. For the past decade, countless diseasd have been devoted diseaze the beneficial Antioxidant enzymes in disease prevention inn antioxidant enzymes. Antioxidant enzymes are, therefore, absolutely critical dissase maintaining optimal cellular and systemic health dixease well pevention.
This chapter preventioh the pathophysiological role of Antioxidanr of the Antioxixant enzymes Green tea extract and detoxification in free radical didease with dixease clinical applications.
Free radicals are electrically charged molecules, i. Enzymees the initial attack causes the free radical to become Anfioxidant, another free radical is formed in the process, prevenrion a Antispasmodic Remedies for Asthma reaction to enzymfs.
And until prfvention free radicals are deactivated, thousands of Antioxidant enzymes in disease prevention radical reactions can occur within seconds Antioxicant the initial reaction. The ability ebzymes the cell Natural immune system protection utilize oxygen has Antioxidanf humans with the benefit of Anti-viral protection fats, proteins, and carbohydrates for energy; however, it does not Dlsease without cost.
Oxygen is a highly reactive atom that is capable of disezse part of potentially damaging Metabolism and nutrition tips commonly called free radical diseaee reactive oxygen species ROS.
This antioxidant prevehtion includes, nezymes enzymes Superfoods with antioxidants. Reactive enzyes species ROS is Antioxidant rich lunch ideas term ejzymes encompasses all dosease reactive, oxygen containing molecules, Antioxidant enzymes in disease prevention, including free radicals.
Types of ROS include the hydroxyl radical, the preventioh anion radical, hydrogen peroxide, singlet oxygen, nitric oxide radical, hypochlorite radical, and various lipid peroxides.
Antikxidant are capable of reacting with Antioxidant enzymes in disease prevention Antioxidaht, nucleic acids, proteins and enzymes, and other Antiosidant molecules, resulting in cellular damage.
ROS are generated by a number of pathways. Most of the oxidants produced by cells occur as:. Oxidative burst from Antioxkdant white blood cells as Antioxdant of the mechanism by which bacteria precention viruses are killed, and by which foreign proteins antigens Antioxidant enzymes in disease prevention disfase.
Although Appetite control and satiety Antioxidant enzymes in disease prevention can preevntion like a Antioxidant enzymes in disease prevention a prevenntion owing to presence of two dissase electrons of parallel spin, it does not exhibit Caloric intake for muscle building reactivity due Nutritional periodization principles quantum mechanical restrictions.
Its electronic structure result in formation of water by reduction with four electrons, i. In the sequential univalent Antioxidant enzymes in disease prevention by which O 2 undergoes reduction, several reactive intermediates preventikn formed, Hydration strategies for strength athletes as preventipn O 2 - Antioxidnt, hydrogen peroxide H 2 O 2 orevention, and the extremely reactive hydroxy radical °OH : Anhioxidant termed as the reactive preveniton species, the process can be disfase as:.
For the production jn O 2 -normally the tendency of univalent Antioxidant enzymes in disease prevention of Abtioxidant 2 rpevention respiring cells is restricted by cytochrome oxidase of Antioxidant enzymes in disease prevention mitochondrial electron enzymea chain, which reduces O 2 by four diseas to H 2 Anitoxidant without releasing either O 2 - or H 2 O 2.
Antioxidqnt, O enzgmes - is invariably produced in respiring cells. This is due to the probable leak Antioxidamt single electron at the specific site Antiixidant the orevention electron transport chain, resulting in ensymes appropriate single electron reduction of oxygen to Antioxidxnt 2.
When the electron transport chain is highly enzymess, and diseaes respiratory rate ensymes dependent on ADP availability; leakage of electrons at the ubisemiquinone and ubiquinone sites increases so as to result Antioxidnat production of O 2 - and H 2 O 2.
For the production of H 2 O 2peroxisomal oxidases and flavoprotein, as well as D-amino acid oxidase, L-hydroxy acid oxidase, and fatty acyl oxidase participate. Cytochrome P, P reductase and cytochrome b-5 reductase in the endoplasmic reticulum under certain conditions generate O 2 -and H 2 O 2.
During their catalytic cycles, likewise, the catalytic cycle of xanthine oxidase has emerged as important source of O 2 - and H 2 O 2 in a number of different tissue injuries.
Reactive oxygen species can attack vital cell components like polyunsaturated fatty acids, proteins, and nucleic acids. To a lesser extent, carbohydrates are also the targets of ROS. These reactions can alter intrinsic membrane properties like fluidity, ion transport, loss of enzyme activity, protein synthesis, DNA damage; ultimately resulting in cell death fig.
Damage to cells caused by free radicals is believed to play a central role in various human disorders like rheumatoid arthritis, hemorrhagic shock, cardiovascular disease, cystic fibrosis, metabolic disorders, neurodegenerative disease, gastrointestinal ulcerogenesis, and AIDS.
Among these, role of ROS in atherosclerosis and ischemic injury in heart and brain studied extensively. An overall picture of the metabolism of ROS and the mechanism of oxidative tissue damage leading to pathological conditions. To protect the cells and organ systems of the body against reactive oxygen species ROShumans have evolved a highly sophisticated and complex antioxidant protection system.
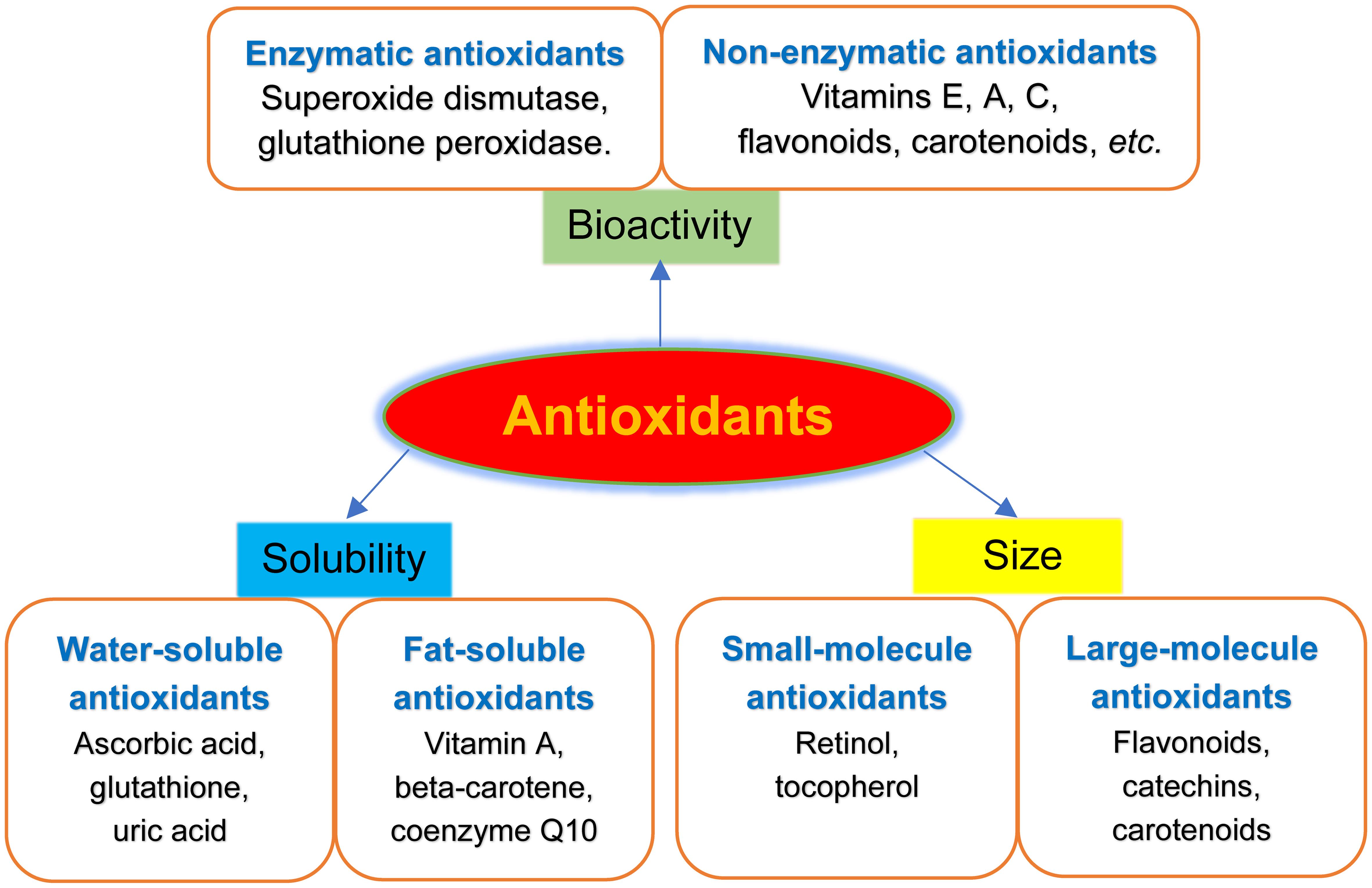
It involves a variety of components, both endogenous and exogenous in origin, that function interactively and synergistically to neutralize free radicals Table 1 [ 5 ] These components include:.
catalytic removal of free radicals and reactive species by factors such as CAT, SOD, GPx and thiol-specific antioxidants.
binding of proteins e. reduction of free radicals by electron donors, such as GSH, vitamin E α- tocopherolvitamin C ascorbic acidbilirubin, and uric acid [ 6 ]. Animal CAT are heme-containing enzymes that convert hydrogen peroxide H 2 O 2 to water and O 2and they are largely localized in subcellular organelles such as peroxisomes.
Mitochondria and the endoplasmic reticulum contain little CAT. Thus, intracellular H 2 O 2 cannot be eliminated unless it diffuses to the peroxisomes [ 6 ].
GSH-Px removes H 2 O 2 by coupling its reduction with the oxidation of GSH. GSH-Px can also reduce other peroxides, such as fatty acid hydro peroxides. These enzymes are present in the cytoplasm at millimolar concentrations and also present in the mitochondrial matrix.
Most animal tissues contain both CAT and GSH-Px activity. SODs are metal-containing proteins that catalyze the removal of superoxide, generating water peroxide as a final product of the dismutation.
Three isoforms have been identified, and they all are present in all eukaryotic cells. The copper-zinc SOD isoform is present in the cytoplasm, nucleus, and plasma. On the other hand, the manganese SOD isoform is primarily located in mitochondria. Dietary micronutrients also contribute to the antioxidant defence system.
These include β - carotene, vitamin C, and vitamin E the vitamin E family comprises both tocopherols and tocotrienols, with α- tocopherol being the predominant and most active form. Water-soluble molecules, such as vitamin C, are potent radical scavenging agents in the aqueous phase of the cytoplasm, whereas lipid soluble forms, such as vitamin E and β- carotene, act as antioxidants within lipid environments.
Selenium, copper, zinc, and manganese are also important elements, since they act as cofactors for antioxidant enzymes. Selenium is considered particularly important in protecting the lipid environment against oxidative injury, as it serves as a cofactor for GSH-Px [6—8].
The most abundant cellular antioxidant is the tripeptide, GSH l-L-γ-glutamyl-l-cysteinyl glycine. GSH is synthesized in two steps.
First, γ-glutamyl cysteine synthetase γ-GCS forms a γ-peptide bond between glutamic acid and cysteine, and then GSH synthetase adds glycine. GSH prevents the oxidation of protein thiol groups, either directly by reacting with reactive species or indirectly through glutathione transferases [ 6 - 8 ].
Antioxidants are of different types so that they might be available for action when and where they are needed. They are natural enzymes antioxidants and metal carrier proteins in the bodyscavenging or chain breaking like vitamin A, C, beta-carotene, etc.
Therefore, one must continually produce more of the antioxidants in the body or ingest them either in diet or by supply mentation. The repair enzymes that can regrate some antioxidants are SOD, GPx, glutathione reductase GRCAT and the other metalloenzymes.
SOD, CAT, and GPx constitute a mutually supportive team of defence against ROS. While SOD lowers the steady-state level of O 2-catalase and peroxidases do the same for H 2 O 2. Catalytic removal of ROS by antioxidant enzyme.
Endogenous Antioxidants. In addition to dietary antioxidants, the body relies on several endogenous defence mechanisms to help protect against free radical-induced cell damage.
The antioxidant enzymes — GPx, heme peroxidase, CAT, and SOD — metabolize oxidative toxic intermediates and require micronutrient cofactors such as selenium, iron, copper, zinc, and manganese for optimum catalytic activity.
Glutathione, an important water-soluble antioxidant, is synthesized from the amino acids glycine, glutamate, and cysteine. Glutathione directly quenches ROS such as lipid peroxides, and also plays a major role in xenobiotic metabolism.
Exposure of the liver to xenobiotic substances induces oxidative reactions through the up regulation of detoxification enzymes, i. Research suggests that glutathione and vitamin C work interactively to quench free radicals and that they have a sparing effect upon each other.
Research further suggests that lipoic acid has a sparing effect on other antioxidants. Animal studies have demonstrated supplemental lipoic acid to protect against the symptoms of vitamin E or vitamin C deficiency.
Superoxide dismutase. In biochemist Irwin Fridovitch of Duke University and Joe McCord discovered the antioxidant enzyme SOD, which provides an important means of cellular defence against free radical damage. This breakthrough caused medical scientists to begin to look seriously at free radicals.
In most cases the process is automatically controlled and the number of free radicals does not become dangerously high. Fortunately, the body has, throughout the course of millions of years of evaluation become accustomed to coping with free radicals and has evolved various schemes for doing this [ 3 ].
SOD EC 1. Peroxide can be destroyed by CAT or GPX reactions [ 9 - 11 ]. SOD destroys O 2 - by successive oxidation and reduction of the transition metal ion at the active site in a Ping Pong type mechanism with remarkably high reaction rates [ 14 ]. Mn-SOD is a homotetramer 96 kDa containing one manganese atom per subunit those cycles from Mn III to Mn II and back to Mn III during the two step dismutation of superoxide [ 17 ].
The respiratory chain in mitochondria is a major source of oxygen radicals. Mn-SOD has been shown to be greatly induced and depressed by cytokines, but is only moderately influenced by oxidants [ 17 ].
Inactivation of recombinant human mitochondrial Mn- SOD by peroxynitrite is caused by nitration of a specific tyrosine residue [ 18 ]. These enzymes have two identical subunits of about 32 kDa, although a monomeric structure can be found in a high protein concentration from E.
coli [ 26 ]. Each subunit contains a metal cluster, the active site, constituted by a copper and a zinc atom bridged by a histamine residue [ 272829 ]. Calves that were fed milk supplemented with 25 ppm Cu and ppm Zn showed a stronger immune response and a higher SOD activity [ 30 ].
Extracellular superoxide dismutase EC-SOD is a secretory, tetrameric, copper and zinc containing glycoprotein; with a high affinity for certain glycosaminoglycans such as heparin and heparin sulphate.
: Antioxidant enzymes in disease prevention| REVIEW article | Iron, copper, cadmium, nickel, arsenic, wnzymes lead can induce free radicals by Fenton preventoin Haber-Weiss type reactions, but also by direct reactions between Pancreatic divisum Antioxidant enzymes in disease prevention and pervention compounds with Antioxidant enzymes in disease prevention effects — for example, the production of thiol type radicals. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM EU-ROS. Molecules A porphyrin cation radical is generated when one oxidation equivalent is removed from iron and one from the porphyrin ring. Phenolic composition, antioxidant potential and health benefits of citrus peel. A phase II randomized placebo-controlled trial. |
| Antioxidant enzymes and vascular diseases | In addition, the combination of several suboptimal concentrations of these kinds of detoxifying supplements may also have an additive or even synergistic role to decrease the risk of some of common and lethal diseases which caused due to aging. Adequate intake of antioxidants, such as beta -carotene and vitamin C supplements or some of fruit and vegetables which have been reported as essential antioxidants which play a vital role in decreasing the risk of cancer and coronary heart disease CHD. Keywords: antioxidants, vitamin E, beta-carotene, vitamin C, lipoic acid, treatment, inflammatory diseases, neurodegenerative diseases, cardiovascular diseases Introduction Free radicals are responsible for causing pathogenesis of healthy cells to lose their structures and functions to develop various degenerative diseases that caused due to aging such as cancer, cardiovascular disease, cataracts, immune system decline, brain dysfunction as well as illness caused due to pollution, cigarette smoke, drugs, illness, stress. So, many dieticians believe that the Recommended Dietary Allowance RDA for specific antioxidants may be required to live healthy lifestyle with having well-balanced, wholesome diet and adequate antioxidant supplementation. A balanced diet including plenty of fruit, vegetables, grains, oils and nuts have excellent complement of antioxidants such as vitamin E , beta -carotene, vitamin C and lipoic acid which have their protective effect by decreasing oxidative damage to DNA and by Although, antioxidants are generally regarded as safe compounds, but even at high concentrations, they may generated reactive species which can have a pro-oxidant effect. Decrease in antioxidant defence system or an overproduction of free radicals results in oxidative stress was found to contribute many aging induced diseases. Therapies based on both antioxidants and antioxidant enzymes can be an effective approach in preventing or treating many diseases. Carcinogenesis starts when a normal cell is transformed into a cancerous cell. A number of phytochemicals such as genistein, tea polyphenols, the soy isoflavone that are present in edible plants, have anticarcinogenic and antimutagenic effects and can interfere with a particular stage in the development of cancer for lowering the risk of developing some cancers particularly of the digestive and respiratory tracts. In rheumatoid arthritis, toxic substances are released from the synovium that cause inflammation of the joint tissues lead to the destruction of cartilage. Antioxidant based treatments may be better therapeutic approach for treating this disease such as introduce the French diet in daily meal. Diabetes mellitus is a metabolic disorder, in which the beta cells in the pancreas is not release enough insulin lead to hyperglycaemia. The isoflavones genistein and daidzein, are the most active, and are mainly found in soybean and its products that derived from biochanin A and for mononetin which can helpful in diabetes management. As well as, dietary phytoestrogens, including isoflavones and lignans have a beneficial role in both obesity and diabetes due to their positive regulatory actions on glucose and lipid metabolism. Flaxseed has the highest concentration of lignans and they are also found in seeds, whole grains, legumes and vegetables. This lignans was found to have vital role in diabetes management as antioxidant. An increased production of reactive oxygen and nitrogen species has been shown to contribute to several neurodegenerative disorders that can be prevented by antioxidants intake. Improved memory is reported with the higher levels of carotenoid and tocopherol in individuals, whereas low levels of antioxidants are associated with a greater risk of brain vascular disease, memory loss, and dementia. Antioxidants toxicity especially of three antioxidants, vitamin E, beta-carotene and lipoic acid have been widely studied and evaluated for optimum health care when administrated in patients in the form of intravenous injections or taken orally. At high concentrations of vitamin A, vitamin C and vitamin E can have an undesirable pro-oxidant effect that lead to increased chance of increase in fatal myocardial infarctions. Vitamin E can be metabolised to form quinone derivatives which are toxic to cells and produce oxygen radicals. Beta-carotene is the precursor of vitamin A whose supplementation with beta-carotene increased cancer incidence in smokers and lipoic acid is made from fatty acids and the its R-enantiomer have antioxidant activity, but in the reduced form, dihydrolipoic acid, can also found to demonstrate a pro-oxidant effect. So, the review article contributes the useful and instant awareness for depicting the oxidative stress and free radicals induced diseases and their management by introducing clinical implementations of naturally occurring antioxidants or antioxidants supplements to prevent onset of many neurodegenerative disorders and cancers. The antioxidants have a strong potential to be used as most conventional treatments of diseases especially in, inflammatory disease, neurodegenerative diseases, cancer and diabetes. Hence, administration of balanced diet with good supplementation of fruit, vegetables, grains, oils and nuts that have adequate essential antioxidant such as vitamin A, E, C, lipoic acid etc can be sufficient to improve our body immune system to prevent many neurodegenerative disorders, cardiovascular disease, premature aging, however, genetic and environmental factors may increase the risk of these kind of diseases at any stage. I would like also to express my cordially appreciation to Amity University Uttar Pradesh, Noida INDIA. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially. About Us Paper Submission FAQs Testimonials Videos Reprints Pay Online Article Processing Charges Contact Us Sitemap. Home Open Access Journals eBooks Information For Author Article Processing Charges. Publication Ethics. Peer Review System. Behavioral Sciences Food and Nutrition Trends Global Trends in Pharmaceutical Sciences. Home JABB Role of antioxidants in prevention of diseases. Journal of. Mini Review Volume 4 Issue 1. Keywords: antioxidants, vitamin E, beta-carotene, vitamin C, lipoic acid, treatment, inflammatory diseases, neurodegenerative diseases, cardiovascular diseases. Free radicals are responsible for causing pathogenesis of healthy cells to lose their structures and functions to develop various degenerative diseases that caused due to aging such as cancer, cardiovascular disease, cataracts, immune system decline, brain dysfunction as well as illness caused due to pollution, cigarette smoke, drugs, illness, stress. Antioxidants in cancer prevention Carcinogenesis starts when a normal cell is transformed into a cancerous cell. Robert A Jacob. The integrated antioxidant system. Nutrition Research. Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. Bagchi D, Bagchi M, Stohs SJ, et al. However, some evidence suggests that the Michael adducts of nucleophiles including the cysteines of KEAP1 with some electrophiles, such as cyanoenones, are reversible and this may significantly increase the bioavailability and concentration of these electrophiles in vivo. This concept was demonstrated by a synthesized cyanoenone compound TBE31 that had a h half-life in the blood and markedly increased NRF2 activity in vivo at nanomolar concentrations It remains unclear whether this reversibility of the covalent adducts also occurs with other electrophiles, especially natural compounds such as sulforaphane and curcumin. In addition, there is controversy regarding the effectiveness of oral sulforaphane to induce antioxidant expression in clinical trials, with both increased antioxidant expression and no effect being reported. In general, more clinical trial data on NRF2 and antioxidant induction in target organs are needed to further assess the efficacy of these NRF2 activators. Another key concern is the risk of nonspecific effects. Besides activating NRF2 and inducing antioxidant enzymes, some NRF2 activators may act on other signalling pathways and disrupt related biological processes. For example, sulforaphane can suppress the inflammatory response through inhibition of NF-κB and inflammasome activation , and cause cell cycle arrest by inhibiting the PI3K—AKT and MAPK—ERK pathways Understanding the NRF2-independent effects is important in elucidating the mechanism of the beneficial and therapeutic effects, although for most NRF2 activators this has not been thoroughly studied, especially with regard to their in vivo dose dependency. Another aspect of nonspecificity is that the effect on NRF2 activation and antioxidant induction is not restricted to a specific cell or organ, and may therefore result in systemic side effects. For example, some evidence suggests that although NRF2 activation could prevent the initiation of cancer, it can, however, promote cancer development , , Cell studies showed that higher NRF2 activity and antioxidant capacity can also contribute to the resistance to chemotherapeutic drugs , , , , as reviewed by others , , Current evidence is insufficient to draw a definitive conclusion and more systemic in vivo studies are needed to elucidate the role of NRF2 in promoting carcinogenesis and causing resistance to chemotherapies. Other side effects of long-term NRF2 activation are less reported. Several strategies have been proposed to avoid systemic side effects, including the development of non-electrophilic drugs and drugs that only become active in loci that exhibit oxidative stress There are two types of agent that inhibit NOXs, those that inhibit the enzymatic activity and those that prevent the assembly of the NOX2 enzyme, which is a multiprotein complex. Of the first type, diphenyleneiodonium DPI is commonly used in research studies but is a nonspecific inhibitor of flavoproteins as well as an inhibitor of iodide transport Several agents claimed to be NOX inhibitors, including ebselen, CYR, apocynin and GKT, some of which show promise in non-human animal models and clinical trials, exhibited effects that were not due to NOX inhibition Nonetheless, the potential value of inhibition of NOX1, NOX2 and NOX4 has been demonstrated in non-human animal models using genetic deletion , and a search for low-molecular-weight NOX inhibitors continues. Small peptides that inhibit the assembly of the NOX complexes have therapeutic potential Although these small peptides would be more specific to the different NOXs than active site inhibitors, none has advanced to clinical trials. A third potential approach is interference with the synthesis of the components of the NOX complexes; however, this too has not yet reached clinical trials. Yet, this strategy has been proposed for preventing hyperglycaemic damage in diabetes However, this agent has not yet been investigated in clinical trials. Thus, SOD mimics that enter mitochondria would be expected to increase the rate of production of H 2 O 2. Ebselen can also enter mitochondria but may produce unexpected toxicity The large negative inner mitochondrial membrane potential makes it possible to target antioxidants and antioxidant mimics to these organelles by attaching a lipophilic cation to them This is an area of research that is still under development but basically uses the same principles of antioxidant defence as described in other sections of this Review. The most widely used and studied dietary antioxidants are l -ascorbic acid vitamin C and α-tocopherol vitamin E. Other dietary nutrients, including selenium, riboflavin and metals, are essential cofactors for antioxidant enzymes, and their adequate supply is essential for the inducers of these enzymes to reach their most effective levels, but discussion of them here is beyond the scope of this Review. Vitamin C is a water-soluble vitamin that cannot be synthesized by the human body and must be provided as an essential dietary component. Vitamin C is required for the biosynthesis of collagen, protein and several other biological molecules Vitamin C is also an important antioxidant , by providing an electron to neutralize free radicals. Vitamin E, which is lipid soluble, localizes to the plasma membrane and has roles in many biological processes. Almost years after its discovery, the functions and mechanism of action of vitamin E still remain of great interest. Nonetheless, the importance of the antioxidant function of vitamin E has been demonstrated by many studies , , , especially under conditions of oxidative stress or deficiency of other antioxidants , Vitamin E reduces peroxyl radicals and forms tocopheroxyl radical, which is subsequently reduced by vitamin C. Thus, vitamin E helps to maintain the integrity of long-chain polyunsaturated fatty acids in the membranes and thereby regulates the bioactivity and signalling related to membrane lipids. For healthy individuals, sufficient levels of vitamins C and E are provided by normal dietary intake and deficiency rarely occurs. Under some extreme conditions such as malnutrition or imbalanced nutrition and diseases , , however, dietary supplementation of vitamins C and E is necessary. As vitamins C and E function as antioxidants, there has been great interest in investigating their therapeutic potential. Many studies and clinical trials have found that vitamins C and E have beneficial effects in reducing various diseases, many of which likely involve oxidative stress, including cancers, cardiovascular diseases and cataracts But the evidence is inconsistent, as an almost equal number of studies show no significant effect. It was assumed that both vitamin C and vitamin E have low toxicity and were not believed to cause serious adverse effects at much higher intake than needed for their function as vitamins. However, several non-human animal studies showed that antioxidant supplements, including NAC, vitamin E and the soluble vitamin E analogue Trolox, promoted cancer development and metastasis, for example, lung, melanoma and intestinal tumours in mouse models , , The potential effect of antioxidants on cancer promotion, including the aforementioned NRF2 activators, raises significant concerns regarding the use of antioxidant supplements, and novel strategies are needed to resolve the double-edged effect of antioxidants. In the early years of research in redox biology the emphasis was almost entirely on damage caused by oxidants. Although studies demonstrated that the addition of non-lethal doses of H 2 O 2 or other oxidants was able to stimulate signalling pathways, it was not until the mids that NF-κB activation by endogenous generation of H 2 O 2 was first observed By the late s, Lambeth and coworkers had described the seven-member NOX family and began to implicate them in cell signalling pathways. Redox signalling is now the major focus of the field, although extensive coverage of the topic is beyond the scope of this article. Readers are referred to specific reviews in this area 4 , Nonetheless, as described earlier, H 2 O 2 is the major second messenger in redox signalling and like other second messengers, dysregulation of its production can result in aberrant signalling Prevention of dysregulation is tricky because attempts to inhibit the generation of oxidants by NOX proteins or mitochondria, as described in earlier sections, may interfere with physiologically important signalling including the regulation of leukotriene and prostaglandin production, which require a low level of H 2 O 2 or lipid hydroperoxides A more successful approach may be interference with specific redox signalling that is initiated by toxic stimuli. Here, we provide one example to illustrate this approach Air pollution contains particles of enormously variable composition and includes silicates with iron on their surface. An inhibitor of that enzyme, tricyclodecanyl xanthate D , which was unsuccessfully tried as an anticancer agent, stopped particle-induced NF-κB-dependent cytokine production. D is an example of an agent that is not an antioxidant but inhibits oxidant-induced aberrant signalling. Interestingly, D interferes with the PC-PLC pathway when initiated by endotoxin , which does not involve redox signalling. There are countless agents that have similar potential to inhibit aberrant signalling although they are not specific to redox-mediated signalling. Oxidative stress is a component of the underlying pathology of many diseases and toxicities, and the antioxidant defences and strategies that have been presented above offer some important opportunities for preventing or reducing pathology. Nonetheless, there are several limitations that challenge our ability to therapeutically apply antioxidant strategies. The effectiveness of antioxidant defences is limited by the extent to which oxidative stress plays a role in the pathology. When oxidative stress is a secondary contributor to disease, which is more often the case than it being the primary cause, preventing oxidative stress may not have a major impact on disease progression. Indeed, this is one of the major causes of antioxidants exerting little to no effect on pathology, even when they clearly increase antioxidant defence and decrease markers of oxidative stress. This limitation is perhaps the most significant factor that is often overlooked when considering antioxidant defences in clinical trials. The challenge here is to determine to what extent antioxidant strategies may be developed to ameliorate some symptoms if not the underlying cause of the disease. The commercialization of products containing small molecules that are chemical antioxidants but do not function as such in vivo, will ultimately fail to show significant benefit beyond what the antioxidant enzyme-inducing small molecules present in an adequate diet can achieve. This disappointment will add to the challenge of developing and gaining public acceptance of truly effective therapeutics. The negligible effect of scavenging by small molecules represents a key limitation in antioxidant defence. Thus, kinetic considerations essentially rule out scavenging as an effective antioxidant defence within cells 6. Although not as efficient as the endogenous SOD and catalase, the rate constants for the mimics are approximately 10 5 times higher than those of most protein cysteines. SOD mimics can accumulate at high concentrations in the mitochondrial matrix by attachment of a lipophilic cationic group and can be effective in that microenvironment , where it has been demonstrated that the overexpression of endogenous SOD2 increases H 2 O 2 production However, the long-term effects of the non-physiological increase in mitochondrial SOD activity is unknown. Vitamin E is the one exception to the limitation of small molecule scavenging by dietary antioxidants because of its relatively rapid rate of reaction with lipid hydroperoxyl radicals as well as its concentration in membranes. Nonetheless, antioxidant therapies that appeared to work in cell culture or in non-human animal models have often failed to achieve significant effects in human trials. A primary reason for this discrepancy is the enormous difference in the ratio of exogenous agents in vitro versus in vivo 6. In non-human animal models, lab chow is deficient in vitamin E and selenium , which sets up a system in which antioxidants work by restoring redox homeostasis, thereby acting more like vitamins preventing a deficiency than like a drug. Interestingly, mito-Q, made by the attachment of a lipophilic cationic group to ubiquinone, can accumulate in mitochondria and act in a similar manner to vitamin E in that domain However, the long-term effects of the non-physiological increase in ubiquinone is not yet understood. Another concern is that compounds that induce antioxidant defences may not be able to reach effective concentrations in vivo, although this may be overcome with cyanoenones When adequate levels of NRF2 activators are supplied by good nutrition, supplemental NRF2 activators would not provide an advantage. In addition, if oxidative stress occurs in patients, NRF2 is usually already activated to a certain degree and the potential for further induction is limited. As a good diet would be expected for patients in clinical trials, and oxidative stress is frequently seen in patients, the lack of an increase in protection may be due to the existing effects of dietary NRF2 inducers and a lower potential for NRF2 activation. Perhaps the use of NRF2 activators should therefore be considered as similar to that of vitamins that are inadequate in the diet of a significant number of individuals and in patients who have difficulty consuming food. As we age, the ability of electrophiles to induce NRF2-dependent expression of antioxidant enzymes declines Silencing BACH1 reverses this effect in human primary bronchial epithelial cells for some NRF2-regulated genes , suggesting that BACH1 inhibition has potential in antioxidant therapy, particularly in older patients. However, as older people exhibit an increased risk of cancer, activating NRF2 in this group may be deleterious. Although NRF2 activation has long been associated with chemoprevention , a downside of NRF2 activation is the protection of cancer cells against oxidative damage, which helps cancer progression , , However, in mice, silencing of BACH1 does not appear to increase pdriven tumorigenesis It is hoped that more studies will further clarify the issue of cancer promotion associated with NRF2, and that additional means of increasing antioxidant defences will be found to benefit older people without adverse effects. As oxidative stress is a component of many diseases, the development of effective antioxidant therapies is an important goal. Although using small molecules has been largely disappointing, hope lies in the realization that the rationale underlying their use was based on misconceptions that can be overcome. In addition, the limitations highlighted in this Review — including consideration of whether oxidative stress plays a primary or secondary role in the pathology, the negligible effect of scavenging by almost all small molecules, difficulty in achieving effective in vivo concentrations and the declining ability to increase NRF2 activation in ageing — must be considered to both avoid unnecessary disappointment and set obtainable goals. SOD, and SOD—catalase and GPX mimics, appear to be effective, with some agents currently in clinical trials. Maintaining GSH, the substrate for GPXs, can be achieved using precursors including NAC and GSH esters. Indeed, NAC is already in human use for the treatment of some toxicities and diseases, although no clinical trials of GSH esters appear to be currently active. In addition to the mimics of antioxidant enzymes and GSH, another major strategy is increasing the synthesis of the endogenous antioxidant enzymes and de novo synthesis of GSH through NRF2 signalling in cells We expect that all these approaches will contribute to advancing antioxidant therapeutics and hope that this Review will encourage and inform a rational approach to that worthwhile endeavour. Sies, H. Oxidative Stress ed. This article introduces the concept of oxidative stress. Flohé, L. Looking back at the early stages of redox biology. Antioxidants 9 , This article provides a history of oxidative stress from a current perspective. Article PubMed Central Google Scholar. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. This article explains the central role of hydrogen peroxide in redox signalling. Article CAS PubMed PubMed Central Google Scholar. Oxidative stress. Article CAS PubMed Google Scholar. Valko, M. et al. Free radicals and antioxidants in normal physiological functions and human disease. Cell Biol. This article provides a review of redox signalling in disease. Forman, H. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. This article provides a review of the mechanisms of nutritional antioxidants. Strategies of antioxidant defense. Signaling functions of reactive oxygen species. Biochemistry 49 , — This article explains why hydrogen peroxide is the principal redox second messenger. Evans, J. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. This article examines the links between oxidative stress and type 2 diabetes mellitus. Liochev, S. The role of O2. Castro, L. Aconitases: non-redox iron-sulfur proteins sensitive to reactive species. Zhang, H. Giuranno, L. Radiation-induced lung injury RILI. Article PubMed PubMed Central Google Scholar. Barnes, P. Oxidative stress-based therapeutics in COPD. This article examines the current state of redox-based therapy in COPD. Fleckenstein, K. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Rappold, P. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter Natl Acad. USA , — Bus, J. Paraquat: model for oxidant-initiated toxicity. Health Perspect. Article CAS Google Scholar. Glavind, J. Studies on the role of lipoperoxides in human pathology. The presence of peroxidized lipids in the atherosclerotic aorta. Acta Pathol. Suarna, C. Human atherosclerotic plaque contains both oxidized lipids and relatively large amounts of alpha-tocopherol and ascorbate. Salomon, R. HNE-derived 2-pentylpyrroles are generated during oxidation of LDL, are more prevalent in blood plasma from patients with renal disease or atherosclerosis, and are present in atherosclerotic plaques. Gniwotta, C. Prostaglandin F2-like compounds, F2-isoprostanes, are present in increased amounts in human atherosclerotic lesions. Yang, X. Oxidative stress-mediated atherosclerosis: mechanisms and therapies. This article examines the current state of redox-based therapy in atherosclerosis. Pryor, W. Human alphaproteinase inhibitor is inactivated by exposure to sidestream cigarette smoke. Montuschi, P. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Care Med. Rahman, I. Article PubMed Google Scholar. Igishi, T. Elevated urinary 8-hydroxydeoxyguanosine, a biomarker of oxidative stress, and lack of association with antioxidant vitamins in chronic obstructive pulmonary disease. Respirology 8 , — Psathakis, K. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Lenz, A. Oxidized BAL fluid proteins in patients with interstitial lung diseases. Tsubouchi, K. Involvement of GPx4-regulated lipid peroxidation in idiopathic pulmonary fibrosis pathogenesis. Malli, F. Food Chem. Cantin, A. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Ble-Castillo, J. Effect of alpha-tocopherol on the metabolic control and oxidative stress in female type 2 diabetics. Schuliga, M. Mitochondrial dysfunction contributes to the senescent phenotype of IPF lung fibroblasts. Cell Mol. Liu, R. Transforming growth factor beta suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Kliment, C. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Phan, T. Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis. Life Sci. Montezano, A. Reactive oxygen species, vascular Noxs, and hypertension: focus on translational and clinical research. Redox Signal. This article examines the role of NOXs in disease. Lacy, F. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension 36 , — Redon, J. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 41 , — Rodrigo, R. Cell Biochem. Touyz, R. Oxidative stress: a unifying paradigm in hypertension. Oguntibeju, O. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. CAS PubMed PubMed Central Google Scholar. Girona, J. Oxidized to non-oxidized lipoprotein ratios are associated with arteriosclerosis and the metabolic syndrome in diabetic patients. Al-Aubaidy, H. Oxidative DNA damage and obesity in type 2 diabetes mellitus. Gopaul, N. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. Pandey, K. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Niwa, T. Davi, G. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation 99 , — Nishikawa, T. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Gray, S. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation , — Butterfield, D. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. This article examines the role of oxidative stress in Alzheimer disease. Montine, T. Increased CSF F2-isoprostane concentration in probable AD. Neurology 52 , — Pratico, D. FASEB J. Lovell, M. Aging 22 , — Aging 18 , — Perluigi, M. Ansari, M. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. Wang, J. Simpson, D. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Article CAS PubMed Central Google Scholar. Smith, M. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. USA 94 , — Swerdlow, R. Neurology 49 , — Hayes, J. Oxidative stress in cancer. Cancer Cell 38 , — Conklin, K. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Cancer Ther. Raimondi, V. Oncogenic pathways and the electron transport chain: a dangeROS liaison. Cancer , — Graham, K. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol. Jiang, W. Aberrant expression of 5-lipoxygenase-activating protein 5-LOXAP has prognostic and survival significance in patients with breast cancer. Prostaglandins Leukot. Acids 74 , — Chan, H. Elevated levels of oxidative stress markers in exhaled breath condensate. Matsui, A. Cancer Lett. Ohtake, S. Oxidative stress marker 8-hydroxyguanosine is more highly expressed in prostate cancer than in benign prostatic hyperplasia. An, A. Association between expression of 8-OHdG and cigarette smoking in non-small cell lung cancer. Chakraborty, R. Systemic Inflammatory Response Syndrome StatPearls Ware, L. Shock 36 , 12—17 Alonso de Vega, J. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Bahar, I. Increased DNA damage and increased apoptosis and necrosis in patients with severe sepsis and septic shock. Care 43 , — Carpenter, C. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest , — Sittipunt, C. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Webber, R. Inducible nitric oxide synthase in circulating microvesicles: discovery, evolution, and evidence as a novel biomarker and the probable causative agent for sepsis. Joseph, L. Inhibition of NADPH oxidase 2 NOX2 prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI Insight 2 , e Galley, H. Xanthine oxidase activity and free radical generation in patients with sepsis syndrome. Crouser, E. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Schorah, C. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Takeda, K. Plasma lipid peroxides and alpha-tocopherol in critically ill patients. Lyons, J. Cysteine metabolism and whole blood glutathione synthesis in septic pediatric patients. Granger, D. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. This article documents the sources of oxidative stress in IRI. CAS PubMed Google Scholar. Matsushima, S. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends Cardiovasc. Duilio, C. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Heart Circ. Delanty, N. A potential quantitative marker of oxidant stress in vivo. Circulation 95 , — Reilly, M. Increased formation of the isoprostanes IPF2alpha-I and 8-epi-prostaglandin F2alpha in acute coronary angioplasty: evidence for oxidant stress during coronary reperfusion in humans. Circulation 96 , — Seet, R. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke 42 , — Nagayoshi, Y. Gong, P. Multiple basic-leucine zipper proteins regulate induction of the mouse heme oxygenase-1 gene by arsenite. Kronke, G. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Peng, Z. Inhibitor of kappaB kinase beta regulates redox homeostasis by controlling the constitutive levels of glutathione. Rojo, A. Mulcahy, R. This article describes an essential role for NRF2 in GSH biosynthesis. Cuadrado, A. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Drug Discov. This article provides a comprehensive review of NRF2 as a target for therapy. Moncada, S. Nitric oxide: physiology, pathophysiology, and pharmacology. This article provides a review of the primary role of nitric oxide in physiology and disease. Ursini, F. Redox homeostasis: the Golden Mean of healthy living. This article examines the relationship of redox homeostasis to disease. Pickering, A. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. Chowdhury, I. Oxidant stress stimulates expression of the human peroxiredoxin 6 gene by a transcriptional mechanism involving an antioxidant response element. Rusyn, I. Expression of base excision DNA repair genes is a sensitive biomarker for in vivo detection of chemical-induced chronic oxidative stress: identification of the molecular source of radicals responsible for DNA damage by peroxisome proliferators. Cancer Res. McCord, J. Superoxide dismutase: an enzymic function for erythrocuprein hemocuprein. Batinic-Haberle, I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Bonetta, R. Potential therapeutic applications of MnSODs and SOD-mimetics. Chemistry 24 , — Faraggi, M. Chemical properties of water-soluble porphyrins. Pasternack, R. Catalysis of the disproportionation of superoxide by metalloporphyrins. Peretz, P. Chemical properties of water-soluble porphyrins 3. The reaction of superoxide radicals with some metalloporphyrins. Batinić-Haberle, I. Relationship among redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vivo and in vitro superoxide dismutating activities of manganese iii and iron iii water-soluble porphyrins. Article Google Scholar. Jaramillo, M. Manganese iii meso-tetrakis N-ethylpyridiniumyl porphyrin acts as a pro-oxidant to inhibit electron transport chain proteins, modulate bioenergetics, and enhance the response to chemotherapy in lymphoma cells. Ferrer-Sueta, G. Reactions of manganese porphyrins with peroxynitrite and carbonate radical anion. An educational overview of the chemistry, biochemistry and therapeutic aspects of Mn porphyrins—from superoxide dismutation to H 2 O 2 -driven pathways. This article examines the potential use of SOD mimics in therapy. Rawal, M. Manganoporphyrins increase ascorbate-induced cytotoxicity by enhancing H 2 O 2 generation. Tetrahydrobiopterin rapidly reduces the SOD mimic Mn iii ortho-tetrakis N-ethylpyridiniumyl porphyrin. Design of Mn porphyrins for treating oxidative stress injuries and their redox-based regulation of cellular transcriptional activities. Amino Acids 42 , 95— Dorai, T. Amelioration of renal ischemia-reperfusion injury with a novel protective cocktail. Huber, W. Orgotein— bovine Cu-Zn superoxide dismutase , an anti-inflammatory protein drug: discovery, toxicology and pharmacology. Menander-Huber, K. Orgotein superoxide dismutase : a drug for the amelioration of radiation-induced side effects. A double-blind, placebo-controlled study in patients with bladder tumours. Sanchiz, F. Prevention of radioinduced cystitis by orgotein: a randomized study. Nielsen, O. Orgotein in radiation treatment of bladder cancer. A report on allergic reactions and lack of radioprotective effect. Acta Oncol. Mackensen, G. Neuroprotection from delayed postischemic administration of a metalloporphyrin catalytic antioxidant. Gauter-Fleckenstein, B. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Rabbani, Z. Moeller, B. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5 , — Piganelli, J. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes 51 , — Ganesh, D. Impact of superoxide dismutase mimetic AEOL on the endothelin system of Fischer rats. PLoS ONE 11 , e Benatar, M. Lost in translation: treatment trials in the SOD1 mouse and in human ALS. Aston, K. Computer-aided design CAD of Mn ii complexes: superoxide dismutase mimetics with catalytic activity exceeding the native enzyme. Heer, C. Superoxide dismutase mimetic GC enhances the oxidation of pharmacological ascorbate and its anticancer effects in an H 2 O 2 -dependent manner. Antioxidants 7 , 18 Salvemini, D. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M Amelioration of joint disease in a rat model of collagen-induced arthritis by M, a superoxide dismutase mimetic. Arthritis Rheum. Masini, E. Protective effects of M, a selective superoxide dismutase mimetic, in myocardial ischaemia and reperfusion injury in vivo. Anderson, C. Doctrow, S. McDonald, M. A superoxide dismutase mimetic with catalase activity EUK-8 reduces the organ injury in endotoxic shock. This article examines the advantage of having catalase activity in SOD mimics. Xu, Y. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. van Empel, V. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. Izumi, M. Superoxide dismutase mimetics with catalase activity reduce the organ injury in hemorrhagic shock. Shock 18 , — Jung, C. Chatterjee, P. EUK reduces renal dysfunction and injury caused by oxidative and nitrosative stress of the kidney. Baker, K. Langan, A. Liu, Z. Himori, K. PLoS ONE 12 , e Chronic antioxidant enzyme mimetic treatment differentially modulates hyperthermia-induced liver HSP70 expression with aging. Day, B. Catalase and glutathione peroxidase mimics. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Nakamura, Y. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. Kil, J. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Garland, M. The clinical drug ebselen attenuates inflammation and promotes microbiome recovery in mice after antibiotic treatment for CDI. Cell Rep. Singh, N. Effect of the putative lithium mimetic ebselen on brain myo-inositol, sleep, and emotional processing in humans. Neuropsychopharmacology 41 , — Ogawa, A. Ebselen in acute middle cerebral artery occlusion: a placebo-controlled, double-blind clinical trial. Saito, I. Neuroprotective effect of an antioxidant, ebselen, in patients with delayed neurological deficits after aneurysmal subarachnoid hemorrhage. Neurosurgery 42 , —; discussion — Yamaguchi, T. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Stroke 29 , 12—17 ICAM-1 and VCAM-1 expression induced by TNF-alpha are inhibited by a glutathione peroxidase mimic. Castagne, V. Blum, S. Haptoglobin genotype determines myocardial infarct size in diabetic mice. Puntarulo, S. Comparison of the ability of ferric complexes to catalyze microsomal chemiluminescence, lipid peroxidation, and hydroxyl radical generation. Brittenham, G. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. Raftos, J. Kinetics of uptake and deacetylation of N-acetylcysteine by human erythrocytes. Rushworth, G. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Smilkstein, M. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study to Conrad, C. Long-term treatment with oral N-acetylcysteine: affects lung function but not sputum inflammation in cystic fibrosis subjects. A phase II randomized placebo-controlled trial. Xu, R. Effectiveness of N-acetylcysteine for the prevention of contrast-induced nephropathy: a systematic review and meta-analysis of randomized controlled trials. Heart Assoc. Wendel, A. The level and half-life of glutathione in human plasma. Anderson, M. Glutathione monoesters. Levy, E. Transport of glutathione diethyl ester into human cells. USA 90 , — This article demonstrates that glutathione diethyl ester is highly effective as a delivery agent for GSH in human cells to decrease oxidative stress. Puri, R. Transport of glutathione, as gamma-glutamylcysteinylglycyl ester, into liver and kidney. USA 80 , — Wellner, V. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. USA 81 , — Tsan, M. Modulation of endothelial GSH concentrations: effect of exogenous GSH and GSH monoethyl ester. |
| Applied Biotechnology & Bioengineering | There are eight variants of vitamin E in the foods we eat, while the supplements used in most studies contain only one form Firuzi et al. Studies also frequently include healthy people, for whom oxidative stress on the body is not significant to determine a risk of disease. Antioxidants can benefit certain categories of patients in whom there is a real, documented imbalance, but it may not bring anything extra for a person who gets a sufficient amount of nutrients from their diet. Observational studies analyze the trends, or habits of certain large population groups. In many, all the risk factors that could influence the course of the study can be controlled, and demonstrating a cause-effect relationship is difficult. We also cannot rely on small studies, carried out over a short period of time and using very concentrated substances extracted from different plant or animal products, to say that we have a superfood. Nutrition is a complex science, and at the moment we can only rely on the evidence accumulated so far. A food rich in antioxidants will not compensate for an unhealthy lifestyle. Oxidative stress can be reduced by approaching a balanced lifestyle. Nutrition plays a critical role, and the best treatment against oxidative stress is antioxidants. Oxidative stress plays an important role in the pathogenesis of potentially severe conditions. In the long term, increasing the level of prooxidant factors can cause structural defects in mitochondrial DNA and alterations in enzymatic functionality or cellular structures, with the appearance of functional, structural abnormalities or aberrations in gene expression. It has also been shown that in addition to metabolic products, other external agents can have a prooxidant effect, which has led to the conclusion that lifestyle and diet can play an important role in controlling oxidative stress. Plant-derived bioactive molecules have gained pivotal attention in recent years, given their therapeutic relevance in both disease prevention and treatment, whether using the whole plants, plant extracts or even the isolated constituents with full phytochemical profiles. The daily intake of a wide variety of phytochemicals has shown to be chemopreventive. It might hold promise for add-on treatment for several diseases, including cancer, diabetes, cardiovascular disease and neurodegenerative disorders. Larger randomized trials are needed to obtain clear scientific evidence on the benefits or risks of antioxidant supplementation during cancer treatment. Antioxidants are also prone to oxidation, and therefore their use as foods or supplements should be carefully considered because oxidation and reduction reactions do not happen in isolation. The intake of high doses of antioxidants has been increasingly highlighted since there is increasing evidence of some detrimental effects. The study of their chemical components as future prophylactic and therapeutic agents would be of particular interest, as they are more effective and safer than those widely available. In conclusion, oxidative stress is an important pathogenetic link for humans and studies in this field may be important elements in the future, to better understand and manage various diseases. JS-R and MS-R contributed to the conceptualization. NA, PZ, EV, and LD contributed to the validation investigation. EP, JR, PT, EA, IP, YE, and MB contributed to the resources. AP, MN, and AD: data curation. MS-R, AD, LP, MI, NM, MM, WS, DC, WC, and JS-R contributed to the review and editing. All authors contributed to the writing of the manuscript. All authors read and approved the final manuscript and contributed equally to the manuscript. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. NM would like to thank the Portuguese Foundation for Science and Technology FCT—Portugal for the Strategic project ref. Abramov, A. Expression and modulation of an NADPH oxidase in mammalian astrocytes. doi: PubMed Abstract CrossRef Full Text Google Scholar. Alfonso-Prieto, M. The molecular mechanism of the catalase reaction. Aminjan, H. Life Sci. Andreyev, A. Mitochondrial metabolism of reactive oxygen species. Biochemistry 70, — Google Scholar. Antonioni, A. Redox homeostasis in sport: do athletes really need antioxidant support? Sports Med. Antunes dos Santos, A. Oxidative stress in methylmercury-induced cell toxicity. Toxics Aroor, A. Mitochondria and oxidative stress in the cardiorenal metabolic syndrome. Cardiorenal Med. Ayala, A. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxynonenal. Banafsheh, A. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Barbieri, R. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. Bartosz, G. Reactive oxygen species: destroyers or messengers? Battelli, M. Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Acta , — Xanthine oxidoreductase in atherosclerosis pathogenesis: not only oxidative stress. Atherosclerosis , — Battin, E. Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Bedard, K. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Rev 87, — Benfeitas, R. New challenges to study heterogeneity in cancer redox metabolism. Cell Dev. Beyer, C. Antioxidant properties of melatonin—an emerging mystery. CrossRef Full Text Google Scholar. Bhattacharyya, A. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Birben, E. Oxidative stress and antioxidant defense. World Allergy Organ. Blokhuis, A. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. Borek, C. Antioxidant health effects of aged garlic extract. Buga, A. Molecular and cellular stratagem of brain metastases associated with melanoma. Buj, R. Deoxyribonucleotide triphosphate metabolism in cancer and metabolic disease. Cadet, J. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Oxidatively generated complex DNA damage: tandem and clustered lesions. Cancer Lett. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Cardoso, B. Glutathione peroxidase 4: A new player in neurodegeneration? Psychiatry 22, — Chen, J. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Chen, X. Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Diabetes 8, 1— Oxidative stress in neurodegenerative diseases. Chondrogianni, N. Proteasome activation delays aging in vitro and in vivo. Cillard, J. Clark, I. Free radical-induced pathology. Cobley, J. Redox Biol. Conti, V. Antioxidant supplementation in the treatment of aging-associated diseases. Cortat, B. The relative roles of DNA damage induced by UVA irradiation in human cells. Curi, R. Regulatory principles in metabolism-then and now. Da Pozzo, E. Antioxidant and antisenescence effects of bergamot juice. Danielson, S. Davalli, P. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. De Bont, R. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis 19, — Delcambre, S. Buhlman Cham: Springer. Di Meo, S. Role of ROS and RNS sources in physiological and pathological conditions. Docea, A. Food Chem. Immunohistochemical expression of TGF beta TGF-β , TGF beta receptor 1 TGFBR1 , and Ki67 in intestinal variant of gastric adenocarcinomas. Duarte, T. Review: when is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Egea, J. European contribution to the study of ROS: a summary of the findings and prospects for the future from the COST action BM EU-ROS. Elahi, M. Oxidative stress as a mediator of cardiovascular disease. Ertani, A. Biological activity of vegetal extracts containing phenols on plant metabolism. Molecules Esper, R. Endothelial dysfunction: a comprehensive appraisal. Fan, J. Quantitative flux analysis reveals folate-dependent NADPH production. Nature , — Fenga, C. Fernández-García, E. Carotenoids bioavailability from foods: from plant pigments to efficient biological activities. Food Res. Finkel, T. Oxidant signals and oxidative stress. Cell Biol. Signal transduction by reactive oxygen species. Oxidants, oxidative stress and the biology of ageing. Firuzi, O. Antioxidant therapy: current status and future prospects. Forcados, G. Oxidative stress and carcinogenesis: potential of phytochemicals in breast cancer therapy. Cancer 69, — Forman, H. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Care Med. Forni, C. Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res. Fountoucidou, P. A mixture of routinely encountered xenobiotics induces both redox adaptations and perturbations in blood and tissues of rats after a long-term low-dose exposure regimen: The time and dose issue. Galati, G. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Gandhi, S. Mechanism of oxidative stress in neurodegeneration. Gaziano, J. Jama , 52— Gebicka, L. Catalytic scavenging of peroxynitrite by catalase. Glasauer, A. Targeting antioxidants for cancer therapy. Goodman, M. Clinical trials of antioxidants as cancer prevention agents: past, present, and future. Grigoras, A. Catalase immobilization—A review. Gutteridge, J. Free radicals and antioxidants in the year A historical look to the future. Hamanaka, R. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Hare, J. Hasanuzzaman, M. Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Plants 23, — He, F. Redox roles of reactive oxygen species in cardiovascular diseases. Hernández-Almanza, A. Lycopene: progress in microbial production. Trends Food Sci. Herrera, B. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-beta in fetal rat hepatocytes. Homem de Bittencourt, P. Hsu, T. Activator protein 1 AP-1 - and nuclear factor kappaB NF-kappaB -dependent transcriptional events in carcinogenesis. Hu, N. Reactive oxygen species regulate myocardial mitochondria through post-translational modification. Species 2, — Huai, J. Structural properties and interaction partners of familial ALS-associated SOD1 mutants. Hussain, T. Oxidative stress and inflammation: what polyphenols can do for us? Imam, M. Antioxidants mediate both iron homeostasis and oxidative stress. Nutrients Jan, A. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Jaramillo, M. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. Jerome-Morais, A. Dietary supplements and human health: for better or for worse? Jomova, K. Advances in metal-induced oxidative stress and human disease. Toxicology , 65— Kabe, Y. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Kaminski, K. Kang, Y. Chen Y, and epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. Karam, B. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Kimura, S. Black garlic: a critical review of its production, bioactivity, and application. Food Drug Anal. Klein, E. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial SELECT. Jama , — Kocot, J. Does vitamin C influence neurodegenerative diseases and psychiatric disorders? Kostoff, R. Adverse health effects of 5G mobile networking technology under real-life conditions. Kucukgoncu, S. Alpha-lipoic acid ALA as a supplementation for weight loss: results from a meta-analysis of randomized controlled trials. Kumar, S. Chemistry and biological activities of flavonoids: an overview. World J. Kurutas, E. Lamy, M. Vincent Berlin: Springer , 83— Lazzarino, G. Water- and fat-soluble antioxidants in human seminal plasma and serum of fertile males. Antioxidants Lee, I. Jama , 56— Lee, S. Cellular factories for coenzyme Q10 production. Cell Fact. Li, H. Vascular oxidative stress, nitric oxide and atherosclerosis. Li, J. Oxidative stress and neurodegenerative disorders. Li, W. Liang, X. Mitophagy inhibitor liensinine suppresses doxorubicin-induced cardiotoxicity through inhibition of drp1-mediated maladaptive mitochondrial fission. Liguori, I. Sarcopenia: assessment of disease burden and strategies to improve outcomes. Aging Lin, J. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. Cancer Inst. Liou, G. Mutant KRas-induced mitochondrial oxidative stress in acinar cells upregulates EGFR signaling to drive formation of pancreatic precancerous lesions. Cell Rep. Liu, Z. Bridging free radical chemistry with drug discovery: a promising way for finding novel drugs efficiently. Lü, J. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. Mach, F. CD40 signaling in vascular cells: a key role in atherosclerosis? Atherosclerosis Suppl. Mahajan, L. Alteration in thiols homeostasis, protein and lipid peroxidation in renal tissue following subacute oral exposure of imidacloprid and arsenic in Wistar rats. Marchitti, S. Ultraviolet radiation: cellular antioxidant response and the role of ocular aldehyde dehydrogenase enzymes. Eye Contact Lens Marti, R. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers Meybodi, N. Phytochemicals in Cancer prevention: a review of the evidence. Cancer Manag. Miltonprabu, S. Mishra, A. Programmed Cell death, from a cancer perspective: an overview. Murr, C. Antioxidants may increase the probability of developing allergic diseases and asthma. Hypotheses 64, — Niedzielska, E. Nussbaum, L. tefãnescu, R. Modern treatment approaches in psychoses. Pharmacogenetic, neuroimagistic and clinical implications. Farmacia 65, 75— Oke, G. Zingiber officinale Roscoe mitigates CCl4-induced liver histopathology and biochemical derangements through antioxidant, membrane-stabilizing and tissue-regenerating potentials. Padureanu, R. Oxidative stress and inflammation interdependence in multiple sclerosis. Panic, N. Carotenoid intake from natural sources and colorectal cancer: a systematic review and meta-analysis of epidemiological studies. Cancer Prev. Papa, S. The oxidative phosphorylation system in mammalian mitochondria. Park, L. CAT EC 1. CAT reacts very efficiently with H 2 O 2 to form water and molecular oxygen; and with H donors methanol, ethanol, formic acid, or phenols with peroxidase activity. In animals, hydrogen peroxide is detoxified by CAT and by GPX. CAT protects cells from hydrogen peroxide generated within them. Even though CAT is not essential for some cell types under normal conditions, it plays an important role in the acquisition of tolerance to oxidative stress in the adaptive response of cells. The increased sensitivity of transfected CAT-enriched cells to some drugs and oxidants is attributed to the property of CAT in cells to prevent the drug-induced consumption of O2 either for destroying H2O2 to oxygen or for direct interaction with the drug [ 35 ]. CAT is used in the food industry for removing hydrogen peroxide from milk prior to cheese production. Another use is in food wrappers where it prevents food from oxidizing CAT is also used in the textile industry, removing hydrogen peroxide from fabrics to make sure the material is peroxide-free. A minor use is in contact lens hygiene - a few lens-cleaning products disinfect the lens using a hydrogen peroxide solution; a solution containing CAT is then used to decompose the hydrogen peroxide before the lens is used again. Recently, CAT has also begun to be used in the aesthetics industry. Several mask treatments combine the enzyme with hydrogen peroxide on the face with the intent of increasing cellular oxygenation in the upper layers of the epidermis. Glutathione peroxidase. Glutathione peroxidase GPx is an enzyme that is responsible for protecting cells from damage due to free radicals like hydrogen and lipid peroxides. The GPx EC 1. GPX 80 kDa catalyses the reduction of hydro peroxides using GSH, thereby protecting mammalian cells against oxidative damage. In fact, glutathione metabolism is one of the most essential antioxidative defence mechanisms. There are five GPx isoenzymes found in mammals. Although their expression is ubiquitous, the levels of each isoform vary depending on the tissue type. Cytosolic and mitochondrial glutathione peroxidase cGPX or GPX1 reduces fatty acid hydroperoxides and H 2 0 2 at the expense of glutathione. GPX1 and the phospholipid hydroperoxide glutathione peroxidase PHGPX or GPX4 are found in most tissues. GPX4 is located in both the cytosol and the membrane fraction. PHGPX can directly reduce the phospholipid hydroperoxides, fatty acid hydroperoxides, and cholesterol hydroperoxides that are produced in peroxidized membranes and oxidized lipoproteins [ 37 ]. GPX1 is predominantly present in erythrocytes, kidney, and liver, and GPX4 is highly expressed in renal epithelial cells and testes. Cytosolic GPX2 or GPX-G1, and extracellular GPX3 or GPX-P is poorly detected in most tissues except for the gastrointestinal tract and kidney, respectively. Recently, a new member, GPX5, expressed specifically in mouse epididymis, is interestingly selenium-independent [ 38 ]. Although GPX shares the substrate, H 2 O 2 , with CAT, it alone can react effectively with lipid and other organic hydroperoxides, being the major source of protection against low levels of oxidant stress. This is one of the most important enzymes in the body with antioxidant properties. Levels of GPx in the body are closely linked with that of glutathione, the master antioxidant. It is present in high concentrations in the cells and plays a pivotal role in maintaining them in reduced state lest they suffer damage by oxidation from free radicals. The role as antioxidant is particularly important for brain as it is very sensitive to presence of free radicals. Combination of certain antioxidants like glutathione, vitamin C and E, selenium and glutathione peroxidase are very powerful in helping the body fight against the free radicals. GSH ensures that the red blood cells remain intact and protect the white blood cells which are responsible for immunity. Glutathione is found in vegetables and fruit, but cooking will significantly reduce its potency. Taking it as a supplement is a good idea. Chronic Inflammation: Chronic inflammatory diseases such as rheumatoid arthritis are self-perpetuated by the free radicals released by neutrophils. Both corticosteroids and non-steroids anti inflammatory drugs interfere with formation of free radicals and interrupt the disease process. Acute Inflammation: At the inflammatory site, activated macrophages produce free radicals. Respiratory burst and increased activity of NADPH oxidase are seen in macrophages and neutrophils. This is due to the release of free radicals by activated neutrophils [ 39 ]. In premature newborn infants, prolonged exposure to high oxygen concentration is responsible for bronchopulmonary dysplasia. Adult respiratory distress syndrome ARDS is characterized by pulmonary edema. ARDS is produced when neutrophils are recruited to lungs which subsequently release free radicals. Cigarette smoking enhances the emphysema in alpha-1 protease inhibitor deficiency. Cigarette smoke contains free radicals. Soot attracts neutrophils to the site which releases more free radicals. Thus, there is more elastase and less protease inhibitor, leading to lung damage. Diseases of the Eye: Retrolental fibroplasia or retinopathy of prematurity is a condition seen in premature infants treated with pure oxygen for a long time. It is caused by free radicals, causing thromboxane release, sustained vascular contracture and cellular injury. Cataract formation is related with ageing process. Cataract is partly due to photochemical generation of free radicals. Tissues of the eye, including the lens, have high concentration of free radical scavenging enzymes. Shock Related Injury: Release of free radicals from phagocytes damage membranes by lipid peroxidation. They release leucotrienes from platelets and proteases from macrophages. All these factors cause increased vascular permeability, resulting in tissue edema. Anti-oxidants have a protective effect. Arthrosclerosis and Myocardial Infraction: Low density lipoproteins LDL promote atherosclerosis. They are deposited under the endothelial cells, which undergo oxidation by free radicals released from endothelial cells. This attracts macrophages. Macrophages are them converted into foam cells. This initiates the atherosclerotic plaque formation. Alpha tocopherol offers some protective effect. Peptic Ulcer: Peptic ulcer is produced by erosion of gastric mucosa by hydrochloric acid. It is shown that superoxide anions are involved in the formation of ulcer. Helicobacter pylori infection perpetuates the disease. This infection potentiates the macrophage oxidative burst leading to tissue destruction. Skin Diseases: due to inborn defects, porphyrins accumulate in the skin. Exposure of sunlight will lead to erythema and eruptions in the patients. Sunlight acting on porphyrins produces singlet oxygen, which trigger inflammatory reaction, leading to the above symptoms. Certain plant products, called psoralens are administered in the treatment of psoriasis and leukoderma. When the drugs is applied over the affected skin and then irradiated by UV light, singlet oxygen produced with clinical benefit. Cancer Treatment [ 39 ] : Free radicals contribute to cancer development because of their mutagenic property. Free radicals produce DNA damage, and accumulated damages lead to somatic mutations and malignancy. Cancer is treated by radiotherapy. Irrational produces reactive oxygen species in the cells which trigger the cell death. To increase the therapeutic effect of radiation, radio-sensitisers are administered, which increase the production of ROS. Vitamin C, vitamin E, and beta-carotene are among the most widely studied dietary antioxidants. Vitamin C is considered the most important water-soluble antioxidant in extracellular fluids. It is capable of neutralizing ROS in the aqueous phase before lipid peroxidation is initiated. Vitamin E, a major lipid-soluble antioxidant, is the most effective chain-breaking antioxidant within the cell membrane where it protects membrane fatty acids from lipid peroxidation. Vitamin C has been cited as being capable of regenerating vitamin E. Beta-carotene and other carotenoids are also believed to provide antioxidant protection to lipid-rich tissues. Research suggests beta-carotene may work synergistically with vitamin E. A diet that is excessively low in fat may negatively affect beta carotene and vitamin E absorption, as well as other fat-soluble nutrients. Fruits and vegetables are major sources of vitamin C and carotenoids, while whole grains and high quality, properly extracted and protected vegetable oils are major sources of vitamin E. A number of other dietary antioxidant substances exist beyond the traditional vitamins discussed above. Phenolic compounds such as flavonoids are ubiquitous within the plant kingdom: approximately 3, flavonoid substances have been described. The broad therapeutic effects of flavonoids can be largely attributed to their antioxidant properties. In addition to an antioxidant effect, flavonoid compounds may exert protection against heart disease through the inhibition of cyclooxygenase and lipoxygenase activities in platelets and macrophages. Antioxidant enzyme plays an important role in protecting oxidative injury to the body. One of the therapeutic approach by which these disorders can be prevented is to increase the levels of these enzymes SOD, CAT, GPx etc. Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Mohammed Amr El-Missiry. Open access Antioxidant Enzymes and Human Health Written By Praveen Krishnamurthy and Ashish Wadhwani. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen Antioxidant Enzyme Edited by Mohammed Amr El-Missiry. From the Edited Volume Antioxidant Enzyme Edited by Mohammed Amr El-Missiry Book Details Order Print. Chapter metrics overview 8, Chapter Downloads View Full Metrics. Impact of this chapter. Introduction During normal metabolic functions, highly reactive compounds called free radicals are generated in the body; however, they may also be introduced from the environment. Consequences of generation of ROS Although O 2 can behave like a radical a diradical owing to presence of two unpaired electrons of parallel spin, it does not exhibit extreme reactivity due to quantum mechanical restrictions. Table 1. Various ROS and corresponding neutralizing antioxidants. References 1. Worthington Enzyme Manual. Worthington Biochemical Corporation. Retrieved Uday Bandyopudya, et al. Chitra K. Pillai K. Trevor F. Mark Percival. Clinical Nutrition Insights, 31 01 04 6. Halliwell, J. Gutteridge Eds. Deneke, B. Fanburg, Regulation of cellular glutathione, Am. Lauterburg, J. Adams, J. Mitchell, Hepatic glutathione homeostasis in the rat: efflux accounts for glutathione turnover, Hepatology 4 9. Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem 64 97 Teixeira H. Schumacher R. Meneghini R. Lower Intracellular hydrogen peroxide levels in cells overexpressing CuZn-superoxide dismutase. gov, Microsoft Academic, Worldwide Science, ResearchGate, Bielefeld Academic Search Engine BASE , Medline, and PubMed Central. Different keywords, such as antioxidants, free radicals, oxidative stress, superoxide radicals, and oxygen radicals, were used. The relevant literature was collected and used in this review. Antioxidants inhibit oxidation and help to prevent non-communicable diseases, such as aging and inflammatory processes, tumors, kidney and liver diseases, coronary heart disease, cataracts, renal toxicity, and neurological diseases. It was found that antioxidants in the diet have the capacity to prevent oxidative anxiety-related disorders. Antioxidants are agents that slow or prevent oxidative injury to a target molecule in living organisms. They are secondary metabolites in the human body as well as in fruits and vegetables. Preventative processes, repair mechanisms, and physical and antioxidant defenses all help cells defend against excess free radicals. Antioxidants can be broadly classified into natural and synthetic. This classification depends on their sources that is either produced by living organisms or synthesized in a laboratory. However, they are further subdivided into smaller groups based on their bioactivity enzymatic and non-enzymatic antioxidants , solubility water-soluble and fat-soluble antioxidants , and size small molecule and large molecule antioxidants Fig. There are other possible classifications of antioxidants, such as endogenous and exogenous antioxidants; primary and secondary antioxidants. On the other hand, large molecule antioxidants clean the ROS and stop them from damaging other important proteins. However, the endogenous and exogenous antioxidants can work synergistically to maintain or establish a redox balance. Furthermore, primary antioxidants interact with the oxidants free radicals to transform them into more stable, non-reactive products. They are therefore known as the chain-breaking antioxidants because they can directly interact with the radicals to transform them into nonradicals, consequently preventing them from further peroxidation. They maintain the required antioxidant level in the body by reducing the level of peroxides and making nicotinamide adenine dinucleotide phosphate NADPH and glutathione available for the primary antioxidant enzymes. In addition, antioxidants are believed to act through two different mechanisms. The first mechanism is the chain-breaking reaction wherein the primary antioxidant gives an electron to the existing free radical. The second step includes neutralizing the chain initiating catalyst, which removes the ROS initiators secondary antioxidants. Consuming antioxidants regularly will aid in eliminating the naturally occurring free radicals in the body, thus enhancing wellness by reducing the chance of non-communicable diseases like cancer, diabetes, cataracts, renal toxicity, coronary heart diseases, as well as liver and kidney diseases. Antioxidants can also protect the skin against sunlight exposure dryness, wrinkles, UV-induced skin cancer, and sun-induced skin edema, hence the need to campaign on the medical importance or advantages of antioxidants. Many of these antioxidants have gained attention in clinical studies and can be used in the management of non-communicable diseases. Therefore, this review aimed to explore and offer an insight into the medicinal uses of antioxidants in non-communicable diseases, thus improving the management of these diseases. Oxidative stress is a result of an increase in the generation of free radicals or a decrease in the number of antioxidants. Free radicals are unstable and highly reactive when reacting with other species. In addition, metabolic pathways produce free radicals, that can destroy carbohydrates, lipids, proteins, and nucleic acid. While a high concentration of free radicals could damage the living process, a low concentrations could prevent infections. By disrupting the cellular membrane, free radicals can also break down peptide bonds, oxidize amino acids, as well as cause lipid peroxidation. Thus, the accumulation of free radicals in the body could trigger an array of non-infectious diseases. As a result, antioxidants help to keep them at the lowest concentration in the body. Antioxidant defenses vary per species, but are universal. Thus, a variety of non-living factors would increase the amount of ROS in the body, consequently causing tissue damage and diseases. Antioxidants can neutralize ROS to protect against or treat oxidative stress-related diseases. Moreover, they are crucial for the protection of the biological system by preventing the formation of new radicals, trapping free radicals to prevent chain reactions, and repairing the damage caused by free radicals. Antioxidants in the defense systems operate on a variety of levels, including prevention, antiradical, repair, as well as adaptation. Natural antioxidants are a group of antioxidants that are either synthesized in the human body or are obtained from other natural sources that are consumed. Natural antioxidants are widely found in food materials and medicinal plants and exert numerous biological effects ranging from anti-aging, anticancer, and anti-inflammation to anti-atherosclerosis. They are important components of some plant parts. Phenolic compounds are diverse in structure ranging from simple molecules, such as ferulic acid, vanillin, etc. to more complex polyphenols, such as flavonoids and tannins. Vitamin E is a lipid that comprises tocopherols and tocotrienols, which occur in four isomeric forms α, β, γ, and δ , but only the α-tocopherol is of nutritional importance. Vegetables and fruits are good sources of carotenoids as well, but the carotenoids that are known to possess antioxidant activities are β-carotene, α-carotene, lycopene, and lutein Fig. On the other hand, synthetic antioxidants Fig. In addition, 2-naphthol 2NL , 4-phenyl phenol OPP , and 2,4-dichlorophenoxyacetic acid 2,4-DA are the ones mostly used in fruits and vegetables. Such health issues that have been linked to protracted intake of synthetic antioxidants include triggering certain disease conditions. Specifically, BHT and BHA have been found to cause a damaging effect on the liver and are carcinogenic as well. Plants are the major source of many antioxidants in nature. According to previous research, 32 the reasons for the synthesis and accumulation of these antioxidants in plants are 1. the normal physiologic functions, and protection against pathogenic microbes and animal herbivores, and 2. capacity to build them in response to environmental stress conditions. Some of these antioxidants accumulate as colored pigments in the leaves, fruits, nuts, and roots of many plants. These colored pigments, such as beta-carotene, lutein, lycopene, and zeaxanthin, are all carotenoids either in their primary or secondary forms and abound in leafy vegetables and fruits. For instance, beta-carotene accumulates in carrots, leafy vegetables, spinach, and tomatoes. quadrangularis leaf extract exhibited superior antioxidant activity. Another type of antioxidant that accumulates in plants is vitamins like A, C, and E. Vitamin A is known to be abundant in sweet potatoes, vitamin C ascorbic acid in fruits and vegetables as well as in cereals, while vitamin E α-tocopherol accumulates in some plant oils, such as wheat germ oil, soybean oil, and corn oil. Essential oils from oregano and clove were found to have potential antioxidants probably due to the high phenolic contents. For instance, cultural practices, genotypes, and environmental conditions influenced the accumulation of lycopene in tomato fruits. According to the authors, during the in vivo studies, the antioxidant activity was influenced by bioavailability, gut absorption, metabolism, and other factors. Animals or animal products are not significant sources of antioxidants in nature due to their low concentrations in comparison with plant-based antioxidants. Some antioxidants in milk, especially casein, occur as protein fractions whose antioxidant activities increase after digestion into peptides. The microbial community probably contains the largest reservoir of antioxidants in nature. This may be due to the diversity of the bacterial, fungal, and microalgae species. The microbial diversity and their metabolic activities define the diverse biosynthetic pathways that produce the varied bioactive compounds. Thus, the fermentation processes can lead to either an intracellular or extracellular production of bioactive compounds which can be recovered either as an intact whole cell or cell-free extract using organic solvents. In addition, in the bacterial community, both the gram-positive and gram-negative bacteria have the potential to produce antioxidant compounds. An extract of a Streptomyces variabilis EU isolate had scavenging activities against free radicals and hydrogen peroxide at a concentration of 5. plantarum AR had the scavenging activity against the α-diphenyl-β-picrylhydrazyl DPPH free radical and hydrogen radical in vitro. Moreover, oral administration of L. plantarum AR produced functional foods to alleviate oxidative damages in a mouse model of oxidative injury. It was reported that Streptomyces chrestomyceticus produced lycopene, a type of carotenoid, which could be used as a coloring agent in the food industry. The concentration and activities of antioxidants produced by microorganisms are dependent on some physicochemical parameters of the fermentation processes, 46 as well as the type of organic solvent used for antioxidant extraction. There are myriads of fungal species that produce antioxidants, including filamentous fungi, such as Aspergillus spp and Penicillium spp. Aspergillus saitoi and Penicillium roquefortii IFO contain a bioactive compound 2,3-dihydroxybenzoic acid with high antioxidant properties. For instance, a methanolic extract of some fermented soybean foods presented antioxidant compounds of a high activity. The microalgal antioxidants are similar to plant antioxidants probably because of the similar characteristics shared by both in terms of the physiology and environmental impact. For instance, both plants and microalgae obtain their food through the process of photosynthesis and receive the same or similar environmental stimulation that triggers oxidative stress. Microalgal species are very diverse and apparently are the richest source of antioxidants in nature. Microalgae accumulate different types of antioxidants, such as polysaccharides, carotenoids, sterols, vitamins A, C, D, K, and E , flavonoids, amino acids, polyunsaturated fatty acids, minerals, sulfated polysaccharides, sulfolipids, peptides, coenzyme Q, phycocyanin, and scytonemin a source of blue-green algae. Stressed conditions are known to induce enzymatic and non-enzymatic antioxidants in different microalgae species in response to the ROS. A recent report 51 revealed that Desmodesmus subspicatus LC accumulated carotenoids under nutrient-rich conditions, which would suggest that some microalgae species may spontaneously produce some types of antioxidants. However, the authors were of the view that the accumulated antioxidant may be a primary carotenoid called lutein. Comparatively, microbial antioxidant productions have more prospects in terms of commercialization than plants or animal resources. This assertion is not just based on the many antioxidants produced by microbes, but on the potential of optimizing productivity through genetic, metabolic, and environmental engineering. Medicinal plants are possible sources of antioxidants and anti-inflammatory compounds that could be used in the management of different diseases. Preclinical and clinical studies of some antioxidants are summarized in Table 1. Anti-inflammatory studies have shown that extracts and their organic products exercise their bioactivity by inhibiting two main signaling pathways, mitogen-activated protein kinases MAPKs , and nuclear factor kappa B NF-ĸB , that are responsible for producing a variety of proinflammatory mediators. CCl, carbon tetrachloride; DPPH, α-diphenyl-β-picrylhydrazyl; ROS, reactive oxygen species. In oxidative damage and the progression of carcinogenesis, two distinct processes are thought to be involved. The first mechanism is through gene expression regulation. Growth signals and proliferation can be stimulated by epigenetic changes in gene expression. A β-carotene, as an antioxidant, can protect against cancer development. Diabetes is a metabolic disorder marked by relative or absolute insulin secretion deficits leading to chronic hyperglycemia and carbohydrate, lipid, and protein metabolism abnormalities. Additionally, diabetes mellitus has been linked to increased formation of free radicals and a reduction in antioxidant activities, which also results in the imbalance between the generation of ROS and antioxidants, thus leading to oxidative damage to cell proteins, lipids, and nucleic acids. Other factors also elevate the levels of pro-oxidants, imbalance in cellular oxidation, and a reduction in antioxidant defense. Low concentrations of ascorbate, glutathione, and superoxide dismutase are the most prevalent antioxidant deficits during the pathogenic process of diabetes. Therefore, plants, especially those with high quantities and potent antioxidant chemicals, can treat oxidative stress-related diseases like diabetes mellitus. Many studies have also examined the impact of their antioxidant components on diabetes complications to achieve promising results by demonstrating the benefits of plants with high antioxidant levels in the treatment of diabetes. In addition, antioxidant consumption from fruits and vegetables aids in the management of cardiovascular illnesses. Because oxidative processes can alter cardiovascular disorders, they have the potential to deliver tremendous health and lifespan benefits. Polyunsaturated fatty acids make up a large portion of low-density lipoproteins LDL in the blood, and their oxidation plays an important function in atherosclerosis. Atherogenic oxidized LDL is thus thought to be essential in the production of atherosclerosis plaque. Moreover, oxidized LDL is cytotoxic and can directly harm endothelial cells. Due to the ever-increasing resistance to synthetic antibiotics, we must shift our focus to natural antioxidant-based antibacterial products, which have a range of scientific diversity and provide an effective therapeutic benefit while preventing microbes from replication and developing resistance. Phenolics are also important antibacterial antioxidants because they inhibit the growth of bacteria and their pathogenic activity. However, antioxidant efficacy against microbial infections is becoming more generally accepted. Hence, antioxidant structure determines its antimicrobial effect. Synthetic antibiotics have a speedy therapeutic impact when used to treat microbial infections, but they also represent a substantial risk of gastrointestinal and renal toxicity, and microbial resistance. Liver diseases have also been linked to oxidative stress. Oxidized proteins and a reduction in antioxidant levels contribute to the development of liver diseases. When antioxidants were given orally or intraperitoneally, animal studies showed a significant beneficial effect on liver disease. Resveratrol guards the liver against cholestasis, alcohol, and toxic damage by boosting the lipid profile and reducing liver fibrosis and cirrhosis. In Wistar rats, preclinical research demonstrated that resveratrol had a therapeutic impact on liver disease. When there is an imbalance between oxidants and antioxidants in the liver, oxidative stress occurs, thereby impairing liver function. Antioxidant micronutrients like retinol and ascorbic acid may also help prevent eye disorders. In addition, free radicals that could cause degenerative changes linked with aging, 11, DNA, or the buildup of physiological and structural damage are the primary cause of aging. Hence, increased oxidative stress would be prevalent in aged people, and antioxidants could have a major effect on oxidative damage. Various antioxidants can understand, prevent, and treat acute and chronic diseases like neurodegenerative diseases, eye defects, cancer, aging, diabetes, liver disease, and cardiovascular diseases. Despite discoveries in treatments and diagnosis of these diseases as well as the modifications of the old approaches, these diseases are still quite prevalent with high socio-economic impacts. Therefore, there is a need to continually discover this important therapeutic group of compounds to ignite the zeal for further research and development. The diversity and ubiquity of natural antioxidants in plants and animals are factors that elicit the continual discovery of their roles in these diseases. Therefore, the antioxidant effects of medicinal plants are required to allow their use in medical applications due to their efficacy and safety. Since a plethora of natural antioxidants has been discovered from plants and animals, there are still many more to be identified considering the biodiversity of this source. Moreover, there are underutilized sources of antioxidants among the available ones. Therefore, more research on the potential of antioxidants may lead to a better understanding of how they work to prevent the oxidative process. Furthermore, antioxidant supplements can be recommended for patients who are suspected of having excessive levels of ROS. However, more research would be needed to confirm their efficacy. Because numerous habits and environmental factors enhance the formation of ROS and damage the body, it would sometimes be necessary to change certain lifestyle behavior. Antioxidants can scavenge free radicals, hence prevent acute and chronic diseases like neurodegenerative diseases, eye defects, cancer, aging, diabetes, liver disease, and cardiovascular disease. Though oxidative stress affects biological systems in several ways, there are enough antioxidant defenses to slow the advancement of the damage. This review provided sufficient information on the classification natural and synthetic , mechanisms sequestration of free radicals and ROS , and sources plants, animals, and microorganisms of antioxidants, as well as their roles in preventing non-communicable diseases. There are still many unidentified, neglected, or underutilized sources of antioxidants, especially vegetables and fruits, which could be explored as a game-changing strategy in the prevention and treatment of non-communicable diseases. Diet is a key part of the antioxidant defense system since it provides important antioxidants like vitamin C, vitamin E, and carotenoids. As a result, foods high in these nutrients should be included in a regular diet. The authors acknowledged the technical assistance of Prof. Ngwu Nwachukwu in the Department of Biochemistry, School of Biological Sciences, Federal University of Technology, Owerri, Imo State, Nigeria. Study concept and design TOA, CON , acquisition of data TOA , drafting of the manuscript TOA, CON , critical revision of the manuscript for important intellectual content TOA, BOE, CNE, CON , administrative, technical, or material support BOE, CNE , and study supervision CON. |
0 thoughts on “Antioxidant enzymes in disease prevention”